Abstract
IL-15 is an important cytokine for the function of the immune system, but the form(s) of IL-15 produced in the human body are not fully characterized. Coexpression of the single-chain IL-15 and the IL-15 receptor alpha (IL-15Rα) in the same cell allows for efficient production, surface display, and eventual cleavage and secretion of the bioactive IL-15/IL-15Rα heterodimer in vivo, whereas the single-chain IL-15 is poorly secreted and unstable. This observation led to the hypothesis that IL-15 is produced and secreted only as a heterodimer with IL-15Rα. We purified human IL-15/IL-15Rα complexes from overproducing human cell lines and developed an ELISA specifically measuring the heterodimeric form of IL-15. Analysis of sera from melanoma patients after lymphodepletion revealed the presence of circulating IL-15/IL-15Rα complexes in amounts similar to the total IL-15 quantified by a commercial IL-15 ELISA that detects both the single-chain and the heterodimeric forms of the cytokine. Therefore, in lymphodepleted cancer patients, the serum IL-15 is exclusively present in its heterodimeric form. Analysis of the form of IL-15 present in either normal or lymphodepleted mice agrees with the human data. These results have important implications for development of assays and materials for clinical applications of IL-15.
Introduction
IL-15 is a member of the common γ-chain family of cytokines and was initially characterized as a T-cell proliferation factor.1,2 IL-15 is a growth, mobilization, and activation factor for important lymphocyte populations, including NK, CD8, and intraepithelial lymphocytes.3–6 IL-15 and IL-2 share the same IL-2/IL-15βγ receptor (IL-2/IL-15Rβγ),7 but their biologic effects at the level of organism are different, as shown by studies in knockout mice. The lack of IL-15 in vivo results in severe defects in the development and function of the immune system.8,9 Although lymphocytes from IL-2 knockout mice fail to proliferate in response to polyclonal T-cell mitogens in vitro,10 the common features developing in these mice are lymphocyte activation and autoimmunity.11 In humans, IL-2–receptor α (IL-2Rα) deficiency has been linked to a paradoxical combination of immunodeficiency and autoimmunity in 2 patients.12,13
The biologic differences of IL-2 and IL-15 are determined by their different production sites,5,14 their strength of association with the membrane proteins IL-2Rα and IL-15Rα,15 respectively, and the regulation of these receptor molecules. IL-2 is produced by activated lymphocytes, whereas IL-15 is produced by stromal cells of several tissues, and by antigen-presenting cells. IL-15Rα has a high affinity for IL-15 (Kd ∼ 10−11M).15 In contrast, IL-2Rα has lower affinity for IL-2 (Kd ∼ 10−8M) and is expressed mainly on activated T cells and persistently on Treg.
IL-15 is known to act on the surface of the cell in complex with IL-15Rα to engage the IL-2/IL-15Rβγ complex in nearby cells, a process termed trans-presentation.16 Genetic and cell transfer experiments have shown that IL-15 and IL-15Rα need to reside in the same cell for appropriate function.17–20 We previously demonstrated that coexpression of the 2 molecules in the same cell leads to rapid intracellular association of IL-15 and IL-15Rα in the endoplasmic reticulum, stabilization of both molecules, and the generation of a stable complex that can translocate to the cell membrane, where it is bioactive.21,22 In addition, the surface heterodimer is rapidly cleaved and released in the plasma as bioactive cytokine.22 Our experiments using IL-15 complexed to a C-terminal deletion of IL-15Rα containing only the soluble extracellular fragment demonstrated that this complex is bioactive in vivo in the absence of any membrane-bound form of IL-15.22 These results explain the necessity for the coordinate expression of IL-15 and IL-15Rα in the same cell for physiologic function,17–19 and predict that the circulating form of IL-15 in biologic fluids is in complex with soluble IL-15Rα (sIL-15Rα).
Although these results suggested that the main bioactive form of IL-15 is in a complex with IL-15Rα,21–26 the actual form of IL-15 produced in humans has not been identified. The levels of IL-15 in normal human serum are close to the limit of detection of the existing assay (∼ 1 pg/mL). Therefore, we studied the nature of the circulating form of IL-15 in sera of metastatic melanoma patients shortly after lymphodepleting treatment. Such patients are known to have increased plasma levels of the homeostatic cytokines IL-7 and IL-15,27 facilitating accurate determinations. Development of a new heterodimer-specific ELISA allowed distinguishing between the single-chain IL-15 and the IL-15 in complex with the IL-15Rα and revealed that the IL-15 is indeed found in the heterodimeric form in human sera.
Methods
Human serum collection
Serum samples collected after lymphodepleting treatment were stored at −80°C and thawed at the time of IL-15 assay. The patient population and cytokine levels have been reported previously.27 Collection of human samples was approved by the National Cancer Institute ethical review board.
Purification and analysis of human glycosylated IL-15/sIL-15Rα complexes
DNA vectors optimized for the efficient expression of human IL-15 and IL-15Rα have been previously described.22,28 HEK293-derived human cell lines expressing high levels of secreted IL-15/IL-15Rα heterodimers were generated after stable transfection, hygromycin selection, and maintenance in serum-free media in a hollow fiber system (FiberCell System). The glycosylated IL-15/sIL-15Rα complexes were separated by denaturing RP-HPLC chromatography. Briefly, samples were disrupted in 8M Guanidine-HCl (Pierce Chemical), under nonreducing conditions, and fractionated by HPLC performed at a flow rate of 300 μL/min on 2.1 × 100 mm Poros R2/H narrow bore column (Boehringer Mannheim), using aqueous acetonitrile/trifluoroacetic acid solvents. A temperature of 55°C was maintained during HPLC separation. Peaks were detected by UV absorption at 280 nm. Quantitation of purified proteins was performed by amino acid analysis using a Hitachi L-8800 Amino Acid Analyzer. Purified IL-15 and sIL-15Rα subunits were mixed at molar ratio 1:1 to allow complex reassociation. IL-15/IL-15Rα heterodimer analysis was performed in both denaturing and nondenaturing conditions. Protein samples were dissolved in a buffer with or without SDS, loaded on polyacrylamide gels (12% and 4%-20% gradient, respectively), and visualized by Coomassie blue staining. Formation of the complexes was confirmed by Western immunoblot analysis, after proteins were transferred onto Immobilon-P membrane (Millipore) using anti–human IL-15 or IL-15Rα antibodies (AF315 and AF247, respectively; R&D Systems).
