Abstract
An electronic survey was used to assess the training needs of clinical and public health researchers who have been involved, and/or plan to become involved, in clinical trials of herbal medicines in Africa. Over 90 researchers were contacted through pre-existing networks, of whom 58 (64%) responded, from 35 institutions in 14 African countries. Over half (57%) had already been involved in a clinical trial of an herbal medicine, and gave information about a total of 23 trials that have already been completed. Of these, only five had been published, and only one had resulted in a licensed product. Fifty-four (54) of the researchers were planning to conduct a clinical trial of an herbal medicine in the future, and gave information about 54 possible trials. Respondents outlined the following most commonly encountered difficulties when conducting clinical trials: resource constraints (including lack of funding, equipment, staff, and infrastructure); social acceptance of the clinical trial (including difficulty recruiting enough patients, poor rapport with traditional healers, and willingness of biomedical staff to be involved); herbal medicine supply (including insufficient cultivation, production, and quality control); lack of trained staff; and logistical issues in conducting trials. The topics in which researchers were least confident were Intellectual Property Rights issues, statistical issues, and issues related to Good Clinical Practice guidelines.
Introduction
There is much research on traditional herbal medicines in Africa, but very little of it is clinical. For example, of over 1200 plant species reportedly used for the treatment of malaria, only 13 have undergone clinical trials, although hundreds have been tested in the laboratory.1 Of the few clinical trials that have been conducted, few are of adequate quality.1,2 In an effort to address this situation, the Multidisciplinary University Traditional Health Initiative (MUTHI)* in collaboration with the Research Initiative on Traditional Antimalarial Methods (RITAM), conducted a training needs assessment for clinical and public health researchers in African universities who have conducted, or are planning to conduct, clinical research on herbal medicines.
Methods
A questionnaire was designed asking researchers about their past experience of clinical trials of herbal medicines and future plans to conduct such trials. Participants were selected purposively according to their previously expressed interest in clinical trials, through several networks such as MUTHI, ANDI (African Network for Drugs and Diagnostics Innovation, http://apps.who.int/tdr/svc/partnerships/initiatives/andi), and RITAM (Research Initiative on Traditional Antimalarial Methods, www.gifts-ritam.org). In addition, requests were also sent to the European and Developing Country Clinical Trial Networks of Excellence and the researchers enrolled in the Cochrane African HIV/AIDS Mentoring Programme.
The questionnaire was sent and returned by e-mail. In the e-mail, it was requested that the survey be forwarded on to other interested parties, thereby using a snowballing technique to increase the surveyed population. The questionnaire was also translated into French to maximize the participation of francophone researchers.
Respondents were asked about their own background and training. They were asked to give information about any clinical trials of herbal medicines in which they had been involved in the past or present, and any future trials they were planning to do. Their consent was sought to publish a summary of information on trials that had already finished. They were asked to list any difficulties they had encountered, or that they anticipated would be a problem in future trials. Respondents were asked to rate the importance of various aspects of clinical trial design, conduct, and reporting, and then to rate their confidence in these aspects (on a scale of 0–5, with 0=no confidence and 5=very confident).
Responses were entered into a Microsoft Access database. Both quantitative and qualitative methods were used to analyze the results. Regarding the difficulties encountered, the responses were grouped into general themes; the frequency with which each theme was mentioned was counted, but some illustrative quotations were also cited. The difficulties were also grouped according to whether or not they could be addressed through further training.
Results
The first request was sent in February 2011 and reminder e-mails were sent in March 2011. In total, the questionnaire was distributed to at least 91 researchers in 18 African countries; 58 of the researchers responded by the deadline (response rate=63%). The respondents came from 35 institutions in 14 African countries (Table 1). Twenty-nine percent (29%) of the respondents were women. The average age was 39.9 years (range 24–64 years). Ninety-one percent (91%) had a Bachelor's degree or higher and 31% already had a PhD. Several were in the process of studying for a PhD. However, only 19 (34%) had a clinical background; the others were nonclinical scientists (9 pharmacists, 9 pharmacologists, 5 biochemists, 5 ethnobotanists†). Most of the clinicians were general physicians (seven) or public health doctors (five); there were also three herbalists,‡ two specialists (a gastroenterologist and a psychiatrist), two research nurses, and a psychologist.
Table 1.
