Abstract
One of the manifestations of X-linked lymphoproliferative disease (XLP) is progressive agammaglobulinemia, caused by the absence of a functional signaling lymphocyte activation molecule (SLAM)–associated protein (SAP) in T, invariant natural killer T (NKT) cells and NK cells. Here we report that α-galactosylceramide (αGalCer) activated NKT cells positively regulate antibody responses to haptenated protein antigens at multiple checkpoints, including germinal center formation and affinity maturation. Whereas NKT cell–dependent B cell responses were absent in SAP−/−.B6 mice that completely lack NKT cells, the small number of SAP-deficient NKT cells in SAP−/−.BALB/c mice adjuvated antibody production, but not the germinal center reaction. To test the hypothesis that SAP-deficient NKT cells can facilitate humoral immunity, SAP was deleted after development in SAPfl/fl.tgCreERT2.B6 mice. We find that NKT cell intrinsic expression of SAP is dispensable for noncognate helper functions, but is critical for providing cognate help to antigen-specific B cells. These results demonstrate that SLAM-family receptor-regulated cell-cell interactions are not limited to T-B cell conjugates. We conclude that in the absence of SAP, several routes of NKT cell–mediated antibody production are still accessible. The latter suggests that residual NKT cells in XLP patients might contribute to variations in dysgammaglobulinemia.
Introduction
Invariant natural killer T (NKT) cells represent a unique subpopulation of T cells with a highly restricted T-cell receptor (TCR) repertoire, expressing Vα14/Jα18 or Vα24/Jα18 rearranged genes in mice and humans, respectively.1 On activation by glycolipid antigens presented on CD1d molecules, NKT cells respond rapidly, secreting high levels of Th1 and Th2 cytokines.2 Despite their limited TCR repertoire, NKT cells can activate antibody responses against T cell–dependent and T cell–independent antigens by α-galactosylceramide (αGalCer) coadministration.3–5 It is plausible that NKT cells influence more than 1 step of the precisely regulated cascade of cellular networking events that gives rise to T cell–dependent B-cell immune responses directed against protein antigens.6 NKT cells are also capable of providing cognate help for B cells, eliciting antibody production through extrafollicular plasma cell formation and atypical germinal center (GC) reaction.7,8
Mutations of the SH2D1A gene, encoding the signaling lymphocyte activation molecule (SLAM) associated protein (SAP), impair T cell–dependent humoral responses in patients with X-linked lymphoproliferative syndrome (XLP), as well as in SAP−/− mice.9–12 In addition to this defect of conventional CD4+ T cells affecting T follicular helper cell–B cell interactions, both XLP patients and SAP−/− mice have reduced numbers of NKT cells.13–15 NKT cells develop in the cortex of the thymus as a result of homotypic interactions between thymocyte precursors carrying the NKT cell–specific αβTCR with thymocytes expressing CD1d/ligands.16 In this process, homotypic interactions mediated by the cooperative engagement of the self-ligand receptors Slamf1 and Slamf6 provide “second signals” that drive maturation of NKT cells in the thymus followed by migration to the medulla and exit into the periphery.17
Because GC formation collapses in XLP patients and SAP−/− mice during infections, especially in a recall response, the relationship between the absence of NKT cells and the dysfunctional antibody responses in XLP patients and SAP−/− mice remains unknown. To this end we used conventional SAP−/− mouse and a novel conditional SAPfl/fl mouse strain together with T-cell transfers to follow antigen-specific antibody responses in the absence of NKT cells because of SAP deficiency or in the presence of NKT cells lacking functional SAP, respectively. These experiments demonstrate that SAP expression in NKT cells is dispensable for their effective response to lipid antigens, including cytokine production and providing noncognate support to protein-specific antibody responses. By contrast, cognate NKT cell help for B cells in response to lipid-antigens requires SAP expression, corresponding to direct T-B cell interactions. These findings suggest a more fundamental role of SLAM-family receptor signaling in providing cognate help to B cells that is not limited to CD4+ T cells.
Methods
Mice
Wild-type (WT), C57BL/6 (B6), and BALB/c mice, as well as tgTCR-OT-II (OT-II) mice (B6) were obtained from The Jackson Laboratory. Transgenic tgCreERT2.B6 mice were purchased from Taconic.18 SAP−/− mice were previously described and backcrossed to the B6 and BALB/c backgrounds for at least 7 generations.19 Jα18−/−.B6 and Jα18−/−.BALB/c mice, originally from Dr M. Taniguchi (Riken, Yokohama, Japan) were provided by Dr M. Exley (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA) and Dr D. T. Umetsu (Children's Hospital, Harvard Medical School, Boston, MA), respectively. Animal studies were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Generation of SAPfl/fl mice
SAPfl/fl NeoΔ ES cells were generated by 2 rounds of homologous recombination in Bruce4 ES cells (B6). In the first round, WT Bruce4 ES cells were transfected with a targeting construct in which SAP exon 1 has been flanked by 2 loxP sites and a Frt-flanked neomycin resistance gene has been placed downstream to exon 1. Homologous recombinants were selected in the presence of G418 and ganciclovir. DNA from each colony was digested with BamH1 and analyzed by Southern blotting using probe 1 as shown in supplemental Figure 1A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). SAPfl/fl Neo+ cells were then transfected with a vector transiently expressing the Flp recombinase. Clones in which SAP exon 1 is floxed and the neomycin deleted (SAPfl/fl NeoΔ) were identified by polymerase chain reaction (PCR) using primers P1 and P2 (supplemental Figure 1A). SAPfl/fl NeoΔ clones were injected into BALB/c blastocysts, chimeric mice were generated, mated with B6 females, and the offsprings were interbred to generate SAPfl/fl NeoΔ mice. SAPfl/fl NeoΔ female mice were subsequently bred with homozygous tgCreERT2 males to generate SAPfl/fl.tgCreERT2 mice that are hemizygous for the transgene.
