Abstract
Despite the frequency and morbidity of venous thromboembolism (VTE) development after traumatic brain injury (TBI), no national standard of care exists to guide TBI caregivers for the use of prophylactic anticoagulation. Fears of iatrogenic propagation of intracranial hemorrhage patterns have led to a dearth of research in this field, and it is only relatively recently that studies dedicated to this question have been performed. These have generally been limited to retrospective and/or observational studies in which patients are classified in a binary fashion as having the presence or absence of intracranial blood. This methodology does not account for the fact that smaller injury patterns stabilize more rapidly, and thus may be able to safely tolerate earlier initiation of prophylactic anticoagulation than larger injury patterns. This review seeks to critically assess the literature on this question by examining the existing evidence on the safety and efficacy of pharmacologic VTE prophylaxis in the setting of elective craniotomy (as this is the closest model available from which to extrapolate) and after TBI. In doing so, we critique studies that approach TBI as a homogenous or a heterogenous study population. Finally, we propose our own theoretical protocol which stratifies patients into low, moderate, and high risk for the likelihood of natural progression of their hemorrhage pattern, and which allows one to tailor a unique VTE prophylaxis regimen to each individual arm.
Key words: anticoagulant, prophylaxis, review, traumatic brain injury, venous thromboembolism
Background
Approximately 235,000 Americans are hospitalized every year with traumatic brain injury (TBI; Langlois et al., 2004), and preventing the formation of blood clots in the extremities and clot migration to the lungs is an important yet controversial aspect of their care. In the absence of any intervention, 54% of civilian TBI patients will develop deep vein thrombosis (DVT) and/or pulmonary embolism (PE; Geerts et al., 1994), and this striking figure is likely to be even higher for brain-injured soldiers due to their prolonged exposure to a hypoxic/hypobaric environment, and prolonged immobilized state during evacuation from the theater of injury to the continental U.S. This risk of DVT development after prolonged air transport has been best demonstrated in the civilian setting through a series of large randomized trials known as the Prevention of Venous Thrombosis in Long-Haul Flights (LONFLIT) series (Belcaro et al., 2001, 2002, 2003, 2004; Cesarone et al., 2003a,2003b; Scurr et al., 2001). In the LONFLIT studies, subjects had bilateral lower extremity ultrasounds performed within 90 min of lift-off, and within 24 h of landing, after undergoing a nonstop commercial flight at least 7 h in duration. The rates of development of new DVTs were 4.5–10.3% in untreated high-risk subjects (Cesarone et al., 2003a; Belcaro et al., 2001,2003,2004; Scurr et al., 2001), 0–3.4% in untreated medium-risk subjects taking 7- to 8-h flights (Belcaro et al., 2002), and 4.2–6% in untreated medium-risk subjects taking 11- to 12-h flights (Cesarone et al., 2003b). Global evacuations taking 24 h or longer and potentially spanning three continents are routine for wounded warriors, highlighting their increased risk for DVT.
The development of venous thromboembolism (VTE) after TBI carries with it the potential for significant complications and morbidity. DVT can cause local extremity problems such as limb swelling, skin ulceration, venous incompetence, or thrombophlebitis. These sequelae can subsequently lead to post-thrombotic syndrome, which will develop in approximately 30% of all patients with DVT (Kahn et al., 2008). Patients with mild post-thrombotic syndrome have been shown to have a quality of life that is poorer than patients of similar age with arthritis, chronic lung disease, or diabetes (Kahn et al., 2008). If the post-thrombotic syndrome progresses to become severe, quality of life drops off to below levels seen in age-matched subjects with angina, cancer, and congestive heart failure (Kahn et al., 2008). Of even more concern, however, is that even small PEs typically cause some degree of hypoxia. This complication has the potential to be disastrous, since even a single, transient episode of oxygen desaturation below 90% has been shown to be associated with a significant increase in mortality early after TBI (Chesnut et al., 1993). If large, PE can cause hemodynamic instability, cardiac arrest, and sudden death. In short, VTE is a common problem seen after a common injury, and defining an optimal VTE prevention regimen after TBI has the potential to decrease morbidity for tens of thousands of patients annually.
The Controversy
Nationally, the centerpiece of VTE prevention strategies for non-brain-injured trauma patients is the initiation of the low-molecular-weight heparin enoxaparin (30 mg subcutaneously every 12 h) at 24 h after injury due to strong Level I evidence demonstrating safety and efficacy for this practice (Geerts et al., 1996; Knudson et al., 1996). Despite the centrality of enoxaparin's use in general trauma patients, however, the extension of its indications to include use in brain-injured subpopulations has been controversial, even in the face of the ubiquity of the problem of TBI, and the significant complications that arise from VTE formation. While enoxaparin's advantages as an effective, noninvasive method of decreasing VTE occurrence after trauma (Geerts et al., 1996; Knudson et al., 1996) are well documented, the administration of a low-dose anticoagulant to a patient with TBI has the potential to iatrogenically propagate intracranial hemorrhage and result in neurologic deterioration. Fear of this complication has historically caused reticence about the practice among TBI caregivers.