IL-15 and IL-15/IL-15Rα ELISA
The concentration of IL-15 in serum of cancer patients was evaluated using a chemiluminescent immunoassay (Quantiglo Q1500B; R&D Systems), according to the manufacturer's recommendations. This ELISA can detect both single-chain IL-15 and IL-15/IL-15Rα heterodimers. To measure exclusively the heterodimers, an ELISA specific for the heterodimeric IL-15/IL-15Rα was developed as follows: ELISA plates were precoated with the same monoclonal anti–human IL-15 antibody used in the Quantiglo Q1500B assay (R&D Systems). Bound heterodimers were detected after incubation with biotin-conjugated polyclonal anti–human IL-15Rα antibody (0.1 μg/mL; BAF847; R&D Systems). HRP-streptavidin (R&D Systems) was added at a dilution of 1:200. After washes to remove unbound reagents, an enhanced luminol/peroxide substrate solution (Quantiglo; R&D Systems) was added to the wells and relative light units (RLUs) emitted were measured using a Dynex Technologies MLX luminometer. The reference standard for the heterodimeric IL-15/IL-15Rα ELISA was 3-fold serial dilutions of purified human IL-15/IL-15Rα heterodimers quantified by amino acid analysis. The standard curve was determined by cubic-spline curve fit, using a Prism 4.0a software package. Intra-assay precision was evaluated by testing 6 samples of known quantity in triplicate. Interassay precision was evaluated by testing 6 samples of known quantity in 5 independent assays.
Mice and cytoreductive regimens
C57BL/6 mice were obtained from Charles River Laboratory. IL-15Rα−/− mice were purchased from The Jackson Laboratory. Cyclophosphamide (CTX; Sigma-Aldrich) was dissolved in pyrogen-free saline solution at a concentration of 20 mg/mL. Six- to 8-week-old female mice were treated with PBS or with CTX at 200 mg/kg of body weight intraperitoneally. Alternatively, lymphopenia was induced by sublethal whole-body irradiation (6 Gy; x-ray source; 1.29 Gy/min, 137-cesium chloride irradiator). All animal experiments were approved by the National Cancer Institute Animal Care and Use Committee.
IL-15 measurements in mice
Mice were bled at different time points after CTX treatment (days 1, 3, 7, 8, 12, and 19) or whole-body irradiation (days 1, 3, 4, 7, and 14). Serum levels of murine IL-15/IL-15Rα heterodimers were measured in untreated and lymphopenic mice by ELISA, using the Mouse IL-15R/IL-15 Complex ELISA Ready-SET-Go (eBioscience). In some experiments, serum samples were incubated with recombinant mouse IL-15Rα-Fc (R&D Systems) before IL-15R/IL-15 Complex ELISA evaluation. Detection of single-chain murine IL-15 was evaluated using Mouse ELISA Ready-SET-Go (eBioscience).
Lymphocyte analysis
Mice were killed at different time points after CTX treatment (days 1, 7, and 12) or whole-body irradiation (days 3, 7, and 14), and spleens and blood were collected. For splenocyte purification, organs were gently squeezed through a 100-μm cell strainer (Thomas) and washed in RPMI (Invitrogen) to remove any remaining organ stroma. The cells were resuspended in RPMI containing 10% FCS and counted. For phenotyping, the cells were incubated with the following mixture of directly conjugated anti–mouse antibodies (BD Biosciences PharMingen): APCCy7-CD3 (145-2C11), PerCP-CD4 (RM4-5), Pacific Blue-CD8 (53-6.7), FITC-CD49b (DX5). Splenocytes were evaluated in a LSR II Flow Cytometer (BD Biosciences), and data were analyzed using FlowJo 8.8.7 software (TreeStar). Complete blood counts were obtained using a Hemavet Multispecies Hematology Analyzer.
Statistical analysis
Correlation of circulating IL-15 and IL-15/IL-15Rα levels in serum was determined by linear regression, using Prism 4.0a software package.
Results
IL-15 ELISAs for the detection of human IL-15 single-chain and IL-15/sIL-15Rα heterodimer
For the quantification of IL-15 levels, we used a commercial ELISA (R&D Systems; Quantiglo Q1500B), in which both the capture and detection antibodies are mouse monoclonal antibodies against 2 distinct regions in human IL-15 (Figure 1A). This ELISA does not distinguish between the single-chain IL-15 and its heterdimeric form with the IL-15Rα, as verified by assays using pure IL-15 monomer or heterodimer (see “Results”). To distinguish the 2 forms, in addition, we established an ELISA detecting only the IL-15/IL-15Rα heterodimer using an anti–IL-15 antibody to bind IL-15 to the plate and an anti–IL-15Rα antibody to detect heterodimers (Figure 1B).
Figure 1.
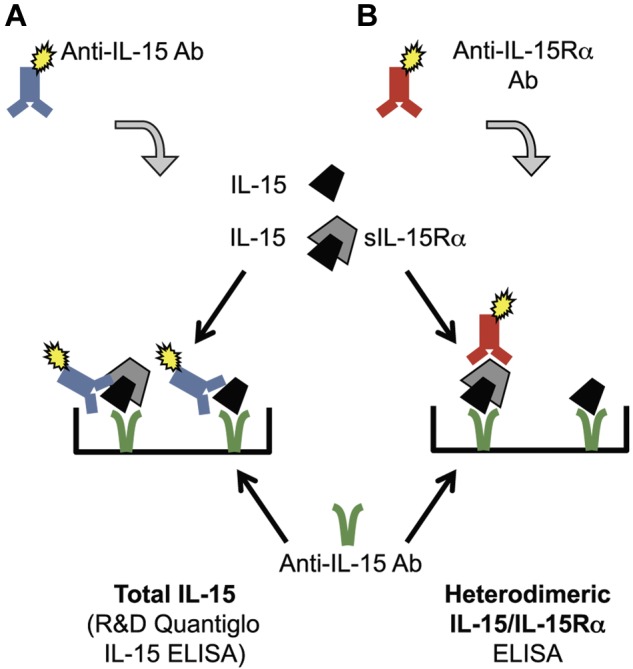
Schematic representation of ELISAs for detection of human IL-15. (A) Total IL-15 was detected by a commercially available IL-15 ELISA (R&D Systems, Quantiglo kit). The capture and detection monoclonal anti–human IL-15 antibodies target different accessible regions of IL-15 molecule. (B) IL-15/IL-15Rα heterodimers were detected using a monoclonal anti–human IL-15 as capture antibody and a polyclonal anti–human IL-15Rα as detection antibody. Single-chain IL-15 or purified sIL-15Rα tested separately were not detected by this heterodimeric IL-15/IL-15Rα ELISA.