Number of Institutions and Respondents per Country
| Country | No. of institutions | No. of respondents |
|---|---|---|
| Uganda | 11 | 18 |
| Nigeria | 6 | 12 |
| Cameroon | 5 | 5 |
| Mali | 2 | 5 |
| Sudan | 2 | 3 |
| South Africa | 1 | 3 |
| Burkina Faso | 1 | 3 |
| Democratic Republic of Congo | 1 | 2 |
| Tanzania | 1 | 2 |
| Gabon | 1 | 1 |
| Kenya | 1 | 1 |
| Madagascar | 1 | 1 |
| Mozambique | 1 | 1 |
| Zambia | 1 | 1 |
Thirty-two (32; 57%) of the respondents had already been involved in conducting a clinical trial of an herbal medicine. Information was reported about 23 completed and 5 ongoing clinical trials of herbal medicines; 18 researchers gave consent to publish a summary of their completed trials, which appears in Table 2 (an additional 4 did not respond to this request, and 1 did not give consent). By far the most common conditions for which herbal medicines had been studied were malaria (nine trials) and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) (six trials). Not all of the trials named the herb that had been studied, but the herbs most commonly quoted were Artemisia annua (five trials) and Vernonia amygdalina (two trials). Only five trials (21%) had been published,3–7 and only two of these in a peer-reviewed, MEDLINE®-listed journal. Only one had resulted in a product with an official marketing authorization: Niprisan for the prevention of crises in patients with sickle cell disorder.4,8 Another product (Faradin, also for sickle cell disease) had been licensed although the trial had not been published.
Table 2.
Clinical Studies Already Conducted on Herbal Medicines in Africa
| Type of condition | Participants | Intervention | Comparison | Outcomes | Study type | No. of patients | Country | Ref. |
|---|---|---|---|---|---|---|---|---|
| Dermatological | Fungal skin disease and acne | “Natural plus” ointment: petroleum jelly with extracts of Warbugia ugandensis, Psorospermum febrifugium, and Zanthoxylum chalybeum | Dettol, petroleum jelly | Clearance of rashes, incidence of adverse effects | Observational | 20 | Uganda | U |
| Hepatitis | Asymptomatic chronic hepatitis B | Entada africana; Entada africana+Combretum micranthum; Cochlospermum tinctorium | None | Clearance of HBeAg and HBsAg | Observational | 360 | Mali | U |
| HIV/AIDS | HIV/AIDS patients, CD4<250, not on ARVs | Undisclosed herbal formula | None | CD4 count | Observational | 12 | Uganda | U |
| Itchy skin rashes in HIV/ AIDS patients | Albizia coriaria, Erythrina abbyssinica, Psorospermum febrifugum | None | Clinical resolution of itchy rashes | Observational | 15 | Uganda | U | |
| HIV / AIDS | PMG151 (4-plant mixture) | PMG+ARVs | CD4 count, symptoms | Controlled | 372 | Burkina Faso | U | |
| HIV / AIDS | Spirulina+ARVs | ARV only | Interactions, adverse events | Controlled | 309 | Burkina Faso | U | |
| Asymptomatic HIV-infected patients not on ARVs | Polysacharride extracts of Agaricus Blazei Mycelia | Starch | Clinical condition; CD4 count; viral load | Controlled | Zambia | 3 | ||
| Malaria | Malaria treatment | “Ajadilopia” (subsequently discovered to contain CQ) | CQ | Controlled | Nigeria | U | ||
| Uncomplicated malaria (adults only) | Artemisia annua | AS/SP | Parasite clearance, pharmacokinetics | RCT | 151 | Mozambique | U | |
| Uncomplicated falciparum malaria (adults and children) | Artemisia annua | Artesunate; AS/AQ | ACR, adverse events | RCT | 73 | Cameroon | 6 | |
| Healthy volunteers | Nauclea pobeguinii | Nil | Adverse events | Phase I | 15 | DRC | 5 | |
| Uncomplicated malaria (adults only) | Nauclea pobeguinii | AS/AQ | ACPR, FCT, adverse events | Phase IIb | 65 | DRC | Submitted | |
| Healthy volunteers | Artemisia annua | Thea sinensis | Incidence of malaria, adverse effects | Observational | 191 | Uganda | 7 | |
| Uncomplicated malaria | Vernonia amygdalina | None | Parasite clearance | Observational | 15 | Uganda | U | |
| Uncomplicated malaria | Artemisia annua | SP, quinine, co-artem | Symptom clearance | Observational | Uganda | U | ||
| Uncomplicated malaria (adults and children) | NIPRD 92/001/1-1 | CQ, SP | Parasite clearance | RCT | Nigeria | U | ||
| Respiratory | Children and adults with cough | Syrup containing Guiera senegalensis (leaves) and Vetiveria nigritiana Stapf (rhizomes) | Codeine | Cough frequency | Controlled | 16 | Mali | U |
| Sickle cell anemia | Sickle cell anemia (Hb SS) patients who had experienced at least 3 crises in the past year | Niprisan (Sorghum bicolor, Eugenia caryophyllum, Pterocarpus osun, Piper guineensis) | Placebo | Outcomes include frequency and severity of major crises, blood transfusion, complete blood count | Randomized crossover trial | 69 | Nigeria | 4, 8 |
