Abstract
Rationale
Previous research suggests that the vesicular monoamine transporter-2 (VMAT2) is a novel target for the treatment of methamphetamine (METH) abuse.
Objective
The effects GZ-793A, a novel, selective, and potent lobelane analog, on the rewarding effects of METH, cocaine, and palatable food in rats were determined.
Method
GZ-793A (3–30 mg/kg, s.c.) was administered 20 min prior to each session in which the groups of rats pressed a lever for infusions of METH (0.03 mg/kg/infusion), cocaine (0.3 mg/kg/infusion), or food pellets. Tolerance to repeated GZ-793A (15 mg/kg, s.c. for 7 days) on METH self-administration and food-maintained responding was determined. The ability of increasing doses of METH (0.001–0.56 mg/kg, i.v.) to surmount inhibition produced by GZ-793A (15 mg/kg, s.c.) was determined. Self-administration of GZ-793A (0.01–0.3 mg/kg/infusion, i.v.) was tested as a substitute for METH infusion. GZ-793A (15 mg/kg, s.c.) was administered 20 min prior to METH or saline conditioning in a place preference test.
Results
GZ-793A specifically decreased METH self-administration, without the development of tolerance. Increasing the unit dose of METH did not surmount the inhibition produced by GZ-793A on METH self-administration. GZ-793A did not serve as a substitute for self-administered METH. GZ-793A blocked METH-induced conditioned place preference (CPP) and did not induce CPP alone.
Conclusions
These results indicate that VMAT2 is a viable target for pharmacological inhibition of METH reward and that GZ-793A represents a new lead in the discovery of a treatment for METH abuse.
Keywords: VMAT2, Methamphetamine, Self-administration, CPP, Lobeline, Lobelane, Abuse, Addiction
Introduction
Methamphetamine (METH) abuse is a serious public health concern. According to the National Institute on Drug Abuse (NIDA), as of 2007, 1.3 million people over the age of 12 reported using METH within a 12-month period. Currently, treatment for METH abuse relies on cognitive–behavioral therapies, 12-step programs, and contingency management. While psychosocial therapies are effective, evidence suggests that they are significantly more effective in treating drug abuse in conjunction with pharmacological treatment (e.g., Anton et al. 1999; Evins et al. 2001). Unfortunately, there are no FDA-approved medications available to treat METH abuse.
Drug and natural rewards (e.g., food) are mediated primarily by mesocorticolimbic dopamine (DA) function (e.g., Wise and Rompre 1989). METH alters numerous components of DA system function including decreasing DA metabolism by inhibition of monoamine oxidase, reversing DA translocation by the dopamine transporter (DAT), augmenting DA release from synaptic vesicles, increasing cytosolic DA concentrations for DAT reverse transport, and indirectly enhancing DA synthesis via activation of tyrosine hydroxylase (Fleckenstein et al. 2009; Guillot and Miller 2009). The outcome is a METH-induced increase in extracellular DA, critically involved in its rewarding effects (Vollm et al. 2004). Thus, pharmacotherapies for METH abuse have focused on targets that decrease METH-induced DA release (Vocci and Appel 2007).
Pharmacological inhibition of the vesicular monoamine transporter-2 (VMAT2) may be an effective strategy to attenuate METH-induced increases in extracellular DA and diminish METH reward (Dwoskin and Crooks 2002; Zheng et al. 2006). Lobeline, an alkaloid from Lobelia inflata and VMAT2 inhibitor (Teng et al. 1997), reduces amphetamine- and METH-induced DA release from rat striatal slices (Miller et al. 2001; Nickell et al. 2010). Lobeline attenuates METH self-administration in rats (Harrod et al. 2001) and reduces amphetamine- and METH-induced hyperactivity (Miller et al. 2001). Phase 1b clinical trials found lobeline to be safe in METH-abusing individuals (Jones 2007). However, lobeline is not selective for VMAT2, acting as an antagonist at nicotinic receptors (nAChRs; Dwoskin and Crooks 2002), prompting the search for more selective VMAT2 inhibitors as potential efficacious therapeutics for METH abuse.
Lobelane, a defunctionalized lobeline analog (Fig. 1; Zheng et al. 2006), with little affinity for nAChRs (Miller et al. 2004; Nickell et al. 2010) attenuates METH self-administration specifically and blocks METH-induced hyperactivity; however, tolerance develops to the effects of lobelane (Neugebauer et al. 2007). UKCP-110, a pyrrolidine nor-lobelane analog, potently and selectively inhibits VMAT2 function, inhibits METH-evoked striatal DA release, exhibits low affinity for nAChRs, and specifically attenuates METH self-administration, without producing tolerance (Beckmann et al. 2010). These findings indicate that novel compounds that inhibit VMAT2 have a preclinical profile supporting their development as METH abuse treatments.
