Abstract
Intrauterine growth retardation (IUGR) is associated with neurological deficits including cerebral palsy and cognitive and behavioral disabilities. The pathogenesis involves oxidative stress that leads to periventricular white matter injury with a paucity of mature oligodendrocytes and hypomyelination. The molecular mechanisms underlying this damage remain poorly understood. We employed a rat model of IUGR created by bilateral ligation of the uterine artery at embryonic day 19 that results in fetal growth retardation and oxidative stress in the developing brain. The IUGR rat pups showed significant delays in oligodendrocyte differentiation and myelination that resolved by 8 weeks. Bone morphogenetic protein 4 (BMP4), which inhibits oligodendrocyte maturation, was elevated in IUGR brains at postnatal time points and returned to near normal by adulthood. Despite the apparent recovery, behavioral deficiencies were found in 8-week-old female animals, suggesting that the early transient myelination defects have permanent effects. In support of these in vivo data, oligodendrocyte precursor cells cultured from postnatal IUGR rats retained increased BMP4 expression and impaired differentiation that was reversed with the BMP inhibitor noggin. Oxidants in oligodendrocyte cultures increased BMP expression, which decreased differentiation; however, abrogating BMP signaling with noggin in vitro and in BMP-deficient mice prevented these effects. Together, these findings suggest that IUGR results in delayed myelination through the generation of oxidative stress that leads to BMP4 upregulation.
Keywords: Bone morphogenetic protein (BMP), Intrauterine growth retardation, Myelin, Oligodendrocytes, Oxidative stress, Periventricular white matter injury
INTRODUCTION
Neurological disabilities ranging from behavioral and motor deficits to cerebral palsy are commonly associated with intrauterine growth retardation (IUGR) that is secondary to uteroplacental insufficiency. A significant factor in the pathogenesis of these disabilities is white matter injury characterized by damage to oligodendrocytes, impaired myelination and astrogliosis (1). The cause of the white matter injury is complex and presumed to be combination of hypoxia/ischemia, inflammation, and excitotoxicity associated with oxidative stress (2).
Oligodendrocyte precursors (OPCs) are the predominant cell type in the developing white matter during the period of greatest risk for the development of IUGR. OPCs are particularly vulnerable to oxidative stress due to their low endogenous glutathione levels, high iron content and high oxidative metabolism (3). As they mature and myelinate axons, oligodendrocytes become more resistant to injury. Although OPC cell death has been observed in perinatal white matter injury (4, 5), oligodendrocyte maturation can also be arrested (6–8), thereby rendering OPCs vulnerable to further damage from oxidative stress.
Oligodendrocyte differentiation during development is controlled by a balance of inductive and repressive factors. Among these is bone morphogenetic protein (BMP) signaling, which inhibits oligodendrocyte differentiation and promotes the formation of astrocytes (9–12). BMPs are increased in models of demyelinating disease and multiple sclerosis (MS) (13–15), and treatment of animal models of white matter injury with BMP-inhibitors have resulted in functional improvement, increased numbers of oligodendrocytes and enhanced myelination (16–20). The mechanism by which BMP is upregulated has not been determined.
Here, we used a rat model of uteroplacental insufficiency induced by bilateral ligation of the uterine artery at embryonic day 19 that results in fetal growth retardation, mitochondrial dysfunction and oxidative stress. We found that IUGR rats exhibit significant developmental delays in oligodendrocyte maturation and myelin protein formation during the neonatal period, coincident with increases in expression of bone morphogenetic protein 4 (BMP4). To understand why BMP signaling is upregulated in this model, we examined the potential role oxidative stress plays in increasing oligodendrocyte BMP4 expression and the inhibition of oligodendrocyte differentiation.
MATERIALS AND METHODS
IUGR Surgery
All experiments were performed in accordance with the guidelines set forth by the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee. Our surgical methods have previously been described (21). In brief, time-dated Sprague-Dawley pregnant rats (Charles River Laboratories, Wilmington, MA) were individually housed under standard conditions and allowed free access to standard rat chow and water. On day 19 of gestation (term is 22 days), the maternal rats were anesthetized with i.p. xylazine (8 mg/kg) and ketamine (40 mg/kg), and both uterine arteries were ligated. Sham operated dams served as controls. Rats recovered within a few hours and had ad libitum access to food and water. Animals were allowed to deliver spontaneously and litters were culled to 7 to 8 at birth to ensure uniformity of litter size between IUGR and control litters.
Immunohistochemistry
To prepare sections of IUGR and sham brains, rats were killed at postnatal day (P)14, P21 and 8 weeks of age (adult) by perfusion in 4% paraformaldehyde. Frozen sections were prepared and cut on a Leica cryostat at 12-μm thicknesses, all according to established protocols (15, 22). The antibodies used were as previously described (22, 23). To label mature oligodendrocytes in vivo, we used anti-myelin proteolipid protein (PLP) (1:2, rat hybridoma AA3 gift of Dr. Dr. Alex Gow, Wayne State University, Detroit, MI), anti-aspartoacylase ([ASPA], 1:1000 dilution, gift of Dr. James Garbern, University of Rochester), the oligodendrocyte marker anti-APC (1:20, Millipore, Billerica, MA), and anti-myelin oligodendrocyte glycoprotein ([MOG], 1:10, [24]). For labeling OPCs, we employed anti-NG2 (1:100, Chemicon International, Temecula, CA) and anti-platelet-derived growth factor receptor-α ([PDGFRα], 1:250, BD Biosciences, PharMingen, San Diego, CA). Astrocytes were labeled with anti-glial fibrillary acidic protein ([GFAP], 1:100, gift of Dr. Virginia Lee, University of Pennsylvania). Axons were labeled using anti-neurofilament antibody, RMO 24 (1:100, gift of Dr. Virginia Lee).
Cell death was assayed using the TUNEL method as well as staining for activated caspase (1:300, Cell Signaling, Beverly, MA), as previously described (25, 26).
To count cells from frozen sections, IUGR and sham animals from at least 3 litters were used. Digital images were taken at 20x magnification from sections at the level of the anterior part of the corpus callosum, counting 5 150μm2 regions of interest per section, at least 2 sections per animal. Statistical significance was calculated using Student t test.