U, unpublished; HBeAg, hepatitis e antigen; HBsAg, hepatitis B surface antigen; HIV/AIDS, human immunodeficiency virus/acquired immune deficiency syndrome; ARVs, antiretrovirals; CQ, chloroquinine; AS, artesunate; AQ, Amodiaquine; ACPR, adequate clinical and parasitologic response; FCT, fever clearance time; SP, sulfadoxine-pyrimethamine; RCT, randomized controlled trial.
Reasons for not publishing trials varied. Some were still in the process of writing up the research. One respondent mentioned that their institute's policy was not to publish clinical results until phase III trials were completed and an application for registration had been filed. However, in most cases it seems that other reasons account for the lack of publication.
Diverse difficulties were quoted; for example, in one case ethical approval had not been sought; in another the study clinician had moved to another project before the trial was completed; the computer containing the trial data had been stolen and there was no backup for one trial; in another case the product had been discovered to be a fraud (adulterated with chloroquine).
Only one of these trials had not encountered any difficulties. Table 3 summarizes the main themes of difficulties cited, and their frequency. Most trials had encountered more than one difficulty. The most frequently cited were constraints in resources (particularly funding, but also lack of infrastructure, equipment, and staff). Social acceptance of the clinical trials was the second most frequent theme. For example, six projects had found it difficult to recruit enough patients, four had found that compliance with the treatment was problematic, and four had found it difficult to involve biomedical health workers in the research. For example, a research nurse quoted “negative attitudes of biomedical professionals on herbal medicine study.” A pharmacologist who had conducted a study of herbal prophylaxis for malaria reported “some opposition from allopathic medicine practitioners resulting from standard government policies of management of malaria using ACT combination therapies.” A research nurse reported that in two trials, traditional healers were “reserved with their information regarding herbal formula before initiating the study.” Logistics (particularly loss to follow-up of patients, and long distance to study sites) and supply of herbal medicines were the next most frequent themes. For example, an ethnobotanist reported difficulty in “acquiring sufficient plant materials for use which are mainly wild crafted.”
Table 3.
Difficulties Encountered in Past or Ongoing Clinical Trials, or Anticipated for Future Clinical Trials of Herbal Medicines
| Frequency in past/ ongoing trials (n=28) | Frequency anticipated in future trials (n=54) | Broad category | Examples |
|---|---|---|---|
| 19 | 35 | Resource constraints | Lack of funding; lack of equipment; lack of human resources |
| 18 | 25 | Social acceptance of the clinical trials | Rapport with traditional healers, willingness of healers to cooperate; difficulty involving biomedical doctors; patient recruitment; patient compliance |
| 9 | 8 | Logistical constraints | Loss to follow-up of patients; distance to study sites; trial management |
| 9 | 19 | Herbal medicine supply | Cultivation, sustainable harvesting and production of herbal medicine; formulation and quality control |
| 6 | 12 | Need for training or support | Lack of trained staff |
| 3 | 3 | Trial design | Protocol design, blinding, data management and analysis |
| 2 | 5 | Ethics | Obtaining ethical approval |
Fifty-four (54; 96%) of the researchers were planning future clinical trials of herbal medicines, and gave their ideas or plans for 54 possible future trials. A few of these were well developed, with a detailed protocol already prepared, whereas most were simply ideas for future research. As with existing trials, the majority of future trials were planned for the treatment of HIV/AIDS (15 trials) or malaria (13 trials). Apart from infectious diseases, the other areas of greatest interest were primary care (including diabetes, hypertension, obesity, low back pain, fever—nine trials), and mental health, gastroenterology, and dermatology (four trials each). Although many respondents did not disclose the names of the herbs they were planning to study, those most often quoted were well-known herbs that have already been studied: Aloe vera (four trials), Phyllanthus spp (four trials), Artemisia annua (three trials), and Moringa oleifera (three trials). Another 22 plant species were named once each.