While this complication sounds disastrous, it is important to keep in mind that the hemorrhage patterns seen after TBI stabilize with time as they begin the healing process, and there is general agreement among TBI caregivers that low-dose anticoagulants are safe once this has occurred. However, this consensus that an early time period exists in which the risk for renewed bleeding in TBI patients is prohibitive for anticoagulant use, and a late time period exists during which the bleeding concern is negligible and anticoagulation is safe, has not been quantitatively translated into practice. National guidelines have not been useful on this point, as the Brain Trauma Foundation, which arguably promulgates the highest-quality evidence-based guidelines in the world for TBI care, simply states that anticoagulation should be used, but that inadequate evidence exists to make recommendations about timing (Bratton et al., 2007). What would seem to be a simple decision to delay anticoagulant initiation in favor of certainty about intracranial stabilization is not such a simple choice, as VTE development is well known to be a time-dependent phenomenon. The longer a caregiver chooses to wait to start an anticoagulant after TBI, the higher the likelihood that VTE will develop, and/or an invasive procedure for PE prevention will be considered via the placement of a prophylactic vena cava filter. These filters are expensive (the patient charge at Parkland is $7256), are associated with caval perforation (0–9%; Ferris et al., 1993; Greenfield et al., 1991; Simon et al., 1989), and can migrate toward or into the chest (0–11%; Ferris et al., 1993; Messmer and Greenfield, 1985; Simon et al., 1989). Additionally, descriptive studies have found acute DVT rates at the site of insertion of 9–33% (Greenfield et al., 1991; Mewissen et al., 1989; Pais et al., 1988), while a trial that randomized subjects with proximal DVT to filter placement or anticoagulation saw significantly higher recurrent DVT rates in the filter arm at 2 years (20% versus 11%; Decousus et al., 1998) and 8 years (36 versus 28%; PREPIC Study Group, 2005) after placement, presumably due in part to the venous endothelial damage that occurs during insertion. Further, filters are usually left in permanently despite the fact that the longest mean follow-up for any of these devices is 9 years (Greenfield and Proctor, 1995; Langan et al., 1999; Patton et al., 1996; Phelan et al., 2009; Rogers et al., 1998). As some patients can be expected to live far beyond this period, those receiving prophylactic filters incur an unknown lifetime risk of unforeseen complications. Taken together, these facts show why most TBI caregivers view the prophylactic vena cava filter as a last resort for patients who will require an excessively long period of intracranial stabilization before being eligible for anticoagulants. Clearly the consequences of delaying anticoagulant use are real.
The crux of the controversy over the use of anticoagulants for VTE prevention after TBI lies in the definitions of these time frames. Translating the qualitative consensus around “early” and “late” time points for the safety of anticoagulant use after TBI into quantitative guidelines will bring order to a process that to date has been largely driven by dogma.
Existing Evidence
Anticoagulation after elective neurosurgery
The perception of this research question as a high-risk endeavor has led to a limited number of studies on anticoagulant administration after TBI. The closest model for making inferences about anticoagulation after TBI is that of elective craniotomy. Extrapolation from this population to that of the brain-injured patient is especially tempting, given that a larger body of work has been done in that field. Seven randomized trials on the question of VTE prevention after elective craniotomy are available to guide practice. Randomized studies have demonstrated the efficacy (Cerrato et al., 1978), and safety (Constantini et al., 2001), of unfractionated heparin relative to placebo, low-molecular-weight heparins plus mechanical compression devices over compression devices alone (Agnelli et al., 1998; Nurmohamed et al., 1996), and low-molecular-weight heparin versus placebo (Melon et al., 1991), for VTE prevention. These results were not universal, as one randomized trial of low-molecular-weight heparin versus mechanical compression devices had to be stopped early due to excessive intracranial bleeding complications in the drug arm (Dickinson et al., 1998). Additional data suggesting that the type of anticoagulant used seems to be less important exists, as a pilot trial randomizing subjects to unfractionated heparin or low-molecular-weight heparin failed to show a difference in either VTE prevention or intracranial bleeding complications (MacDonald et al., 2003). Taken together, a recent meta-analysis calculated that for every 1000 patients undergoing craniotomy who receive pharmacologic VTE prophylaxis, 91 VTE events will be prevented, while 7 episodes of iatrogenic hemorrhage expansion will occur (Hamilton et al., 2011). Unfortunately, the authors were not able to comment on the severity and clinical significance of these intracranial bleeding episodes.
While these data are helpful, they must be interpreted with consideration of its limitations for applicability to trauma patients. Most importantly, the hemorrhage control techniques that are the hallmark of an operative intervention are not present during the expectant management of a head injury. This would presumably put traumatic lesions at higher risk for spontaneous expansion. Additionally, all but one (MacDonald et al., 2003) of the randomized trials had VTE prevention as their primary end-point, and were therefore not powered to primarily assess worsening of intracranial hemorrhage rates. Finally, elective craniotomy patients are often at higher risk for VTE than trauma patients, as they often have preoperative malignancy, long-standing leg weakness, more advanced age, and longer operative procedures. These shortcomings all serve to limit the generalizability of these results to VTE prevention after TBI.
Anticoagulation after TBI as a homogenous population
The field of pharmacologic VTE prevention after TBI is a young one. Geerts' landmark 1996 randomized trial of enoxaparin versus unfractionated heparin after trauma excluded “frank intracranial bleeding,” but allowed contusions and petechial hemorrhages. These criteria resulted in only 13 patients with TBI being randomized (of 265 total subjects), with one experiencing progression (Geerts et al., 1996). The next description of the practice was reported in the context of a 2000 validation study of VTE risk factors in 102 trauma patients, in which the authors described in passing that anticoagulation was started in 26 patients in whom some degree of intracranial blood was present without any instances of TBI worsening (Gearhart et al., 2000). While the authors did not comment on the amount of blood present or on the timing of treatment initiation, this study was notable for its challenge to the standard at the time, which saw no role for pharmacologic prophylaxis in this setting. It would be another 2 years before the first report dedicated to this question would be published, when 76 patients with severe TBI were retrospectively examined for the incidence of progression after the initiation of unfractionated heparin for VTE prevention (Kim et al., 2002). The timing of heparin initiation was arbitrarily dichotomized for the purpose of analysis at 72 h post-admission, and the authors found that there were no increases in intracranial bleeding complications between the early and late groups. Interestingly, equivalent rates of VTE development were also seen between the groups, although this was in part attributable to the fact that symptomatic criteria (which miss subclinical events) were used as a trigger for VTE testing.