Western Blotting
Brain tissue samples from P14, P21 and 8-week-old adult rats were dissected, protein was prepared, and gels were run as previously described (22, 23). Membranes were incubated with the following primary antibodies: anti-myelin basic protein ([MBP], rat hybridoma supernatant, gift of Dr. Virginia Lee, 1:500) with anti-rat IgG, anti-MOG, with anti-mouse IgG, anti-PLP with anti-rat IgG, anti-BMP4 (R&D Systems, Minneapolis, MN, 1:1000) with anti-mouse IgG, anti-GFAP with anti-rat IgG, anti-Phospho-Smad1, 5, 8 (Cell Signaling) with goat anti-rabbit IgG, and anti-4-hydroxy-2-nonenal ([4HNE], Alpha Diagnostic International, San Antonio, TX) with goat anti-rabbit IgG. All secondary antibodies were conjugated with horseradish peroxidase and used at 1:100. The membranes were imaged using ECL reagents (Amersham, Piscataway, NJ) and hyperfilm (Amersham). Blots were stripped and reprobed for glyceraldehyde 3-phosphate dehydrogenase ([GAPDH], 1:2,500, Chemicon International) or tubulin (1:1,000, Sigma, St. Louis, MO) as a loading control for protein quantification. The PLP antibody requires non-reducing conditions and therefore cannot be quantified. Statistical significance for the protein quantification was calculated using Student t test.
Semi-thin Sections
P14 rats were killed and processed for semi-thin sections according to established protocols (27). Three sham and 3 IUGR rats were perfused with PBS followed by 2% paraformaldehyde and 2% glutaraldehyde in 0.1M phosphate buffer. The brains were removed and placed in fixative overnight. The brains were cut to visualize the entire corpus callosum, which was then removed. The corpus callosum was post fixed in 4% OsO4 in 0.1 M phosphate buffer for 2 hours, rinsed in 0.1M phosphate buffer, and dehydrated in ascending concentrations of ethanol. Tissue was then embedded in ascending concentrations of Epoxy and semi-thin sections were stained with toluidine blue. Photographs were taken of the entire corpus callosum beginning 70 μm from the most rostral end, where the first myelinating axons appeared at P14. A montage was created. Four 225 μm2 regions of interest were marked in identical areas in each animal and the myelinating axons in each area counted. The number of myelinating axons was compared by analysis of variance (ANOVA).
Cell Culture Generation and Treatment
To generate cultures of purified OPCs from newborn IUGR and sham rats and from BMPR knockout and wild type mice, a mixed population of cells was harvested from neonatal brain and seeded on 100-mm Petri dishes in serum-containing medium, as previously described (12). After 1 to 2 hours, the cell cultures were switched to a serum-free growth medium containing Neurobasal medium (Invitrogen, Life Technologies, Grand Island, NY) with B27 supplement (1:50; Life Technologies), 10 ng/ml basic fibroblast growth factor, 2 ng/ml platelet-derived growth factor (both from R&D Systems), and 1 ng/ml neurotrophin-3 (Peprotech, Rocky Hill, NJ). Cultures were purified using a gentle modified wash-down procedure (22). Within 3 days, the cells were confluent and could be subcultured into polylysine-coated flasks, 12-mm polylysine-coated coverslips for immunofluorescence, or 100-mm polylysine-coated Petri dishes for Western blotting. These cells could be passaged 3 to 4 times and generally contained no more than 5% to 10% astrocytes.
To determine the ability of IUGR OPCs to differentiate, IUGR and sham cultures were established at P2 as described above and grown until 75% confluent, approximately 1 week. Some cultures were collected at this point for Western blotting to detect phospho-Smad signaling. Growth medium was removed from cultures and cells were fed with “differentiation medium” (DM), consisting of 50% Dulbecco’s modified eagle medium, 50% Ham’s F12 with 50 μg/ml transferrin, 5 μg/ml putrescine, 3 ng/ml progesterone, 2.6 ng/ml selenium, 12.5 μg/ml insulin, 0.4 μg/ml T4, 0.3% glucose, 2 mM glutamine, 10 ng/ml and biotin. Some cultures were treated with 500 ng/ml noggin (R&D Systems).
To generate oxidative stress, normal rat OPCs were generated as described above, seeded on coverslips and 100-mm dishes, and placed in DM with either 5 μm tert-butyl hydroperoxide ([tBOOH], Sigma), 100 μm L-buthionine sulfoximine ([BSO], Sigma), or no extra treatment. Some coverslips also received 500 ng/ml noggin at the same time as the tBOOH or BSO. Cells were collected from 100 mm plates at 72 hours and mRNA was isolated for quantitative polymerase chain reaction (Q-PCR). Coverslips were also stained to determine the number of oligodendrocytes at 72 hours.
The Bmpr1 double knockout mice (Bmpr1 DKO) were generated as previously described (23). All mice were killed at P1 and OPC cultures were harvested as described above (28). Individual mouse brains were cultured separately until mice were genotyped by PCR of tail DNA, at which point cultures from mice of identical genotypes were then combined.
Immunofluorescence
Cells on coverslips were processed for detection of specific antigens as described previously (12, 29). Antibodies used for cell cultures were anti-galactocerebroside (GalC) (30) and anti-phospho-Smad 1, 5, 8 (1:3,000, Cell Signaling).
To count cells expressing antigens in culture, antigen-positive and 4′,6-diamidino-2-phenylindole (DAPI)-positive cells were counted in 10 fields in each of 2 coverslips from at least 3 separate preparations of cells using a Leica DM6000B fluorescence microscope at 63x magnification. Approximately 1000 cells were counted per condition. Statistical significance was calculated using Student t test.
Q-PCR
OPC cultures were grown on 50-mm dishes and harvested after 72 hours after treatment with tBOOH or BSO. Cells were extracted with Trizol (Molecular Research Center, Inc., Cincinnati, OH) and Q-PCR was performed using SYBR Green (Applied Biosystems, Life Technologies) or Taq Man as previously described (7, 28). Samples were measured in triplicate for each experiment and normalized to either GAPDH (cyber green) or actin (Taq Man).
Behavioral Testing
Grip strength was used as a measure of motor performance. IUGR and sham rats grasped a metal bar attached to a strain gauge (Columbus Instruments, Columbus OH). The rats were pulled back gently by the tail until the bar was released. The maximum tension was recorded over 3 trials. A rat rotorod (Ugo Basile, Collegeville PA) was used to test coordination and motor learning. The rats walked on a rotating rod suspended 25 cm above a platform. Time until falling off the rod was measured. The initial speed of the rod was 5 rpm, accelerating to 40 rpm over a 3-minute interval. Each rat was given 3 trials/day for 5 days with 15-minute inter-trial intervals. Daily means and standard error were determined. An ANOVA was used to test for significant differences between sex, group and day using the ANOVAN function in Matlab (Mathworks, Natick MA). A post-hoc Tukey-Kramer mutiple comparison test was performed to assess for individual differences among groups.
RESULTS
Uteroplacental Insufficiency Leads to Decreased Numbers of Oligodendrocytes, Fewer Myelinating Axons and Reduced Myelin Protein Levels at P14
Compared to sham (n = 10), the corpus callosum from IUGR animals (n = 13) had large PLP deficits that were most severe around the ventricles (Fig. 1A, B). The anterior commissure and the pencil fibers of the basal ganglia also exhibited a reduction in PLP staining (Fig. 1C, D and data not shown). In contrast, the optic tracts had normal levels of PLP staining (not shown). The decreased immunohistochemistry (IHC) staining for PLP was paralleled by similar reductions in MOG (Fig. 1E, F) and APC (Fig. 1G, H).
