Abstract
Environmental enrichment (EE) and serotonin1A (5-HT1A)-receptor agonists provide significant benefit after experimental traumatic brain injury (TBI). The aim of this study was to test the hypothesis that combining these therapies would produce an effect that is more robust than either therapy alone. Anesthetized adult male rats received a cortical impact or sham injury and then were randomly assigned to EE or standard (STD) housing where they received either buspirone (0.3 mg/kg) or vehicle (1.0 mL/kg) once daily for 3 weeks. Motor and cognitive assessments were conducted on post-injury days 1–5 and 14–19, respectively. CA1/3 neurons were quantified at 3 weeks. No differences were observed among buspirone and vehicle sham groups in any task regardless of housing condition and thus the data were pooled. CA3 cell loss was reduced in the TBI+EE+buspirone and TBI+EE+vehicle groups. Motor recovery, spatial learning, and memory retention were enhanced in the TBI+EE+buspirone, TBI+EE+vehicle, and TBI+STD+buspirone groups versus the TBI+STD+vehicle group (p≤0.005). Moreover, spatial learning was significantly better in the TBI+EE+buspirone group versus the TBI+STD+buspirone group (p<0.0001). No differences were revealed between the buspirone and vehicle EE groups. These data show that EE and buspirone benefit functional outcome after TBI, but their combination is not more robust than either alone, which does not support the hypothesis. The lack of an additive effect may be due to the early-and-continuous EE paradigm on its own producing marked benefits, resulting in a ceiling effect. The evaluation of buspirone in a delayed-and-abbreviated EE paradigm is ongoing in our laboratory.
Key words: behavioral outcome, controlled cortical impact, functional recovery, hippocampus, learning and memory, Morris water maze, serotonin (5-HT1A)-receptor agonist, traumatic brain injury
Introduction
An estimated 1.7 million Americans suffer significant neurological disabilities resulting from traumatic brain injury (TBI) each year (Faul et al., 2010; Selassie et al., 2008; Sosin et al., 1995; Summers et al., 2009; Thurman et al., 1999). The cost of medical and rehabilitative care as well as that associated with the loss of productivity results in a significant monetary burden that is conservatively estimated to be more than $50 billion per year (Max et al., 1991). Consequently, the need for development, refinement, and effective implementation of treatment strategies capable of producing neurobehavioral and cognitive recovery after TBI is high.
Several treatment approaches have been implemented after experimental TBI, leading to numerous pre-clinical studies demonstrating improvement in behavioral and histological outcome (Bales et al., 2009; Garcia et al., 2011; Kokiko and Hamm, 2007; McIntosh et al., 1998; Wheaton et al., 2009). While many of these interventions have produced benefits in the laboratory, the same cannot be said for the clinic (Doppenberg et al., 2004; Menon, 2009). The overall lack of translational success has evolved into the current thinking by many in the TBI community that mono-therapies may be insufficient to produce a translatable product, and that a more dedicated effort toward poly-therapies is necessary. Indeed, a National Institutes of Health (NIH) TBI workshop convened to discuss the opportunities and challenges associated with conducting research studies using multiple drug interventions (Margulies et al., 2009). The workshop leaders focused exclusively on the potential for combinatorial pharmacological agents to promote recovery. While this is a commendable start, there is certainly real potential and value in including non-invasive therapies, such as environmental enrichment (EE) or exercise (Gomez-Pinilla et al., 2011; Griesbach et al., 2009; Hamm et al., 1996; Hicks et al., 2002; Hoffman et al., 2008; Kline et al., 2007,2010; Matter et al., 2011; Passineau et al., 2001; de Witt et al., 2011), in combinational therapy paradigms for promoting recovery and perhaps augmenting the pharmacological effects, particularly in rehabilitation settings.
With this mindset, the current study was designed to test the hypothesis that combining two therapies that have been shown to produce benefits on their own would result in a more robust effect when combined after TBI. The end-point measures are standard in the laboratory and the TBI field in general, and include motor, cognitive, and histological outcomes. The first therapeutic approach is EE, which consists of increased living space, complex stimuli, and social interaction that promotes exploration of the surroundings (Sozda et al., 2010). This paradigm has consistently shown improvements in behavioral and histological outcome after brain trauma and can therefore be considered a reasonable approach to pre-clinical rehabilitation. Importantly, the benefits conferred by EE span clinically-relevant models of experimental TBI, such as controlled cortical impact (CCI; Chen et al., 2005; de Witt et al., 2011; Hoffman et al., 2008; Kline et al., 2007,2010; Matter et al., 2011; Sozda et al., 2010) and fluid percussion (Hamm et al., 1996; Hicks et al., 2002; Gaulke et al., 2005; Giza et al., 2005; Maegele et al., 2005; Passineau et al., 2001). The second therapy in this combined treatment approach is the systemic administration of the serotonin1A (5-HT1A)-receptor agonist buspirone. A recent dose-response study from our laboratory showed that buspirone facilitated spatial learning in a well-established water maze task and reduced the size of TBI-induced cortical lesions (Olsen et al., 2012), which replicates the results of studies evaluating other 5-HT1A-receptor agonists, such as 8-OH-DPAT (Cheng et al., 2007,2008; Kline et al., 2002c,2004,2007,2010), and repinotan HCl (Kline et al., 2001). The advantage of evaluating buspirone in an experimental model of TBI is that it is used clinically as a treatment for anxiety and depression, and therefore safety and tolerability issues are well known (Chew and Zafonte, 2009). Furthermore, because of its widespread clinical applicability, its translation from bench to bedside could be expedited if it is shown to be efficacious in pre-clinical models of TBI.