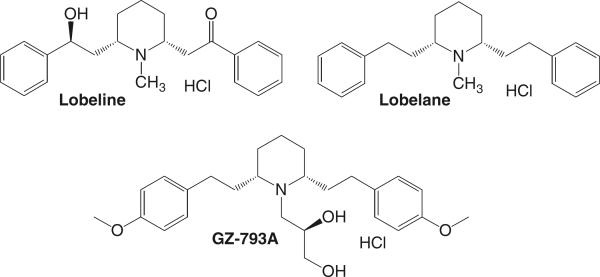
Fig. 1.
Chemical structures for lobeline, lobelane, and GZ-793A
In the current study, the ability of a novel N-dihydroxyl lobelane analog, N-(1,2R-dihydroxylpropyl)-2,6-cis-di-(4-methoxyphenethyl)piperidine hydrochloride, GZ-793A (Fig. 1), to block METH reward was examined. GZ-793A is a potent and selective VMAT2 inhibitor that eliminates METH-induced striatal DA release (Horton et al. 2011b). These promising neurochemical results provide the impetus to determine the ability of GZ-793A to block METH reward via self-administration and conditioned place preference (CPP) assays. GZ-793A specificity was evaluated by determining its ability to block cocaine and food reward via self-administration and food-maintained operant responding, respectively. The development of tolerance to these effects of GZ-793A was determined. To evaluate potential for abuse liability, GZ-793A was assessed for its ability to maintain self-administration and to establish CPP.
Materials and methods
Subjects
Adult male Sprague–Dawley rats (Harlan Inc., Indianapolis, IN), 2–4 months of age, were housed individually in a temperature- and humidity-controlled environment with a 14:10 h light/dark cycle; experimentation was conducted during the light phase. The rats were acclimated to the colony and handled daily for 1 week. Rats were maintained on an ad libitum feeding schedule. Experimental protocols were in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
Operant conditioning experiments were conducted in operant conditioning chambers (ENV-008, MED Associates, St. Albans, VT), enclosed within sound-attenuating compartments (ENV-018M, MED Associates). Each chamber was connected to a PC interface (SG-502, MED Associates) and operated using MED-PC™. Within each chamber, there was a 5×4.2-cm recessed food tray, two extended retractable levers mounted on either side of the recessed food tray (7.3 cm above metal rod floor), and a 28-V, 3-cm diameter, white cue light mounted 6 cm above each lever. Drugs (0.1 ml over 5.9 s) were infused via syringe pump (PHM-100, MED Associates) through a water-tight swivel attached to a head-mounted cannula.
CPP experiments were conducted in rat CPP chambers (MED-CPP-RS, MED Associates), enclosed in sound-attenuating compartments. Each chamber contained three compartments separated by manual guillotine doors and illuminated with white 28-V lights: two 28-cm long compartments (black with steel rod floor or white with wire mesh floor) separated by a 12-cm long compartment (gray with smooth plastic floor).
Drugs
d-Methamphetamine HCl was purchased from Sigma-Aldrich (St. Louis, MO), and cocaine HCl was a gift from the NIDA (Bethesda, MD). N-(1,2R-Dihydroxylpropyl)-2,6-cis-di-(4-methoxyphenethyl)piperidine HCl (GZ-793A, Fig. 1) was synthesized according to reported methods (Horton et al. 2011b). Compounds were dissolved in sterile saline (0.9% NaCl) and administered as salt weights.
METH and cocaine self-administration
METH and cocaine self-administration were carried out according to previous studies (Beckmann et al. 2010; Green et al. 2010; Harrod et al. 2001; Neugebauer et al. 2007), including training, drug dose, and stability criteria. Rats were trained briefly to respond on one lever for food reinforcement (active lever) on an FR 1 schedule, while responding on the other lever had no consequence (inactive lever; counterbalanced). The rats were anesthetized (100 mg/kg ketamine and 5 mg/kg diazepam, i.p.) and catheters were implanted into the right jugular vein. Following a 1-week recovery period, two groups of rats were trained to press the active lever for either an infusion of METH (0.03 mg/kg/infusion) or cocaine (0.3 mg/kg/infusion). Each infusion was followed by a 20-s signaled (illumination of both lever lights) time out, and the response requirement was gradually increased to a terminal fixed ratio 5 (FR 5) schedule of reinforcement over nine sessions. Each daily session lasted 60 min, and training continued until responding stabilized, with 20% or less variability in the number of infusions earned across three successive sessions, at least a 2:1 ratio of active to inactive lever responses, and at least ten infusions earned per session. Once rats reached the terminal FR 5, the average number of sessions to criteria was not different between METH (8.0±1.39) and cocaine (6.57±0.20) groups. GZ-793A (3, 10, 15, or 30 mg/kg, s.c.) or saline was administered acutely 20 min prior to the session, according to a Latin square design. At least two maintenance sessions (i.e., no treatment) were included between each test session to ensure stable responding. To investigate tolerance to the effects of GZ-793A on METH self-administration, a separate group of rats was administered GZ-793A (15 mg/kg, s.c.) or saline 20 min prior to each of seven consecutive METH self-administration sessions.