The only interventional trial in this field followed, with a Turkish study from 2004 which randomized 120 subjects to enoxaparin 40 mg once a day or mechanical compression devices on the lower extremities (Kurtoglu et al., 2004). Enthusiasm for the finding that the rates of worsening of intracranial hemorrhage were low and equivalent between the groups (1.6% in each) were tempered by the serious methodologic shortcomings of this trial with regard to TBI exacerbation, as the dose of enoxaparin used was not the standard of care for American trauma patients, and the actual timing of anticoagulant initiation was never clearly delineated. An additional problem with the study design contributed to the otherwise surprising finding of fatal PE rates of 3.3% in the compression arm and 6.6% in the anticoagulant arm, in that the use of enoxaparin alone as an intervention is not appropriate, since the standard of care is to use it in conjunction with mechanical compression devices. This study was important in one respect, however, in that it demonstrated that subjects or their surrogates would consent to what was perceived as a high-risk interventional trial in this area.
The only reports on this field over the next several years would be descriptive studies in 2007 (Cothren et al., 2007) and 2008 (Depew et al., 2008). Cothren and colleagues published their experience with a new anticoagulant, dalteparin, and its safety and efficacy in VTE prevention in 743 polytrauma patients after its initiation a mean of 3.3 days after injury. TBI was not an exclusion criterion (an exception to the rule in most studies at the time of anticoagulation after trauma), and the authors mentioned in passing that none of the 174 subjects with intracranial hemorrhage had worsening of their bleeding (Cothren et al., 2007). The generalizability of this study was limited by decreased compliance with the anticoagulation regimen in the sample (only 74%), a lack of reporting on the timing of dalteparin use for the TBI subgroup, and the fact that this low-molecular-weight heparin has not been adopted as a standard pharmacologic intervention for VTE prevention in non-brain-injured trauma patients. Similarly, the Depew group found TBI progression rates of 3%, whether anticoagulation with enoxaparin or unfractionated heparin was started before or after 72 h in 124 TBI patients, and nearly identical rates of VTE of 13% and 11%, respectively (Depew et al., 2008). The exact times of anticoagulant initiation were not described, so it is impossible to say if they were close enough to have resulted in similar point estimates.
The importance of stability of the hemorrhage pattern prior to initiating anticoagulants was demonstrated in a 2010 report, which showed that TBI patients who had worsening of their hemorrhage patterns between their first and second CT scans (i.e., an unstable TBI pattern), followed by enoxaparin initiation had a 13-fold higher rate of continued hemorrhage progression (Levy et al., 2010). Importantly, stable scan results were not found to be significantly associated with expansion of injury after enoxaparin initiation. This makes intuitive sense and factored heavily into our work in this area. Using stability of CT scan injury patterns as an inclusion criterion, a Canadian study reported on the safety and efficacy of enoxaparin versus dalteparin in 287 TBI patients with moderately to severely depressed levels of consciousness. After starting anticoagulation between 48 and 72 h after injury, identical symptomatic VTE rates of 7% were found between the groups (Dudley et al., 2010). Additionally, only one symptomatic intracranial hemorrhage expansion was found in the entire cohort. By using decreased level of consciousness and not presence of hemorrhage as the main inclusion criterion, however, this study potentially skewed toward under-estimation of the true expansion rate, as some of these subjects were enrolled despite not having intracranial bleeding.
In 2011, Koehler and associates reported on their experience with 669 TBI patients in whom enoxaparin was used (Koehler et al., 2011). After defining early and late anticoagulation as before or after 72 h after injury, the authors reported no significant reductions in symptomatic VTE complications between the arms, and no differences in symptomatic hemorrhage expansion. By relying solely on clinical manifestations rather than performing scheduled screening to detect DVT, however, the authors surely underestimated the true incidence of thrombotic complications. In the same year, Minshall and colleagues retrospectively reviewed their institution's use of enoxaparin and unfractionated heparin for VTE prevention after TBI. While that report demonstrated that enoxaparin had significantly better protective effects against VTE and lower intracranial bleeding complications compared to unfractionated heparin, the heparin group also had significantly more severe brain injuries, making these results unsurprising (Minshall et al., 2011). The importance of compliance with uninterrupted pharmacologic prophylaxis has recently been illustrated by the finding that VTE development after TBI was significantly associated not with the timing of anticoagulant administration, but rather with the completeness of the regimen. Salottolo and co-workers retrospectively showed that when anticoagulant administration was interrupted by as little as a single missed dose, a sevenfold increase in the odds of VTE development resulted (Salottolo et al., 2011).
Two recent reports on the effectiveness of VTE prophylaxis after TBI have produced conflicting results despite large sample sizes. The Wisconsin group retrospectively examined 812 TBI patients, of whom 402 received anticoagulation at a mean of 94 h after injury with either enoxaparin or unfractionated heparin. The authors found that despite equivalent intracranial injury burdens and significantly higher overall injury severity, the anticoagulation group had a significantly lower VTE rate with a similar rate of intracranial hemorrhage progression (Scudday et al., 2011). This stands in contrast to a retrospective study of 2000 TBI patients, which showed that TBI was associated with a three- to fourfold increase in the risk of VTE, regardless of the presence or timing of anticoagulation (Reiff et al., 2009).