Methods
Subjects
Sixty adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were initially housed in standard steel-wire mesh cages and maintained in a temperature-controlled (21±1°C) and light-controlled (on 7:00 am to 7:00 pm) environment with food and water available ad libitum. After 1 week of acclimatization all rats underwent a single day of beam-walk training, which consisted of 3–5 trials to traverse the beam. Following training the rats were randomly assigned to one of the following group conditions: TBI+EE+buspirone (0.3 mg/kg; n=10), TBI+EE+vehicle (1.0 mL/kg; n=10), TBI+STD+buspirone (n=10), TBI+STD+vehicle (n=10), sham+EE+buspirone (n=5), sham+EE+vehicle (n=5), sham+STD+buspirone (n=5), or sham+STD+vehicle (n=5). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Every attempt was made to limit the number of rats used and to minimize suffering.
Surgery
A CCI injury was produced as previously described (Kline et al., 2002a,b,c,2004,2007,2010). Briefly, surgical anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane, respectively, in 2:1 N2O:O2. After endotracheal intubation the rats were secured in a stereotaxic frame, ventilated mechanically, and temperature was maintained at 37±0.5°C with a heating blanket. Utilizing aseptic procedures a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy was made in the right hemisphere (encompassing bregma and lambda, between the sagittal suture and the coronal ridge) with a hand-held trephine. The bone flap was removed and the craniectomy was enlarged further with rongeurs. Subsequently, the impacting rod was extended and the impact tip (6 mm, flat) was centered and lowered through the craniectomy until it touched the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8-mm tissue deformation at 4 m/sec). Immediately after the CCI, anesthesia was discontinued and the incision was promptly sutured. The rats were subsequently extubated and assessed for acute neurological outcome. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
Acute neurological evaluation
Hindlimb reflexive ability was assessed immediately following the cessation of anesthesia by gently squeezing the rats' paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to the prone position.
Drug administration
Buspirone was purchased from Sigma-Aldrich (St. Louis, MO), and was prepared daily by dissolving in sterile saline, which also served as the vehicle. Buspirone (0.3 mg/kg), or a comparable volume of vehicle (1.0 mL/kg), was administered intraperitoneally beginning 24 h after CCI or sham injury and once daily for 3 weeks. The dose of buspirone and route of administration were selected based on previous data from our laboratory showing this regimen to be optimal after chronic treatment (Olsen et al., 2012).
Housing conditions: Environmental manipulation
Following surgery, and after the effects of anesthesia abated (as evidenced by spontaneous movement in the holding cage), the rats were returned to the colony where those designated for EE were immediately placed in specifically designed 36×30×20-inch steel-wire cages that consist of three levels with ladders to ambulate from one level to another, and contain various toys (e.g., balls, blocks, and tubes), nesting materials (e.g., paper towels), and ad libitum food and water (for depiction of the EE cage, see Kline et al., 2007 and Sozda et al., 2010). To maintain novelty, the objects were rearranged every day and changed each time the cage was cleaned, which was twice per week. Groups of 10–12 rats, which included subsets of each of the conditions (i.e., buspirone- and vehicle-treated TBI and sham controls), were housed together to minimize variability. Rats in the STD conditions were placed in standard steel-wire mesh cages (2 rats per cage) with only food and water.
Motor performance
Beam-balance and beam-walk tasks were used to assess motor function. Briefly, the beam-balance task consists of placing the rat on an elevated and narrow beam (1.5 cm wide), and recording the time it remains on up to a maximum of 60 sec. The beam-walk task, modified from that originally devised by Feeney and colleagues (1982), consists of training/assessing rats using a negative-reinforcement paradigm to escape ambient light and white noise by traversing an elevated narrow beam (2.5×100 cm), and entering a darkened goal box situated at the opposite end. When the rat enters the goal box the adverse stimuli (light and noise) are terminated, thus serving as reinforcement (reward) for completing the task. Performance was assessed by recording the elapsed time to traverse the beam. Rats were tested for beam-balance and beam-walk performance prior to surgery to establish a baseline measure, and again on post-operative days 1–5. Testing consisted of three trials (60 sec allotted time with an inter-trial interval of 30 sec) per day on each task. If the rat was unable to traverse the entire length of the beam the maximum allowed time of 60 sec was recorded. The average daily scores for each subject were used in the statistical analyses.
Cognitive function: Acquisition of spatial learning
Spatial learning was assessed in a Morris water maze (Morris, 1984), a task that has been shown to be sensitive to cognitive function after TBI (Dixon et al., 1999; Hamm et al., 1992; Kline et al., 2002a,b,c,2010; Matter et al., 2011; Scheff et al., 1997). Briefly, the maze consisted of a plastic pool (180 cm diameter, 60 cm high) filled with tap water (26±1°C) to a depth of 28 cm, that was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear acrylic glass stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning began on post-operative day 14, and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water's surface (i.e., invisible to the rat). On day 19 the platform was raised 2 cm above the water's surface (i.e., visible to the rat) as a control procedure to determine the contributions of non-spatial factors (e.g., sensorimotor performance, motivation, and visual acuity) on cognitive performance. For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
Cognitive function: Probe trial (memory retention)
One day after the final acquisition training session (i.e., day 19), all rats were given a single probe trial to measure retention. Briefly, the platform was removed from the pool and the rats were placed in the maze from the location point most distal to the quadrant where the platform was previously situated (i.e., “target quadrant”) and allowed to freely explore the pool for 30 sec. Typically, rats that have learned the specific location of the escape platform exhibit a spatial bias and spend significantly more time in the target quadrant. The percent time spent in the target quadrant was used in the statistical analysis.
