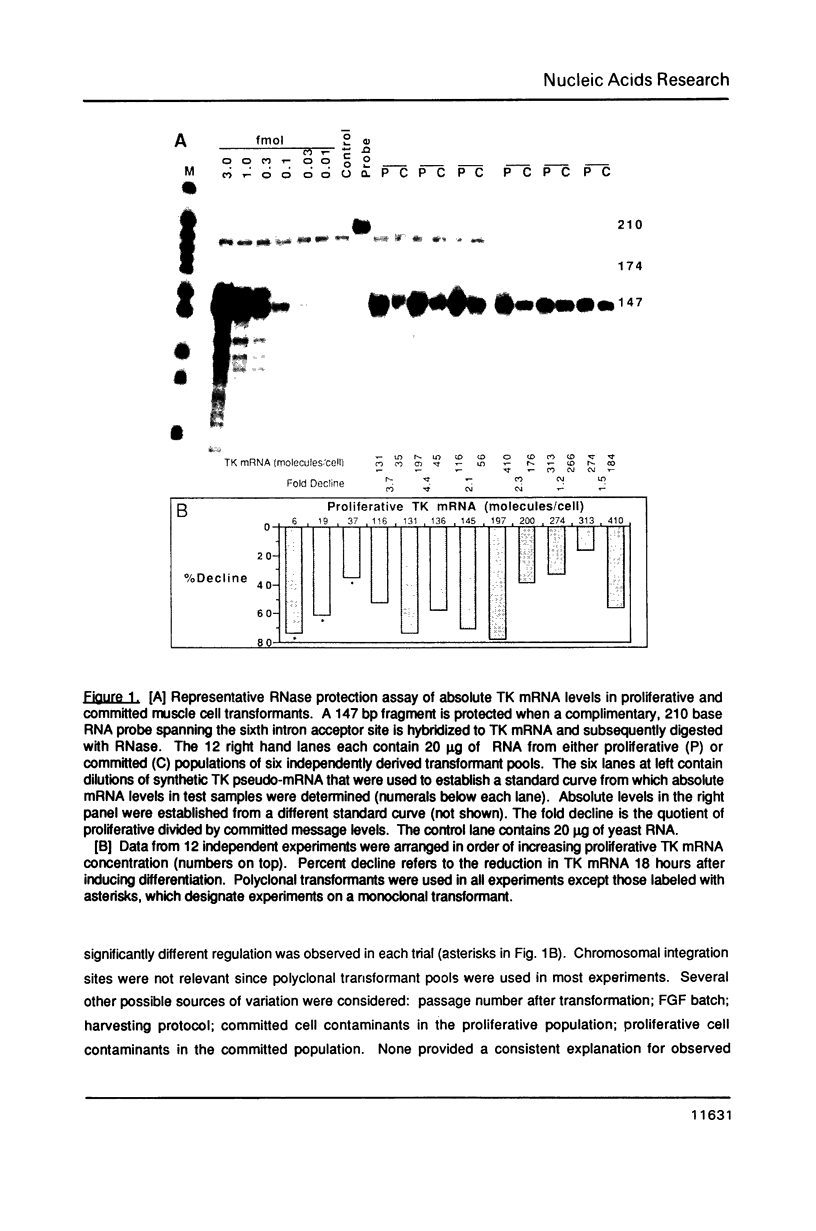
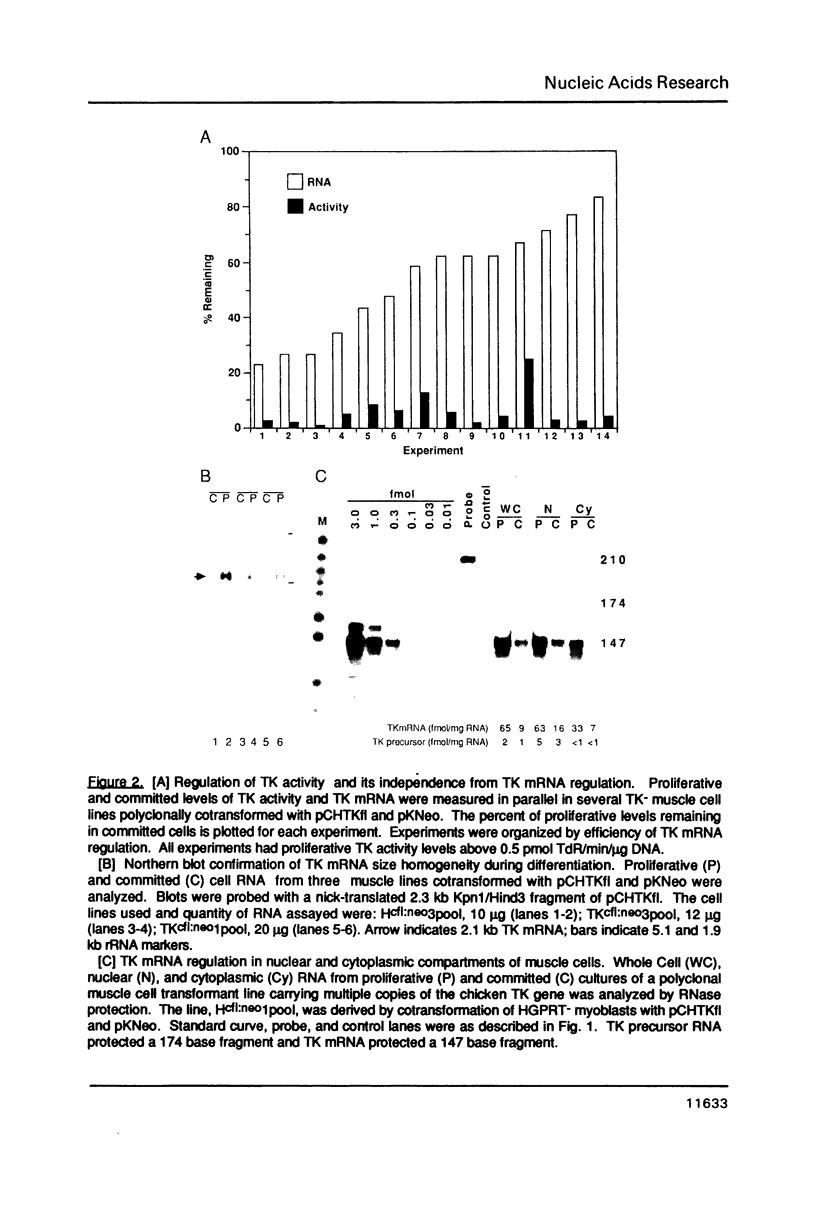
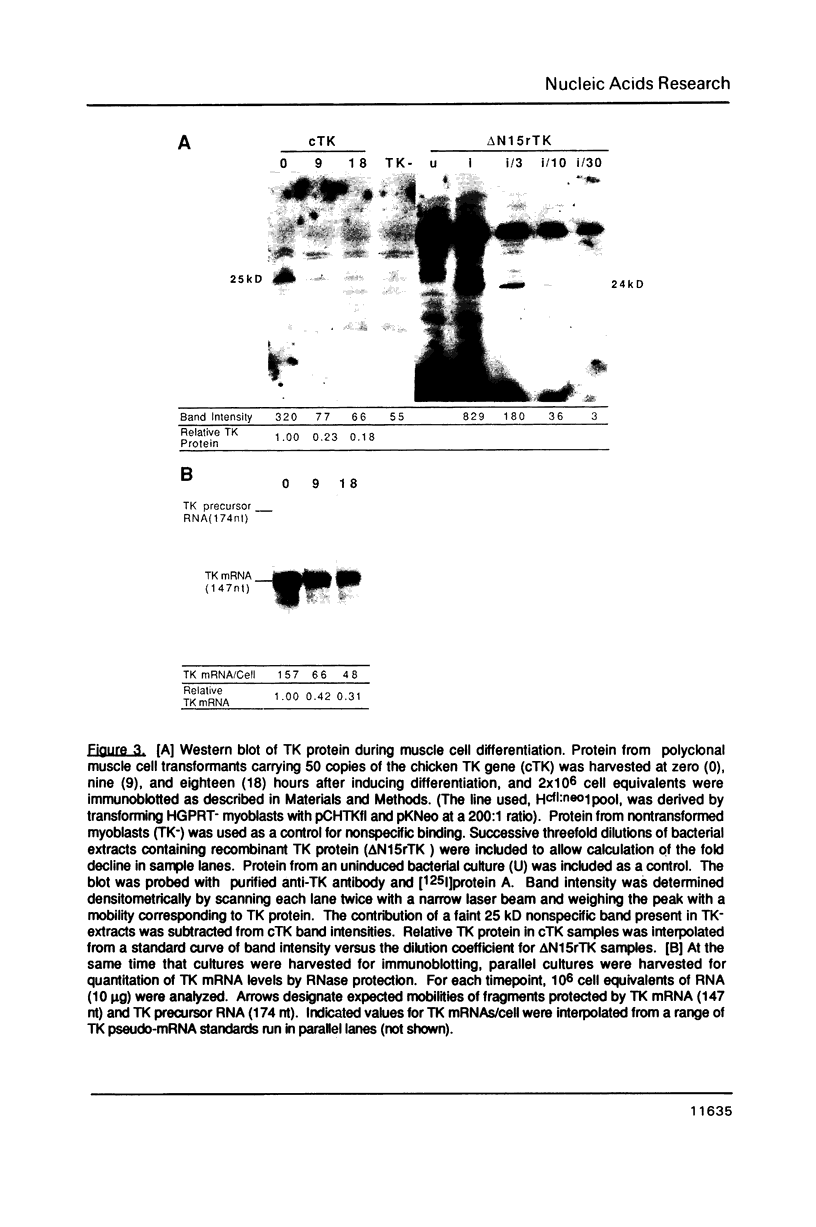
Abstract
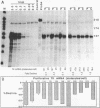
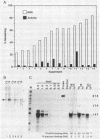
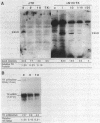
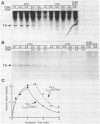
Replication-dependent changes in levels of enzymes involved in DNA precursor biosynthesis are accompanied frequently by changes in levels of cognate mRNA. We tested the common assumption that changes in mRNA levels are responsible for growth-dependent expression of these enzymes using a line of mouse muscle cells that irreversibly withdraws from the cell cycle as part of its terminal differentiation program. Thymidine kinase (TK) mRNA, activity, and protein levels were quantitated in cells transformed with multiple copies of the chicken TK gene. The decline in TK mRNA (both whole cell and cytoplasmic) during myogenesis was poor (2-fold average) and variable (1.2 to 8-fold). In contrast, TK activity always was regulated efficiently (20-fold), even in cells which regulated TK mRNA very poorly. Thus, regulation of TK activity was independent of TK mRNA regulation as myoblasts withdrew from the cell cycle. A TK/beta-galactosidase fusion protein was used to derive an antibody against chicken TK. Immunoblot and immunoprecipitation analyses demonstrated TK protein levels, like TK activity levels, declined to a greater extent than TK mRNA levels. Thus, TK activity likely was regulated by a mechanism involving either decreased translation of TK mRNA or increased degradation of TK protein in committed muscle cells.
