Abstract
The aim of this study was to evaluate the potential efficacy of the serotonin1A (5-HT1A) receptor agonist buspirone (BUS) on behavioral and histological outcome after traumatic brain injury (TBI). Ninety-six isoflurane-anesthetized adult male rats were randomized to receive either a controlled cortical impact or sham injury, and then assigned to six TBI and six sham groups receiving one of five doses of BUS (0.01, 0.05, 0.1, 0.3, or 0.5 mg/kg) or saline vehicle (VEH, 1.0 mL/kg). Treatments began 24 h after surgery and were administered intraperitoneally once daily for 3 weeks. Motor function (beam-balance/beam-walk tests) and spatial learning/memory (Morris water maze) were assessed on post-operative days 1–5 and 14–19, respectively. Morphologically intact CA1/CA3 cells and cortical lesion volume were quantified at 3 weeks. No differences were observed among the BUS and VEH sham groups in any end-point measure and thus the data were pooled. Regarding the TBI groups, repeated-measures ANOVAs revealed that the 0.3 mg/kg dose of BUS enhanced cognitive performance relative to VEH and the other BUS doses (p<0.05), but did not significantly impact motor function. Moreover, the same dose conferred selective histological protection as evidenced by smaller cortical lesions, but not greater CA1/CA3 cell survival. No significant behavioral or histological differences were observed among the other BUS doses versus VEH. These data indicate that BUS has a narrow therapeutic dose response, and that 0.3 mg/kg is optimal for enhancing spatial learning and memory in this model of TBI. BUS may have potential as a novel pharmacotherapy for clinical TBI.
Key words: behavioral outcome, controlled cortical impact, functional recovery, 5-HT1A-receptor agonist, hippocampus, learning and memory, Morris water maze, traumatic brain injury
Introduction
Traumatic brain injury (TBI) affects 1.4 to 2 million Americans each year and results in approximately 52,000 deaths and 120,000 cases of long-term motor and cognitive disabilities (Faul et al., 2010; Goldstein, 1990; Selassie et al., 2008; Sosin et al., 1995; Summers et al., 2009). The most commonly reported ailments by patients following TBI are cognitive in nature and consist of persistent deficits in both learning and memory, which are manifest in the inability to acquire new information (Horneman and Emanuelson, 2009). Thus these individuals tend to become easily confused and forgetful, and many complain of an inability to organize thoughts or acquire spatial awareness, which subsequently delays their reintegration into society (Binder, 1996; Millis et al., 2001). The acute and chronic medical and rehabilitative care required because of these TBI-induced deficits, along with the loss of productivity, results in injury-related costs that exceed $50 billion per year (Max et al., 1991). As such, TBI is a significant health care issue with limited treatment options.
In order to combat this problem, a variety of therapeutic approaches have been investigated. One strategy that has received considerable attention is the administration of pharmacological agents, acting either as agonists or antagonists, on various neurotransmitter systems. Of these, the catecholaminergic, cholinergic, and glutamatergic neurotransmitter systems have received overwhelming consideration as potential targets for therapeutic intervention (Bales et al., 2009; Dixon et al., 1997,1999; Kline et al., 2002a,2004b; Kokiko and Hamm, 2007; McDowell et al., 1998; Parton et al., 2005; Sutton and Feeney, 1992; Temple and Hamm, 1996; Verbois et al., 2003; also see reviews by Garcia et al., 2011 and Wheaton et al., 2009). In contrast, the serotonergic (5-hydroxytryptamine; 5-HT) system has received sparse attention after TBI, with just a few reports describing the behavioral consequences of altering 5-HT neurotransmission (Baños et al., 2010; Wang et al., 2011; Wilson and Hamm, 2002). The paucity of TBI data exists despite the vigorous study in non-TBI models demonstrating that the 5-HT system, and in particular the 5-HT1A subtype, is an integral component of cognitive processing (Barnes and Sharp, 1999; Meneses, 1999; Meneses and Perez-Garcia, 2007), and in clinical psychopathologies, such as anxiety and depression (Albert and Lemonde, 2004; Koen and Stein, 2011). Given the cognitive deficits observed after TBI, these studies suggest that 5-HT1A receptors could be an important target to investigate after TBI.