A spontaneous motor activity recording and tracking (SMART) system (San Diego Instruments, San Diego, CA) was used to record the data, which included time to locate the platform, time in the target quadrant, and swim speed.
Histology: Quantification of hippocampal neurons
Three weeks after CCI or sham injury the rats were anesthetized with pentobarbital (50 mg/kg IP), and then perfused transcardially with 200 mL heparinized 0.1 M phosphate-buffered saline (pH 7.4), followed by 300 mL 10% buffered formalin. The brains were extracted, post-fixed in 10% buffered formalin for 1 week, dehydrated with alcohols, and embedded in paraffin. Seven-micrometer-thick coronal sections were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on gelatin-coated glass microscope slides. After drying at room temperature, the sections were deparaffinized in xylenes, rehydrated, and stained with cresyl violet. An observer blinded to experimental conditions analyzed one coronal section underlying the area of contusion (3.5 mm posterior to the bregma) from the rats in each group for determination of treatment efficacy on selectively vulnerable hippocampal CA1 and CA3 neurons. To reduce counting errors associated with false-positive identification of dying neurons, the total number of CA1 and CA3 morphologically-intact neurons (i.e., those with a clearly defined cell body and nucleus) were counted using a Nikon Eclipse E600 microscope with a 40× objective. For consistency and replication, the data are presented as the percent of total neurons in the ipsilateral (injured) CA1 and CA3 regions relative to the contralateral (uninjured) hippocampus, as previously reported (Cheng et al., 2007; Dixon et al., 1999; Hoffman et al., 2008; Kline et al., 2004,2010; Sozda et al., 2010).
Data analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions using StatView 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). The acute neurological, probe trial, swim speed, and histological data were analyzed by one-factor ANOVAs. When the ANOVA showed a significant effect, the Bonferroni/Dunn post-hoc test was utilized to determine specific group differences. The results are expressed as the mean±standard error of the mean (SEM), and were considered significant when p values were ≤0.05 or as determined by the Bonferroni/Dunn statistic after correcting for multiple comparisons, which according to the number of groups was revealed to be p≤0.005.
Results
There were no significant differences in any assessment among the sham control groups, regardless of treatment (buspirone or housing), and thus the data were pooled into one group (denoted as Sham).
Acute neurological function
No significant differences were observed among the TBI groups in time to recover the hindlimb withdrawal reflex in response to a brief paw pinch (left range=176.0±3.4 sec to 190.0±4.8 sec, p>0.05; right range=170.7±3.0 sec to 177.0±4.1 sec, p>0.05), or for return of righting ability (range 358.9±24.3 sec to 401.2±27.2 sec, p>0.05) following the cessation of anesthesia. The lack of significant differences with these acute neurological indices suggests that all groups experienced an equivalent level of injury and anesthesia.
Motor function: Beam-balance
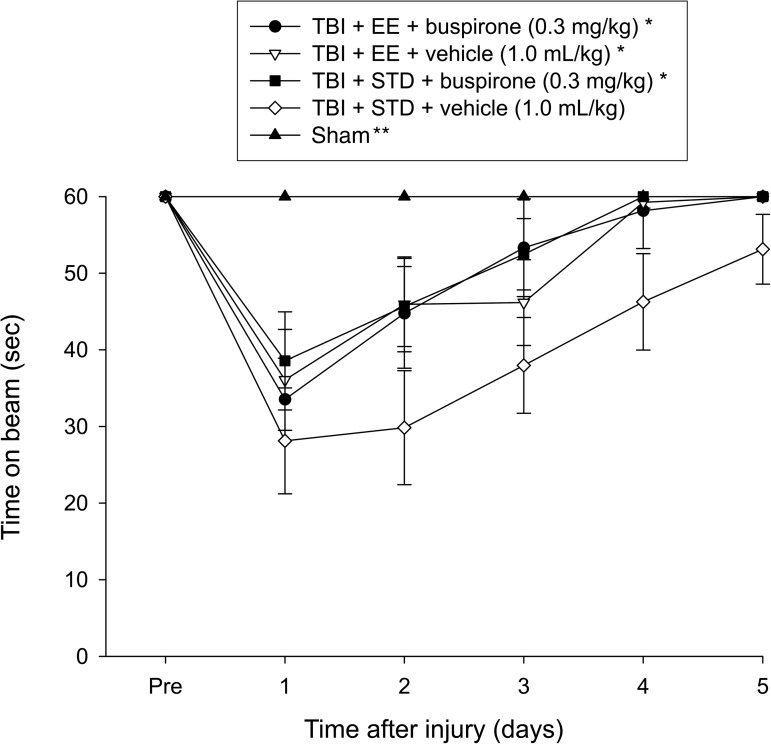
No pre-surgical differences were observed among groups, as all rats were capable of balancing on the beam for the allotted 60 sec (Fig. 1). Following the cortical impact all groups were significantly impaired relative to the sham group (p≤0.001), which was able to maintain balance for the full 60 sec. The repeated-measures ANOVA revealed significant group (F4,55=9.772, p<0.0001), and day (F5,275=38.834, p<0.0001) differences, as well as a significant group×day interaction (F20,275=4.485, p<0.0001). The post-hoc test revealed that the TBI+EE+buspirone, TBI+EE+vehicle, and TBI+STD+buspirone groups were able to regain their beam-balancing ability significantly quicker than the TBI+STD+vehicle group, which did not return to baseline over the 5 days of testing (p≤0.0009). No significant differences were revealed among the three therapy groups (p≥0.47).