Table 3 also lists the difficulties anticipated by researchers in running future trials; these are similar to those encountered in past trials. Among the social issues mentioned was rapport with traditional healers (for example, a research nurse anticipated “traditional healers concealing information about their herbs if not well prepared and issues of intellectual property right handled” [sic]). More respondents considered that supply of the herbal medicine would be an issue for future trials (for example, an herbalist wrote “some of the plants we may want to test are now difficult to find because of deforestation/over-harvesting.”)
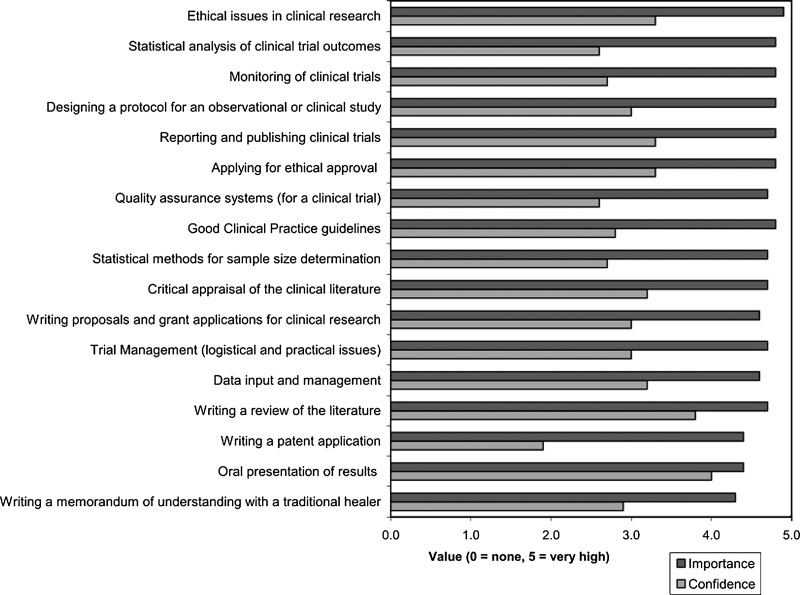
The respondents' average ratings of the importance of various aspects of clinical trials, and their confidence in dealing with them, are given in Figure 1. Almost all aspects were rated as important or very important by most respondents. The aspects that were rated as most important were ethical issues, statistical analysis, protocol design, monitoring, reporting, and publishing trial results. Intellectual property rights (IPR) issues such as writing a memorandum of understanding with a traditional healer, or a patent application, were rated as least important. The items in which researchers were least confident were IPR issues, statistical issues, and issues related to Good Clinical Practice guidelines (GCP). Regarding the method of training, 51 of the 58 respondents (87%) were English-speaking or said they were able to train in English, and 48 (84%) said they would be able to participate in online training modules.
FIG. 1.
Average ratings of the importance attached to, and respondents' confidence in dealing with, different aspects of clinical trials.
Discussion
The responses confirm that the questionnaire reached the target group: clinical and public health researchers in African institutions with an interest in conducting clinical trials of herbal medicines. The majority were from a nonclinical background, however, and already had a high level of academic qualifications. There are of course other African institutions conducting clinical trials of herbal medicines that did not respond; in some cases this may be because they felt they already have sufficient expertise and do not need further training. There was a wide range of expertise among the respondents, ranging from very junior staff with no experience of clinical research to a few senior academics with extensive experience of clinical trials of herbal medicines.
It was surprising to find out that many clinical trials had been conducted, and that very few of these had been published. This highlights the need to strengthen capacity for conducting clinical trials in Africa, and to improve the quality of trials so that they are conducted to a high, publishable standard, and so that effort is not wasted.