Food-maintained responding
The methods for food-maintained responding were identical to those for METH and cocaine self-administration, with two exceptions; rats did not undergo catheter implantation surgery and rats responded for food pellets [45-mg pellets (BIO-SERV, #F0021, Frenchtown, NJ] throughout the experiment.
METH dose–effect
The METH dose–effect experiments were conducted using published methods (Beckmann et al. 2010; Harrod et al. 2001). A group of rats was trained initially to self-administer METH (0.03 mg/kg/infusion). Following stable responding, METH unit dose (0.001–0.1 mg/kg/infusion, including saline) was varied across sessions, according to a Latin square design. Then, to determine if higher doses of METH would surmount the effect of GZ-793A, GZ-793A (15 mg/kg, s.c.) was administered 20 min prior to METH self-administration sessions across METH doses (0.001–0.56 mg/kg/infusion, including saline). Each METH dose was tested on two consecutive 60-min sessions and data averaged for statistical analysis. At least two maintenance sessions, in which rats self-administered the training dose (0.03 mg/kg/infusion), were included between each test session to ensure stable responding.
GZ-793A substitution
Rats were trained to self-administer METH (0.03 mg/kg/infusion), and following stability, assigned to the saline or GZ-793A group matched for baseline responding. GZ-793A (0.01, 0.03, 0.1, and 0.3 mg/kg/infusion; ascending dose order) or saline was substituted for METH over an additional 20 sessions, with each dose tested for five consecutive days. Then, the rats resumed self-administration of the training dose of METH (0.03 mg/kg/infusion) for five sessions.
METH-conditioned place preference
CPP procedures were based on published methods (Neugebauer 2008). A single preconditioning day was followed by eight conditioning days and a post-conditioning test day. During the preconditioning day, rats had free access for 15 min to all CPP chamber compartments (black, white, and gray). The rats were randomly assigned to four groups (saline–saline, saline–METH, GZ-793A–saline, and GZ-793A–METH). On four of the eight conditioning days, rats were injected with saline or GZ-793A (15 mg/kg, s.c.) 20 min prior to an injection of saline or METH (0.5 mg/kg, s.c.) and were confined immediately to a side compartment (black or white, counterbalanced). On alternating conditioning days, the rats were injected with saline and confined to the alternate compartment. During the post-conditioning day, rats were had free access for 15 min to all compartments. A difference score (time spent in drug-associated compartment during post-conditioning day minus time spent in drug-associated compartment during preconditioning day) defined CPP. No injections were given on the pre- or postconditioning days.
Statistical analyses
Separate one-way repeated-measures analysis of variance (ANOVA) determined the acute effects of GZ-793A on METH self-administration, cocaine self-administration, food-maintained responding, and inactive lever pressing. Separate two-way mixed-factors ANOVAs determined the effects of repeated GZ-793A on METH self-administration, food-maintained responding, inactive lever pressing, and body weight. Data were analyzed as percent baseline for the acute and repeated GZ-793A experiments. Baseline was defined as the two baseline days preceding treatment (acute effects) or treatment session 1 (repeated effects). Separate two-way mixed-factors ANOVAs determined the effects of GZ-793A on METH self-administration and food-maintained responding across time, on the METH dose–effect curve, and on the effect of substituting GZ-793A for METH during self-administration. A one-way ANOVA determined the effect of GZ-793A on METH-induced CPP. Post hoc analyses were performed using Bonferroni-corrected pairwise comparisons.
Results
Effects of acute GZ-793A on METH self-administration, cocaine self-administration, and food-maintained responding
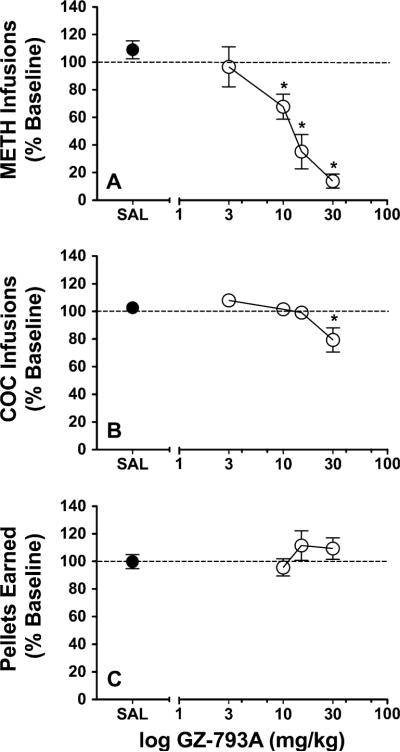
GZ-793A dose-dependently decreased the number of METH infusions, expressed as a percent change from baseline (baseline mean ± SEM=18.5±1.47 infusions; Fig. 2a). One-way ANOVA revealed a main effect of dose [F(4, 20)=13.99, p<0.01]. Post hoc analyses indicated that GZ-793A (10, 15, and 30 mg/kg) significantly decreased METH infusions. In contrast, one-way ANOVA revealed no main effect of GZ-793A dose on inactive lever presses during METH self-administration (data not shown; baseline mean ± SEM=5.83±3.51 inactive lever responses).