FIG. 1.
Mean (±standard error of the mean) time (sec) maintaining balance on an elevated narrow beam before and after TBI or sham injury (*p≤0.0009 versus TBI+STD+vehicle; **p≤0.001 versus all TBI groups). No other group comparisons were significant (p≤0.47; TBI, traumatic brain injury; EE, environmental enrichment; STD, standard environment).
Motor function: Beam-walk (time to traverse)
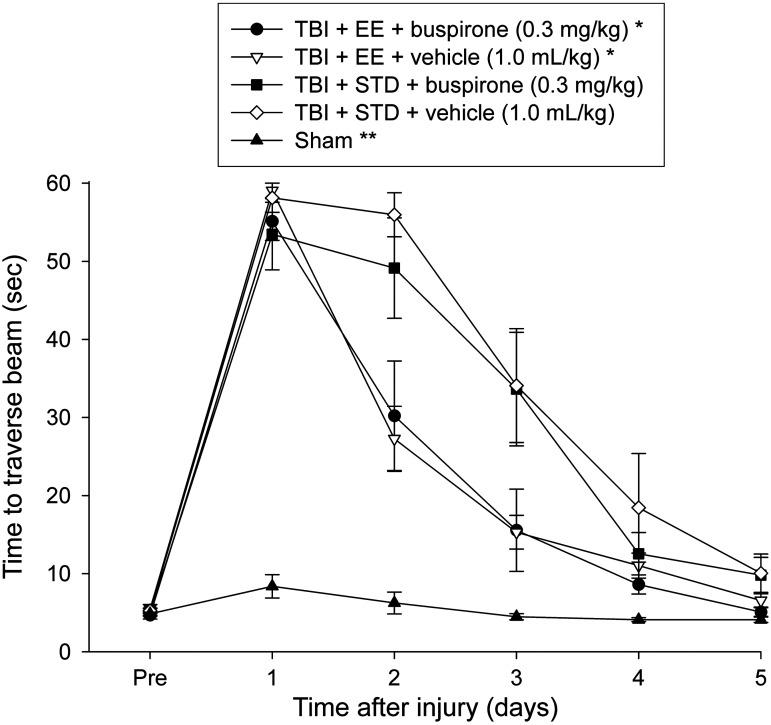
No pre-surgical differences in time to traverse the beam were revealed among groups, as all rats were fully trained and became proficient at reaching the goal box in approximately 5 sec (Fig. 2). After TBI, the ANOVA revealed significant group (F4,55=36.754, p<0.0001), and day (F5,275=198.098, p<0.0001) differences, as well as a significant group×day interaction (F20,275=18.968, p<0.0001). The post-hoc test showed that all TBI groups differed from the sham controls (p<0.0001). Additionally, the TBI+EE+buspirone and TBI+EE+vehicle groups were able to traverse the beam significantly quicker than the TBI+STD+vehicle group (p≤0.005), but did not differ from one another (p=0.79). No other group comparisons were significant.
FIG. 2.
Mean (±standard error of the mean) time to traverse an elevated narrow beam after TBI or sham injury (*p≤0.005 versus TBI+STD+vehicle; **p≤0.0001 versus all TBI groups). No other group comparisons were significant (EE, environmental enrichment; STD, standard environment; TBI, traumatic brain injury).
Cognitive function: Acquisition of spatial learning
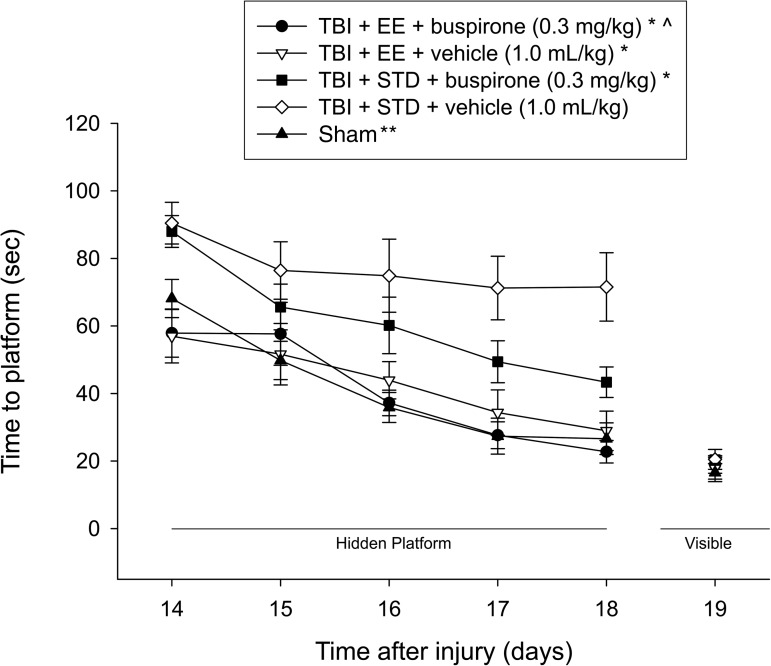
Analysis of the water maze data revealed several significant findings. A single-day analysis on the first day of training confirmed that both TBI+EE groups, regardless of treatment, were able to find the platform significantly quicker than both TBI+STD groups (p≤0.0042; Fig. 3). The repeated-measures ANOVA revealed significant group (F4,55=11.379, p<0.0001), and day (F4,220=29.826, p<0.0001) differences. The post-hoc test revealed that the TBI+EE+buspirone, TBI+EE+vehicle, and TBI+STD+buspirone groups located the escape platform significantly faster over the 5 days of training versus the TBI+STD+vehicle group (p<0.0001). Moreover, the TBI+EE+buspirone group also performed significantly better than the TBI+STD+buspirone group (p<0.0001), but did not differ from the TBI+EE+vehicle group (p=0.63) or the sham controls (p=0.83). In addition, the TBI+EE+vehicle group performed better than the TBI+STD+buspirone group (p=0.006), but like the TBI+EE+buspirone group, it also did not differ from the sham controls (p=0.72). The sham group was significantly better than the TBI+STD+buspirone and TBI+STD+vehicle groups (p<0.0001). No significant differences in swim speed (range 28.3±1.0 cm/sec to 31.5±1.4 cm/sec) or visible platform acquisition (Fig. 3), were observed among the groups, suggesting that neither motor nor visual impairments influenced the assessment of place learning.