Immunizations
Animals were immunized intraperitoneally with 40 or 50 μg NP-keyhole limpet hemocyanin (NP-KLH; Biosearch Technologies) or with NP-KLH plus 4 μg αGalCer (Alexxis) in 200 μL phosphate-buffered saline (PBS). OT-II T-cell transferred animals were immunized intraperitoneally with 50 μg NP-ovalbumin (NP-OVA; Biosearch Technologies) and 4 μg αGalCer in 200 μL PBS 24 hours after cell transfers. For cognate interactions 2 to 4 μg NP-αGalCer (synthesized as described)20 was injected intraperitoneally in 200 μL PBS. For other immunization protocols Imject Alum (Thermo Scientific) was added to NP-KLH solution dropwise with constant mixing in final volume ratio of 1:2. After precipitating for 30 minutes 200 μL solution containing 50 μg NP-KLH was injected intraperitoneally.
ELISA
High binding plates (Costar) were coated overnight at 4°C with [NP(3)-BSA] or [NP(23)-BSA] (50 μg/mL; Biosearch Technologies). Horseradish peroxidase–conjugated sheep anti–mouse IgG antibody (Amersham) was used for detection. Relative affinity of the NP-specific IgG antibodies was calculated from the ratio of antibody binding to low-density hapten [NP(3)-BSA] versus high-density hapten [NP(23)-BSA]–coated plates.
IFNγ and IL-4 production was determined by BD OptEIA enzyme-linked immunosorbent assay (ELISA) sets.
Flow cytometry
Single-cell suspensions of splenocytes, thymocytes, and liver lymphocytes were stained with the following antibodies and reagents after blocking nonspecific binding with CD16/32 (93) and 15% rabbit-serum: αCD8α (53-6.7), αCD24 (M1/69), αCD44 (IM7), αCD69 (H1.2F3), αCD138 (281-2), αB220 (RA3-6B2), αDX5 (DX5), αFas (Jo2), αT- and B-cell activation antigen (GL-7), αIgD (11-26), αNK1.1 (PK136), and αTCRβ (H57-597) purchased from eBioscience, BD Pharmingen, or BioLegend. PBS57-loaded CD1d tetramer was provided by the National Institutes of Health tetramer facility. NP-APC was conjugated as reported.21 Data were acquired with LSRII cytometer (BD Pharmingen) and analyzed using FlowJo Version 8.8.6 software (TreeStar). Dead cells were excluded by 4,6-diamidino-2-phenylindole (DAPI) uptake. For intracellular cytokine staining αIFNγ (XMG1.2) and αIL-4 (11B11) antibodies were used (Biolegend and BD Pharmingen, respectively) after surface staining of relevant markers followed by cell permeabilization (Biolegend kit).
Immunohistochemistry and microscopy
Snap-frozen spleens in Tissue-Tek optimal cutting temperature (OCT) embedding medium were cryosectioned and stained with FITC-conjugated PNA (Vector Laboratories) and Alexa 633 (Invitrogen)–conjugated anti–mouse IgD as described.22 Images were taken at room temperature using Olympus Fluoview FV1000 confocal system with 40× magnification lenses (Olympus) equipped with Bio-Rad's image acquisition software.
Tamoxifen administration
An aliquot of 100 mg tamoxifen-free base (T5648; Sigma-Aldrich) was suspended in 100 μL ethanol and dissolved in 1 mL sunflower oil (Sigma-Aldrich). This tamoxifen solution was sonicated for 1 to 2 minutes, diluted to 5 mg/mL in sunflower oil and 200 μL (1 mg) was administrated for 4 consecutive days per os using a feeding needle (Fisher).
Adoptive cell transfers
Single-cell suspensions from the spleen of OT-II and SAP−/− × OT-II donor mice were negatively selected for CD4+ T cells followed by positive selection for CD62Lhi naive cells using a magnetic cell sorting kit (Miltenyi Biotec). Recipient mice were injected intravenously with 2 × 106 CD4+ naive T cells in 200 μL PBS.
Statistics
Statistical analyses were performed with GraphPad Prism 4, using 2-tailed unpaired t tests. Graphs show mean values, error bars indicate SEM.
Results
Activated NKT cells positively regulate B-cell responses at multiple checkpoints
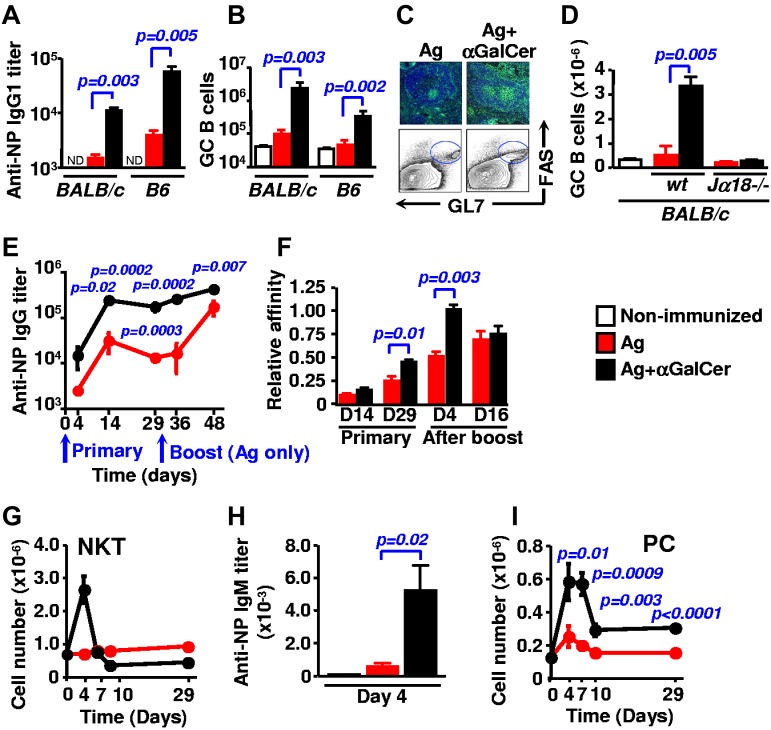
To evaluate at which checkpoints during B-cell responses NKT cells function, mice were injected with a suboptimal concentration of haptenated NP-KLH and αGalCer. After 9 days hapten-specific IgG antibody titers in the serum of BALB/c and B6 mice that had been coinjected with αGalCer were higher than in the serum of mice challenged with the NP-KLH antigen alone (Figure 1A). In addition to an increase in antibody responses, the number of splenic GL7+ Fas+ B cells, a hallmark of a vigorous GC reaction, was higher in the presence of αGalCer (Figure 1B). The latter was confirmed by immunohistochemical staining of PNA+ IgD− cells (Figure 1C). To verify that αGalCer affects GC formation exclusively through the activation of NKT cells, Jα18−/− mice, which do not express this cell population, were immunized in an identical fashion. As expected, Jα18−/− mice were unable to mount a significant αGalCer-dependent GC response (Figure 1D).