Figure 1.
P14 Rats with intrauterine growth retardation (IUGR) have fewer mature oligodendrocytes than sham rats. (A) Sections of corpus callosum and striatum from IUGR and sham rats labeled with antibodies to myelin proteins illustrating fewer oligodendrocytes in IUGR sections. Nuclei are labeled with DAPI. (A, B) Myelin proteolipid protein (PLP) staining is clearly decreased in the midline of the corpus callosum of IUGR animals compared to sham (5x magnification, higher magnification inserts, 40x magnification). (C, D) Pencil fibers in the striatum show greatly decreased labeling for PLP in sections from IUGR animals (10x magnification). (E, F) myelin oligodendrocyte glycoprotein (MOG)-positive cells are much less numerous in the IUGR animals (20x magnification). (G, H) Many fewer APC-positive oligodendrocytes are present in the corpus callosum of the IUGR compared to sham rats (20x magnification). (I) There is an overall decrease in DAPI-positive cells in the corpus callosum of IUGR rats of 7% compared to sham but this is not statistically significant (NS, p < 0.41). (J) The percentage of PLP-positive cells per DAPI-positive cells is decreased by 46% in the corpus callosum of IUGR compared to sham rats (***, p < 0.002).
We performed cell counts on PLP-labeled sections from 7 IUGR and 10 sham animals from multiple litters that had received surgery on different days to correct for any variability in the IUGR. At P14, oligodendrocytes are in sufficiently low numbers and are sufficiently discrete to use the anti-PLP antibody for counting (22). To ensure that any differences observed were not due to a general reduction in numbers of cells in white matter tracts, total numbers of cells were determined using DAPI-labeled nuclei (n = 10 IUGR and 7 sham). The IUGR animals had 7% fewer DAPI-positive cells than the shams although this was not statistically significant (Fig. 1I). Correcting for this difference in total cells, the change in PLP-positive cells was calculated as a percent of total DAPI-positive nuclei and was found to be decreased by 42% in rats with IUGR vs. shams (Fig. 1J), although some IUGR rats had as much as a 70% decrease in PLP-positive cells compared to sham (Fig. 1B).
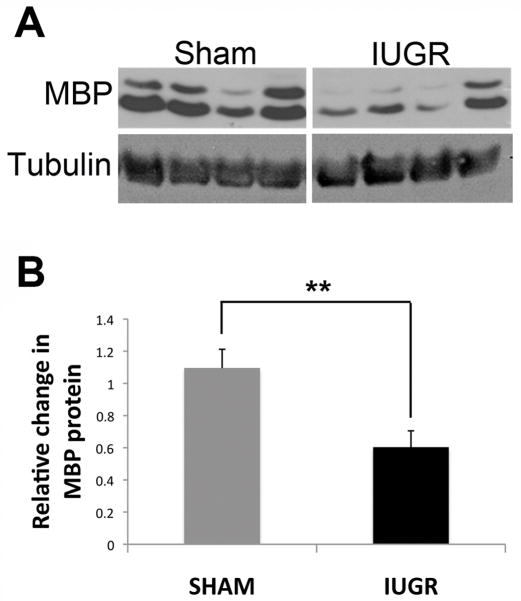
To complement the cell counting and qualitative IHC analysis, we performed Western blots using antibody to MBP on whole brain protein from IUGR and shams at P14. Because of variability among the animals (Fig. 2A), we quantified blots performed on multiple litters receiving surgery on different days and used 12 sham and 12 IUGR rats. Quantification of the blots revealed an overall 45% decrease in MBP with individual variations as shown in the representative blot (Fig. 2B).
Figure 2.
Western blots from brains of 4 sham and 4 intrauterine growth retardation (IUGR) rats show decreases in myelin basic protein (MBP). (A) There is some variability among the animal samples. (B) Quantification of the blots shows a significant decrease in MBP in the IUGR rats (**p < 0.02).
To examine myelination, semi-thin sections of corpus callosum were prepared from 3 IUGR and 3 sham animals at P14 and myelinating axons were counted (Fig. 3A). The IUGRs had on average 46% fewer myelinating axons than the shams (Fig. 3B).
Figure 3.

Rats with intrauterine growth retardation (IUGR) at P14 had markedly reduced myelinating axons vs. sham rats. Semi-thin sections were prepared from IUGR and sham rats and counterstained with toluidine blue. (A) Representative 100x photomicrographs of myelinating axons from an identical area of the corpus callosum in sham and IUGR animals. (B) Myelinating axons were counted in four 225-μm2 regions of interest placed in identical areas of the corpus callosum and there were significantly fewer myelinating axons in all 4 areas in the IUGR vs. sham rats (p < 0.001).
To assess for the role of oxidative stress leading to the changes in white matter development, Western blots were prepared from 4 IUGR and 4 shams and were probed with antibody to 4-HNE, a bi-product of polyunsaturated fatty acids produced by lipid peroxidation. HNE can react with sulfhydryl groups, histidine and lysine residues of proteins to form covalently modified proteins that can be detected in immunoblots using an antibody directed against the HNE moiety. Detection of HNE-protein adducts is, therefore, accepted as presumptive evidence of oxidative stress (31). Western blot analysis revealed multiple bands in the IUGR rats, suggesting that a number of proteins were modified by HNE in IUGR animals (Fig. 4A).
Figure 4.
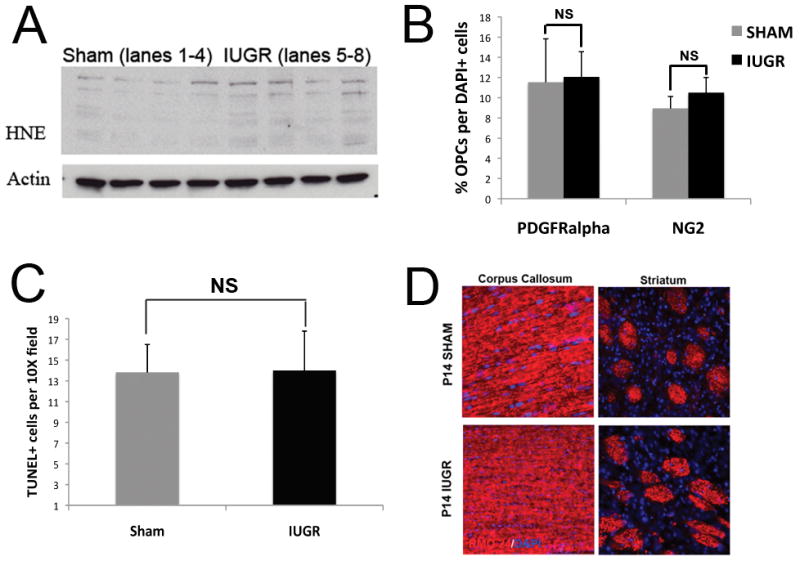
Although oxidative stress is present, the number of oligodendrocyte precursors (OPCs), TUNEL-positive cells and the amount of neurofilament staining is not altered in rats with intrauterine growth retardation (IUGR). (A) Western blots prepared from brain tissue from IUGR and sham rats and probed for the oxidative stress marker 4-hydroxy-2-nonenal (4HNE). All 4 IUGR samples show increased expression of HNE-modified proteins vs. controls. Actin was used as a loading control. (B) Sections of P14 IUGR and sham brains were labeled with antibody to NG2 or platelet-derived growth factor receptor–α (PDGFR-alpha) to detect OPCs and counted. There were no significant differences using either antibody. (C) The TUNEL assay was performed on sister sections. Quantification of TUNEL-positive cells showed no significant differences between IUGR and sham rats. (D) Neurofilament staining (red) was similar in sections of corpus callosum and striatum in IUGR and sham rats at P14. Note the presence of neurofilament labeling in the pencil fibers despite the lack of oligodendrocytes for myelination (Fig. 1D).