FIG. 3.
Mean (±standard error of the mean) time (sec) to locate a hidden (submerged) platform in a water maze (*p≤0.0001 versus TBI+STD+vehicle, 1.0 mL/kg; **p≤0.0001 versus TBI+STD+buspirone, 0.3 mg/kg, and TBI+STD+vehicle, 1.0 mL/kg; ^p≤0.0001 versus TBI+STD+buspirone, 0.3 mg/kg). No other group comparisons were significant (EE, environmental enrichment; STD, standard environment; TBI, traumatic brain injury).
Cognitive function: Probe trial
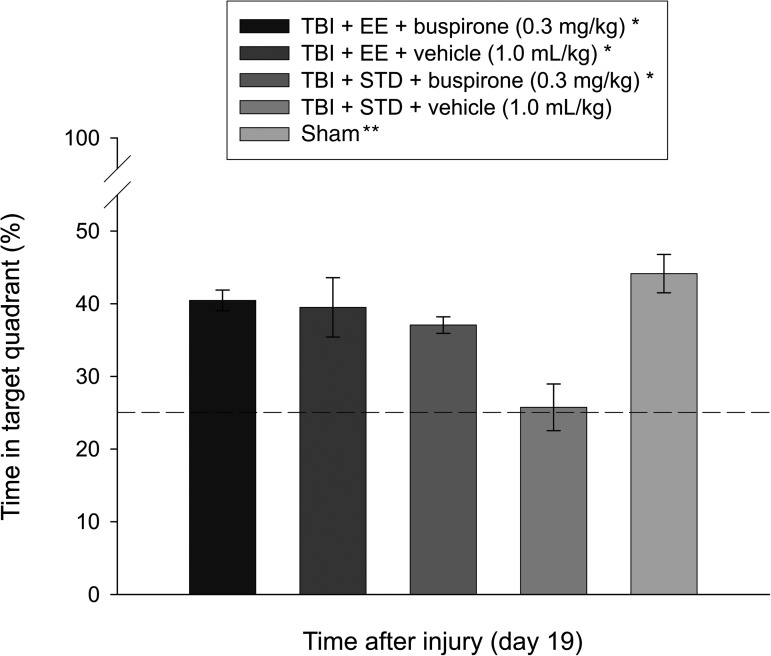
Analysis of the probe data revealed intact memory, as evidenced by a greater percentage of the allotted time spent searching in the target quadrant, for the TBI+EE+buspirone (40.5±1.4%), TBI+EE+vehicle (39.5±4.1%), and TBI+STD+buspirone (37.1±1.1%) groups versus the TBI+STD+vehicle group (25.7±3.2%; p≤0.003), and no differences from the sham (44.1±2.6%) controls (p≥0.07). In addition, as shown in Figure 4, the shams were significantly better than the TBI+STD+vehicle group (p<0.0001).
FIG. 4.
Mean (±standard error of the mean) percentage of time spent in the target quadrant (i.e., where the platform was previously located), following a single-probe trial 19 days after TBI or sham injury (*p≤0.003 versus TBI+STD+vehicle, 1.0 mL/kg; **p<0.0001 versus TBI+STD+vehicle, 1.0 mL/kg). No other group comparisons were significant. The dotted line represents performance at the chance level (25%; EE, environmental enrichment; STD, standard environment; TBI, traumatic brain injury).