Anticoagulation after TBI as a heterogenous population
A commonality of the studies to this point has been inclusion criteria that generally consist of the presence of intracranial blood alone. By choosing to study their TBI populations as homogenous groups in which intracranial hemorrhage was either present or absent, however, the researchers in this field have made important implicit concessions. Inherent in this methodologic construct is the idea that the risks for spontaneous progression and the times needed for stabilization of hemorrhage patterns are equivalent across all sizes and scopes of injury, and therefore that a single VTE prevention regimen can be crafted for all TBI patients. This is contrary to what is seen in clinical practice, however, as the association between increasing severity of intracranial injury and higher rates of spontaneous progression has been well documented (Beaumont et al., 2006; Bee et al., 2009; Chang et al., 2006; Chiergato et al., 2005; Park et al., 2009; Velmahos et al., 2006). It is due to this systematic weakness in most studies on VTE prevention after TBI that the works of Berne and Norwood stand apart by virtue of their recognition that brain injury exists as a spectrum of disease. In recognizing this heterogeneity, they characterized a set of highly-specific injury patterns that merited a method of VTE prevention all their own (Norwood et al., 2001, 2002, 2008). After defining these injuries (subdural or epidural hemorrhages thinner than 9 mm, parenchymal contusions smaller than 2 cm, and a single contusion per lobe), the authors demonstrated that patients with these small injury patterns, which were radiographically unchanged 24 h after injury, could have low-dose enoxaparin started with a subsequent TBI progression rate of 3.4%. Further, of the 525 subjects in their series, 10 of 18 TBI progressions occurred in patients with protocol violations, as enoxaparin was started within 20 h of admission. In those patients who did not get enoxaparin started until a full 24 h had elapsed after injury (and were thus per protocol), the progression rate was 1.8%, with only two patients requiring a change in their care beyond simply stopping the enoxaparin. Both rates were comparable to anticoagulant-naive historic controls, and Berne and Norwood argued that the benefits of early aggressive VTE prevention with enoxaparin balanced the very small risk of worsened TBI. While these works were important in their inherent recognition of (1) different injury patterns having different degrees of risk for spontaneous progression, and (2) the need for precise descriptions of eligible injury patterns to assist promulgation and adoption, they were not without their shortcomings. Most importantly, the authors did not attempt to elucidate management strategies for what constitutes the large majority of TBI patients, and the only controls were historical.
Future Directions
By now it should be clear that the binary dichotomization of brain injuries as present or absent, while methodologically simple, is lacking as a basis for generating guidelines for VTE prevention strategies. Additionally, while limited work has been done on a specific TBI subpopulation, no effort has been made to create recommendations that apply to the entire spectrum of injury seen in clinical practice. Such an effort will have to recognize that different levels of risk for spontaneous expansion of hemorrhage exist, and that the different time frames needed for stabilization of intracranial injury patterns across the entire spectrum of brain injuries will necessitate VTE prevention regimens that are tailored to each arm. A large part of this work will be elucidating the natural evolution of the brain injuries in each category, so that the absolute earliest time point for safe initiation of anticoagulants can be identified. Finally, once the risk categories and their individualized VTE prevention strategies are created, the efficacy of these recommendations will have to be demonstrated. The task is analogous to building a house on the edge of a cliff to take advantage of the view: the closer one gets to the edge, the greater the reward, but the consequences of going too far can be disastrous.
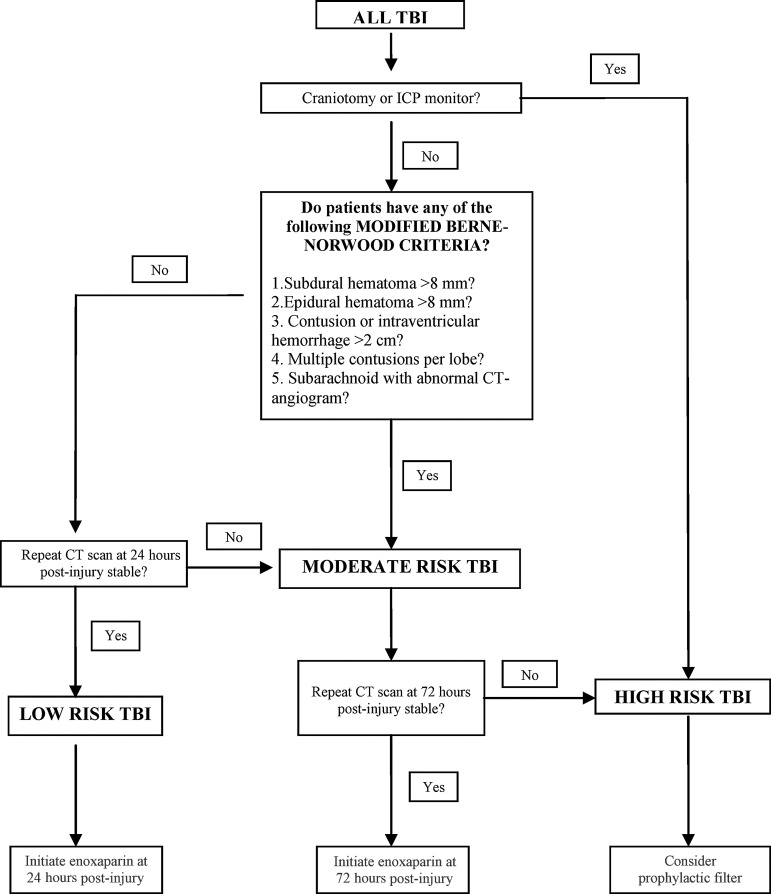
Our group has attempted to address these deficiencies through the creation of just such an algorithm, known as the “Parkland Protocol” (Fig. 1). We began by modifying the original Berne-Norwood criteria to include patients with any amount of subarachnoid hemorrhage, with a normal CT-angiogram or an area of intraventricular hemorrhage (IVH) less than 2 cm in size, and used these as the basis for an algorithm that categorizes patients by the likelihood of natural progression of their brain hemorrhage pattern. Patients meeting these modified Berne-Norwood criteria, and with stable CT scans at 24 h after injury, are considered to be low-risk for spontaneous enlargement of their brain injury, while those not meeting the modified Berne-Norwood criteria are categorized as moderate-risk for spontaneous TBI progression. Patients undergoing a craniotomy or placement of an intracranial pressure monitor are categorized as high-risk for spontaneous TBI progression. After categorizing TBI by risk, the protocol then makes individualized recommendations for VTE prevention for each arm. The low-risk and moderate-risk arms (which roughly correspond with mild and moderate TBI), have recommendations for the timing of pharmacologic prophylaxis initiation. The high-risk arm (which corresponds with severe TBI) does not have a hard time point, and instead relies on daily reassessments of the risk:benefit ratio for starting anticoagulation, while considering the placement of a prophylactic vena cava filter.