Indeed, previous studies from our laboratory have demonstrated that the selective and high-affinity 5-HT1A receptor agonists repinotan HCl and 8-OH-DPAT enhance spatial learning and memory performance in a Morris water maze (MWM) task, reduce hippocampal neuron loss, and decrease cortical lesion size after controlled cortical impact (CCI) injury (Cheng et al., 2007,2008; Kline et al., 2001,2002b,2004a,2007b,2010). Beneficial effects of 5-HT1A-receptor agonists have also been reported in other models of central nervous system (CNS) injury, such as focal or global cerebral ischemia (De Vry and Jentzsch, 1998; De Vry et al., 1997,1998; Prehn et al., 1991,1993; Semkova et al., 1998), and acute subdural hematoma (Alessandri et al., 1999). Thus, the aim of the current study was to determine whether the 5-HT1A-receptor agonist buspirone (BUS) will also facilitate better neurobehavioral outcomes and attenuate histological damage after CCI injury. A significant advantage in investigating BUS is that it is used routinely as a treatment for anxiety and depression (Chew and Zafonte, 2009), and therefore safety and tolerability issues are well known. Furthermore, because of its widespread clinical applicability, its translation from bench to bedside could be expedited if it is shown to be efficacious in pre-clinical models of TBI.
Methods
Subjects
Ninety-six adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were housed in standard steel wire-mesh cages and maintained in an environment with controlled temperature (21±1°C) and light (lights on 7:00 am to 7:00 pm), with food and water available ad libitum. After 1 week of acclimatization the rats underwent a single day of beam-walk training, which consisted of 3–5 trials to traverse the beam. When the rats were able to perform the task in under 5 sec, they were randomly assigned to one of the following conditions: TBI + BUS (0.01 mg/kg; n=13), TBI + BUS (0.05 mg/kg; n=13), TBI + BUS (0.1 mg/kg; n=13), TBI + BUS (0.3 mg/kg; n=13), TBI + BUS (0.5 mg/kg; n=13), and TBI + VEH (1.0 mL/kg; n=13), plus sham controls representing each BUS dose and vehicle (VEH; n=18). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize suffering.
Surgery
A CCI injury was produced as previously described (Cheng et al., 2008; Hoffman et al., 2008; Kline et al., 2001,2010). Briefly, surgical anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane, respectively, in 2:1 N2O:O2 mixture. After endotracheal intubation the rats were secured in a stereotaxic frame, ventilated mechanically, and temperature was maintained at 37±0.5°C with a heating blanket. Utilizing aseptic procedures a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy was made in the right hemisphere (between the bregma/lambda and the sagittal suture/coronal ridge) with a hand-held trephine. The bone flap was removed and the craniectomy was enlarged further with cranial rongeurs. Subsequently, the impacting rod was extended and the impact tip (6 mm, flat) was centered and lowered through the craniectomy until it touched the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8 mm tissue deformation at 4 m/sec). Immediately after surgery anesthesia was discontinued, the incision was sutured, and the rats were extubated and then neurologically evaluated. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
Acute neurological evaluation
Hindlimb reflexive ability was assessed by squeezing the rat's paw every 5 sec following the discontinuation of anesthesia and recording the time to elicit a withdrawal reflex. Righting reflex was determined by measuring the time required for rats to successfully turn from a supine to a prone position.