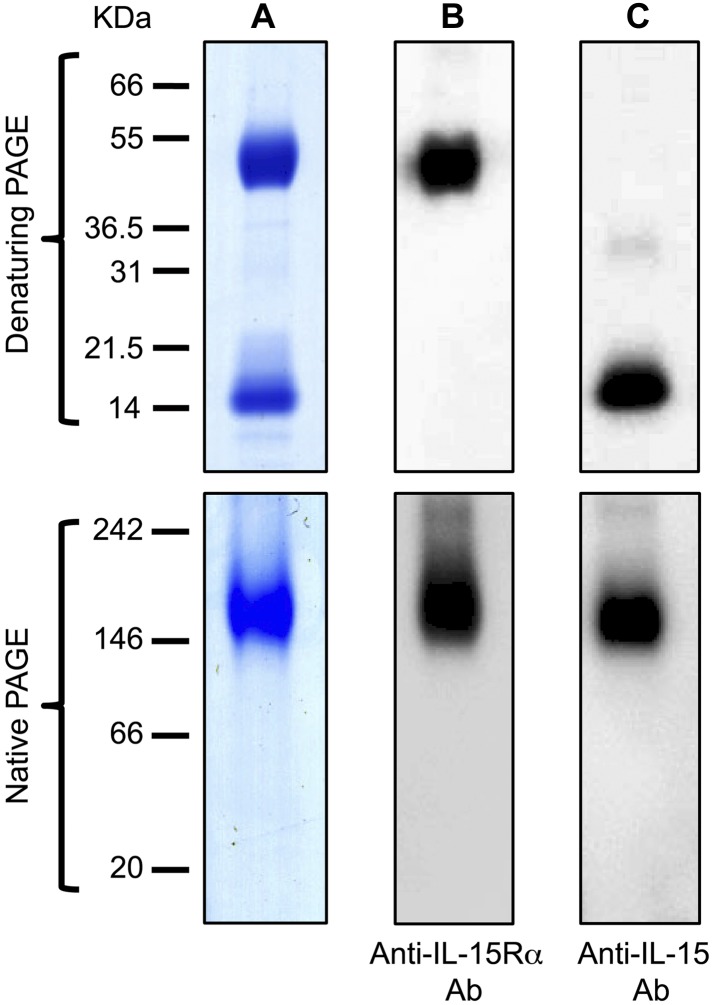
The development of the assay detecting exclusively the IL-15 heterodimer required a suitable IL-15/IL-15Rα standard for calibration, similar to the heterodimer produced in the body. Both IL-15 and IL-15Rα are glycosylated.29,30 These modifications, as well as the masking of the IL-15/IL-15Rα interaction sites resulting from the heterodimer formation, may affect the ability of antibodies generated against Escherichia coli, yeast, or insect cell-derived IL-15 monomers to recognize antigenic epitopes on human IL-15 compared with the heterodimeric complex, resulting in inaccurate measurements. Therefore, we purified the authentically glycosylated human IL-15/IL-15Rα heterodimer from human overproducing cell lines as an improved standard for IL-15 assays. High-level production of IL-15 in mammalian systems has been challenging in the past. We have previously described methods to develop efficient expression vectors for IL-15, combining mRNA optimization (RNA/codon optimization) of the IL-15 coding sequences and substitution of the signal peptide with other efficient secretory signals.28 We also produced vectors expressing IL-15 in combination with IL-15Rα, which resulted in stable, secreted IL-15/IL-15Rα heterodimers, with a final improvement in secreted IL-15 of more than 1000-fold compared with vectors expressing the wild-type IL-15 cDNA.22 Taking advantage of these optimized vectors, we developed cell lines based on human HEK-293 clones that can produce up to 200 mg/L of IL-15/IL-15Rα (C.B. and G.N.P., unpublished data). Secreted IL-15/sIL-15Rα complexes are correctly processed and glycosylated and are suitable as IL-15/IL-15Rα ELISA reference standard. Human IL-15 and sIL-15Rα, purified by HPLC from supernatants of such cell lines (purity, 98%-99%), were quantified by amino acid mass analysis, mixed in a molar ratio 1:1 and allowed to reassociate. IL-15/IL-15Rα complexes were analyzed by electrophoresis on polyacrylamide gels and visualized by Coomassie blue staining (Figure 2A) and by Western immunoblot using anti–IL-15Rα (Figure 2B) or anti–IL-15 antibodies (Figure 2C). Under denaturing conditions, 2 bands were visible, corresponding to sIL-15Rα and IL-15 of approximately 50 and approximately 15 kDa, respectively. Western immunoblot analysis confirmed that the 2 bands correspond to human IL-15Rα and IL-15, respectively (Figure 2A-C top panels). Under native conditions, both anti–human IL-15Rα and anti–human IL-15 antibodies detected the same approximately 140 to 160 kDa form, compatible with the size of the authentically glycosylated, IL-15/sIL-15Rα heterodimers (Figure 2B-C bottom panels). No single bands for IL-15 or IL-15Rα were present, indicating that all the IL-15 and IL-15Rα proteins were in complexes.
Figure 2.
Polyacrylamide gel analysis of IL-15/sIL-15Rα complex purified from an overproducing human cell line. (A) HPLC-purified human IL-15 and sIL-15Rα produced by a HEK293-derived cell line were mixed in a molar ratio 1:1, and IL-15/IL-15Rα complexes were visualized in Coomassie blue-stained denaturing SDS-polyacrylamide gel (top panel) and in Coomassie blue-stained nondenaturing polyacrylamide gel (bottom panel). (B-C) IL-15/IL-15Rα shown in panel A was detected by Western immunoblot. Complexes were analyzed under denaturing condition (top panels) or under nondenaturing condition (bottom panels) using a goat anti–human IL-15Rα antibody (B) or a goat anti–human IL-15 antibody (C).
The purified IL-15/IL-15Rα heterodimers were used in 3-fold serial dilutions as reference standard for IL-15/IL-15Rα heterodimer ELISA. The ELISA showed a log-linear relation of RLU to IL-15/IL-15Rα amounts from 27 to 2250 pg/mL (r2 = 0.93, P < .0001; Figure 3).
Figure 3.