Fig. 2.
Acute effects of GZ-793A on METH self-administration, cocaine self-administration, and food-maintained responding. a Relative to SAL control, GZ-793A treatment (s.c.) dose-dependently decreased METH infusions, n=6. b GZ-793A treatment (s.c.) dose-dependently decreased cocaine infusions, n=7. c GZ-793A treatment (s.c.) did not alter pellets earned, n=12. Asterisk denotes significant difference relative to SAL control
GZ-793A also dose-dependently decreased the number of cocaine infusions, expressed as percent change from baseline (baseline mean ± SEM=33.3±1.46 infusions; Fig. 2b). One-way ANOVA revealed a main effect of dose [F(4, 24)=5.69, p<0.01]. Post hoc analyses indicated that only the highest dose of GZ-793A (30 mg/kg) significantly reduced cocaine infusions. In contrast, one-way ANOVA revealed no main effect of GZ-793A on inactive lever presses during cocaine self-administration (data not shown; 0.54±0.18 inactive lever responses).
GZ-793A did not alter the number of pellets earned, expressed as a percent change from baseline (baseline mean ± SEM=49.4±3.71 pellets; Fig. 2c). One-way ANOVA revealed no main effect of dose on the number of pellets earned [F(3, 33)=1.26, p>0.05]. In addition, like METH and cocaine self-administration, one-way ANOVA revealed no main effect of dose on inactive lever presses during food-maintained responding [F(3, 33)=2.60, p>0.05]; data not shown; 1.50±0.57 inactive lever responses).
Effects of repeated GZ-793A on METH and food-maintained responding
Since the 15 mg/kg dose of GZ-793A reduced METH infusions by ~50% (Fig. 2), this dose was used in subsequent experiments. Figure 3 (top panel) illustrates that across seven consecutive sessions, GZ-793A (15 mg/kg) administration continued to decrease METH self-administration, expressed as a percent change from baseline (baseline mean ± SEM for GZ-793A group=17.5±2.0 infusions; saline group=19.3±1.6 infusions). Two-way ANOVA revealed a main effect of treatment [F(1, 6)=45.24, p<0.01], but no main effect of session or treatment × session interaction, indicating that repeated GZ-793A reduced METH self-administration across the seven consecutive sessions. Post hoc analyses indicated that, relative to saline control, METH infusions were significantly decreased following GZ-793A for each of the seven consecutive sessions. In contrast, a two-way ANOVA revealed no main effect of GZ-793A on inactive lever presses during METH self-administration (data not shown; GZ-793A group, 4.90±1.05 inactive lever responses; saline group, 5.75±1.27 inactive lever responses).
Fig. 3.
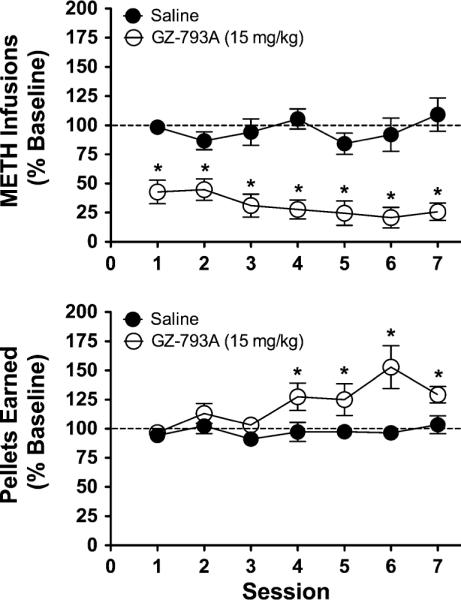
Effect of repeated GZ-793A on METH self-administration and food-maintained responding. Top panel Relative to saline control, repeated GZ-793A (15 mg/kg; s.c.) treatment significantly decreased METH infusions, without the development of tolerance, n=4/group. Bottom panel Repeated GZ-793A (15 mg/kg; s.c.) treatment significantly increased pellets earned, n=6/group. Asterisk denotes significance relative to saline control
Figure 3 (bottom panel) illustrates that GZ-793A (15 mg/kg) administration across seven consecutive sessions increased the number of pellets earned across sessions, expressed as percent change from baseline (baseline mean ± SEM for GZ-793A group=48.3±4.66 pellets; saline group=46.0±2.39 pellets). Two-way ANOVA revealed a main effect of treatment [F(1, 10)=15.31, p<0.01], a main effect of session [F(6, 60)=2.93, p<0.05], and a trend toward a significant treatment × session interaction [F(6, 60)=2.16, p=0.05], demonstrating that GZ-793A increased the number of pellets earned and that this increase varied as a function of repeated treatments. Post hoc analyses indicated that GZ-793A significantly increased pellets earned on sessions 4–7. In contrast, like METH self-administration, a two-way ANOVA revealed no significant effects of GZ-793A on inactive lever presses during food-maintained responding (data not shown; GZ-793A group, 0.57±0.11 inactive lever presses; saline group, 0.71±0.16 inactive lever presses).