FIG. 1.
The Parkland Protocol. This algorithm categorizes TBI patterns as low-risk, moderate-risk, or high-risk for spontaneous expansion, and tailors VTE prophylaxis to each type (TBI, traumatic brain injury; VTE, venous thromboembolism; ICP, intracranial pressure; CT, computed tomography).
We have completed a prospective study defining the natural history of the brain injuries in each of these arms (Phelan et al., in press [a]), and are now turning our attention to examining the safety of our theoretical recommendations through a series of randomized trials known as the “Delayed vs. Early Enoxaparin Prophylaxis (DEEP)” studies (Phelan et al, in press [b]).
Conclusion
While VTE prophylaxis after TBI is a common problem with the potential for considerable morbidity, no national standard of care for this practice exists, and research on it has generally been limited to retrospective and/or observational studies in which patients are dichotomized as having the presence or absence of intracranial blood. This approach seems counter-intuitive, as smaller injury patterns would seem to stabilize more rapidly, and potentially be eligible for different timing of pharmacologic prophylaxis than more severe injuries. In fact, this general approach has likely hindered the development and promulgation of clinically-useful guidelines. In contrast, our group has created the Parkland Protocol, which stratifies patients into low-risk, moderate-risk, and high-risk candidates for the likelihood of natural progression of their hemorrhage pattern. By creating these three tiers of brain injury and tailoring a unique prophylaxis regimen to each individual arm, one of the greatest obstacles to the generation of quality guidelines has been overcome. We are in the midst of a series of projects designed to assess the safety of the Parkland Protocol's recommendations, and in doing so we hope to define a best practice for VTE prophylaxis for those suffering TBI.
Acknowledgments
I would like to thank Kendra Armijo for her administrative support in the preparation of this manuscript.
Author Disclosure Statement
No competing financial interests exist. I receive support from NIH grant no. 1 KL2 RR024983-01, titled, “North and Central Texas Clinical and Translational Science Initiative” (Robert Toto, M.D., PI) from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. The contents herein are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or the NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp
References
- Agnelli G. Piovella F. Buoncristiani P. Severi P. Pini M. D'Angelo A. Beltrametti C. Damiani M. Andrioli G. Pugliese R. Iorio A. Brambilla G. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N. Engl. J. Med. 1998;339:80–85. doi: 10.1056/NEJM199807093390204. [DOI] [PubMed] [Google Scholar]
- Beaumont A. Gennarelli T. CT prediction of contusion evolution after closed head injury: the role of pericontusional edema. Acta. Neurochir. Suppl. 2006;96:30–32. doi: 10.1007/3-211-30714-1_7. [DOI] [PubMed] [Google Scholar]
- Bee T. Magnotti L. Croce M. Maish G. Minard G. Schroeppel T. Zarzaur B. Fabian T. Necessity of repeat head CT and ICU monitoring in patients with minimal brain injury. J. Trauma. 2009;6:1015–1018. doi: 10.1097/TA.0b013e31819adbc8. [DOI] [PubMed] [Google Scholar]
- Belcaro G. Cesarone M. Nicolaides A. Ricci A. Geroulakos G. Shah S. Ippolito E. Myers K. Bavera P. Dugall M. Moia M. Di Renzo A. Errichi B. Brandolini R. Dugall M. Griffin M. Ruffini I. Ricci A. Acerbi G. Prevention of venous thrombosis with elastic stockings during long-haul flights: the LONFLIT 5 JAP study. Clin. Appl. Thromb. Hemostasis. 2003;9:197–201. doi: 10.1177/107602960300900303. [DOI] [PubMed] [Google Scholar]
- Belcaro G. Cesarone M. Rohdewald P. Ricci A. Ippolito E. Dugall M. Griffin M. Ruffini I. Acerbi G. Vinciguerra M. Bavera P. Di Renzo A. Errichi B. Cerritell F. Prevention of venous thrombosis and thrombophlebitis in long-haul flights with pycnogenol. Clin. Appl. Thromb. Hemostasis. 2004;10:373–377. doi: 10.1177/107602960401000410. [DOI] [PubMed] [Google Scholar]
- Belcaro G. Cesarone M. Shah S. Nicolaides A. Geroulakos G. Ippolito E. Winford M. Lennox A. Pellegrini L. Brandolini R. Myers K. Simeone E. Bavera P. Dugall M. Di Renzo A. Moia M. Prevention of edema, flight microangiopathy and venous thrombosis in long flights with elastic stockings. A randomized trial: The LONFLIT 4 Concorde Edema-SSL Study. Angiology. 2002;53:635–645. doi: 10.1177/000331970205300603. [DOI] [PubMed] [Google Scholar]