Drug administration
BUS was purchased from Sigma-Aldrich (St. Louis, MO) and was prepared daily by dissolving in sterile saline, which also served as the vehicle. BUS (doses in mg/kg of 0.01, 0.05, 0.1, 0.3, or 0.5) or saline vehicle (1.0 mL/kg) was administered intraperitoneally once daily beginning 24 h after surgery, and once daily until sacrificed for histology (3 weeks). On days of motor or cognitive testing the injections were administered 1 h prior to testing to avoid behavioral symptoms of the serotonin syndrome (i.e., flat body posture) that could affect performance. The narrow range of doses was selected based on pilot data from our laboratory showing that 0.3 mg/kg of BUS was beneficial, and also from a previous study showing that the 5-HT1A-receptor agonist 8-OH-DPAT has a narrow dose profile.
Motor performance
Motor function was assessed with the well-established beam-balance and beam-walk tasks (Cheng et al., 2007; Hoffman et al., 2008; Kline et al., 2004a,2004b; Matter et al., 2011; Sozda et al., 2010). Briefly, the beam-balance task consists of placing the rat on a narrow (1.5-cm-wide) and elevated (90 cm) wooden beam and recording the time it remains on the beam. The beam-walk task, modified from that originally devised by Feeney and colleagues (1982), consists of training/assessing rats using a negative-reinforcement paradigm to escape bright light and white noise by traversing an elevated narrow beam (2.5×100 cm) and entering a darkened goal box situated at the opposite end. When the rat enters the goal box the adverse stimuli (light and noise) are terminated, which reinforces task completion. Performance is assessed by recording the elapsed time to traverse the beam. The rats were assessed on both beam tasks prior to surgery to establish baseline performance, and again on post-operative days 1–5. Testing consisted of three trials (60-sec maximum with a 30-sec inter-trial interval) per day on each task. If the rats were unable to traverse the entire length of the beam the maximum allotted time was recorded. The average daily scores for each subject were used in the statistical analyses.
Cognitive function: Acquisition of spatial learning
Spatial learning was assessed in a Morris water maze (MWM; Morris, 1984), which has been shown to be sensitive to assess cognitive function after TBI (Hamm et al, 1992; Kline et al., 2002a,b,2010; Scheff et al., 1997). Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26±1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear acrylic glass stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for 5 consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water's surface (i.e., invisible to the rat). On day 19 the platform was raised 2 cm above the water's surface (i.e., visible to the rat) as a control procedure to determine the contributions of non-spatial factors (e.g., sensorimotor performance, motivation, and visual acuity) on cognitive performance. For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times and swim distances of the four daily trials for each rat were averaged and used in the statistical analyses.
Cognitive function: Probe trial (memory retention)
One day after the final acquisition training session (i.e., day 19), all rats were given a single probe trial to measure retention. Briefly, the platform was removed from the pool and the rats were placed in the maze from the location point most distal to the quadrant where the platform was previously situated (i.e., the target quadrant) and allowed to freely explore the pool for 30 sec. The rationale for the probe trial is that rats that have learned the location of the escape platform exhibit a spatial bias and spend significantly more time searching for it in the target quadrant after it has been removed. The percent time spent in the target quadrant was used in the statistical analysis.
A spontaneous motor activity recording and tracking system (San Diego Instruments, San Diego, CA) was used to record the data, which included time to locate the platform, distance to the platform, time in the target quadrant, and swim speed.
Histology: Quantification of hippocampal neurons
Three weeks after CCI or sham injury the rats were anesthetized with pentobarbital (50 mg/kg IP), and then perfused transcardially with 200 mL heparinized 0.1 M phosphate-buffered saline (pH 7.4), followed by 300 mL 10% buffered formalin. The brains were extracted, post-fixed in 10% buffered formalin for 1 week, dehydrated with alcohols, and embedded in paraffin. Seven-micrometer-thick coronal sections were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on gelatin-coated glass microscope slides. After drying at room temperature, the sections were deparaffinized in xylene, rehydrated, and stained with cresyl violet. An observer blinded to experimental conditions analyzed tissue from five rats in each group to determine treatment efficacy on selectively vulnerable hippocampal CA1 and CA3 neurons. To reduce counting errors associated with false-positive identification of dying neurons, the total number of CA1 and CA3 morphologically intact neurons (i.e., those with a clearly defined cell body and nucleus) were counted using a Nikon Eclipse E600 microscope (Nikon Corporation, Tokyo, Japan) with a 40×objective. For consistency and replication, the data are presented as the percent of total neurons in the ipsilateral (injured) CA1 and CA3 regions relative to the contralateral (uninjured) hippocampus, as previously reported (Cheng et al., 2007; Dixon et al., 1999; Hoffman et al., 2008; Kline et al., 2004a,2004b; Matter et al., 2011; Sozda et al., 2010).