The time course of effect for GZ-793A to decrease METH infusions during the last session of repeated treatment (session 7) is illustrated in Fig. 4. Two-way ANOVA revealed a main effect of treatment [F(1, 6)= 16.61, p<0.01] and time [F(11, 66)=3.17, p<0.01] and a time × treatment interaction [F(11, 66)=2.62, p<0.01], demonstrating that GZ-793A treatment reduced METH infusions throughout the last session (Fig. 4). Post hoc analyses indicated that GZ-793A significantly reduced METH infusions at 5–15, 25–35, and 45 and 60 min time points.
Fig. 4.
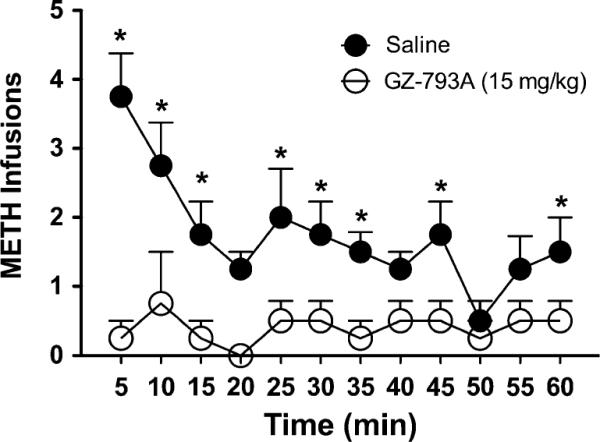
GZ-793A time course. GZ-793A (15 mg/kg; s.c.) treatment significantly reduced METH infusions throughout the last session of repeated treatment (session 7), n=4. Asterisk denotes significance relative to saline control
Two-way ANOVA revealed a main effect of session [F (6, 36)=25.84, p<0.01], but no main effect of GZ-793A treatment and no session × treatment interaction on body weight across METH self-administration sessions. As expected, weight increased across sessions, and repeated GZ-793A treatment had no effect on weight gain (data not shown). Regarding the effect of repeated GZ-793A treatment on body weight across food-maintained sessions, twoway ANOVA revealed a main effect of session [F(6, 60)= 34.43, p<0.01], as well as a treatment × session interaction [F(6, 60)=7.51, p<0.01]. Weight increased across sessions; however, rats treated with repeated GZ-793A gained weight at a significantly higher rate than the saline control group (GZ-793A group=4% increase; saline group=2% increase; data not shown).
Effect of GZ-793A on METH dose–effect curve
GZ-793A treatment produced a downward shift in the METH dose–effect curve (Fig. 5). Two-way ANOVA revealed a main effect of METH dose [F(5, 45)=6.77, p< 0.01] and GZ-793A treatment [F(1, 9)=20.76, p<0.01], as well as a dose × treatment interaction [F(5, 45)=8.40, p< 0.01] on number of METH infusions, demonstrating that varying the METH unit dose altered the number of METH infusions and that GZ-793A treatment reduced METH infusions across unit doses. Post hoc analyses indicated that GZ-793A treatment significantly reduced METH infusions at the 0.01 and 0.03 mg/kg/infusion unit doses of METH.
Fig. 5.
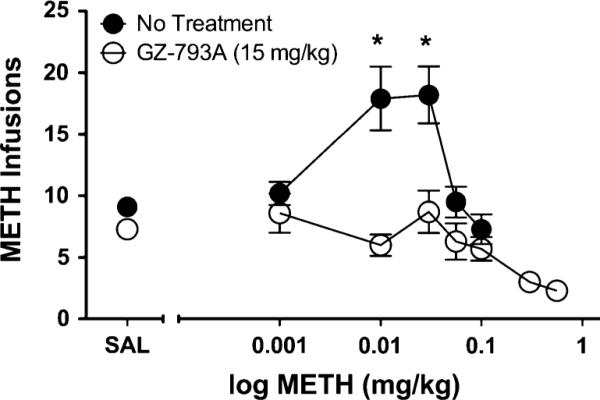
Effect of GZ-793A on METH dose-effect. The reduction in METH infusions following GZ-793A treatment (15 mg/kg; s.c.) was not surmounted by increasing the self-administered METH unit dose, n=10. Asterisk denotes significance relative to no pretreatment
GZ-793A substitution
Baseline levels of METH self-administration did not differ between the GZ-793A and saline groups (Fig. 6). Following stability, either GZ-793A (0.01–0.3 mg/kg/infusion) or saline was substituted for METH (0.03 mg/kg/infusion) on sessions 2–21. The number of infusions did not differ between the GZ-793A and saline groups. On sessions 22–26, when METH self-administration was reinstated, the number of METH infusions earned by the GZ-793A group was not different from that earned by the saline group. Two-way ANOVA revealed a main effect of session [F(24, 288)=6.83, p<0.01], but no effect of substitution group or session × substitution group interaction. When either GZ-793A or saline was substituted for METH (session 21), the number of infusions was decreased compared to baseline [t (24)=−8.32, p<0.01]. When METH was reinstated (session 26), the number of infusions was increased compared to the last session in which GZ-793A or saline was substituted for METH (session 21) [t(24)=4.32, p<0.01]. Thus, when GZ-793A or saline was substituted for METH, the level of extinction was not different between GZ-793A and saline control groups, and the extinguished responding was reinstated in both groups by reinstating METH self-administration.