The predominance of HIV/AIDS and malaria among the conditions studied may reflect the areas of interest of the networks that were used for disseminating the questionnaires, but they undoubtedly also represent two of the greatest public health problems facing sub-Saharan Africa. Although the difficulties cited in running trials were mainly social, financial, and logistical, it is estimated that 57% of these could be addressed through training. Resource constraints were categorized as not easily addressed by training, although training in completing grant applications may help. However, it was judged that some aspects of social acceptability of the trial can be improved through training: A good literature review and protocol design will encourage biomedical staff to participate, and a memorandum of understanding will improve rapport with traditional healers. Good participant information sheets and community relations will help to improve recruitment. Other issues amenable to training are herbal medicine production and formulation, clinical trial design, and ethics, analysis, and reporting.
The questionnaires were not anonymized, which may have biased the responses, particularly regarding confidence in dealing with certain aspects of clinical trials (for example, some respondents rated themselves as “very confident” in “reporting and publishing clinical trials” although they had never published a clinical trial report). Many also rated their confidence as high in writing proposals and grant applications, yet cited lack of funding as one of the difficulties they face. Writing a memorandum of understanding with a traditional healer was rated (on average) as less important than other aspects, although difficulties in rapport with traditional healers were cited for several past and future trials.
The MUTHI project hopes to address many of the outlined difficulties in focused training workshops, online activities, and mentoring of researchers. An initial workshop was held at the University of the Western Cape (South Africa) in November 2011, addressing many of the priority issues. Further workshops are planned in Mali and Uganda. Online resources will be made available, and an open online discussion group has been started at http://globalhealthtrials.tghn.org/community/groups/group/traditional-medicine/topics/125/
Conclusions
There is much interest, but insufficient capacity, in African universities for clinical research on traditional African herbal medicines. Improving the quality of trials should increase the number of publications and so the impact of the research. Some proposed trials had already been planned very carefully and were close to fruition, whereas others were no more than very preliminary ideas. Areas for training prioritized by respondents are ethical issues, statistical analysis, protocol design, GCP, reporting and publishing trial results, and IPR issues. These areas have been prioritized for developing training materials.
Footnotes
Botanists specialising in studying the traditional uses of plants
Traditional healers specialising in the prescription of herbal remedies
Acknowledgments
This work was funded by the European Union Research Directorate through the MUTHI project, FP7 Grant Agreement No.: 266005. Members of the MUTHI group helped to identify respondents and conduct this work, in particular Prof. Bernard Kiremire (Makerere University) and Prof. Drissa Diallo (University of Bamako).
Disclosure Statement
No financial conflicts exist.
References
- 1.Willcox ML. Bodeker G. Traditional herbal medicines for malaria. BMJ. 2004;329:1156–1159. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willcox M, et al. Do ethnobotanical and laboratory data predict clinical safety and efficacy of anti-malarial plants? Malaria J. 2011;10(suppl 1):S7. doi: 10.1186/1475-2875-10-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handema R. Terunuma H. Kasolo F. A prospective study of Agaricus blazei mycelia compound administration in asymptomatic HIV-infected patients in Lusaka, Zambia. Med J Zambia. 2007;8:1–4. [Google Scholar]
- 4.Wambebe C, et al. Double-blind, placebo-controlled, randomised cross-over clinical trial of niprisan in patients with sickle cell disorder. Phytomedicine. 2001;8:252–261. doi: 10.1078/0944-7113-00040. [DOI] [PubMed] [Google Scholar]
- 5.Mesia K, et al. Assessment of the short-term safety and tolerability of a quantified 80% ethanol extract from the stem bark of Nauclea pobeguinii (PR 259 CT1) in healthy volunteers: A clinical phase I study. Planta Med. 2011;77:111–116. doi: 10.1055/s-0030-1250134. [DOI] [PubMed] [Google Scholar]
- 6.Kengne RDC, et al. Comparative study of the quality and efficiency of artemisinin drug based and Artemisia annua grown in Cameroon. 5th MIM Pan-African Malaria Conference; Kenya: Nairobi; 2009. p. 87. [Google Scholar]
- 7.Ogwang PE, et al. Use of Artemisia annua L. Infusion for Malaria Prevention: Mode of action and benefits in a Ugandan community. Br J Pharm Res. 2011;1:124–132. [Google Scholar]
- 8.Wambebe CO, et al. Efficacy of niprisan in the prophylactic management of patients with sickle cell disease. Curr Ther Res. 2001;62:26–34. [Google Scholar]