Sham and IUGR Rats Had Equal Numbers of Oligodendrocyte Precursors at P14 and Did Not Demonstrate Increased Apoptotic Cell Death
To quantify the number of OPCs at P14, cell counts were performed on sections from IUGR and sham animals immunolabeled for the OPC makers NG2 or PDGFRα and counterstained with DAPI. Although slightly higher numbers of NG2-positive and PDGFRα cells were found in IUGR vs. sham animals, the differences were not significant, suggesting that precursor numbers were unaffected by IUGR (Fig. 4B; n = 3 for each group).
To determine if there was an increase in cell death, TUNEL assays were performed on P14 sections (n = 4 for each group). Occasional TUNEL-positive cells were seen in the corpus callosum of both groups; concentrations of TUNEL-positive cells were only identified in the subventricular zone. Cell counts performed on sections that included the SVZ reveal no statistically significant differences between the 2 groups (Fig. 4C). We also performed staining for activated caspase 3 and the oligodendrocyte marker APC on P14 sections and counted the number of oligodendrocytes with and without caspase labeling in regions of interest in the corpus callosum and found no significant differences (data not shown). These data suggest that cell death at P14 cannot explain the significant lack of mature oligodendrocytes, although they do not rule out cell death at earlier time points.
Myelin Proteins at P21
Western blots were also performed on brain from IUGR and sham animals at P21. IUGRs continued to show a significant decreased in the amount of myelin protein as shown by MBP, MOG, and PLP labeling (Fig. 5). However, these differences were less dramatic than those seen at P14.
Figure 5.
Level of myelin proteins continue to be decreased in brains from the intrauterine growth retardation (IUGR) compared to sham animals at P21 but the difference is not as marked as at P14. Western blots were prepared from brains of IUGR and sham rats at P21 and probed with antibody to myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and myelin proteolipid protein (PLP). All 3 myelin proteins were decreased (A) but quantification showed that those differences were diminishing compared to P14 (B). GAPDH = glyceraldehyde 3-phosphate dehydrogenase. (*p < 0.01)
P14 IUGR Animals Showed Normal Axonal Labeling but Marked Astrogliosis
Myelination is dependent on the presence of axons to myelinate, thus reduced numbers or absence of axons would also result in an oligodendrocyte maturation defect. To exclude this possibility we evaluated the axon network present at P14. Neurofilament labeling revealed qualitatively similar axonal labeling in both IUGR and sham operated groups (Fig. 4D; n = 3 for both groups).
Astrogliosis often accompanies the white matter injury associated with IUGR. To determine if astrogliosis was present in our model, sections through the corpus callosum of P14 IUGR and sham rats were labeled for GFAP by IHC. Enlarged and prominent GFAP-positive astrocytes were found throughout the corpus callosum (Fig. 6A). GFAP-positive cell counts in the corpus callosum show a trend towards increased numbers of GFAP-positive cells that was not statistically significant (Fig. 6B; n = 3 for each group). However, western blots of whole brain protein probed with anti-GFAP antibody show increased GFAP expression in all 4 IUGR animals when compared to 4 shams (Fig. 6C, D), indicating that astrocytes undergo both hypertrophy and hyperplasia in response to IUGR.
Figure 6.
Brains of rats with intrauterine growth retardation (IUGR) exhibit astrogliosis at P14. (A) Sections of corpus callosum from IUGR rats show larger and more intensely stained glial fibrillary acidic protein (GFAP)-positive astrocytes in IUGR vs. sham animals. (B) GFAP-positive and DAPI-positive cells were counted. There was a non-significant trend to increased GFAP-positive cells in IUGR animals (p < 0.096). (C) Western blots from brains of IUGR and sham rats show increases in GFAP in all IUGR animals. (D) Quantification of the western blot demonstrates a significant increase in GFAP in the IUGR vs. sham rats (*p < 0.05).
Myelin Protein and GFAP Levels at 8 Weeks
IHC with the anti-PLP antibody showed the white matter to be similar in the 2 groups at 8 weeks of age (Fig. 7A; n = 7 for each group). Mature oligodendrocyte counts were performed on sections using antibody to ASPA, which labels oligodendrocyte cell bodies thereby facilitating cell counting. In the corpus callosum there were similar numbers of oligodendrocytes in IUGR and sham animals (Fig. 7B; n = 3 for each group). We also determined the levels of MBP, PLP, MOG (not shown) and GFAP by Western blotting (Fig. 7C). Quantitative analysis of the blots revealed no statistically significant differences between the sham and IUGR samples (Fig. 7D). Together these data indicate that the delay in oligodendrocyte differentiation and accompanying astrogliosis following IUGR normalizes by adulthood.
Figure 7.
Numbers of oligodendrocytes and astrocytes and oligodendrocyte and astroglial proteins are the same in rats with intrauterine growth retardation (IUGR) and shams at 8 weeks. (A) Sections of the midline of the corpus callosum of sham and IUGR rats labeled with antibody to myelin proteolipid protein (PLP) and DAPI. No significant differences are seen between the sections. (B) Cell counts of aspartoacylase (ASPA)-positive oligodendrocytes in regions of interest in the corpus callosum of three IUGR and 3 sham rats at 8 weeks show no differences. (C) Western blots of 4 brains each from IUGR and sham rats demonstrate that expression of myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) are the same at 8 weeks. (D) Quantification of the western blots shows no significant differences in MBP or GFAP between IUGR and sham brains. NS = not significant.