Histology: Cortical lesion volume
Cortical lesion volumes (mm3) were determined by first calculating the area of the lesion (mm2), which was done by outlining the area of missing cortical tissue for each section taken at 1-mm intervals (MCID software; Imaging Research, Ontario, Canada), and then multiplying the sum of the lesions obtained from each section by the distance between sections (1 mm).
Statistical analysis
Statistical analyses were performed on data collected by observers blinded to treatment conditions using StatView 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). The acute neurological assessments, probe trial, swim speed, and histological data were analyzed by one-factor ANOVAs. When the ANOVA showed a significant effect, the Bonferroni/Dunn post-hoc test was utilized to determine specific group differences. The results are expressed as the mean±standard error of the mean (SEM), and are considered significant when p≤0.05, or as determined by the Bonferroni/Dunn statistic after correcting for multiple comparisons.
Results
Statistical analyses were performed on a total of 94 rats, as two were removed from the study (1 each from the TBI + VEH and TBI + BUS (0.5 mg/kg) groups) due to their inability to locate the visible platform, which may indicate visual acuity deficits. No significant differences (p>0.05) were observed in any outcome measure among the sham groups, regardless of treatment or dose, and thus their data were pooled.
Acute neurological function
No significant differences were observed among the TBI groups in time to elicit a hindlimb paw pinch-induced withdrawal reflex in either limb (left: range 169.2±3.2 sec to 181.5±3.6 sec, p>0.05; right: range 162.2±3.1 sec to 174.1±3.4 sec, p>0.05), or return of righting ability (range 345.0±14.7 sec to 398.6±10.9 sec, p>0.05), following the cessation of anesthesia. The lack of significant differences with these acute neurological indices suggests that all groups experienced an equivalent level of injury and anesthesia.
Motor function: Beam-balance
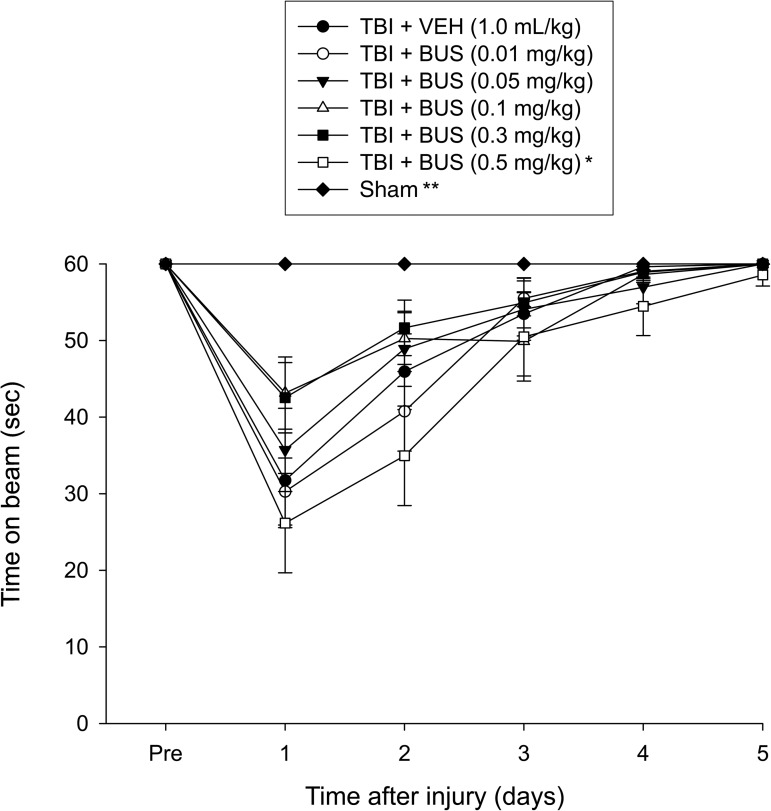