- Belcaro G. Geroulakos G. Nicolaides A. Myers K. Winford M. Venous thromboembolism from air travel: the LONFLIT study. Angiology. 2001;52:369–374. doi: 10.1177/000331970105200601. [DOI] [PubMed] [Google Scholar]
- Bratton S. Chestnut R. Ghajar J. McConnell Hammond F. Harris O. Hartl R. Manley G. Nemecek A. Newell D. Rosenthal G. Schouten J. Shutter L. Timmons S. Ullman J. Videtta W. Wilberger J. Wright D. The Brain Trauma Foundation Guidelines for the Management of Severe Traumatic Brain Injury: Deep Vein Thrombosis Prophylaxis. J. Neurotrauma. 2007;24:S32–S36. doi: 10.1089/neu.2007.9991. [DOI] [PubMed] [Google Scholar]
- Cerrato D. Ariano C. Fiacchino F. Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J. Neurosurg. 1978;49:378–381. doi: 10.3171/jns.1978.49.3.0378. [DOI] [PubMed] [Google Scholar]
- Cesarone M. Belcaro G. Errichi B. Nicolaides A. Geroulakos G. Ippolito E. Winford M. Lennox A. Pellegrini L. Myers K. Ricci A. Hans C. Simeone E. Bavera P. Dugall M. Moia M. Stuard S. The LONFLIT4—Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology. 2003b;54:143–154. doi: 10.1177/000331970305400202. [DOI] [PubMed] [Google Scholar]
- Cesarone M. Belcaro G. Nicolaides A. Ricci A. Geroulakos G. Ippolito E. Brandolini R. Vinciguerra G. Dugall M. Griffin M. Ruffini I. Acerbi G. Corsi M. Riordan N. Stuard S. Bavera P. Di Renzo A. Kenyon J. Errichi B. Prevention of venous thrombosis in long-haul flights with Flite Tabs: the LONFLIT-FLITE randomized, controlled trial. Angiology. 2003a;54:531–539. doi: 10.1177/000331970305400502. [DOI] [PubMed] [Google Scholar]
- Chang E. Meeker M. Holland M. Acute traumatic intraparenchymal hemorrhage: risk factors for progression in the early post-injury period. Neurosurgery. 2006;58:647–656. doi: 10.1227/01.NEU.0000197101.68538.E6. [DOI] [PubMed] [Google Scholar]
- Chesnut R. Marshall L. Klauber M. Blunt B. Baldwin N. Eisenberg H. Jane J. Marmarou A. Foulkes M. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Chiergato A. Fainardi E. Morselli-Labate A. Antonelli V. Compagnone C. Targa L. Kraus J. Servadei F. Factors associated with neurological outcome and lesion progression in traumatic subarachnoid hemorrhage patients. Neurosurgery. 2005;56:671–680. doi: 10.1227/01.neu.0000156200.76331.7a. [DOI] [PubMed] [Google Scholar]
- Constantini S. Kanner A. Friedman A. Shoshan Y. Israel Z. Ashkenazi E. Gertel M. Even S. Shevach Y. Shalit M. Umansky F. Rappaport Z. Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J. Neurosurg. 2001;94:918–921. doi: 10.3171/jns.2001.94.6.0918. [DOI] [PubMed] [Google Scholar]
- Cothren C. Smith W. Moore E. Morgan S. Utility of once-daily dose of low molecular weight heparin to prevent VTE in multisystem trauma patients. World J. Surg. 2007;31:98–104. doi: 10.1007/s00268-006-0304-1. [DOI] [PubMed] [Google Scholar]
- Decousus H. Leizorovicz A. Parent F. Page Y. Tardy B. Girard P. Laporte S. Faivre R. Charbonnier B. Barral F. Huet Y. Simonneau G. A clinical trial of vena cava filters in the prevention of pulmonary embolism in patients with proximal DVT. N. Engl. J. Med. 1998;338:409–415. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- Depew A. Hu C. Nguyen A. Driessen N. Thromboembolic prophylaxis in blunt traumatic intracranial hemorrhage: A retrospective review. Am. Surg. 2008;74:906–911. [PubMed] [Google Scholar]
- Dickinson L. Miller L. Patel C. Gupta S. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery. 1998;43:1074–1081. doi: 10.1097/00006123-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Dudley R. Aziz I. Bonnici A. Saluja R. Lamoureux J. Kalmovitch B. Gursahaney A. Razek T. Maleki M. Marcoux J. Early venous thromboembolic event prophylaxis in traumatic brain injury with low-molecular-weight heparin: Risks and benefits. J. Neurotrauma. 2010;27:2165–2172. doi: 10.1089/neu.2010.1366. [DOI] [PubMed] [Google Scholar]
- Ferris E.J. McCowan T.C. Carver D.K. McFarland D.R. Percutaneous inferior vena caval filters: Follow-up of seven designs in 320 patients. Radiology. 1993;188:851–856. doi: 10.1148/radiology.188.3.8351361. [DOI] [PubMed] [Google Scholar]
- Gearhart M. Luchette F. Proctor M. Lutomski D. Witsken C. James L. Davis K. Johannigman J. Hurst J. Frame S. The risk assessment profile score identifies trauma patients at risk for deep vein thrombosis. Surgery. 2000;128:631–640. doi: 10.1067/msy.2000.108224. [DOI] [PubMed] [Google Scholar]