Histology: Quantification of hippocampal neurons
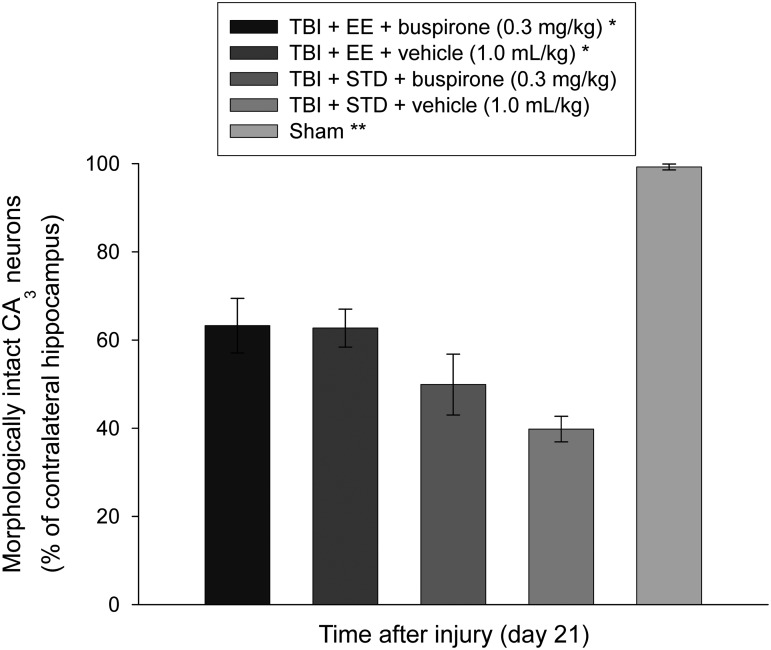
The CCI injury resulted in a significant reduction of morphologically intact (i.e., normal-appearing) CA1/3 neurons in the hippocampus ipsilateral to the impact. In the CA1 all TBI groups differed from the sham control group (p<0.0001). The percentage of normal-appearing neurons in the sham group was 100.7±1.0, while in the TBI+EE+buspirone, TBI+EE+vehicle, TBI+STD+buspirone, and TBI+STD+vehicle groups, the percentages were 51.5±7.1, 49.6±6.3, 47.0±2.6, and 36.3±4.8 neurons, respectively. While the TBI+EE+buspirone group exhibited a greater percentage of cells versus the TBI+STD+vehicle group, the post-hoc statistic did not reveal significance, as a corrected p value of 0.005 was required, and only a p of 0.04 was attained. Due to the lack of statistically significant differences among the treatment groups, these data are not depicted graphically. Comparable to the CA1 data, all TBI groups differed from the sham control group in the CA3 region (p<0.0001). The mean ratio of morphologically-intact neurons in the sham group was 100.8±0.7, while in the TBI+EE+buspirone, TBI+EE+vehicle, TBI+STD+buspirone, and TBI+STD+vehicle groups, the percentages were 63.3±6.2, 62.8±4.3, 50.1±7.0, and 39.8±3.0 neurons, respectively (Fig. 5). Post-hoc analyses on these data revealed that the TBI+EE+buspirone and TBI+EE+vehicle groups were significantly better than the TBI+STD+vehicle group (p=0.002 and 0.003, respectively), but did not differ from each other (p=0.94). The TBI+STD+buspirone group did not differ from any of the TBI groups (p≥0.064).
FIG. 5.
Mean (±standard error of the mean) morphologically-intact CA3 neurons (% of contralateral hippocampus) quantified 3 weeks after TBI or sham injury. (*p≤0.003 versus TBI+STD+vehicle, 1.0 mL/kg; **p<0.0001 versus all TBI groups). No other group comparisons were significant (EE, environmental enrichment; STD, standard environment; TBI, traumatic brain injury).
Discussion
To date there is only one study reporting on the beneficial effects of a delayed (24 h after injury) and chronic (21 days) treatment regimen with the 5-HT1A-receptor agonist buspirone after CCI injury (Olsen et al., 2012). The goal of the current, and novel, study was to determine whether the combination of EE and buspirone would result in greater behavioral recovery and histological protection after CCI injury than that produced by either treatment individually. The rationale for the combinational therapy paradigm is based on the plethora of single-therapy studies showing beneficial laboratory results with limited clinical translation. The data showed that EE, buspirone, and their combination, facilitated beam-balance recovery and enhanced spatial learning and memory relative to vehicle-treated STD-housed controls after TBI. Moreover, regardless of whether combined with buspirone or vehicle, both EE groups accelerated beam-walk performance and attenuated CA3 cell loss similarly. The only difference between the TBI+EE+vehicle and TBI+EE+buspirone groups is that the latter was significantly better than the TBI+STD+buspirone group in the water maze. Overall, while significant benefits were produced by all three treatment conditions, our hypothesis that the combination of EE and buspirone would be more efficacious than either therapy alone was not supported.
A salient explanation for the lack of an additive effect is that EE produced robust effects leading to a ceiling effect that curtailed any opportunity for buspirone to confer additional statistically significant improvement. As seen in the behavioral figures, the EE groups reached the level of sham controls by the last day of testing on motor function, and did not differ from the onset in the spatial learning paradigm. With this level of improvement, it is impossible to achieve an additive effect by combination treatments. Similar findings have been reported from studies in our laboratory evaluating the potential efficacy of single (Kline et al., 2007) or chronic (Kline et al., 2010) administration of the 5-HT1A-receptor agonist 8-OH-DPAT plus EE. We have previously discussed that if the inability to achieve statistically significant differences with combinational therapies is limited by ceiling effects, that alterations in the current testing paradigms or injury severity are warranted (Kline et al., 2010). At that time examples for potential changes were to (1) choose more rigorous behavioral tasks such that neither therapy (EE or pharmacotherapy) would reach the level of uninjured controls, thus affording the opportunity for additional recovery; (2) produce a more severe TBI such that the animals are more impaired and do not recover as efficiently; or (3) administer sub-therapeutic doses of pharmacotherapies and/or EE, with the rationale that neither therapy would be effective on its own, but when combined would produce a synergistic effect.