Figure 1.
αGalCer-activated NKT cells promote antigen-specific antibody production and GC formation. (A-D) B6 or BALB/c mice (n = 5) were immunized intraperitoneally with 40μg NP-KLH (red) or with NP-KLH plus 4 μg αGalCer (black) and analyzed after 9 days. Representative of 3 to 4 independent experiments with 4 to 5 animals per group. (A) Hapten-specific IgG1 titers in the serum of WT B6 and BALB/c mice. (B) Total numbers of B220+ GL7+ Fas+ GC B cells in the spleen of WT B6 and BALB/c animals. (C) Representative immunohistologic and flow cytometry staining of splenic GCs of WT B6 mice. Green: PNA, blue: IgD. (D) Total numbers of GC B cells (B220+ GL7+ Fas+) in the spleen of WT and Jα18−/− animals (BALB/c). (E) Kinetics of hapten-specific IgG response of WT B6 mice immunized with NP-KLH (red) or NP-KLH plus αGalCer (black). Mice were bled 4, 14, and 29 days after the primary injection, then both groups were challenged with antigen alone on day 30 and were bled 4 and 16 days after. (F) Affinity of NP-specific IgG in immune-sera collected as in (E). (G) B6 mice were immunized with NP-KLH (red) or NP-KLH plus αGalCer (black) and groups of 3 mice were killed at each time point. The number of TCRβ+ CD1d TET+ NKT cells was determined in the spleen. (H) NP-specific IgM titers in the serum of B6 mice 4 days after immunization with NP-KLH with our without coinjecting αGalCer. (I) The number of plasma cells (PC; CD138+B220int) was determined in the spleen of mice that were immunized as in panel G.
When antibody responses by B6 mice immunized with either NP-KLH alone or together with αGalCer were followed during a period of 29 days, we found that these NKT-elicited GCs not only supported high titers of NP-specific IgG production, but also facilitate the process of affinity maturation during late primary responses (Figure 1E-F). Early primary responses (at day 4), preceding the GC reaction, were also affected by the presence of αGalCer. At the time of the brief expansion of NKT cells at day 4 (Figure 1G), the NP-specific IgM and IgG titers were enhanced (Figure 1H-E, day 4), together with a transient rise of CD138+B220int plasma cells in the spleen at day 4 and day 7 (Figure 1I). Consistent with previous observation,4,7,23 the effect of αGalCer was sustained during the memory responses after rechallenging mice with antigen alone (Figure 1E-F).
Taken together, these data demonstrate that αGalCer-activated NKT cells provoke high titers of antibody production through enhancing humoral responses at distinct checkpoints, including the induction of short-lived plasma cells and GCs as well as eliciting stronger memory reactions.
Residual NKT cells in SAP deficient BALB/c mice support antibody responses
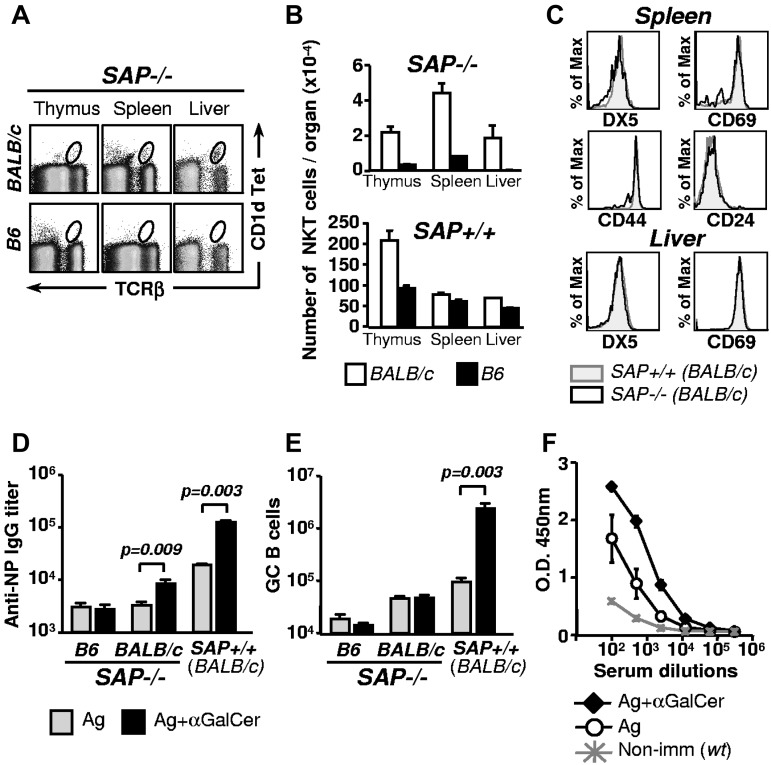
Patients with XLP develop dysgammaglobulinemia with variable severity and also have reduced numbers of NKT cells caused by a defective SH2D1A [SAP] gene.24,25 We and others previously found that SAP in conventional CD4+ T cells affects antibody responses, albeit to a different degree in B6 and BALB/c mice.26–29 Here we evaluate whether defective NKT cell development is a contributing factor to the variably impaired antibody responses in SAP−/− mice. First, we determined that in contrast to SAP−/−.B6 mice the thymus, spleen and liver of SAP−/−.BALB/c mice contain small but detectable numbers of NKT cells (1%-4% of WT; Figure 2A-B). The peripheral NKT cells in SAP−/−.BALB/c mice express the same cell surface markers as in WT mice (Figure 2C). The difference between the 2 mouse strains could not be attributed to polymorphism of the Slamf locus on chromosome 1, because SAP−/−.Sle1b.B6 mice, which similarly to BALB/c mice, carry Slamf-haplotype 2 genes, do not express NKT cells (data not shown). Most importantly, the NKT cells in SAP−/−.BALB/c mice are functionally competent, as judged by their ability to enhance antibody production to NP-KLH in response to αGalCer (Figure 2D). By contrast, these αGalCer activated NKT cells do not partake in the GC reaction (Figure 2E).
Figure 2.