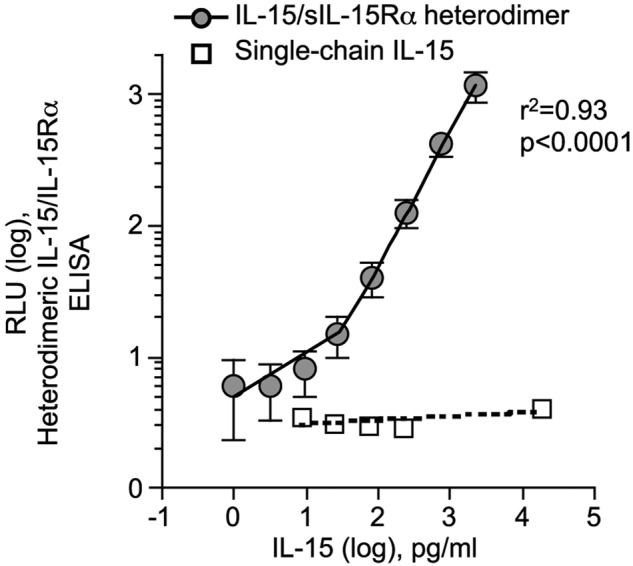
Soluble IL-15/IL-15Rα protein standard for the heterodimeric IL-15/IL-15Rα ELISA. Standard curve for human heterodimeric IL-15/IL-15Rα ELISA. HPLC-purified human IL-15 and sIL-15Rα were quantified by amino acid mass analysis and mixed in a molar ratio 1:1. Triplicates of 3-fold serial dilutions of IL-15/IL-15Rα complexes were assayed in the heterodimeric IL-15/IL-15Rα ELISA. Circles represent RLU average for each IL-15/IL-15Rα concentration tested from 5 independent assays; SD for the 5 assays is shown. The intra-assay variability was 3% to 12% coefficient of variation (CV), and the interassay variability was 18% to 33% CV. Open squares represent RLU values of the indicated amounts of single-chain IL-15 tested in the heterodimeric IL-15/IL-15Rα ELISA (negative control). Linear regression analysis showed no significant correlation (r2 = 0.32, P = .32)
Control experiments using human IL-15, sIL-15Rα, and IL-15/sIL-15Rα heterodimers purified from human cells verified the specificity of the 2 described ELISAs. The commercial IL-15 ELISA is able to detect both single-chain IL-15 and heterodimeric IL-15 complexed to the IL-15Rα, whereas the heterodimeric IL-15/IL-15Rα ELISA detected exclusively the heterodimeric form of IL-15. Purified single-chain IL-15 was quantified by amino acid analysis and tested in the heterodimeric IL-15/IL-15Rα ELISA. RLU readings for all the single-chain IL-15 concentrations used (up to 20 000 pg/mL) were below the limit of the detection of the assay (RLU < 10) and not dose-dependent (linear regression, r2 = 0.32, P = .32; Figure 3 open squares). Similarly, sIL-15Rα was not detected by the heterodimeric ELISA described herein, even if used in high concentration up to 20 000 pg/mL (data not shown). We used these 2 ELISAs to quantify the different forms of IL-15 in human sera.
Circulating IL-15 in serum of cancer patients is associated with sIL-15Rα
We wished to study the molecular form of IL-15 in human serum. In humans, the levels of circulating IL-15 under normal conditions are low or undetectable (∼ 1 pg/mL). However, lymphodepleting chemoradiation regimens result in higher serum IL-15 levels in metastatic melanoma patients.27 Therefore, we used sera from such patients to investigate the form of the circulating IL-15. First, the commercially available ELISA detecting all forms of IL-15 was used (Figure 1A). Eight serum samples showed detectable IL-15 levels, ranging from 45.3 ± 8 pg/mL to 242.4 ± 26 pg/mL (Figure 4A filled bars). In 2 samples (S3, S6), the serum IL-15 levels were low (∼ 2 pg/mL).
Figure 4.
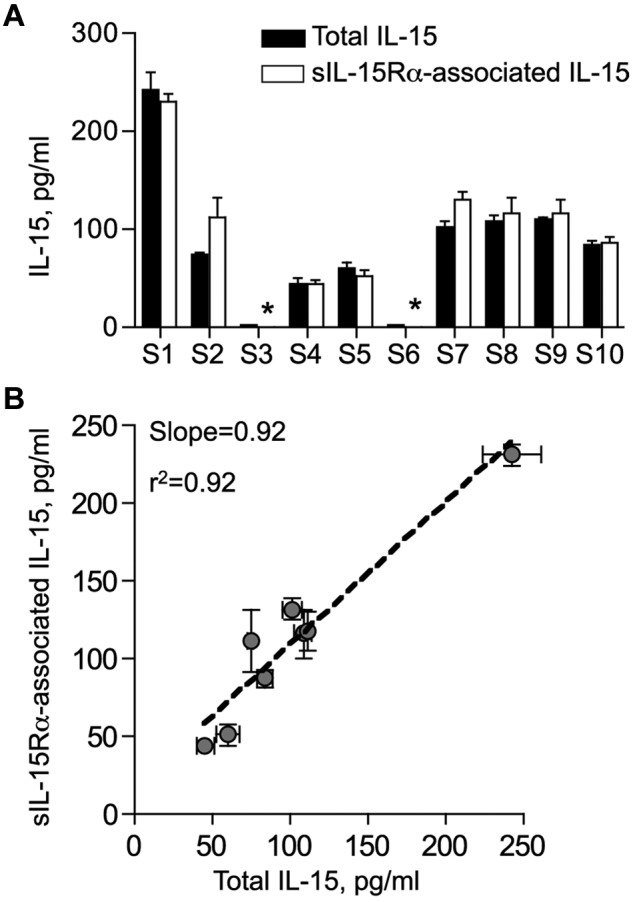
Detection of IL-15 and heterodimeric IL-15/IL-15Rα complexes in human sera. (A) Total IL-15 (filled bars) and sIL-15Rα–associated IL-15 (open bars) in sera of 10 lymphodepleted melanoma patients. Total IL-15 was evaluated using the commercially available IL-15 ELISA (Quantiglo Q1500B). sIL-15Rα–associated IL-15 was detected by the heterodimeric IL-15/IL-15Rα ELISA using capture anti–IL-15 antibody and detection anti–IL-15Rα antibody, as described in “IL-15 and IL-15/IL-15Rα ELISA.” Data are mean ± SEM of 2 to 4 independent measurements. *Value below the limit of detection of the assay. (B) Correlation of total IL-15 and sIL-15Rα–associated IL-15 levels in the sera of lymphodepleted patients. Graph represents mean ± SEM from 2 to 4 independent measurements for each sample. Data were fitted to a linear regression curve using Prism 4.0a software package (correlation coefficient r2 = 0.92; slope = 0.92).
Second, the heterodimeric IL-15/IL-15Rα ELISA was used to detect specifically the IL-15 associated with sIL-15Rα in the same sera. In this assay, IL-15/sIL-15Rα complexes are bound to plate through anti–IL-15 antibody and detected with anti–IL-15Rα antibody (Figure 1B). The 8 patient samples revealed detectable levels of sIL-15Rα–associated IL-15, ranging from 43.5 ± 9 pg/mL to 231.1 ± 14 pg/mL. Two samples (S3, S6) were below the detection limit of the assay (Figure 4A open bars). To establish the fraction of circulating IL-15 that is associated with sIL-15Rα, serum levels of IL-15 obtained by the commercial IL-15 ELISA were compared with the serum levels of sIL-15Rα–associated IL-15 obtained by the heterodimeric IL-15/IL-15Rα ELISA. Figure 4B shows the correlation of the values measured in both ELISAs. The slope of the linear regression curve has an r2 value of 0.92 ± 0.11, supporting the model that essentially all of the IL-15 in human serum is present as heterodimeric complex with sIL-15Rα.