No pre-surgical differences were observed among groups, as all rats were capable of balancing on the beam for the allotted 60 sec (Fig. 1). Following the CCI all groups were markedly impaired relative to the sham group, which was able to maintain pre-surgery balancing ability. The ANOVA revealed significant group (F6,87=5.153, p=0.0001) and day (F5,435=70.695, p<0.0001) differences, as well as a significant group×day interaction (F30,435=3.858, p<0.0001), which was attributed to the sham animals performing significantly better than all TBI groups. None of the BUS groups differed from the VEH controls, but the post-hoc analysis revealed a significant difference between the TBI + BUS (0.3 mg/kg) and the TBI + BUS (0.5 mg/kg) groups (p=0.0014), with the latter performing worse. No other comparisons were significantly different.
FIG. 1.
Mean time (sec) (±standard error of the mean) maintaining balance on an elevated narrow beam before and after TBI or sham injury (*p=0.0014 versus TBI + BUS 0.3 mg/kg; **p<0.0001 versus all TBI groups). No other group comparisons were significant (TBI, traumatic brain injury; BUS, buspirone; VEH, vehicle).
Motor function: Beam-walk
No pre-surgical differences in time to traverse the beam were revealed among groups, as all rats were proficient and reached the goal box in approximately 5 sec. After CCI, the ANOVA revealed significant group (F6,87=14.105, p<0.0001) and day (F5,435=269.938, p<0.0001) differences, as well as a significant group×day interaction (F30,435=9.401, p<0.0001), which was attributed to all TBI groups, differing from the sham controls (p<0.0001). No statistically significant differences were observed among the TBI groups, regardless of treatment or dose (data not presented graphically due to lack of treatment significance).
Cognitive function: Acquisition of spatial learning (time to platform)
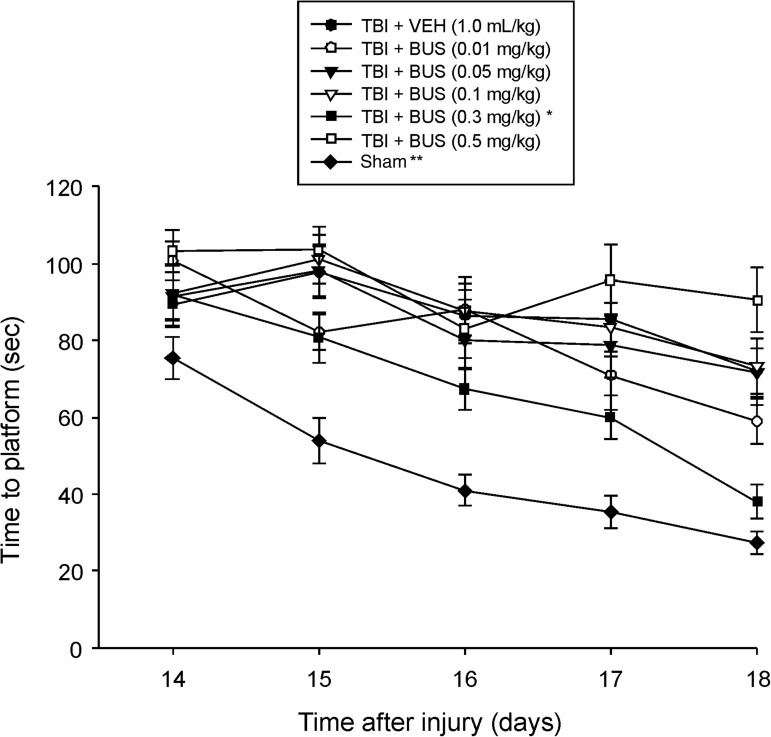
Analysis of the MWM data revealed significant group (F6,87=21.359, p<0.0001), day (F4,348=29.205, p<0.0001), and group×day (F24,348=1.759, p=0.016) differences. The post-hoc analysis revealed that the TBI + BUS (0.3 mg/kg) group was able to locate the escape platform significantly quicker over time than all other TBI groups, regardless of treatment or dose (p<0.0024; Fig. 2). Moreover, the TBI + BUS (0.5 mg/kg) group performed worse than the TBI + BUS (0.01 mg/kg) group (p=0.0014). No other TBI group comparisons were significantly different. The sham group was significantly better than all TBI groups (p<0.0001).