Fig. 6.
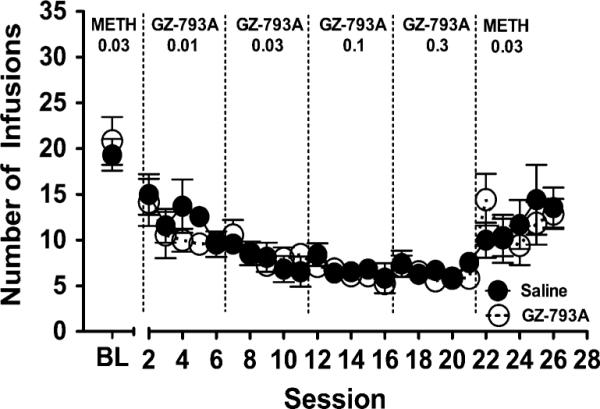
GZ-793A substitution. Rats did not differentially self-administer GZ-793A (0.01–0.3 mg/kg/infusion) relative to saline control. Baseline infusions represent self-administration of METH (0.03 mg/kg/infusion); sessions 1–21 represent substitution of varying unit doses of GZ-793A or saline; sessions 22–26 represent reinstatement of METH (0.03 mg/kg/infusion) self-administration, n=7/group
Effect of GZ-793A on METH CPP
GZ-793A treatment blocked the development of METH CPP, while having no effect (no preference or aversion) when given alone (Fig. 7). One-way ANOVA revealed a main effect of treatment group [F(3, 24)=7.02, p<0.01]. Post hoc analyses indicated that the difference score was greater in the saline (SAL)–METH group compared to all other groups. No other between-group differences were found.
Fig. 7.
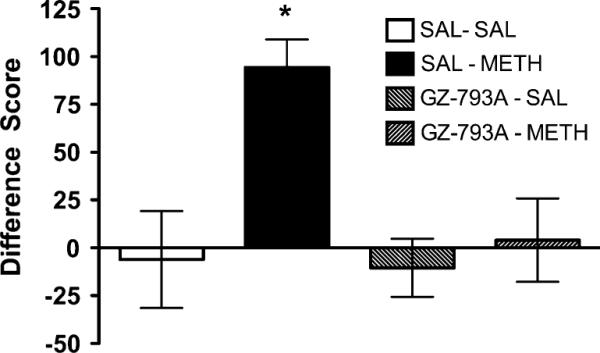
Effect of GZ-793A on METH CPP. Treatment with GZ-793A (15 mg/kg; s.c.) blocked METH CPP, while GZ-793A treatment alone had no effect (no preference or aversion), n=7/group. Asterisks indicate significance relative to SAL-SAL control
Discussion
The current study was a comprehensive investigation of the ability of GZ-793A to specifically block METH primary and conditioned reward. GZ-793A decreased METH self-administration throughout the 60-min session. Importantly, both METH self-administration and CPP were blocked at GZ-793A doses that did not produce a corresponding decrease in cocaine self-administration, food-maintained responding, or conditioned place aversion. The specificity of GZ-793A for METH reward, relative to cocaine, is likely due to differences in mechanisms of action, where METH directly interacts with VMAT2 and cocaine does not (Fleckenstein et al. 2009; Wilson and Kish 1996). Furthermore, tolerance did not develop to the specific effects of GZ-793A on METH self-administration. The ability of GZ-793A to decrease METH self-administration was not surmounted by increasing METH unit doses, suggesting noncompetitive inhibition. Given that stimulant pretreatments are known to decrease stimulant self-administration (Caine et al. 1999, 2000; Wilson and Schuster 1973), the effects of GZ-793A on METH self-administration could be interpreted as a substitution effect, with GZ-793A serving as a substitute reinforcer to reduce METH intake. However, this interpretation is unlikely because GZ-793A was not self-administered and did not produce CPP, indicating that GZ-793A treatment does not decrease METH self-administration through substitution; these results also suggest that GZ-793A would have low abuse liability. The emergence of GZ-793A from an iterative drug discovery approach (Harrod et al. 2001; Neugebauer et al. 2007; Beckmann et al. 2010) demonstrates that potent and selective inhibition of VMAT2 function translates to optimal behavioral characteristics that are requisite for a candidate pharmacotherapy to treat METH abuse.