To further investigate whether any free single-chain IL-15 exists in human sera, the patient sera were preincubated with excess of purified sIL-15Rα to allow the formation of heterodimeric complexes by any single-chain IL-15 potentially present in the sample. After a one-hour incubation at room temperature, the heterodimer levels were quantified by the heterodimeric IL-15/IL-15Rα ELISA. Comparisons with the measurements before addition of sIL-15Rα showed that the values measured for each sample did not increase on addition of excess of exogenous sIL-15Rα, indicating that no single-chain IL-15 is present in the patient sera (Figure 5). In control experiments, we showed that increasing amounts of purified sIL-15Rα mixed with known amount of purified single-chain IL-15 resulted in increasing heterodimer values detected by this assay, as expected. These data suggest that IL-15 is exclusively present in association with sIL-15Rα as IL-15/sIL-15Rα heterodimers in sera from lymphodepleted patients.
Figure 5.
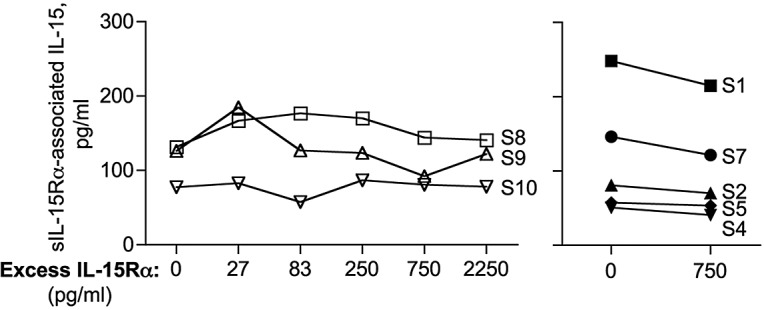
Circulating human IL-15 is exclusively present as heterodimeric IL-15/sIL-15Rα complex. Serum samples from lymphodepleted cancer patients were preincubated with exogenous sIL-15Rα to allow the in vitro formation of IL-15/sIL-15Rα complexes from any free IL-15 potentially present in the sample. Serum samples were next evaluated for the amount of sIL-15Rα–associated IL-15 by ELISA. Serum sample S8, S9, or S10 was preincubated with increasing sIL-15Rα concentrations (0-2250 pg/mL; left panel). Serum samples S1, S2, S4, S5, and S7 were preincubated with a fixed amount of sIL-15Rα (750 pg/mL; right panel). The assay was repeated twice.
Detection of IL-15/IL-15Rα heterodimers in mouse serum
Additional supporting evidence for the presence of IL-15 in the heterodimeric form came from our analysis of mouse sera. A newly developed commercial ELISA (Mouse IL-15R/IL-15 Complex ELISA Ready-SET-Go), using antibodies specific for murine IL-15/sIL-15Rα complexes but unable to recognize the individual molecules, was used to measure IL-15 in mouse sera. The basal levels of IL-15/IL-15Rα detected in normal C57BL/6 mice were 12.2 ± 4.3 pg/mL (Figure 6). On CTX treatment, the mice showed elevated level of IL-15/IL-15Rα, up to day 12 after a single CTX administration, with a peak 5-fold increase at day 3 (53.9 ± 4.4 pg/mL; Figure 6A). The same serum samples tested negative in a different mouse IL-15 ELISA (Mouse ELISA Ready-SET-Go; data not shown), suggesting the inability of the mouse anti–IL-15 antibodies used in the latter assay to recognize IL-15 in complex with sIL-15Rα. To investigate whether all mouse IL-15 is bound to sIL-15Rα, serum samples from untreated and CTX-treated mice were preincubated with excess recombinant mouse IL-15Rα-Fc before IL-15/IL-15Rα heterodimer evaluation by ELISA, in a procedure similar to the one described for human sera. The values measured for each sample did not increase on addition of recombinant mouse IL-15Rα-Fc in normal sera (Figure 6B) or on CTX treatment (Figure 6C), suggesting that no single-chain IL-15 is detectable in mouse serum. These results reinforce the conclusions that IL-15 is normally produced in the body as heterodimeric IL-15/IL-15Rα complex and indicates that lymphodepleting regimens affect the level but not the form of circulating IL-15.
Figure 6.
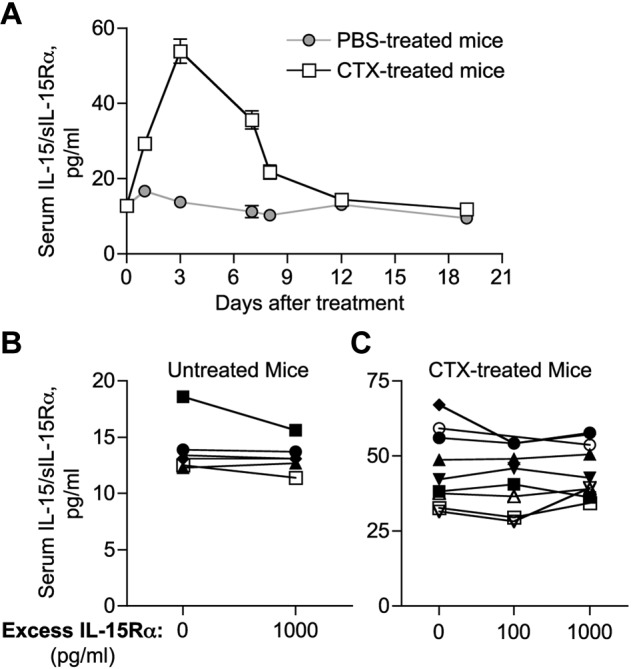
Demonstration of IL-15/sIL-15Rα heterodimers in normal and lymphodepleted mice. (A) Serum levels of IL-15/IL-15Rα heterodimer in normal mice ( ) and on CTX treatment (□). Mice were bled at different time points (days 1-19) after CTX or placebo administration. Circulating IL-15/IL-15Rα was assessed by ELISA (eBioscience) and reported as mean ± SEM of 6 to 33 mice per time point. (B-C) Serum samples from 6 untreated (B) and 9 CTX-treated (day 3 after treatment; C) mice were preincubated with exogenous recombinant mouse IL-15Rα-Fc (100-1000 pg/mL, as indicated) to allow the in vitro formation of IL-15/sIL-15Rα complexes with any free IL-15 potentially present in the sample. Serum samples were next evaluated for the amount of sIL-15Rα–associated IL-15 by ELISA.
) and on CTX treatment (□). Mice were bled at different time points (days 1-19) after CTX or placebo administration. Circulating IL-15/IL-15Rα was assessed by ELISA (eBioscience) and reported as mean ± SEM of 6 to 33 mice per time point. (B-C) Serum samples from 6 untreated (B) and 9 CTX-treated (day 3 after treatment; C) mice were preincubated with exogenous recombinant mouse IL-15Rα-Fc (100-1000 pg/mL, as indicated) to allow the in vitro formation of IL-15/sIL-15Rα complexes with any free IL-15 potentially present in the sample. Serum samples were next evaluated for the amount of sIL-15Rα–associated IL-15 by ELISA.