IUGR Rats Demonstrate Strength and Coordination Deficiencies at P14 that Persist into Adulthood
To determine if IUGRs have strength deficits, we first assessed grip strength at P14. IUGR rats were found to be significantly weaker then sham rats (Fig. 8A, n = 15 IUGR and 17 sham from 7 litters). When grip strength was retested on the same population at 6.5 weeks of age, IUGR rats were slightly weaker but this was not significant (Fig. 8A). Because grip strength is a relatively coarse measure of strength, and possibly not sensitive enough in the adults, we performed rotorod testing at 8 weeks on the same groups of rats (Fig. 8B). Female rats on the whole were better at this task than males (n = 8 female IUGR, 7 male IUGR, 10 female sham, 7 male sham). The female sham animals remained on the rotorod for a significantly longer time than the IUGR females on days 3 to 5, but there was no difference in males on any day (Fig. 8B). In addition, the performance of sham females improved significantly more over the 5-day testing period vs. the performance of IUGR females. We also performed open-field testing as a measure of anxiety in the rats but no difference was detected in the maximum speed or distance traveled between IUGR and sham animals (data not shown). These data suggest that IUGR rats have strength and coordination deficiencies that begin during development and persist to a lesser degree in adulthood despite the development of apparently normal myelination.
Figure 8.
Rats with intrauterine growth retardation (IUGR) show motor abnormalities at P14 continuing into adulthood. (A) Grip strength testing was performed at 14 days and at 6.5 weeks (adult). At P14, the IUGR animals were significantly weaker than the shams (**p < 0.004), but this difference disappeared in the adults (p < 0.23) (NS). (B) Rotorod testing was performed at 8 weeks. The males clearly performed worse than the females without regard to treatment (p < 0.01). Within a specific gender, female sham rats remained on the rotorod longer than female IUGR rats on days 3 to 5 and showed greater improvement (p < 0.05). There were no differences in the males on any day.
BMP4 is Significantly Increased in IUGR Animals During the Period of Myelination but Returns to Near Control Levels in the Adult
Because increased expression of BMPs is associated with demyelination in adult disorders such as spinal cord injury and models of MS (14, 15), we hypothesized that BMPs would be increased in IUGR animals. Our previous experiments as well as those of others have shown that the most abundant BMP isoform present after injury is BMP4 (15, 32). Q-PCR was performed on whole brain from 5 sham and 5 IUGR animals at P14 and at 8 weeks. BMP4 mRNA was increased 388% over sham at P14 but was only increased 20% over shams by 8 weeks, which was not statistically significant (Fig. 9A). Western blots to detect BMP4 protein performed on whole brain from IUGR and sham (n = 4 from both groups) demonstrated a 3.5-fold increase in BMP expression in the IUGRs over the shams at P14, and a 2.5-fold increase at P21, although there was some animal-to-animal variability (Fig. 9B). By 8 weeks of age, although BMP4 levels appeared somewhat higher in the IUGRs than the shams, the differences were not statistically significant when normalized to the loading controls (Fig. 9C). These data indicate that BMP4 is abundant in IUGR rats during the period of delayed oligodendrocyte differentiation and myelination, but decreases as the numbers of mature oligodendrocytes and amount of myelin approaches that of the sham rats.
Figure 9.
Bone morphogenetic protein (BMP) mRNA and protein are increased at P14 and P21 in intrauterine growth retardation (IUGR) vs. sham rats but returns to the same level as the shams by 8 weeks. (A) BMP4 mRNA was measured by Q-PCR at P14 and 8 weeks (adult). There was a 3.9-fold increase in BMP4 expression in the IUGR compared to sham rats at P14 (**p < 0.0003) but only a 20% at 8 weeks, which was not significant (NS, p < 0.63). (B) Western blots of brain samples show variability in level of expression among animals but BMP4 is greater at p14 and P21 in the IUGR vs. sham rats. (C) Quantification of the Western blots shows an increase in BMP expression of 3.5-fold at P14 (p < 0.007), 2.5-fold at P21 (p < 0.001), but a 25% increase at 8 weeks of age that was not significant (p < 0.08). GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
OPCs Isolated from IUGR Rats Demonstrate a Differentiation Defect that is mediated through BMP Signaling
To test whether BMP signaling is essential for the delayed differentiation phenotype, we cultured OPCs from IUGR and sham animals at P2. To determine if BMP4 signaling is active in these cells, we examined phosphorylated Smad, an obligate downstream component for conical BMP signaling, by Western blotting. OPCs from shams had minimal phosphoSmad signaling whereas the IUGR OPC cultures showed strong phospho-Smad immunoreactivity, indicating that increases in BMP4 signaling persist in culture with no added BMP or serum (n = 2 from each group; Fig. 10A).
Figure 10.
Oligodendrocyte precursors (OPCs) isolated from animals with intrauterine growth retardation (IUGR) express phospho-Smad protein and do not differentiate as well as OPCs from shams but differentiation can be rescued with the bone morphogenetic protein (BMP) inhibitor, noggin. (A) OPCs were isolated from the brains of P2 IUGR and sham animals and grown in culture for 1 week. Western blots performed on OPC protein demonstrate significant phospho-Smad labeling in the IUGR cultures indicating BMP signaling through the canonical pathway. (B) OPC cultures were placed in differentiation medium (DM) with and without noggin for 72 hours and labeled with antibody to galactocerebroside (GalC) and DAPI. IUGR cultures in DM without noggin had fewer GalC-positive cells than comparable Sham cultures or cultures with noggin (63x magnification). (C) The number of oligodendrocytes was quantified by counting cells labeled with antibody to GalC and DAPI. The OPCs from IUGR animals differentiated only half as well as the OPCs from the sham animals (n = 4, *p < 0.01). Treatment of IUGR OPCs with noggin (500 ng/ml) increased differentiation to the level of the shams (n = 4, *p < 0.03), whereas treatment of sham OPCs with noggin had no significant effect.
To determine if increased BMP signaling could inhibit the differentiation of OPCs cultured from IUGR animals, IUGR and Sham OPCs were seeded on coverslips and placed in DM. Separate sets of cultures were established from 4 different litters, each using brains from 2 IUGRs and 2 shams. After 72 hours, the cells were labeled with an antibody to GalC, which identifies the first stage in oligodendrocyte differentiation. Differentiation of OPCs from IUGR animals was decreased 40% compared to shams (Fig. 10B, C; p < 0.01). When sham and IUGR cultures were placed in differentiation medium together with the BMP inhibitor noggin and labeled with an antibody to GalC 72 hours later, the percentage of GalC-positive cells in IUGR cultures was increased to the levels of sham cells (Fig. 10B, C; p < 0.03). Noggin treatment of the sham cells had no significant effect on the percentage of cells that differentiated (Fig. 10C). Thus, the persistence of BMP signaling in OPCs in culture inhibits differentiation that can be rescued by the specific BMP inhibitor noggin. These data suggest that cells cultured from IUGR and sham animals accurately preserve the in vivo milieu and that BMP is responsible for the developmental delay.