Within the dose range evaluated, GZ-793A did not decrease food-maintained responding. On the contrary, food intake was increased as a function of repeated GZ-793A treatment, and relative to saline control, was accompanied by a marginal body weight gain (4% increase from baseline weight). Although speculative, a number of underlying mechanisms could be responsible for the increase in responding for food pellets and weight gain, including perseverative responding on the food-reinforced lever, a general increase in motivation for palatable food, alterations in metabolic rate, and elevated hypothalamic noradrenergic function which could contribute to weight gain (Shimazu et al. 1986). A comparison of the effects on food-maintained responding of GZ-793A with its parent compounds, lobeline and lobelane, reveals that only GZ-793A increased responding for food. However, several procedural differences between these studies limit direct comparisons. For example, the parent compounds were tested under restricted post-session access to food, whereas the current study evaluating GZ-793A employed unrestricted post-session food access throughout experimentation. Furthermore, lobeline was tested in 15-min food-maintained responding sessions without a time-out following pellet delivery (Harrod et al. 2001). Lobelane was tested in 60-min sessions with a 100-s time-out following each pellet delivery (Neugebauer et al. 2007). Schedules used in the studies evaluating the parent compounds may have artificially suppressed the number of pellets earned within a session. In the current study, GZ-793A was tested on food-maintained responding using a schedule identical to that for METH self-administration, i.e., FR5 with 20-s time-out following pellet delivery. In any case, the current finding that repeated GZ-793A treatment did not decrease food-maintained behavior indicates that GZ-793A does not produce general response suppression or toxic effects within the dose range evaluated.
A growing body of evidence, including the current findings, demonstrates that inhibition of VMAT2 function specifically blocks METH reward (Harrod et al. 2001; Neugebauer et al. 2007; Beckmann et al. 2010). Furthermore, VMAT2 inhibition selectively reduces METH-induced striatal DA release (Miller et al. 2004; Beckmann et al. 2010; Nickell et al. 2010; Horton et al. 2011a, b), consistent with the critical involvement of DA function in reward (Berridge 2007; Wise and Rompre 1989; Everitt and Robbins 2005). The effect of GZ-793A to inhibit METH-evoked DA release is selective, since GZ-793A does not alter nicotine-evoked or field stimulation-evoked striatal DA release (unpublished results). The inability of GZ-793A to serve as a reinforcer is likely due to its inability to elevate extracellular DA when administered alone (Horton et al. 2011b). Similar to self-administration, CPP is dependent on DA function (Bardo and Bevins 2000). Thus, the inability of GZ-793A to elevate extracellular DA offers an explanation as to why GZ-793A does not establish a place preference.
GZ-793A has marked advantages as a clinical candidate for the treatment of METH abuse relative to its parent compounds (lobeline and lobelane). First, GZ-793A exhibited greater efficacy in decreasing METH self-administration, while also demonstrating greater specificity than its parent compounds (present results; Harrod et al. 2001; Neugebauer et al. 2007; Beckmann et al. 2010). However, direct comparisons of the present results with previous findings are limited due to several procedural differences instituted while evaluating the parent compounds relative to GZ-793A. A second advantage of GZ-793A is that it exhibits a longer duration of action in decreasing METH self-administration than either lobeline or lobelane (Harrod et al. 2001; Neugebauer et al. 2007), with inhibition throughout the entire 60-min session. The increase in the duration of action of GZ-793A compared to its parent compounds suggests that GZ-793A has an improved pharmacokinetic profile (i.e., longer plasma half-life and/or lower clearance rate) and improved drug-like properties, and may be more amenable to the development of clinical dosage formulations. Finally, GZ-793A is more selective inhibiting VMAT2 function than either lobeline or lobelane (Horton et al. 2011b), suggesting that GZ-793A may have fewer side effects.
A controversy surrounding the development of VMAT2 inhibitors as pharmacotherapies for METH abuse is the potential for enhancement of METH-induced neurotoxicity. The neurotoxicity with repeated administration of high doses of METH in rats is associated with a decrease in VMAT2 protein immunoreactivity and function (Eyerman and Yamamoto 2007), which may result in an elevation of cytosolic DA, leading to DA auto-oxidation and neurotoxicity. Further, VMAT2 knockout mice show increased METH neurotoxicity relative to wild-type mice (Fumagalli et al. 1999; Guillot and Miller 2009; Larsen et al. 2002; Vergo et al. 2007). However, reversible pharmacological inhibition of VMAT2 function has not been reported to exacerbate METH neurotoxicity in knockout or wild-type animals. On the contrary, lobeline protects against METH neurotoxicity (Eyerman and Yamamoto 2005), and lobeline decreases METH-evoked striatal DA release (Nickell et al. 2010). Supporting evidence of the safety of VMAT2 inhibitors includes that tetrabenazine has FDA approval for the treatment of Huntington's chorea (Bohnen et al. 2000; Suzuki et al. 2001), and that METH-addicted individuals given lobeline in phase II clinical trials exhibited no adverse effects (Jones 2007). Based on observations that GZ-793A is a more potent and selective inhibitor of VMAT2 than lobeline, neuroprotection from METH-induced toxicity would be predicted for GZ-793A. Thus, while METH-induced neurotoxicity may be enhanced in VMAT2 knockout mice, reversible pharmacological inhibition of VMAT2 appears to protect against METH neurotoxicity.