Residual NKT cells in SAP−/−.BALB/c are competent stimulators of antibody production. (A) NKT cells in thymus, spleen and liver isolated from SAP−/−.BALB/c or SAP−/−.B6 mice, were identified with CD1d tetramer loaded with the αGalCer analog PBS57 and anti-TCRβ. (B) The number of NKT cells in different organs of SAP−/−.BALB/c and SAP−/−.B6 mice (n = 3). NKT cells in control WT mice are indicated in the bottom panel. (C) Comparison of activation/differentiation markers on splenic and liver NKT cells (CD1d TET+ TCRβ+) of SAP+/+ and SAP−/−.BALB/c mice. (D) Anti-NP IgG titers in the serum of SAP−/−.BALB/c or SAP−/−.B6 mice 9 days after intraperitoneal immunization with NP-KLH alone or with NP-KLH plus αGalCer. Hapten-specific serum IgG titers were determined by ELISA. Representative of at least 2 independent experiments with 5 animals per group. (E) The number of GC B cells (B220+ Fas+ GL7+) in the spleen was determined by flow cytometry 9 days after immunization as in (D). Representative of at least 2 independent experiments with 5 animals per group. (F) SAP−/−.BALB/c mice were immunized with NP-KLH with or without αGalCer. Forty days after primary injections both groups were rechallenged with NP-KLH, and after 5 days anti-NP IgG levels were determined.
Interestingly, however, when SAP−/−.BALB/c mice received a boost with NP-KLH 40 days after the first immunization, mice that had originally received NP-KLH together with αGalCer had higher anti-NP IgG titers than the animals that were immunized without αGalCer (Figure 2F). Thus, the activation of NKT cells may elicit memory-like responses independent of GC formation in SAP−/−.BALB/c mice.
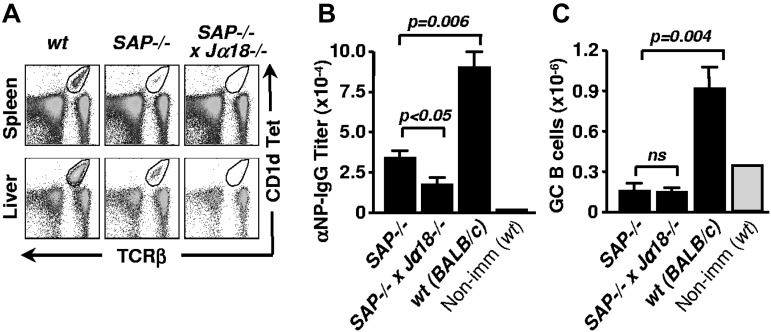
Because not all pathogens directly trigger NKT-cell activation, we investigated the contribution of NKT cell–mediated help in the presence of other adjuvants. To this end, we first eliminated the residual NKT cells in the SAP−/−.BALB/c mice by introducing the Jα18−/− mutation (Figure 3A). Then, single and double-mutant mice were immunized with NP-KLH in alum and NP-specific antibody titers in the serum were determined after 13 days. In the presence of residual NKT cells, the titers of NP-specific IgG were significantly higher in SAP−/−.BALB/c mice compared with SAP−/− × Jα18−/− mice (Figure 3B). As previously shown, and as in the case of αGalCer, GC B cells could not be detected in any of the SAP-deficient animals (Figure 3C).27,28
Figure 3.
Residual NKT cells in SAP−/−.BALB/c mice support NP-specific IgG response in the presence of Alum. (A) NKT cells of SAP−/−.BALB/c mice were eliminated by crossing with Jα18−/−.BALB/c mice. NKT cells in spleen and liver were identified with CD1d tetramer loaded with the αGalCer analog PBS57 and anti-TCRβ. (B) NP-specific serum IgG titers in SAP−/−.BALB/c and SAP−/− x Jα18−/−.BALB/c mice 13 days after immunization with NP-KLH in alum. (C) The number of splenic GC B cells was determined by flow cytometry in mice immunized as in panel B.
Collectively, a small residual number of SAP-deficient NKT cells can support humoral immunity to some extent. Activated NKT cells might not efficiently support GC formation in the SAP−/−.BALB/c mouse because of a lack of (1) a sufficient number of NKT cells, (2) cell intrinsic SAP expression, or (3) an interplay with SAP-positive T helper cells.
Intact responsiveness of NKT cells after tamoxifen-induced deletion of SAP in adult SAPfl/fl.tgCreERT2 mice
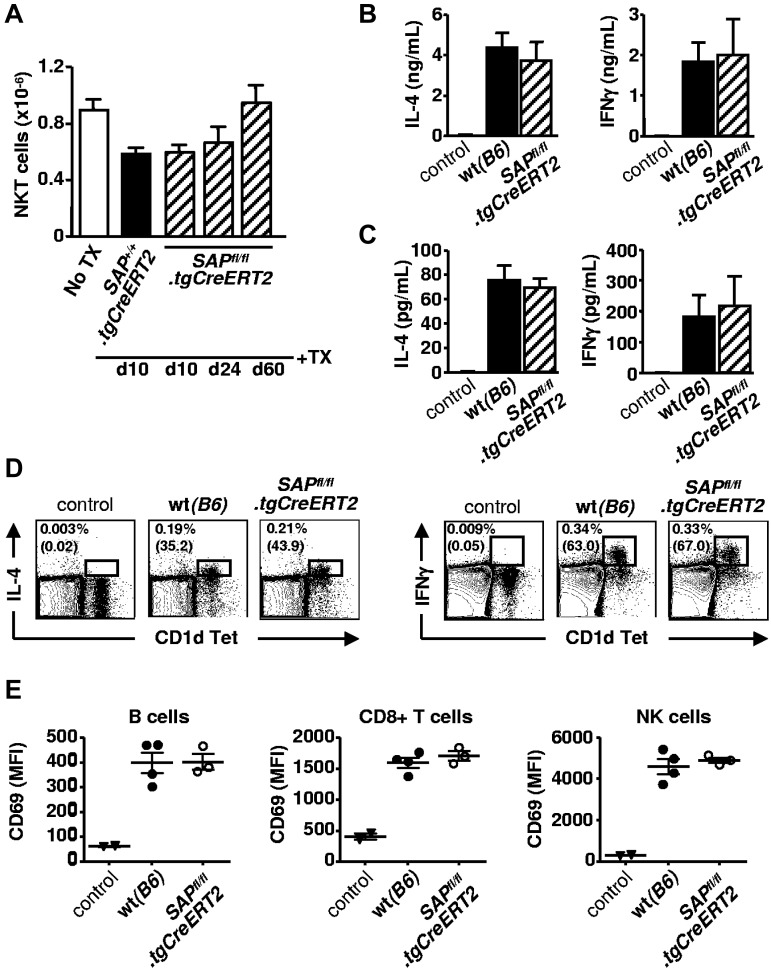
To further evaluate the requirements for NKT cell–helped GC development, we used a conditional SAP knockout mouse, SAPfl/fl.NeoΔB6, which was crossed with a tgCreERT2.B6 mouse for temporally controlled gene inactivation (supplemental Figure 1A). Disruption of the SAP gene in the resulting SAPfl/fl.tgCreERT2 mice was achieved by tamoxifen treatment of adult animals thus bypassing the developmental arrest of NKT cells. After determining the amount of tamoxifen required to abolish SAP expression without detectable background activities (supplemental Figure 1B), we investigated possible changes in the peripheral NKT-cell compartment. As shown in Figure 4A, the number of splenic NKT cells and other cells in tamoxifen-injected SAPfl/fl.tgCreERT2 mice was identical to that in SAP+/+.tgCreERT2 animals, which had been treated in a similar fashion, and remained constant over a period of 2 months.