BMP is Increased in the IUGR Rats by Oxidative Stress
Although BMP upregulation coinciding with demyelination or lack of remyelination disease has been demonstrated in a variety of neonatal injury models as well as adult demyelinating disease and MS models (14, 15, 19, 20), what causes the increases in BMP is not known. One of the most significant consequences of uteroplacental insufficiency is the induction of oxidative stress, which we have previously shown to inhibit oligodendrocyte differentiation (7). To test the hypothesis that oxidative stress drives the elevation of BMP, we treated normal neonatal OPCs with BSO (an inhibitor of glutathione reductase, which diminishes the ability to reduce oxidants) and tBOOH (which leads to lipid peroxidation, consumption of reduced glutathione and NADPH depletion) to induce oxidative stress. Both agents generated a significant reduction in total glutathione at 24 hours (7). Both agents reduce oligodendrocyte differentiation without increasing cell death and increase expression of Id2 and Id4 mRNA, which can be upregulated by BMP expression (7). In the present study, OPC cultures were treated separately with BSO and tBOOH. Q-PCR, performed after 72 hours showed that BMP4 mRNA was increased 2.5- to 5-fold (n = 3 separate cultures; Fig. 11A). Immunostaining for phospho-Smad performed at 6 hours after treatment showed increased nuclear labeling in both tBOOH (Fig. 11B) and BSO (not shown) when compared to controls.
Figure 11.
Oxidant treatment of cells decreases oligodendrocyte differentiation but inhibition of bone morphogenetic protein (BMP) signaling can rescue this effect. (A) Q-PCR of 3 separate cultures of oligodendrocyte precursors (OPCs) treated with the oxidants L-buthionine sulfoximine (BSO) or tert-butyl hydroperoxide (tBOOH) for 72 hours shows increases in bone morphogenetic protein 4 (BMP4) mRNA averaging 5.12-fold and 2.2-fold, respectively. (B) p-SMAD staining downstream of BMP is visible 3 hours after treatment with tBOOH but not in control conditions. BMP treatment (50 ng/ml) is used as a positive control (C) OPC cultures were treated with BSO or tBOOH and 500 ng/ml noggin simultaneously and labeled with anti-galactocerebroside (GalC) after 3 days. tBOOH and BSO reduced the percentage of GalC-positive cells by approximately 40% relative to differentiating medium (DM) alone. When noggin was added at the same time as the oxidative agents, the percentages of GalC-positive cells returned to control levels (*p < 0.05 vs. cultures without noggin). (D) Mouse OPC cultures were established from wild type (WT) and mutant mice lacking BMPR 1a and 1b in the neural tube (DKO) and labeled with anti-GalC after 3 days. tBOOH and BSO treatment were unable to inhibit differentiation in the cells lacking BMP type 1 receptors.*p < 0.05.
To demonstrate that the increase in BMP4 was inhibiting oligodendrocyte differentiation, we added 500 ng/ml noggin to tBOOH or BSO when switching OPC cultures into DM. OPC cultures treated with BSO or tBOOH showed a 44% decrease in differentiation vs. untreated controls at 72 hours (Fig. 11C), as we previously demonstrated (7). When noggin was added noggin along with the tBOOH or BSO, differentiation was the same as in the untreated controls, indicating that the noggin prevented the observed impairment in OPC differentiation (n = 3 separate cultures; Fig. 11C). Noggin added to untreated cells had no effect. Thus, a pharmacological blockage of BMP signaling permitted full differentiation even in the presence of oxidative stress.
To demonstrate further that BMP4 is responsible for the decrease in oligodendrocyte differentiation caused by oxidative stress, we used OPCs cultures from mice with deletions of both BMPR1a and BMPR1b (BMPR double knockout mice [BMPRdKO]) (33, 34). We previously found that OPCs from BMPRdKO mice do not respond to BMP and do not show phospho-Smad signaling in the nucleus, but do differentiate (23, 28). When OPCs from these BMPRdKO mice were treated with tBOOH or BSO, they differentiated as well as OPCs from the untreated animals (n = 3 separate cultures; Fig. 11D). Wild type mice from these litters had a significant decrease in GalC-positive differentiation in the presence of tBOOH and BSO, also as previously shown. Thus, genetically disrupting BMP signaling ameliorated the oligodendrocyte differentiation defect caused by oxidative stress.
DISCUSSION
Intrauterine growth retardation has been linked to the development of cerebral palsy in children who were born preterm or term. Using a model of uteroplacental insufficiency that is associated with fetal growth retardation, oxidative stress and mitochondrial dysfunction (21, 35), we found oligodendrocyte and myelin maturation defects in addition to motor deficits; features commonly found in affected children (36–38). While the pathogenesis of cerebral palsy in children who were formerly IUGR is complex and poorly understood, our data provide a mechanistic link between oxidative stress and increased BMP4 expression, which inhibits oligodendrocyte maturation and myelination. BMP4 was upregulated during the period of delayed myelination and returned to near normal levels as the rats matured and myelination increased. However, despite near normalization of myelination, mild motor deficits persisted, indicating that defects in myelination induced during fetal life can have life-long consequences.
The IUGR rat model demonstrated typical features of white matter injury, including a myelination defect and astrogliosis. At P14, the midpoint for myelin development in the rat, the myelination abnormality was characterized by a decrease in the number of oligodendrocytes and myelinating axons and a reduction in myelin protein expression. Myelin protein levels were similarly reduced at P21, indicating that the deficiencies in oligodendrocytes and myelin persisted through the end of the myelination period. Astrogliosis was also present at P14. The combination of lack of mature oligodendrocytes and astrogliosis is common in animal models of white matter disease as well as the brains of babies with white matter injury (1, 6, 39).
Despite the paucity of oligodendrocytes in the IUGR animals compared to sham, the number of NG2-positive or PDGFRα-positive OPCs did not differ between the IUGR and sham animals. Thus, precursor number was not affected by the injury and precursors remain in the white matter and may retain their potential to mature and myelinate. One might expect OPCs to accumulate in these animals because many of them are unable to differentiate. However, this is not the case and is comparable to genetic models in which a paucity of mature oligodendrocytes during development does not reflect a change in the number of precursors (12, 22, 40–42). It is unclear if these oligodendrocyte precursors give rise to normally functioning oligodendrocytes. Studies of PWMI in humans as well as experimental animals have shown reduced arborization of processes in oligodendrocytes labeling with mature markers. One study linked this finding to NMDA receptors located on the processes of oligodendrocytes that detach and disintegrate in an oxygen glucose deprivation model due to excitotoxicity (6, 8, 43). This mechanism may play a role in our model as well; indeed, the oligodendrocyte shown in Figure 10B has truncated process growth. Our finding that motor deficits persist in IUGR adults also suggests that these precursors give rise to abnormally functioning oligodendrocytes.