Since our prior reports on combinational therapies (Kline et al., 2007,2010), we have initiated intensive investigations into the sub-therapeutic dosing paradigm, particularly as it relates to EE, but also to pharmacotherapies. In a recently published study we sought to address the disparity between the laboratory, where enrichment (i.e., rehabilitation) is typically provided continuously, and that of the clinic, where rehabilitation is relatively short (Blackerby, 1990; Shiel et al., 2001; Zhu et al., 2007). To this end, we evaluated the effects of an abbreviated enrichment paradigm consisting of 2, 4, or 6 h (i.e., sub-therapeutic doses) of EE per day, which is more in line with clinical rehabilitation. The data showed that the groups receiving 2 h and 4 h of daily EE did not benefit from the limited exposure, and were no different from non-enriched controls, but the 6-h group did benefit. These findings indicate that we have defined a sub-therapeutic dose of EE that we can manipulate in future studies by combining with pharmacotherapies or other therapeutic strategies. This approach would limit the EE-induced ceiling effects and would afford the opportunity for drug+EE synergistic effects.
Despite the lack of an additive effect with the combined therapy paradigm, several important findings were revealed, which lend support for continued combinational approaches after TBI that include EE. First, co-administration of the two therapies did not negate the benefits induced by one or both treatments, as has been reported with other dual-treatment strategies (Faden, 1993; Griesbach et al., 2008; Guluma et al., 1999; Kline et al., 2002a). Second, both EE groups performed significantly better in the water maze versus the STD-housed groups on the first day of training (day 14 post-TBI), despite no difference in the acute neurological assessments that are correlated with injury severity. This finding suggests that in addition to potential plasticity-related reparative responses, such as the attenuation of TBI-induced choline acetyltransferase-positive medial septal cell loss (Kline et al., 2010), or increases in neurogenesis, dendritic growth, synaptophysin, or nerve growth factor (Frick and Fernandez, 2003; Leggio et al., 2005; Olson et al., 2006; Torasdotter et al., 1998; van Praag et al., 2000), EE also conferred cognitive protection. Attributing the benefit to EE is based on the finding that the STD-housed buspirone-treated group did not exhibit the same initial good start. Third, regarding the histological data, the lack of a difference between the buspirone+EE and vehicle+EE groups suggests that EE, but not necessarily buspirone, mediated the reduction in hippocampal cell loss. This finding is consistent with a recent report from our laboratory showing that while buspirone reduced lesion volume, it did not significantly affect hippocampal cell survival (Olsen et al., 2012). When considering these three points, the findings suggest that the two therapies did not antagonize one another, and that further refinement of the model, including changes in doses, timing of administration, and intensity of rehabilitation, may induce different outcomes via additive or synergistic mechanisms. The sub-therapeutic dosing strategy would be clinically relevant, as the potential for pharmacological side effects may be curtailed, while still conferring a beneficial effect on functional recovery. Such studies are currently being conducted in our laboratory.
Acknowledgments
This work was supported, in part, by National Institutes of Health grants HD043851, HD046700, and NS060005 (A.E.K.).
Author Disclosure Statement
No competing financial interests exist.
References
- Bales J.W. Wagner A.K. Kline A.E. Dixon C.E. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackerby W.F. Intensity of rehabilitation and length of stay. Brain Inj. 1990;4:167–173. doi: 10.3109/02699059009026162. [DOI] [PubMed] [Google Scholar]
- Cheng J.P. Aslam H.A. Hoffman A.N. Zafonte R.D. Kline A.E. The neurobehavioral benefit conferred by a single systemic administration of 8-OH-DPAT after brain trauma is confined to a narrow therapeutic window. Neurosci. Lett. 2007;416:165–168. doi: 10.1016/j.neulet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.P. Hoffman A.N. Zafonte R.D. Kline A.E. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. Li Y. Kline A.E. Dixon C.E. Zafonte R.D. Wagner A.K. Gender and environmental effects on regional BDNF expression after experimental traumatic brain injury. Neuroscience. 2005;135:11–17. doi: 10.1016/j.neuroscience.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Chew E. Zafonte R.D. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J. Rehabil. Res. Dev. 2009;46:851–878. doi: 10.1682/jrrd.2008.09.0120. [DOI] [PubMed] [Google Scholar]
- de Witt B.W. Ehrenberg K.M. McAloon R.L. Panos A.H. Shaw K.E. Raghavan P.V. Skidmore E.R. Kline A.E. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil. Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Kraus M.F. Kline A.E. Ma X. Yan H.Q. Griffith R.G. Wolfson B.M. Marion D.W. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 1999;14:285–294. [PubMed] [Google Scholar]
- Doppenberg E.M.R. Choi S.C. Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J. Neurosurg. Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Comparison of single and combined drug treatment strategies in experimental brain trauma. J. Neurotrauma. 1993;10:91–100. doi: 10.1089/neu.1993.10.91. [DOI] [PubMed] [Google Scholar]