Figure 4.
SAP negative NKT cells are functionally active. (A) Tamoxifen (1 mg) was administered for 4 days in SAPfl/fl.tgCreERT2 and SAP+/+.tgCreERT2 mice. Ten, 24, or 60 days after the last injection mice were killed. Total number of CD1d TET+ TCRβ+ NKT cells in the spleen was determined by flow cytometry. Representative of 2 independent experiments with 3 to 4 animals per group. (B) Serum cytokine levels 3 hours after injecting taxomifen-treated WT and SAPfl/fl.tgCreERT2 mice with αGalCer plus NP-KLH. Control group represents WT animals without αGalCer plus NP-KLH stimulation. (C) Ex vivo cytokine production of total splenocytes isolated from mice stimulated as in (B), and cultured for an additional 4 hours. (D) Splenocytes isolated freshly from mice stimulated as in panel B were subject to intracellular IL-4 and IFNγ staining. Percentages of CD1d tetramer+ and cytokine+ cells are calculated after gating on viable cells according to scatter characteristics. Percentages of cytokine-positive cells among CD1d tetramer+ TCRβ+ NKT cells are in brackets. (E) Bystander activation of B cells (B220+CD8−CD4−NK1.1−), CD8+ T cells (CD8+B220−CD4−NK1.1−) and NK cells (NK1.1+CD4−B220−) were determined by CD69 up-regulation in mice injected as in panel B.
To assess whether NKT cells function properly without expressing SAP, tamoxifen treated WT and SAPfl/fl.tgCreERT2 mice were tested for early cytokine production after αGalCer-induced NKT cell stimulation in vivo. Serum samples, collected from WT and SAPfl/fl.tgCreERT2 animals 3 hours after the αGalCer plus NP-KLH injection, contained comparably high levels of IL-4 and IFNγ (Figure 4B). To confirm that NKT-cell activation and cytokine production is intact in the splenic environment, splenocytes were isolated from the αGalCer-injected animals and were cultured for an additional 4 hours for ex vivo IL-4 and IFNγ production (Figure 4C), or were subject to intracellular IL-4 and IFNγ staining (Figure 4D). As the activation of NKT cells have been implicated in quick stimulation of bystander lymphocytes,30 we measured CD69 up-regulation on usbB cells, CD8+ T cells and NK cells, and found the same pattern in the presence or absence of SAP (Figure 4E).
Thus, conditional depletion of SAP in adult mice does not affect the size and the responsiveness of the splenic NKT-cell compartment.
SAP expression in NKT cells is dispensable for supporting B cells in a noncognate manner
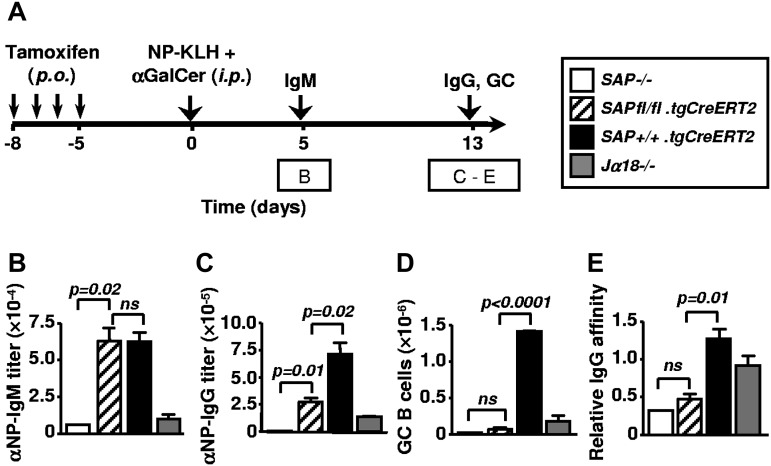
To evaluate the necessity of SAP expression at different stages of NKT cell–promoted B-cell responses, a cohort of SAPfl/fl.tgCreERT2 mice together with SAP+/+.tgCreERT2, SAP−/−.B6 and Jα18−/−.B6 controls were treated with tamoxifen for 4 consecutive days. Five days later, mice were immunized with 50 μg of NP-KLH plus αGalCer (Figure 5A). Five days after immunization, titers of anti-NP IgM antibodies in the serum of SAPfl/fl.tgCreERT2 mice were comparable with titers found in SAP+/+.tgCreERT2 (Figure 5B) and WT B6 mice (not shown). By contrast, SAP−/−.B6 and Jα18−/−.B6 mice had a severe defect in NKT cell–dependent antibody production (Figure 5B). After 13 days SAP-deficient (SAPfl/fl.tgCreERT2) NKT cells still supported IgG production to some extent. However, their responses were clearly impaired compared with SAP+/+.tgCreERT2 mice (Figure 5C). In the absence of SAP, NKT cells did not support GC formation in SAPfl/fl.tgCreERT2 mice (Figure 5D), reminiscent of the result obtained with SAP−/−.BALB/c mice (Figure 2D-E). Moreover, NP-specific IgG antibodies generated in SAPfl/fl.tgCreERT2 mice were found to be low affinity antibodies, supporting the notion that the antibody-producing B cells may not originate in the GCs (Figure 5D). By contrast, in Jα18−/−.B6 mice, αGalCer-activated NKT cells are absent, but because of their intact Th compartment, these mice can mount a modest GC response to NP-KLH with affinity maturation (Figure 5C-E).