Surprisingly, we did not see significant cell death by TUNEL or caspase staining at P14. The small foci of TUNEL-positive cells in the subventricular zone are considered normal and did not differ between IUGR and sham rats. This suggests that cell death at P14 cannot account for the lack of mature oligodendrocytes. Nevertheless, it is possible that cell death occurred much earlier and was cleared by P14 or is cleared at such a high rate that it cannot be appropriately captured by assaying a single time point.
Previous work has implicated oxidative stress as the primary mechanism for white matter injury (4, 44, 45). Our IUGR model features oxidative stress, BMP upregulation, and a lack of oligodendrocytes. To demonstrate that these are linked, we treated OPCs in vitro with agents that cause oxidative stress by 2 different mechanisms and demonstrated that oxidative stress executes this function by upregulating BMP signaling, which in turn inhibits OPC differentiation leading to impaired myelination. We have previously shown that oxidant treatment aborts oligodendrocyte differentiation by downregulating transcription factors and nuclear proteins needed for differentiation such as Olig 2 and Sox 10 while upregulating those that inhibit differentiation such as Ids 2 and 4 and acetylated histone H3 and H4 (7). BMP is known to inhibit oligodendrocyte differentiation through the regulation of key transcription factors and nuclear proteins mentioned above (46). Our present data showing the differentiation and myelination defects can be prevented by blocking BMP signaling even in the presence of oxidative stress validate our model.
Evidence that BMP signaling is an important modulator of oligodendrocyte maturation and myelination is well established as is a role for this signaling pathway in white matter diseases. The function of BMP in oligodendrocyte development has been shown by our laboratory and others to arrest oligodendrocyte precursor maturation, inhibit myelin protein expression and increase astrogliogenesis (9–12, 47). Furthermore, it has been established that levels of BMP decrease around the time of birth, thus permitting OPC differentiation (9). A number of studies have shown increased expression of BMPs, especially BMP4, in spinal cord injury or demyelinating disease in the adult (13–15, 48–51). BMP upregulation has recently been demonstrated in 2 neonatal white matter injury models as well (19, 20). Abrogation of BMP signaling has been shown to improve myelination, decrease astrogliosis, and increase functional recovery (16–20).
It is not yet clear which cell types are making the BMP. Studies, including our own, using demyelinating models, have implicated astrocytes (13, 52), macrophages (15), neurons (52, 53) and oligodendrocytes (15, 53). Because BMP4 is a secreted and diffusible protein, identification of the cell of origin is difficult and it is possible that BMPs are made by multiple cell types. While knowing the cell of origin is of inherent interest, it is not necessary for the consideration of therapy to rescue myelination during IUGR or other conditions in which BMP inhibits myelination. Although our in vitro data indicates that BMP directly affects oligodendrocytes, representing a possible cause of demyelination, we cannot rule out the possibility that other cell types, such as astrocytes, are affected by BMP upregulation and indirectly inhibit myelination. This will be the subject of future research.
Despite the paucity of oligodendrocytes and lack of myelin at P14 and P21, myelin in mature IUGR animals appeared essentially normal by IHC, cell counts, and myelin protein quantification. GFAP expression also returned to normal levels. Thus, OPCs in the IUGR animals are eventually able to overcome the differentiation block and this coincides with decreased BMP4 expression. In contrast, a study of IUGR in which uterine artery ligation was performed much earlier in gestation showed a decrease in the thickness of the corpus callosum in the motor cortex at P60 (54, 55). However, this model results in a significant decrease in overall brain size and thus represents a very severe global phenotype (55).
Of particular importance and relevance to human IUGR and cerebral palsy is the persistence of motor and coordination disturbances found in the adult IUGR animals, even though the myelination differences have resolved. These data suggest a developmental timing requirement for oligodendrocyte differentiation and myelination, which, if delayed, results in persistent defects that are also seen in children who were born growth retarded (36). However, the behavioral differences in the adult rats were more statistically significant in the females. There are several possible reasons for this. First, multiple behavioral studies have shown that there are often differences between genders in behavioral testing, particularly motor performance and anxiety, with females performing better (i.e. more activity) in open field and other motor tasks (56, 57). The reason for these differences not well established but is likely due to the effects of sex hormones on the brain (58). Because the normal females perform better than normal males, differences due to IUGR will be more pronounced in females, as our represented in our data. Second, in premature infants, there is substantial literature about sex differences in outcomes (59, 60). Our behavioral data could be related to the mechanisms underlying these differences, which are not well understood.
In summary, we have provided a mechanism for the increase in BMP4 during demyelinating injury. Oxidative stress is a common and significant component of IUGR, as well as other perinatal injuries causing demyelination and adult demyelinating disease such as MS (4, 44, 45). Our previous work demonstrates that oxidative stress in vitro inhibits differentiation by altering the cellular and molecular program of maturation (7). We now show that BMP is upregulated by oxidative stress and oxidants cannot inhibit oligodendrocyte differentiation when BMP signaling is abrogated. This is the first demonstration that oxidative stress operates through BMP in the nervous system in general, and in oligodendrocytes in particular, and provides a mechanism to explain BMP upregulation in demyelinating diseases.
Acknowledgments
This work was supported by National Multiple Sclerosis Society RG 4105-A7 (to JBG), NIDDK 55704 (to RAS), and by the Cellular Neuroscience Core of the Institutional Intellectual and Developmental Disabilities Research Center (HD26979).
We thank Dr. Steven Scherer (University of Pennsylvania) and the members of his laboratory for assistance with the semi-thin sections.