In addition, analysis of sera from CTX-treated IL-15Rα knockout mice using mouse IL-15 ELISA failed to detect any IL-15 (data not shown), supporting previous conclusions that efficient IL-15 production requires simultaneous expression of IL-15Rα16–19,21,22 and that the 2 molecules are associated in mouse serum.
Different kinetics of appearance of serum IL-15/IL-15Rα heterodimers after CTX administration or whole-body irradiation
To investigate the mechanism(s) leading to elevated levels of circulating IL-15/IL-15Rα heterodimeric complexes in lymphopenic conditions, we compared the effect of 2 different cytoreductive regimens in mice: CTX treatment and whole-body irradiation. Comparison of serum IL-15/IL-15Rα levels over time revealed higher levels of IL-15/IL-15Rα for a prolonged period of time after CTX treatment. Whereas irradiated mice have peak serum IL-15/IL-15Rα levels at day 1 after treatment (3-fold increase), CTX-treated mice have a higher peak at day 3 (5-fold increase). IL-15/IL-15Rα levels declined to pretreatment values at day 7 after irradiation, whereas CTX-treated mice showed persistently elevated levels for up to 12 days after treatment (Figure 7A; P = .007). Mice were killed at different time points after cytoreductive regimens, and the kinetics of lymphocyte depletion and reconstitution were determined by multiparametric flow cytometry and complete blood count. Both cytoreductive treatments had rapid and profound effects, resulting in a severe reduction in the absolute number of NK and CD8+ T cells in spleen (Figure 7B and C, respectively), as well as in the absolute number of lymphocytes in blood (Figure 7D). Lymphocyte depletion was concurrent with the increase in serum IL-15/IL-15Rα levels soon after cytoreductive treatment, suggesting that elimination of cells that normally consume circulating IL-15 is one of the causes for elevated IL-15/IL-15Rα serum levels, as previously suggested.31,32 However, analysis at day 7 after treatment revealed that irradiated mice were characterized by a persistent lymphopenia, which was as severe as in CTX-treated mice, despite their lower levels of circulating IL-15/IL-15Rα (similar to the ones observed in untreated mice; Figure 7).
Figure 7.
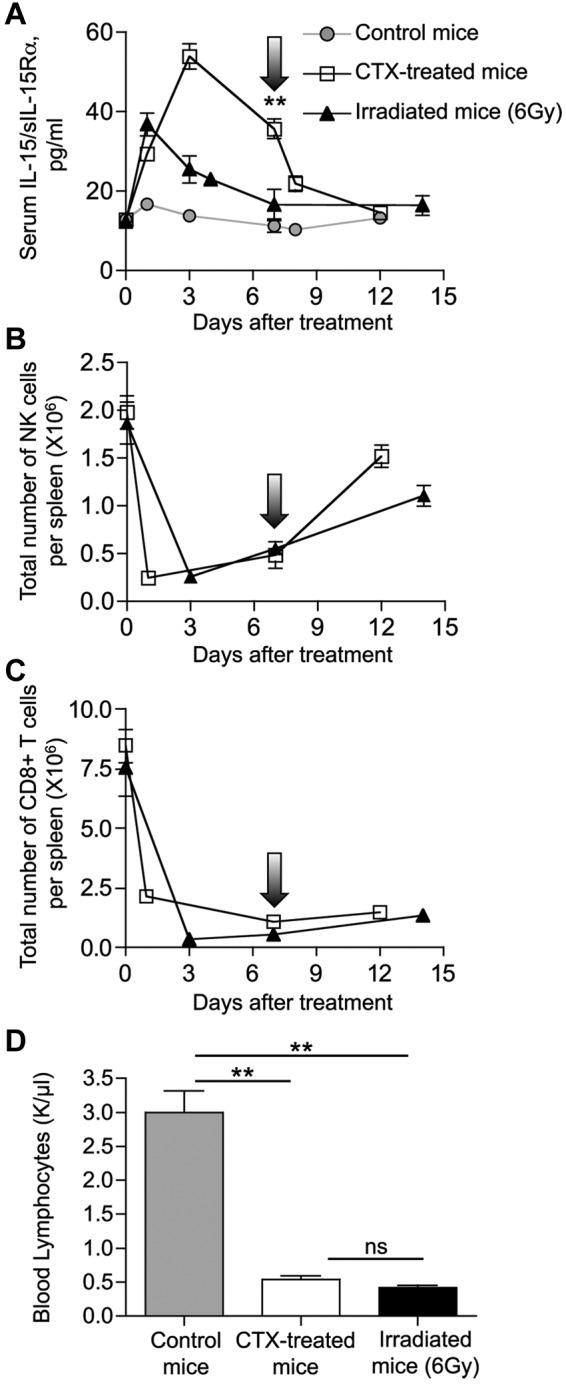
Different kinetics of IL-15/IL-15Rα heterodimers after CTX administration or whole-body irradiation. Six- to 8-week-old C57BL/6 mice were treated with CTX (□) or exposed to 6 Gy of whole-body irradiation (▴). Arrows indicate day 7 after treatment. (A). Mice were bled at the indicated time points after treatment, and the circulating IL-15/IL-15Rα heterodimers were measured by ELISA (eBioscience) and reported as mean ± SEM of 2 to 33 mice per time point.  represents the average level of circulating mouse IL-15/IL-15Rα from 10 normal mice. **P = .007. (B-C) Mice were killed at the indicated time points after treatment, and spleens were collected for analysis. Total number of NK cells (B) and CD8+ T cells (C) per spleen over time was determined by flow cytometry. (D) Absolute count of lymphocytes in blood at day 7 after cytoreductive treatments. Three mice per group were analyzed. Hematologic profile on 10 untreated mice was also performed as control. **P < .01. ns indicates not significant.
represents the average level of circulating mouse IL-15/IL-15Rα from 10 normal mice. **P = .007. (B-C) Mice were killed at the indicated time points after treatment, and spleens were collected for analysis. Total number of NK cells (B) and CD8+ T cells (C) per spleen over time was determined by flow cytometry. (D) Absolute count of lymphocytes in blood at day 7 after cytoreductive treatments. Three mice per group were analyzed. Hematologic profile on 10 untreated mice was also performed as control. **P < .01. ns indicates not significant.
Taken together, these data suggest that IL-15 bioavailability is determined only partially by consumption, as additional mechanisms may contribute to the higher and persistent IL-15/IL-15Rα levels observed in CTX-treated mice. Differential induction of IL-15/IL-15Rα production could explain the difference in IL-15/IL-15Rα serum level observed after CTX treatment and whole-body irradiation.