Figure 5.
Early and late NKT-dependent antibody responses after tamoxifen-induced deletion of SAP in SAPfl/fl.tgCreERT2 mice. (A) Outline of experiments in (B-E). Ten-week-old SAPfl/fl.tgCreERT2 and Sh2d1a−/−, Jα18−/−, SAP+/+.tgCreERT2 mice were treated with tamoxifen for 4 consecutive days and rested for an additional 5 days before being immunized with NP-KLH plus αGalCer. On day 5 and day 13 samples were taken. Data are representative of 3 independent experiments with 3 to 5 animals per group. (B) Early primary NP-specific IgM response 5 days after immunization with NP-KLH plus αGalCer. (C-E) Analysis of late primary response of NP-KLH plus αGalCer-injected mice 13 days after immunization: NP-specific serum IgG titers (D), total number of GC centrocytes (B220+ GL7+ Fas+ IgDlo) in the spleen (E) and the affinity of NP-specific IgG antibodies (F).
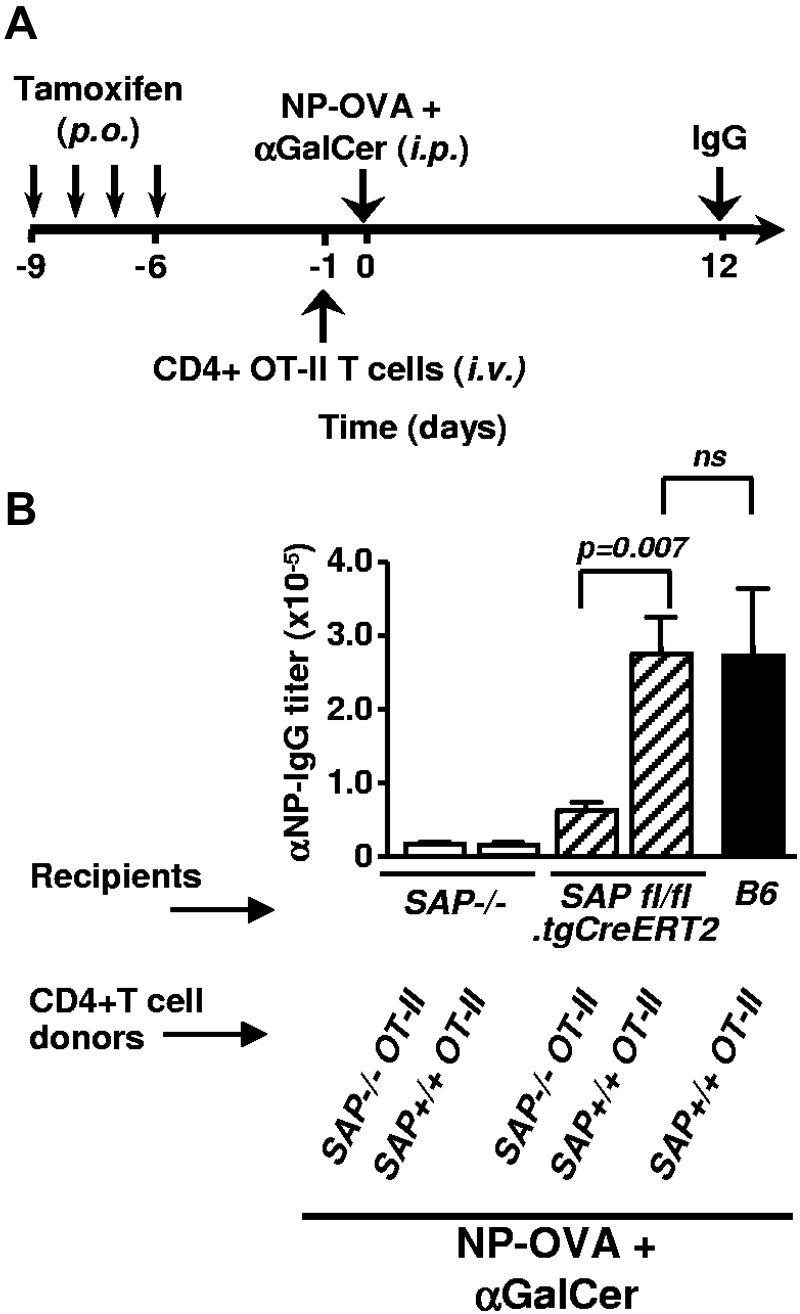
To asses the SAP-dependence of helper activities of NKT cells delivered through Th functions, tamoxifen treated SAPfl/fl.tgCreERT2 mice were adoptively transferred with CD4+ T cells from SAP+/+ or SAP−/− OT-II donors, then immunized with NP-OVA plus αGalCer (Figure 6A). As our data demonstrate, when efficient CD4+ T-cell help is available, SAP deficient NKT cells promote late primary IgG responses equally well to their WT counterparts (Figure 6B).
Figure 6.
SAPfl/fl.tgCreERT2-derived NKT cells assist late primary antibody responses on transfer of SAP+/+ OT-II CD4+ T cells. (A) Outline of CD4+ T cell transfer experiment. SAP−/−, SAPfl/fl.tgCreERT2 and WT control mice were treated with tamoxifen for 4 consecutive days and rested for additional 4 days before being adoptively transferred by 2 × 106 purified CD4+ T cells from SAP positive or negative OT-II mice. Twenty-four hours after cell transfers mice were immunized with NP-OVA plus αGalCer. Result of a single experiment with 3 to 5 animals per group. (B) Twelve days after immunization, NP-specific IgG antibody titers in the serum were determined by ELISA.
Thus, in early and late antibody responses against protein antigens, αGalCer-activated NKT cells provide appropriate B-cell help without intrinsic SAP expression. Contrary to the early waves of NKT-supported antibody production, in GC-dependent late responses the cooperation of SAP-sufficient T helper cells is necessary.