References
- 1.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 2.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–8. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 3.Folkerth RD, Haynes RL, Borenstein NS, et al. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004;63:990–9. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- 4.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levison SW, Rothstein RP, Romanko MJ, et al. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 2001;23:234–47. doi: 10.1159/000046149. [DOI] [PubMed] [Google Scholar]
- 6.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chornic perinatal whilte matter injury. Ann Neurol. 2008;63:520–30. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French H, Reid M, Mamontov P, et al. Oxidative stress disrupts oligodendrocyte maturation. Journal of Neuroscience Research. 2009;87:3076–87. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billiards SS, Haynes RL, Folkerth RD, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18:153–63. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller RH, Dinsio K, Wang R, et al. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- 10.Grinspan JB, Edell E, Carpio DF, et al. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- 11.Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–77. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 12.See J, Zhang X, Eraydin N, et al. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–92. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Fuller ML, Dechant AK, Rothstein B, et al. Bone morphogenetic proteins promote gliosis in demyelinating spinal cord lesions. Ann Neurol. 2007;62:288–300. doi: 10.1002/ana.21179. [DOI] [PubMed] [Google Scholar]
- 14.Cate HS, Sabo JK, Merlo D, et al. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J Neurochem. 2010;115:11–22. doi: 10.1111/j.1471-4159.2010.06660.x. [DOI] [PubMed] [Google Scholar]
- 15.Ara J, See J, Mamontov P, et al. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–35. doi: 10.1002/jnr.21462. [DOI] [PubMed] [Google Scholar]
- 16.Jablonska B, Aguirre A, Raymond M, et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–50. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setoguchi T, Nakashima K, Takizawa T, et al. Treatment of spinal cord injury by transplantation of fetal neural precursors cells engineered to express BMP inhibitor. Exp Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Sabo JK, Aumann TD, Merlo D, et al. Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions. J Neurosci. 2011;31:4504–10. doi: 10.1523/JNEUROSCI.5859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dummula K, Vinukonda G, Chu P, et al. Bone Morphogenetic Protein Inhibition Promotes Neurological Recovery after Intraventricular Hemorrhage. J Neurosci. 2011;31:12068–82. doi: 10.1523/JNEUROSCI.0013-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dizon ML, Maa T, Kessler JA. The bone morphogenetic protein antagonist noggin protects white matter after perinatal hypoxia-ischemia. Neurobiol Dis. 2011;42:318–26. doi: 10.1016/j.nbd.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selak MA, Storey BT, Peterside I, et al. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E130–7. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- 22.Feigenson K, Reid M, See J, et al. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Molecular and Cellular Neuroscience. 2009;42:255–65. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 23.See J, Mamontov P, Ahn K, et al. BMP signaling mutant mice exhibit glial cell maturation defects. Mol Cell Neurosci. 2007;35:171–82. doi: 10.1016/j.mcn.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piddlesden S, Lassman H, Laffafian I, et al. Antibody-mediated demyelination in experimental allergic encephalomyelitis is independent of complement membrane attack complex formation. Clin Exp Immunol. 1991;83:245–50. doi: 10.1111/j.1365-2249.1991.tb05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasuda T, Grinspan JB, Stern JL, et al. Apoptosis occurs in the oligodendroglial lineage, and is prevented by basic fibroblast growth factor. J Neurosci Res. 1995;40:306–17. doi: 10.1002/jnr.490400304. [DOI] [PubMed] [Google Scholar]
- 26.Grinspan JB, Marchionni MA, Reeves MF, et al. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin and the role of neuregulins. J Neurosci. 1996;16:6107–18. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arroyo EJ, Bermingham JR, Jr, Rosenfeld MG, et al. Promyelinating Schwann cells express Tst-1/SCIP/Oct-6. J Neurosci. 1998;18:7891–902. doi: 10.1523/JNEUROSCI.18-19-07891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feigenson K, Reid M, See J, et al. Canonical Wnt signaling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro. 2011;3:e00061. doi: 10.1042/AN20110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinspan JB, Franceschini B. PDGF is a survival factor for PSA-NCAM+ oligodendroglial pre-progenitor cells. J Neurosci Res. 1995;41:540–51. doi: 10.1002/jnr.490410414. [DOI] [PubMed] [Google Scholar]
- 30.Ranscht B, Clapschaw PA, Price J, et al. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA. 1982;79:2709–13. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons RA, Suponitsky-Kroyter I, Selak MA. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to beta-cell failure. J Biol Chem. 2005;280:28785–91. doi: 10.1074/jbc.M505695200. [DOI] [PubMed] [Google Scholar]
- 32.Zhao C, Fancy SPJ, Magy L, et al. Stem Cells, progenitors and myelin repair. J Anat. 2005;207:251–8. doi: 10.1111/j.1469-7580.2005.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn K, Mishina Y, Hanks MC, et al. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–61. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- 34.Wine-Lee L, Ahn KJ, Richardson RD, et al. Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development. 2004;131:5393–403. doi: 10.1242/dev.01379. [DOI] [PubMed] [Google Scholar]
- 35.Peterside IE, Selak MA, Simmons RA. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E1258–66. doi: 10.1152/ajpendo.00437.2002. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsson B, Ahlin K, Francis A, et al. Cerebral palsy and restricted growth status at birth: population-based case-control study. BJOG. 2008;115:1250–5. doi: 10.1111/j.1471-0528.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 37.Guellec I, Lapillonne A, Renolleau S, et al. Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics. 2011;127:e883–91. doi: 10.1542/peds.2010-2442. [DOI] [PubMed] [Google Scholar]
- 38.Carbillon L. Cerebral palsy and restricted growth status at birth: population-based case-control study. BJOG. 2009;116:735–6. doi: 10.1111/j.1471-0528.2008.02081.x. author reply 6. [DOI] [PubMed] [Google Scholar]
- 39.Sizonenko SV, Camm EJ, Dayer A, et al. Glial responses to neonatal hypoxic-ischemic injury in the rat cerebral cortex. Int J Dev Neurosci. 2008;26:37–45. doi: 10.1016/j.ijdevneu.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Stolt CC, Rehberg S, Ader M, et al. Terminal differentiation of mylein-forming oligodendroyctes depends on the transcription factor Sox 10. Genes and Development. 2002;16:165–70. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu QR, Sun T, Zhu Z, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 42.Qi Y, Cai J, Wu Y, et al. Control of oligodendrocyte differentiation by the Nkx2. 2 homeodomain transcription factor. Development. 2001;128:2723–33. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- 43.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 44.Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008;274:48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Back SA, Gan X, Li Y, et al. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–53. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–42. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- 47.Mekki-Dauriac S, Agius E, Kan P, et al. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129:5117–30. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- 48.Cheng X, Wang Y, He Q, et al. Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells. 2007;25:3204–14. doi: 10.1634/stemcells.2007-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hampton DW, Asher RA, Kondo T, et al. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci. 2007;26:3024–35. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- 50.Setoguchi T, Yone K, Matsuoka E, et al. Traumatic injury-induced BMP 7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–25. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Cheng X, He Q, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–8. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Leong SY, Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur J Neurosci. 2005;22:1895–906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- 53.Xiao Q, Du Y, Wu W, et al. Bone morphogenetic proteins mediate cellular response and, together with Noggin, regulate astrocyte differentiation after spinal cord injury. Exp Neurol. 2010;221:353–66. doi: 10.1016/j.expneurol.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Olivier P, Baud O, Bouslama M, et al. Moderate growth restriction: deleterious and protective effects on white matter damage. Neurobiol Dis. 2007;26:253–63. doi: 10.1016/j.nbd.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Olivier P, Baud O, Evrard P, et al. Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol. 2005;64:998–1006. doi: 10.1097/01.jnen.0000187052.81889.57. [DOI] [PubMed] [Google Scholar]
- 56.Rodgers RJ, Cole JC. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol Behav. 1993;54:729–36. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- 57.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–50. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 58.Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Horm Behav. 1979;12:112–63. doi: 10.1016/0018-506x(79)90017-5. [DOI] [PubMed] [Google Scholar]
- 59.Hintz SR, Kendrick DE, Vohr BR, et al. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95:1239–48. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 60.Binet ME, Bujold E, Lefebvre F, et al. Role of Gender in Morbidity and Mortality of Extremely Premature Neonates. Am J Perinatol. 2012;29:159–66. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]