Discussion
In this work, we took advantage of our purified, human cell-produced IL-15/sIL-15Rα heterodimers to establish a reliable and specific assay for the evaluation of the form of circulating IL-15 in human serum. The purified human heterodimers were used as standards in an ELISA to facilitate specific quantification of IL-15/sIL-15Rα heterodimers. The important conclusion from this work is that IL-15 circulates in the plasma as heterodimer associated with sIL-15Rα. Mixing experiments providing additional purified sIL-15Rα suggested that the single-chain IL-15 is not present in detectable amounts in serum, reinforcing the conclusion that IL-15Rα serves as a chaperone molecule rescuing unstable single-chain IL-15 from intracellular degradation and promoting its secretion.21,22,33,34 In agreement with the human data, similar analysis performed in normal, lymphodepleted, or IL-15Rα knockout mice revealed that all detectable IL-15 in mouse serum is bound to sIL-15Rα. The absence of single-chain IL-15 both under physiologic conditions or on lymphopenia induction in mice suggests that lymphodepleting treatments only affect the level but not the form of IL-15 produced in the body (ie, IL-15/IL-15Rα complexes). These findings provide new insights into the mechanism of regulation and action of IL-15 and will directly aid in the development of novel methodologies for the detection and study of human IL-15. At present, the only IL-15 international standard available is a single-chain IL-15 produced in yeast (NIBSC). Assays based on this standard may underestimate IL-15 levels in biologic fluids because of masking by the interaction with IL-15Rα or by glycosylation and other modifications. Therefore, we propose that a standard human IL-15 heterodimer preparation, such as the material used in this study, be used as an improved international standard in IL-15 assays.
The size of the cellular compartment consuming IL-15 through the binding to IL-2/IL-15Rβγ is important for the amount of the free cytokine found in circulation.31,32 Analysis of lymphocyte populations in correlation to IL-15/IL-15Rα serum levels on 2 different cytoreductive regimes in mice (CTX treatment and whole-body irradiation) suggests that elimination of cellular “sinks” contributes but is not the only mechanism leading to elevated level of circulating IL-15/IL-15Rα. Additional mechanisms may also determine the final plasma concentration of heterodimeric IL-15. CTX therapy has been associated with the induction of a proinflammatory microenvironment involving factors, such as lipopolysaccharide, monocyte chemotactic protein-1, and IFN-α,35 known to up-regulate IL-15 and IL-15Rα expression.36 After CTX administration, IL-15 RNA levels in spleen have been reported to increase by 2-fold 2 days after the treatment and to go back to baseline level at day 4,37 suggesting that increased gene expression may also contribute to the overall spike in circulating IL-15/IL-15Rα levels at day 3 after CTX administration. Alternatively, irradiation may result in the elimination of cells producing IL-15/IL-15Rα. Increased shedding of IL-15/IL-15Rα complexes from the cell surface can also contribute to the elevated level of serum IL-15/IL-15Rα after lymphodepleting treatment. Recently, a member of the polytopic membrane family of proteins, iRhom2, has been identified as the molecular regulator of the ADAM family metalloprotease TACE (TNF-α–converting enzyme). On inflammation, iRhom2 binds TACE and promotes its trafficking from the endoplasmic reticulum to the cell surface, the site of cleavage of target transmembrane proteins, such as TNF-α, CD62L, and IL-6R.38 The regulation if IL-15Rα ectodomain shedding is still poorly understood, but a similar mechanism may be involved. Further studies are necessary to elucidate the mechanisms of IL-15/IL-15Rα regulation in the body.
IL-15 is known to act as a cell-associated cytokine. Dubois et al demonstrated that IL-15Rα on the surface of bone marrow–derived cells, such as dendritic cells and activated monocytes/macrophages, presents surface bound IL-15 to nearby cells expressing the IL-2/IL-15Rβγ.16 This process, called trans-presentation, directly sustains maturation, survival, and proliferation of NK, CD8+ T cells and intraepithelial lymphocytes in mice and humans.34,39–43 Several studies have also suggested that the simultaneous expression of IL-15Rα in the same cell is required for the production and secretion of IL-15 under physiologic conditions.16–19 We have previously demonstrated that co-production leads to intracellular association of IL-15 and IL-15Rα in the endoplasmic reticulum, stabilization of both molecules, and efficient transport to the cell surface. We showed that an additional critical step is the rapid cleavage and release of the IL-15/IL-15Rα complex from the cell surface, both in vitro and in vivo, resulting in a soluble, systemically active form of IL-15/IL-15Rα, in addition to the bioactive complex on the cell surface.22 Our experiments using IL-15 complexed to a C-terminal deletion of IL-15Rα consisting only of the soluble receptor-α extracellular fragment demonstrated that the circulating heterodimer is also bioactive in vivo in the absence of any membrane-bound form.22 These results are in agreement with the reported ability of recombinant sIL-15Rα to act as potent agonist of IL-15 function in vivo23–26 and provide the molecular basis to explain some intriguing observations, including the requirement of production of IL-15 and IL-15Rα from the same cells for appropriate function in vivo.18,19 It has also been reported that the cells expressing IL-15 also express IL-15Rα,16 further supporting our findings of IL-15 production as a heterodimer in the body. Interpretation of all data available to date suggests that, in both human and mouse, the IL-15/IL-15Rα heterodimeric form, either cell-associated or soluble, is responsible for IL-15 bioactivity. According to this model, the cell-associated IL-15 heterodimer is the newly produced full complex displayed on the surface of the cell, whereas cleavage of this complex leads to the circulating bioactive form in the plasma, which can act distant to the production site. It remains to be determined whether IL-15 heterodimers on the surface of the cells or in a soluble circulating form act on different lymphocyte populations, deliver different type of signals, or have different roles in physiologic or pathologic conditions. Mortier et al have recently suggested that NK cells and effector/memory CD8+ T-cell subsets differentially use IL-15/IL-15Rα signals delivered by either dendritic cells or macrophages in mice.44 The findings support the hypothesis that IL-15 exerts its physiologic function in a cell-contact-dependent fashion and that plasma membrane-bound IL-15/IL-15Rα signal may synergize with different costimulatory signals specific for different lymphocyte subpopulations.34,44 However, it is conceivable that under certain conditions, such as lymphopenia, the soluble heterodimer has the potential to transmit IL-15 signals at a distance (ie, bone marrow or peripheral tissues), stimulating maturation and differentiation of immature lymphocytes and activation and tissue redistribution of NK cells or resting memory CD8+ T cells. In humans undergoing allogeneic stem cell transplantation, an elevated level of IL-15 favors the engraftment of donor NK with elevated levels of tumor-reactive activating receptors.31 Recent reports demonstrated that human IL-15/sIL-15Rα boosts human T-cell reconstitution in a humanized mouse model.45 In addition, soluble IL-15 heterodimers were reported to support survival and proliferation of immature human thymocyte subsets as well as to accelerate thymopoiesis.46 Taken together, these findings suggest that increased IL-15 bioavailability results in strong systemic growth and mobilization signals for lymphocytes. Therefore, the ability of IL-15 to form stable soluble heterodimers with sIL-15Rα that circulate in the bloodstream and are transported to sites away from the production makes IL-15 a hormone-like molecule, capable to act at distance, in addition to the membrane-bound heterodimeric cytokine with locally restricted functions during cell-to-cell interaction. This hypothesis suggests that the cell-associated form of IL-15/IL-15Rα has local activity at the immunologic synapse, probably in conjunction with codelivered signals, whereas the soluble heterodimer found in the plasma can have systemic functions, such as supporting lymphopenia-induced proliferation or mobilization/trafficking of NK and T cells. We hypothesize that serum IL-15/IL-15Rα can act at a distance also under physiologic conditions. The ability to act at a distance is a function of the superior stability of the IL-15/IL-15Rα complexes. The measured circulating levels of IL-15 represent the small portion that remains free after the interaction with the large number of cells having the IL-2/IL-15Rβγ, which form a “sink” as argued by many investigators.31,32
These results supported previous reports showing that IL-15Rα is part of the active IL-15 cytokine,21,22 and have important implications for development of assays and materials for clinical application of IL-15. Although single-chain IL-15 was shown to be bioactive in primates,47–50 the use of IL-15 heterodimers may provide additional advantages because IL-15/IL-15Rα is the natural IL-15 cytokine produced in human body and circulating in the blood and is more stable and bioactive in vivo compared with monomeric IL-15.22,24,25 The heterodimeric form of IL-15 may allow simpler delivery of bioactive cytokine at appropriate levels for in vivo activity and may minimize the possibility for adverse events.