Cognate NKT-B cell interactions are SAP dependent
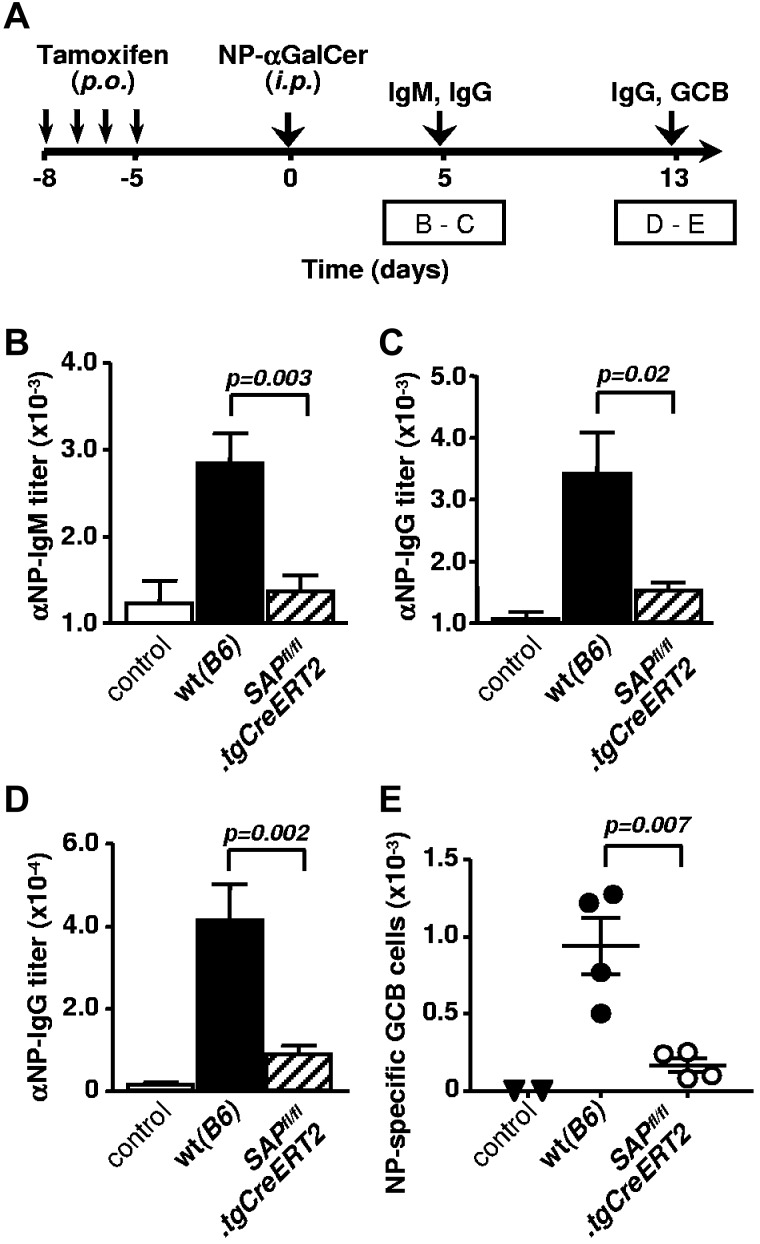
Prompted by recent findings that NKT cells are capable of providing potent, lipid-specific cognate help for B cells,7,8 we set out to investigate the SAP dependence of direct NKT-B cells interactions. To elicit NKT cell mediated cognate help, tamoxifen treated WT and SAPfl/fl.tgCreERT2 mice were immunized with the haptenated lipid antigen NP-αGalCer, then early and late NP-specific B cell responses were evaluated (Figure 7A). Anti-NP IgM and IgG antibody responses were diminished in the absence of SAP (Figure 7B-D); presumably because of inefficient cognate NKT-B cell conjugate formation, similarly to T-B cell interactions. Given that lipid-antigen mediated NKT-cell help also leads to a moderate GC development,7,8 we investigated the presence of NP-reactive GC B cells in the spleen. Small numbers of hapten-specific GC B cells were indeed detectable in NP-αGalCer immunized WT mice, and were completely lacking in those controls, injected with nonconjugated αGalCer or vehicle dimethylsulfoxide (Figure 7E). It is evident the increase in number of antigen-specific GC B cells required signaling through SAP because NP-αGalCer immunized SAPfl/fl.tgCreERT2 mice showed significantly reduced GC B cells (Figure 7E).
Figure 7.
Cognate NKT cell help to B cells requires SAP-dependent signaling. (A) Outline of experiments in panels B through E. SAPfl/fl.tgCreERT2 mice and B6 (WT) counterparts (9 to 10 weeks old) were treated with tamoxifen for 4 consecutive days and rested for an additional 5 days before immunization with 2 to 4 μg NP-αGalCer. Serum samples were analyzed on day 5 and 13, splenocytes on day 13. Antibody titers are combined from 2 independent experiments with 4 to 5 animals per group in each. Controls represent WT animals injected with αGalCer. (B-C) NP-specific IgM (B) and IgG (C) response 5 days after immunization with NP-αGalCer. (D) NP-specific serum IgG titers 13 days after immunization with NP-αGalCer. (E) Total numbers of B220+ GL7+ Fas+ NP-specific GC B cells in the spleen of NP-αGalCer (or αGalCer control) immunized mice. Representative result of 2 independent experiments.
Taken together, when B cells elicit cognate help from NKT cells by lipid-antigen presentation, intrinsic SAP expression of NKT cells is a prerequisite for an effective interaction and B-cell response.
Discussion
We demonstrated that NKT cells can facilitate humoral immunity at multiple checkpoints. We used a haptenated protein antigen coinjected with the NKT cell–activating lipid antigen αGalCer, and found diverse mechanisms by which NKT cells enhance different stages of B cell activation. First, NKT cells could help early waves of antibody production against NP-KLH, even in the absence of SAP, suggesting that additional T cell help is not required for these early antibodies. Contrary to immunizations with particulate antigens or NP-αGalCer conjugates, coinjection of αGalCer and NP-KLH does not force the couptake of the 2 antigens by the same B cell.20–31 Nevertheless, although some B cells could still gather both antigens and directly interact with NKT cells,32 it is more feasible that in these conditions other APCs, probably dendritic cells (DCs), play the major role in activating NKT cells. Hence intensive cytokine production by NKT cells could be sufficient to provoke early plasma cell differentiation without their cognate interaction with B cells. Furthermore, we found a direct link between the activation of NKT cells and GC development, similarly to other recent findings.7 In these processes, in contrast to early plasma cell formation, NKT cells absolutely require the contribution of competent T helper cells. CD40L expression by CD4+ T cells, but not by NKT cells was shown to be required in αGalCer enhanced humoral responses.33,34 Here we have shown a similar pattern for SAP-dependent signaling of Slamf-receptors.