- Geerts W. Code K. Jay R. Chen E. Szalai J. A prospective study of venous thromboembolism after major trauma. N. Engl. J. Med. 1994;331:1601–1606. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- Geerts W. Jay R. Code K. Chen E. Szalai J. Saibil E. Hamilton P. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N. Engl. J. Med. 1996;335:701–707. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- Greenfield L. Chok J. Proctor M. Bonn J. Bookstein J. Castaneda-Zuniga W. Cutler B. Ferris E. Keller F. McGowan T. Results of a multicenter study of the modified hook-titanium Greenfield filter. J. Vasc. Surg. 1991;14:253–257. doi: 10.1067/mva.1991.29913. [DOI] [PubMed] [Google Scholar]
- Greenfield L. Proctor M. Twenty year clinical experience with the Greenfield filter. Cardiovasc. Surg. 1995;3:199–205. doi: 10.1016/0967-2109(95)90895-c. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Yee W. Hull R. Ghali W. Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: A systematic review and meta-analysis. Neurosurgery. 2011;68:571–581. doi: 10.1227/NEU.0b013e3182093145. [DOI] [PubMed] [Google Scholar]
- Kahn S. Shbaklo H. Lamping D. Holcroft C. Shrier I. Miron M. Roussin A. Desmarais S. Joyal F. Kassis J. Solymoss S. Desjardins L. Johri M. Ginsberg J. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J. Thromb. Haemost. 2008;6:1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- Kim J. Gearhart M. Zurick A. Zuccarello M. James L. Luchette F. Preliminary report on the safety of heparin for deep venous thrombosis prophylaxis after severe head injury. J. Trauma. 2002;53:38–43. doi: 10.1097/00005373-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Knudson M. Morabito D. Paiement G. Shackleford S. Use of low molecular weight heparin in preventing thromboembolism in trauma patients. J. Trauma. 1996;41:446–459. doi: 10.1097/00005373-199609000-00010. [DOI] [PubMed] [Google Scholar]
- Koehler D. Shipman J. Davidson M. Guillamondegui O. Is early venous thromboembolism prophylaxis safe in trauma patients with intracranial hemorrhage? J. Trauma. 2011;70:324–329. doi: 10.1097/TA.0b013e31820b5d22. [DOI] [PubMed] [Google Scholar]
- Kurtoglu M. Yanar H. Bilsel Y. Guloglu R. Kizilirmak S. Buyukkurt D. Granit V. Venous thromboembolism prophylaxis after head and spinal trauma: Intermittent pneumatic compression devices versus low molecular weight heparin. World J. Surg. 2004;28:807–811. doi: 10.1007/s00268-004-7295-6. [DOI] [PubMed] [Google Scholar]
- Langan E., 3rd Miller R. Casey W., 3rd Graham R. Taylor S. Prophylactic inferior vena cava filters in trauma patients at high risk: Follow-up examination and risk/benefit assessment. J. Vasc. Surg. 1999;30:484–490. doi: 10.1016/s0741-5214(99)70075-3. [DOI] [PubMed] [Google Scholar]
- Langlois J. Rutland-Brown W. Thomas K. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2004. [Google Scholar]
- Levy A. Salottolo R. Bar-Or R. Offner P. Mains C. Sullivan M. Bar-Or D. Pharmacologic thromboprophlaxis is a risk factor for hemorrhage progression in a subset of patients with traumatic brain injury. J. Trauma. 2010;68:886–894. doi: 10.1097/TA.0b013e3181d27dd5. [DOI] [PubMed] [Google Scholar]
- MacDonald R. Amidei C. Baron J. Weir B. Brown F. Erickson R. Hekmatpanah J. Frim D. Randomized, pilot study of intermittent pneumatic compression devices plus dalteparin versus intermittent pneumatic compression devices plus heparin for prevention of venous thromboembolism in patients undergoing craniotomy. Surg. Neurol. 2003;59:363–374. doi: 10.1016/s0090-3019(03)00111-3. [DOI] [PubMed] [Google Scholar]
- Melon E. Keravel Y. Gaston A. Huet Y. Combes S. The NEUOONOX group. Deep venous thrombosis prophylaxis by low molecular weight heparin in neurosurgical patients. Anesthesiology. 1991;75:A214. [Google Scholar]
- Messmer J.M. Greenfield L.J. Greenfield caval filters: long-term radiographic follow-up study. Radiology. 1985;156:613–618. doi: 10.1148/radiology.156.3.4023218. [DOI] [PubMed] [Google Scholar]
- Mewissen M. Erickson S. Foley W. Lipchik E. Olson D. McCann K. Schreiber E. Thrombosis at venous insertion sites after inferior vena cava filter placement. Radiology. 1989;173:155–157. doi: 10.1148/radiology.173.1.2675181. [DOI] [PubMed] [Google Scholar]
- Minshall C. Eriksson E. Leon S. Doben A. McKinzie B. Fakhry S. Safety and efficacy of heparin or enoxaparin prophylaxis in blunt trauma patients with a head Abbreviated Injury Severity score >2. J. Trauma. 2011;71:396–400. doi: 10.1097/TA.0b013e31822734c9. [DOI] [PubMed] [Google Scholar]
- Norwood S. Berne J. Rowe S. Villarreal D. Ledlie J. Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J. Trauma. 2008;65:1021–1027. doi: 10.1097/TA.0b013e31818a0e74. [DOI] [PubMed] [Google Scholar]