Full text
PDF








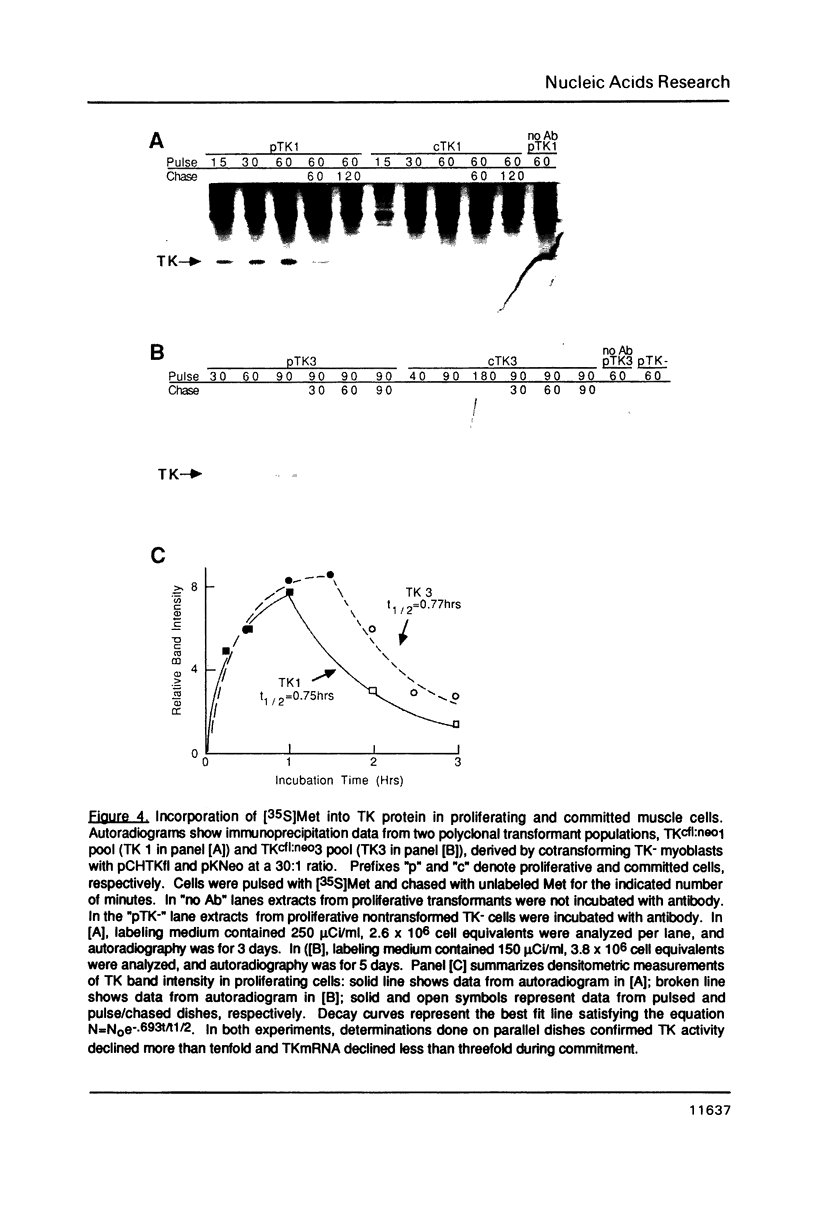

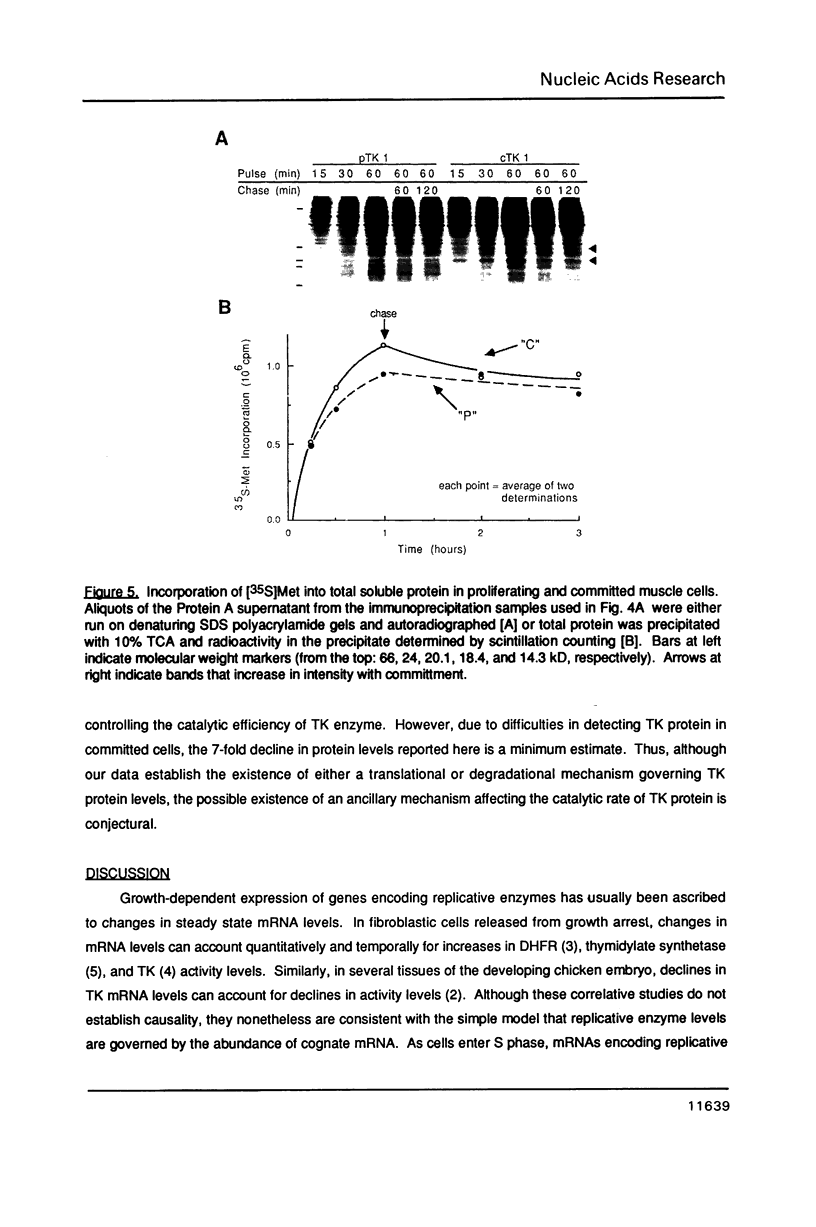




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Shimizu K., Koyama H., Kaneda S., Takeishi K., Seno T. Cell-cycle-directed regulation of thymidylate synthase messenger RNA in human diploid fibroblasts stimulated to proliferate. J Mol Biol. 1986 Aug 20;190(4):559–567. doi: 10.1016/0022-2836(86)90241-x. [DOI] [PubMed] [Google Scholar]
- Bowman L. H., Emerson C. P., Jr Formation and stability of cytoplasmic mRNAs during myoblast differentiation: pulse-chase and density labeling analyses. Dev Biol. 1980 Nov;80(1):146–166. doi: 10.1016/0012-1606(80)90505-9. [DOI] [PubMed] [Google Scholar]
- Bowman L. H. rDNA transcription and pre-rRNA processing during the differentiation of a mouse myoblast cell line. Dev Biol. 1987 Jan;119(1):152–163. doi: 10.1016/0012-1606(87)90217-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Jaynes J. B., Hauschka S. D. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol Cell Biol. 1985 Mar;5(3):484–492. doi: 10.1128/mcb.5.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Schimke R. T. Murine dihydrofolate reductase transcripts through the cell cycle. Mol Cell Biol. 1986 Feb;6(2):365–371. doi: 10.1128/mcb.6.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham P. J., Schimke R. T. Transcriptional regulation of mouse dihydrofolate reductase in the cell cycle. J Biol Chem. 1985 Jun 25;260(12):7675–7680. [PubMed] [Google Scholar]
- Geyer P. K., Meyuhas O., Perry R. P., Johnson L. F. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982 Jun;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. E., Beckman C. A., Cowan K. H. 5' Nucleotide sequences influence serum-modulated expression of a human dihydrofolate reductase minigene. Mol Cell Biol. 1986 Mar;6(3):878–886. doi: 10.1128/mcb.6.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. K., Kainz M. S., Merrill G. F. Introns are inconsequential to efficient formation of cellular thymidine kinase mRNA in mouse L cells. Mol Cell Biol. 1987 Dec;7(12):4576–4581. doi: 10.1128/mcb.7.