Acknowledgments
The authors thank Drs Antonio Valentin, Rashmi Jalah, Viraj Kulkarni, Brunda Ganneru, and Guy Pilkington (Vaccine Branch, Frederick National Laboratory for Cancer Research [FNLCR], Frederick, MD) for critical discussions and technical assistance; Drs Jeffrey Lifson (AIDS and Cancer Virus Program, SAIC-Frederick Inc, FNLCR, Frederick, MD) and Crystal Mackall (Pediatric Oncology Branch, NCI, Bethesda, MD) for critical review of the manuscript; and Terry Jones (Vaccine Branch, FNLCR, Frederick, MD) for editorial assistance.
This work was supported in part by the National Cancer Institute, National Institutes of Health (contract HHSN261200800001E), and by the Research Program of the Center for Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), NIH.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.B., E.C., and G.N.P. designed research; C.B., J.B., M.R., R.K.B., C.A., R.S., and E.C. performed experiments and analyzed data; B.K.F. analyzed data; S.A.R. contributed clinical samples and reviewed the manuscript; and C.B., B.K.F., and G.N.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George N. Pavlakis and Cristina Bergamaschi, Human Retrovirus Section, Vaccine Branch, Center for Cancer Research, FNLCR, PO Box B, Bldg 535, Rm 206, Frederick, MD 21702-1201; e-mail: pavlakig@mail.nih.gov or bergamac@mail.nih.gov.
References
- 1.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91(11):4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 3.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170(10):5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 4.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180(4):1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 7.Giri JG, Ahdieh M, Eisenman J, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13(12):2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 10.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352(6336):621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 11.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 12.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A. 1997;94(7):3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119(2):482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 15.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14(15):3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17(5):537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 17.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koka R, Burkett PR, Chien M, et al. Interleukin (IL)-15Ralpha-deficient natural killer cells survive in normal but not IL-15Ralpha-deficient mice. J Exp Med. 2003;197(8):977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15Ralpha by the same cells. J Immunol. 2004;173(11):6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 20.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 2004;103(3):988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 21.Bergamaschi C, Jalah R, Kulkarni V, et al. Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo. J Immunol. 2009;183(5):3064–3072. doi: 10.4049/jimmunol.0900693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergamaschi C, Rosati M, Jalah R, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283(7):4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 23.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180(4):2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein MP, Kovar M, Purton JF, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc Natl Acad Sci U S A. 2006;103(24):9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177(9):6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortier E, Quemener A, Vusio P, et al. Soluble interleukin-15 receptor alpha (IL-15Ralpha)-sushi as a selective and potent agonist of IL-15 action through IL-15Rbeta/gamma: hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem. 2006;281(3):1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 27.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalah R, Rosati M, Kulkarni V, et al. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol. 2007;26(12):827–840. doi: 10.1089/dna.2007.0645. [DOI] [PubMed] [Google Scholar]
- 29.Dubois S, Magrangeas F, Lehours P, et al. Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. J Biol Chem. 1999;274(38):26978–26984. doi: 10.1074/jbc.274.38.26978. [DOI] [PubMed] [Google Scholar]
- 30.Kurys G, Tagaya Y, Bamford R, Hanover JA, Waldmann TA. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J Biol Chem. 2000;275(39):30653–30659. doi: 10.1074/jbc.M002373200. [DOI] [PubMed] [Google Scholar]
- 31.Boyiadzis M, Memon S, Carson J, et al. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant. 2008;14(3):290–300. doi: 10.1016/j.bbmt.2007.12.490. [DOI] [PubMed] [Google Scholar]
- 32.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duitman EH, Orinska Z, Bulanova E, Paus R, Bulfone-Paus S. How a cytokine is chaperoned through the secretory pathway by complexing with its own receptor: lessons from interleukin-15 (IL-15)/IL-15 receptor alpha. Mol Cell Biol. 2008;28(15):4851–4861. doi: 10.1128/MCB.02178-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205(5):1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salem ML, Kadima AN, El-Naggar SA, et al. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30(1):40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- 36.Neely GG, Robbins SM, Amankwah EK, et al. Lipopolysaccharide-stimulated or granulocyte-macrophage colony-stimulating factor-stimulated monocytes rapidly express biologically active IL-15 on their cell surface independent of new protein synthesis. J Immunol. 2001;167(9):5011–5017. doi: 10.4049/jimmunol.167.9.5011. [DOI] [PubMed] [Google Scholar]
- 37.Bracci L, Moschella F, Sestili P, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13(2):644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 38.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 335(6065):225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huntington ND, Legrand N, Alves NL, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J Immunol. 2009;183(2):1044–1054. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104(2):588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112(12):4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortier E, Advincula R, Kim L, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31(5):811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Huntington ND, Alves NL, Legrand N, et al. IL-15 transpresentation promotes both human T-cell reconstitution and T-cell-dependent antibody responses in vivo. Proc Natl Acad Sci U S A. 108(15):6217–6222. doi: 10.1073/pnas.1019167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huntington N, Alves N, Legrand N, et al. Autonomous and extrinsic regulation of thymopoiesis in human immune system (HIS) mice. Eur J Immunol. 2011;41(10):2883–2893. doi: 10.1002/eji.201141586. [DOI] [PubMed] [Google Scholar]
- 47.Berger C, Berger M, Hackman RC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114(12):2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugli E, Goldman CK, Perera LP, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116(17):3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picker LJ, Reed-Inderbitzin EF, Hagen SI, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116(6):1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]