FIG. 2.
Mean time (sec) (±standard error of the mean) to locate a hidden (submerged) platform in the Morris water maze (*p<0.0024 versus all other TBI groups; **p<0.0001 versus all TBI groups). In addition, aside from the TBI + BUS (0.5 mg/kg) group performing significantly worse than the TBI + BUS (0.01 mg/kg) group (p=0.0014), no other group comparisons were significant (TBI, traumatic brain injury; BUS, buspirone; VEH, vehicle).
Cognitive function: Acquisition of spatial learning (distance traveled)
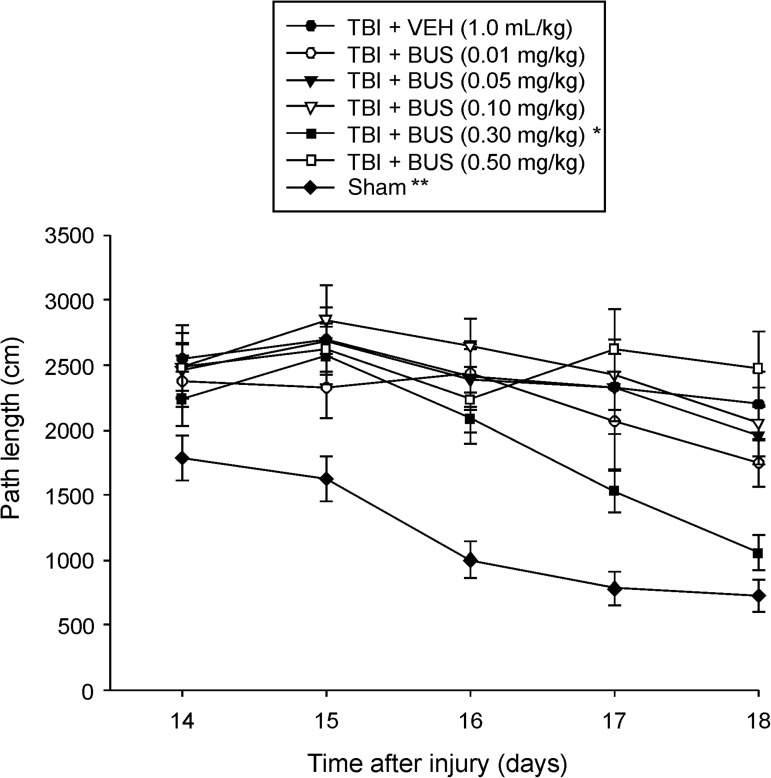
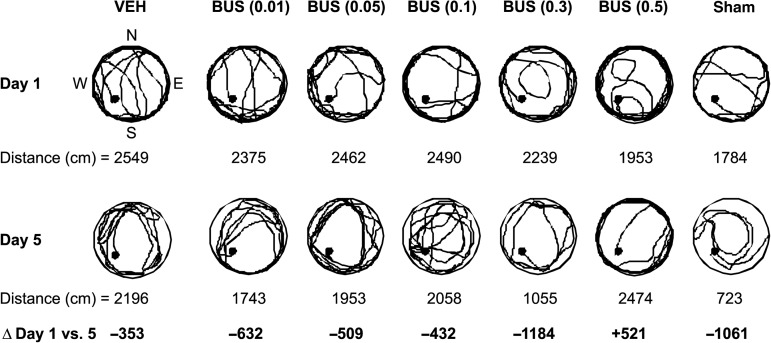
Analysis of distanced traveled (i.e., path length) to locate the escape platform revealed significant group (F6,87=12.040, p<0.0001), day (F4,348=14.744, p<0.0001), and group×day differences (F24,348=1.528, p=0.05). Specifically, all TBI groups swam farther distances to find the platform, which suggests significant impairments in the acquisition of spatial learning versus sham controls (p<0.0001). However, the distances required to locate the platform were progressively shorter over time for the TBI + BUS (0.3 mg/kg) group versus all other TBI groups, regardless of treatment or dose (p<0.0001; Figs. 3 and 4). No other TBI group comparisons were significantly different. The sham group was significantly better than all TBI groups (p<0.0001).