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. K., Kainz M. S., Merrill G. F. The chicken thymidine kinase gene is transcriptionally repressed during terminal differentiation: the associated decline in TK mRNA cannot account fully for the disappearance of TK enzyme activity. Dev Biol. 1987 Aug;122(2):439–451. doi: 10.1016/0012-1606(87)90308-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem. 1985 Oct 15;260(23):12464–12473. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer R., Müllner E., Seiser C., Wintersberger E. Cell cycle regulated synthesis of stable mouse thymidine kinase mRNA is mediated by a sequence within the cDNA. Nucleic Acids Res. 1987 Jan 26;15(2):741–752. doi: 10.1093/nar/15.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Imam A. M., Crossley P. H., Jackman A. L., Little P. F. Analysis of thymidylate synthase gene amplification and of mRNA levels in the cell cycle. J Biol Chem. 1987 May 25;262(15):7368–7373. [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Wiedemann L. M. Regulation of dihydrofolate reductase gene expression in mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):397–306. doi: 10.1002/jcp.1040970314. [DOI] [PubMed] [Google Scholar]
- KREBS E. G., GRAVES D. J., FISCHER E. H. Factors affecting the activity of muscle phosphorylase b kinase. J Biol Chem. 1959 Nov;234:2867–2873. [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Growth-dependent expression of dihydrofolate reductase mRNA from modular cDNA genes. Mol Cell Biol. 1983 Sep;3(9):1598–1608. doi: 10.1128/mcb.3.9.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. K., Wells S., Lau Y. F., Lee A. S. Sequences contained within the promoter of the human thymidine kinase gene can direct cell-cycle regulation of heterologous fusion genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5894–5898. doi: 10.1073/pnas.85.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Matkovich D. A. Genetic determinants of growth phase-dependent and adenovirus 5-responsive expression of the Chinese hamster thymidine kinase gene are contained within thymidine kinase mRNA sequences. Mol Cell Biol. 1986 Jun;6(6):2262–2266. doi: 10.1128/mcb.6.6.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Shimizu K., Zipser D. Isolation and preliminary characterization of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1983 Oct;3(10):1815–1823. doi: 10.1128/mcb.3.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Kellems R. E. Control of dihydrofolate reductase messenger ribonucleic acid production. Mol Cell Biol. 1981 Nov;1(11):961–971. doi: 10.1128/mcb.1.