As NKT cells have been shown to elicit DC maturation and improve their capacity to present protein antigens to T cells, activation of NKT cells may support priming of naive CD4+ T cells into T follicular helper cells (Tfh).33,35,36 We could not detect higher numbers of CXCR5+PD-1hi Tfh cells in αGalCer adjuvated animals during primary responses, however this population was significantly enhanced shortly after the boost immunization (our unpublished observation). Thus, the precise mechanism by which CD4+ T cells primed with NKT cell–experienced DCs may elicit a robust GC reaction remains to be clarified.
Surprisingly, we also observed that NKT cells could affect recall responses even in the absence of functional T helper cells and without GCs. Consistent with this finding, Galli et al reported that αGalCer had an adjuvating effect in MHC-II−/− mice detected after 2 immunizations with the influenza virus protein H3N2.4 Others, however, had to apply an agonistic anti-CD40 to elicit αGalCer-mediated adjuvanticity in MHC-II−/− animals.3 In that study a different immunization schedule was used with more frequent boost immunizations, which may not support this potential NKT-dependent, T-independent alternative memory response.3
SAP−/− animals and XLP patients do not express NKT cells in the periphery because of thymic developmental arrest.13–15,17 By discovering that SAP null animals in BALB/c background express a low numbers of NKT cells and by generating conditional SAP−/−.B6 mice with inducible deletion of the SH2D1A gene, it became possible to study the role of SAP in peripheral NKT-cell functions. Although NKT cells do not require intrinsic SAP to provide noncognate help to B cells, SAP expression in CD4+ T cells is still a critical factor for the development of NKT cell–enhanced GCs. In contrast, cognate NKT cell help, recruited by presentation of haptenated lipid antigens, is dependent on SAP signaling of NKT cells, suggesting a universal mechanism by which Slamf-receptors regulate the communication between B cells and different types of helper lymphocytes.
Taken together, some of the various routes of NKT-supported B-cell responses remain intact in SAP deficient animals with particular genetic backgrounds, and might be present in certain genetic groups of XLP patients. A better understanding of how the αGalCer-activated NKT cells modulate antibody responses should potentially be beneficial for designing novel vaccination strategies. These alternative approaches could be especially advantageous for individuals with different primary immunodeficiencies, including XLP.
Supplementary Material
Acknowledgments
The authors thank Drs Mark Exley, Dale T. Umetsu, and Muriel Pichavant and members of the Terhorst laboratory for thoughtful discussions, reagents, and technical advice.
This study was supported by grants from the National Institutes of Health (PO1 AI 35714 and RO1 AI 065687 to C.T.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.D. and C.T. designed experiments and wrote the paper; C.D., M.K., K.K.-T., N.G.-M., W.C., and A.F.A. performed research and analyzed data; G.S.B. and N.V. synthesized lipid antigens; N.W. assisted with construction of the floxed mouse; M.C.C. and G.C.T. contributed to the design of some of the experiments; E.A.L. provided reagents, lipid antigens, technical and conceptual advice; and C.T. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cynthia Detre, Beth Israel Deaconess Medical Center, 3 Blackfan Cir, CLS-928, Boston, MA 02115; e-mail: cdetre@bidmc.harvard.edu.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3(9):867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 3.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119(1):116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli G, Pittoni P, Tonti E, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104(10):3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang ML. How do natural killer T cells help B cells? Expert Rev Vaccines. 2009;8(8):1109–1121. doi: 10.1586/erv.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 7.King IL, Fortier A, Tighe M, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2011;13(1):44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang PP, Barral P, Fitch J, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2011;13(1):35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 9.Sayos J, Wu C, Morra M, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the coreceptor SLAM. Nature. 1998;395(6701):462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 10.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20(2):129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 11.Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin Immunopathol. 2010;32(2):157–171. doi: 10.1007/s00281-009-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 13.Nichols KE, Hom J, Gong SY, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11(3):340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquier B, Yin L, Fondanèche MC, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201(5):695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174(6):3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7(7):505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 17.Griewank K, Borowski C, Rietdijk S, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seibler J, Zevnik B, Küter-Luks B, et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 2003;31(4):e12. doi: 10.1093/nar/gng012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Nguyen KB, Pien GC, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2(5):410–414. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter EA, Brigl M, Illarionov P, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci U S A. 2008;105(24):8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200(12):1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell compartment and FcgammaRIIB receptors. J Exp Med. 2002;196(9):1189–1199. doi: 10.1084/jem.20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur J Immunol. 2008;38(4):1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morra M, Silander O, Calpe S, et al. Alterations of the X-linked lymphoproliferative disease gene SH2D1A in common variable immunodeficiency syndrome. Blood. 2001;98(5):1321–1325. doi: 10.1182/blood.v98.5.1321. [DOI] [PubMed] [Google Scholar]
- 25.Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol. 2003;3(10):813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- 26.Latour S. Natural killer T cells and X-linked lymphoproliferative syndrome. Curr Opin Allergy Clin Immunol. 2007;7(6):510–514. doi: 10.1097/ACI.0b013e3282f1bad6. [DOI] [PubMed] [Google Scholar]
- 27.Morra M, Barrington RA, Abadia-Molina AC, et al. Defective B cell responses in the absence of SH2D1A. Proc Natl Acad Sci U S A. 2005;102(13):4819–4823. doi: 10.1073/pnas.0408681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421(6920):282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura H, Ohta A, Sekimoto M, et al. alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199(1):37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 31.Barral P, Eckl-Dorna J, Harwood NE, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105(24):8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111(4):2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2009;113(2):370–376. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 34.Shah HB, Joshi SK, Lang ML. CD40L-null NKT cells provide B cell help for specific antibody responses. Vaccine. 2011;29(49):9132–9136. doi: 10.1016/j.vaccine.2011.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198(2):267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.