- Norwood S. McAuley C. Berne J. Vallina V. Kerns D. Grahm T. McLarty J. A potentially expanded role for enoxaparin in preventing venous thromboembolism in high risk blunt trauma patients. J. Am. Coll. Surg. 2001;192:161–167. doi: 10.1016/s1072-7515(00)00802-4. [DOI] [PubMed] [Google Scholar]
- Norwood S. McAuley C. Berne J. Vallina V. Kerns D. Grahm T. Short K. McLarty J. Prospective evaluation of the safety of enoxaparin prophylaxis for venous thromboembolism in patients with intracranial hemorrhagic injuries. Arch. Surg. 2002;137:696–702. doi: 10.1001/archsurg.137.6.696. [DOI] [PubMed] [Google Scholar]
- Nurmohamed M. van Riel A. Henkens C. Koopman M. Que G. d'Azemar P. Buller H. ten Cate J. Hoek J. van der Meer J. van der Heul D. Turpie A. Haley S. Sicurella A. Gent M. Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thromb. Haemost. 1996;75:233–238. [PubMed] [Google Scholar]
- Pais S. Tobin K. Austin C. Queral L. Percutaneous insertion of the Greenfield inferior vena cava filter: experience with ninety-six patients. J. Vasc. Surg. 1988;8:460–464. doi: 10.1067/mva.1988.avs0080460. [DOI] [PubMed] [Google Scholar]
- Park H. Joo W. Chough C. Cho C. Lee K. Rha H. The clinical efficacy of repeat brain computed tomography in patients with traumatic intracranial haemorrhage within 24 hours after blunt head injury. Br. J. Neurosurg. 2009;23:617–621. doi: 10.3109/02688690902999302. [DOI] [PubMed] [Google Scholar]
- Patton J. Fabian T. Croce M. Minard G. Pritchard F. Kudsk K. Prophylactic Greenfield filters: acute complications and long-term follow up. J. Trauma. 1996;41:231–237. doi: 10.1097/00005373-199608000-00006. [DOI] [PubMed] [Google Scholar]
- Phelan H.A. Eastman A. Madden C. Aldy K. Berne J. Norwood S. Scott W. Bernstein I. Pruitt J. Butler G. Rogers L. Minei J. TBI risk stratification at presentation: A prospective study of the incidence and timing of radiographic worsening in the Parkland Protocol. J. Trauma. doi: 10.1097/TA.0b013e3182606327. (In press [a]) [DOI] [PubMed] [Google Scholar]
- Phelan H.A. Gonzalez R. Scott W. White C. McClure M. Minei J. Long-term follow-up of prophylactic permanent vena cava filters in trauma patients. J. Trauma. 2009;67:485–489. doi: 10.1097/TA.0b013e3181ad67c1. [DOI] [PubMed] [Google Scholar]
- Phelan H.A. Wolf S.A. Norwood S.H. Aldy K. Brakenridge S.C. Eastman A.L. Madden C.J. Nakonezny P.A. Yang L. Chason D.P. Arbique G. Berne J. Minei J.P. A randomized, double-blinded, placebo-controlled pilot trial of anticoagulation in low-risk TBI: The Delayed vs. Early Enoxaparin Prophylaxis I (DEEP I) study. J. Trauma. doi: 10.1097/TA.0b013e31825ac49e. (In press [b]) [DOI] [PubMed] [Google Scholar]
- PREPIC Study Group. Eight year follow up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112:416–422. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- Reiff D. Haricharan R. Bullington N. Griffin R. McGwin G. Rue L., 3rd Traumatic brain injury is associated with the development of deep vein thrombosis independent of pharmacologic prophylaxis. J. Trauma. 2009;66:1436–1440. doi: 10.1097/TA.0b013e31817fdf1c. [DOI] [PubMed] [Google Scholar]
- Rogers F. Strindberg G. Shackford S. Osler T. Morris C. Ricci M. Najarian K. D'Agostino R. Pilcher D. Five-year follow-up of prophylactic vena cava filters in high-risk trauma patients. Arch. Surg. 1998;133:406–412. doi: 10.1001/archsurg.133.4.406. [DOI] [PubMed] [Google Scholar]
- Salottolo K. Offner P. Levy A. Mains C. Slone D. Bar-Or D. Interrupted pharmacologic thromboprophylaxis increases VTE in traumatic brain injury. J. Trauma. 2011;70:19–26. doi: 10.1097/TA.0b013e318207c54d. [DOI] [PubMed] [Google Scholar]
- Scudday T. Brasel K. Webb T. Codner P. Somberg L. Weigelt J. Herrmann D. Peppard W. Safety and efficacy of prophylactic anticoagulation in patients with traumatic brain injury. J. Am. Coll. Surg. 2011;213:148–154. doi: 10.1016/j.jamcollsurg.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Scurr J. Machin S. Bailey-King S. Mackie I. McDonald S. Smith P. Frequency and prevention of symptomless deep-vein thrombosis in long-haul flights: a randomised trial. Lancet. 2001;357:1485–1489. doi: 10.1016/S0140-6736(00)04645-6. [DOI] [PubMed] [Google Scholar]
- Simon M. Athanasoulis C. Kim D. Steinberg F. Porter D. Byse B. Kleshinski S. Geller S. Orron D. Waltman A. Simon nitinol inferior vena cava filters: initial clinical experience. Radiology. 1989;172:99–103. doi: 10.1148/radiology.172.1.2662259. [DOI] [PubMed] [Google Scholar]
- Velmahos G. Gervasini A. Petrovick L. Dorer M. Spaniolas K. Alam H. De Moya M. Borges L. Conn A. Routine repeat head CT for minimal head injury is unnecessary. J. Trauma. 2006;60:494–499. doi: 10.1097/01.ta.0000203546.14824.0d. [DOI] [PubMed] [Google Scholar]