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschika S. D. Myogenic differentiation in permanent clonal mouse myoblast cell lines: regulation by macromolecular growth factors in the culture medium. Dev Biol. 1981 Aug;86(1):19–30. doi: 10.1016/0012-1606(81)90311-0. [DOI] [PubMed] [Google Scholar]
- Liu H. T., Gibson C. W., Hirschhorn R. R., Rittling S., Baserga R., Mercer W. E. Expression of thymidine kinase and dihydrofolate reductase genes in mammalian ts mutants of the cell cycle. J Biol Chem. 1985 Mar 25;260(6):3269–3274. [PubMed] [Google Scholar]
- Mariani B. D., Slate D. L., Schimke R. T. S phase-specific synthesis of dihydrofolate reductase in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4985–4989. doi: 10.1073/pnas.78.8.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Harland R. M., Groudine M., McKnight S. L. Genetic and physical analysis of the chicken tk gene. Mol Cell Biol. 1984 Sep;4(9):1769–1776. doi: 10.1128/mcb.4.9.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Witter E. B., Hauschka S. D. Differentiation of thymidine kinase deficient mouse myoblasts in the presence of 5'-bromodeoxyuridine. Exp Cell Res. 1980 Sep;129(1):191–199. doi: 10.1016/0014-4827(80)90342-0. [DOI] [PubMed] [Google Scholar]
- Santiago C., Collins M., Johnson L. F. In vitro and in vivo analysis of the control of dihydrofolate reductase gene transcription in serum-stimulated mouse fibroblasts. J Cell Physiol. 1984 Jan;118(1):79–86. doi: 10.1002/jcp.1041180114. [DOI] [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Stewart C. J., Ito M., Conrad S. E. Evidence for transcriptional and post-transcriptional control of the cellular thymidine kinase gene. Mol Cell Biol. 1987 Mar;7(3):1156–1163. doi: 10.1128/mcb.7.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart P., Ito M., Stewart C., Conrad S. E. Induction of cellular thymidine kinase occurs at the mRNA level. Mol Cell Biol. 1985 Jun;5(6):1490–1497. doi: 10.1128/mcb.5.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Travali S., Lipson K. E., Jaskulski D., Lauret E., Baserga R. Role of the promoter in the regulation of the thymidine kinase gene. Mol Cell Biol. 1988 Apr;8(4):1551–1557. doi: 10.1128/mcb.8.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]