- Faul M. Xu L. Wald M.M. Coronado V.G. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- Feeney D.M. Gonzalez A. Law W.A. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Frick K.M. Fernandez S.M. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol. Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Garcia A.N. Shah M.A. Dixon C.E. Wagner A.K. Kline A.E. Biologic and plastic effects of experimental traumatic brain injury treatment paradigms and their relevance to clinical rehabilitation. PMR. 2011;3:S18–S27. doi: 10.1016/j.pmrj.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulke L.J. Horner P.J. Fink A.J. McNamara C.L. Hicks R.R. Environmental enrichment increases progenitor cell survival in the dentate gyrus following lateral fluid percussion injury. Brain Res. Mol. Brain Res. 2005;141:138–150. doi: 10.1016/j.molbrainres.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza C.C. Griesbach G.S. Hovda D.A. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav. Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Zhuang Y. Feng Z. Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach G.S. Hovda D.A. Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach G.S. Hovda D.A. Gomez-Pinilla F. Sutton R.L. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guluma K.Z. Saatman K.E. Brown A. Raghupathi R. McIntosh T.K. Sequential pharmacotherapy with magnesium chloride and basic fibroblast growth factor after fluid percussion brain injury results in less neuromotor efficacy than that achieved with magnesium alone. J. Neurotrauma. 1999;16:311–321. doi: 10.1089/neu.1999.16.311. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Dixon C.E. Gbadebo D.M. Singha A.K. Jenkins L.W. Lyeth B.G. Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Temple M.D. O'Dell D.M. Pike B.R. Lyeth B.G. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J. Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- Hicks R.R. Zhang L. Atkinson A. Stevenon M. Veneracion M. Seroogy K.B. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience. 2002;112:631–637. doi: 10.1016/s0306-4522(02)00104-5. [DOI] [PubMed] [Google Scholar]
- Hoffman A.N. Malena R.R. Westergom B.P. Luthra P. Cheng J.P. Aslam H.A. Zafonte R.D. Kline A.E. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci. Lett. 2008;431:226–230. doi: 10.1016/j.neulet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A.E. Bolinger B.D. Kochanek P.M. Carlos T.M. Yan H.Q. Jenkins L.W. Marion D.W. Dixon C.E. Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res. 2002a;937:22–31. doi: 10.1016/s0006-8993(02)02458-7. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Dixon C.E. Zafonte R.D. Bolinger B.D. The therapeutic efficacy conferred by the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma. 2004;21:175–185. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Marion D.W. Dixon C.E. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma. 2002b;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kline A.E. McAloon R.L. Henderson K.A. Bansal U.K. Ganti B.M. Ahmed R.H. Gibbs R.B. Sozda C.A. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A.E. Wagner A.K. Westergom B.P. Malena R.R. Zafonte R.D. Olsen A.S. Sozda C.N. Luthra P. Panda M. Cheng J.P. Aslam H.A. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A.E. Yu J. Horváth E. Marion D.W. Dixon C.E. The selective 5-HT1A receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–555. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Yu J. Massucci J.L. Zafonte R.D. Dixon C.E. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci. Lett. 2002c;333:179–182. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- Kokiko O.N. Hamm R.J. A review of pharmacological treatments used in experimental models of traumatic brain injury. Brain Inj. 2007;21:259–274. doi: 10.1080/02699050701209964. [DOI] [PubMed] [Google Scholar]
- Leggio M.G. Mandolesi L. Federico F. Spirito F. Ricci B. Gelfo F. Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav. Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Maegele M. Lippert-Gruener M. Ester-Bode T. Garbe J. Bouillon B. Neugebauer E. Klug N. Lefering R. Neiss W.F. Angelov D.N. Multimodal early onset stimulation combined with enriched environment is associated with reduced CNS lesion volume and enhanced reversal of neuromotor dysfunction after traumatic brain injury in rats. Eur. J. Pharmacol. 2005;21:2406–2418. doi: 10.1111/j.1460-9568.2005.04070.x. [DOI] [PubMed] [Google Scholar]
- Margulies S. Hicks R. The Combination Therapies for Traumatic Brain Injury Workshop Leaders. Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter A.M. Folweiler K.A. Curatolo L.M. Kline A.E. Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair. 2011;25:558–564. doi: 10.1177/1545968310397206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max W. Mackenzie E.J. Rice D.P. Head injuries: costs and consequences. J. Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- McIntosh T.K. Juhler M. Wieloch T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J. Neurotrauma. 1998;15:731–769. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- Menon D.K. Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 2009;37:S129–S135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Olsen A.S. Sozda C.N. Cheng J.P. Hoffman A.N. Kline A.E. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A receptor agonist buspirone. J. Neurotrauma. 2012 doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A.K. Eadie B.D. Ernst C. Christie B.R. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Passineau M.J. Green E.J. Dietrich W.D. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 2001;168:373–384. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Baldwin S.A. Brown R.W. Kraemer P.J. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Selassie A.W. Zaloshnja E. Langlois J.A. Miller T. Jones P. Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Shiel A. Burn J.P. Henry D. Clark J. Wilson B.A. Burnett M.E. McLellan D.L. The effects of increased rehabilitation therapy after brain injury: results of a prospective controlled trial. Clin. Rehabil. 2001;15:501–514. doi: 10.1191/026921501680425225. [DOI] [PubMed] [Google Scholar]
- Sosin D.M. Sniezek J.E. Waxweiler R.J. Trends in death associated with traumatic brain injury, 1979 through 1992. JAMA. 1995;273:1778–1780. [PubMed] [Google Scholar]
- Sozda C.N. Hoffman A.N. Olsen A.S. Cheng J.P. Zafonte R.D. Kline A.E. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C.R. Ivins B. Schwab K.A. Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 2009;76:105–110. doi: 10.1002/msj.20100. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Torasdotter M. Metsis M. Henriksson B.G. Winblad B. Mohammed A.H. Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res. 1998;93:83–90. doi: 10.1016/s0166-4328(97)00142-3. [DOI] [PubMed] [Google Scholar]
- van Praag K. Kempermann G. Gage F.H. Neural consequences of environmental enrichment. Nat. Rev. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Wheaton P. Mathias J.L. Vink R. Impact of early pharmacotherapy treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J. Clin. Psychopharmacol. 2009;29:468–477. doi: 10.1097/JCP.0b013e3181b66f04. [DOI] [PubMed] [Google Scholar]
- Zhu X.L. Poon W.S. Chan C.C.H. Chan S.S.H. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Inj. 2007;21:681–690. doi: 10.1080/02699050701468941. [DOI] [PubMed] [Google Scholar]