In summary, the current comprehensive investigation of the behavioral pharmacology of GZ-793A reveals that this novel compound specifically blocks METH primary and conditioned reward and has low abuse liability. The current research demonstrates that potent and selective inhibition of VMAT2 function translates to optimal behavioral characteristics that are requisite for a candidate pharmacotherapy to treat METH abuse.
Acknowledgments
We thank Kate Fischer for technical assistance. This work was supported by National Institutes of Health National Institute on Drug Abuse (R01 DA13519 and T32DA01617). The University of Kentucky holds patents on lobeline, lobelane, UKCP-110, and GZ-793A. A potential royalty stream to Dwoskin, Crooks, and Zheng may occur consistent with University of Kentucky policy.
References
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Siripurapu KB, Nickell JR, Denehy ED, Vartak AP, Crooks PA, et al. The pyrrolidine analog of nor-lobelane, cis-2,5-di-(2-Phenethyl)-pyrrolidine hydrochloride, inhibits VMAT2 function, dopamine release, and methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2010;335(3):841–851. doi: 10.1124/jpet.110.172742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Koeppe RA, Meyer P, Ficaro E, Wernette K, Kilbourn MR, et al. Decreased striatal monoaminergic terminals in Huntington disease. Neurology. 2000;54(9):1753–1759. doi: 10.1212/wnl.54.9.1753. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291(1):353–360. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D1-like and D2-like agonists in rats trained to discriminate cocaine from saline: influence of experimental history. Exp Clin Psychopharmacol. 2000;8(3):404–414. doi: 10.1037//1064-1297.8.3.404. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63(2):89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(1):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine Tob Res. 2001;3(4):397–403. doi: 10.1080/14622200110073920. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J Pharmacol Exp Ther. 2005;312(1):160–169. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103(3):1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology. 2009;56(Suppl 1):133–138. doi: 10.1016/j.neuropharm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19(7):2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67(1):28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 2009;39(2):149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298(1):172–179. [PubMed] [Google Scholar]
- Horton DB, Siripurapu KB, Norrholm SD, Culver JP, Hojahmat M, Beckmann JS, et al. meso-Transdiene analogs inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release. J Pharmacol Exp Ther. 2011a;336(6):940–951. doi: 10.1124/jpet.110.175117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DB, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. Novel N-1,2-dihydroxypropyl analogs of lobelane inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release. J Pharmacol Exp Ther. 2011b doi: 10.1124/jpet.111.184770. doi:10.1124/jpet.111.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. Double-blind, placebo-controlled, cross-over assessment of intravenous methamphetamine and sublingual lobeline interactions. 2007 NCT00439504. ClinicalTrials.gov.
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22(20):8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, et al. Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther. 2001;296(3):1023–1034. [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Zheng G, Grinevich VP, Norrholm SD, Dwoskin LP. Lobeline analogs with enhanced affinity and selectivity for plasmalemma and vesicular monoamine transporters. J Pharmacol Exp Ther. 2004;310(3):1035–1045. doi: 10.1124/jpet.104.068098. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM. Doctor of Philosophy Thesis. University of Kentucky; Lexington, KY: 2008. The effects of lobeline on methamphetamine-induced conditioned place preference and dopaminergic alterations in the nucleus accumbens shell. [Google Scholar]
- Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, Bardo MT. Lobelane decreases methamphetamine self-administration in rats. Eur J Pharmacol. 2007;571(1):33–38. doi: 10.1016/j.ejphar.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell JR, Krishnamurthy S, Norrholm S, Deaciuc G, Siripurapu KB, Zheng G, et al. Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J Pharmacol Exp Ther. 2010;332(2):612–621. doi: 10.1124/jpet.109.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Noma M, Saito M. Chronic infusion of norepinephrine into the ventromedial hypothalamus induces obesity in rats. Brain Res. 1986;369(1-2):215–223. doi: 10.1016/0006-8993(86)90530-5. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Desmond TJ, Albin RL, Frey KA. Vesicular neurotransmitter transporters in Huntington's disease: initial observations and comparison with traditional synaptic markers. Synapse. 2001;41(4):329–336. doi: 10.1002/syn.1089. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. Lobeline and nicotine evoke [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. J Pharmacol Exp Ther. 1997;280(3):1432–1444. [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102(Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Vollm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, et al. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29(9):1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kish SJ. The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci. 1996;16(10):3507–3510. doi: 10.1523/JNEUROSCI.16-10-03507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Schuster CR. The effects of stimulants and depressants on cocaine self-administration behavior in the rhesus monkey. Psychopharmacologia. 1973;31(4):291–304. doi: 10.1007/BF00421274. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: role as a novel target for drug development. AAPS J. 2006;8(4):E682–E692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]