FIG. 3.
Mean (±standard error of the mean) path length (cm) during acquisition of spatial learning in the Morris water maze (*p<0.0001 versus all other TBI groups; **p<0.0001 versus all TBI groups; TBI, traumatic brain injury; VEH, vehicle).
FIG. 4.
Representative swim paths showing the mean path length (i.e., distance traveled) on training days 1 and 5 (i.e., post-operative days 14 and 18) in the Morris water maze (•=location of the platform in the southwest quadrant; Δ=mean change in swimming distance from training day 1 to day 5). Note that the TBI + BUS (0.3 mg/kg) group had a larger decrease in distance traveled than the other TBI groups (TBI, traumatic brain injury; BUS, buspirone; VEH, vehicle).
Cognitive function: Probe trial (memory retention)
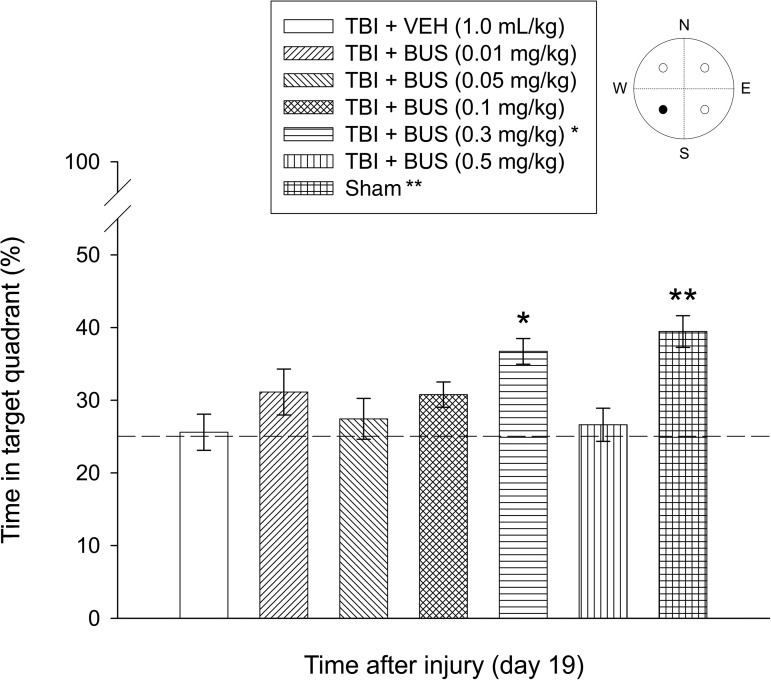
Analysis of the probe trial data showed a significant group effect (F6,87=5.336, p=0.0001). Briefly, the TBI + BUS (0.3 mg/kg) group spent a greater percentage of the 30 sec allotted time in the target quadrant than the TBI + VEH group (36.7±1.7% versus 25.6±2.4%; p=0.0013). Moreover, the TBI + BUS (0.3 mg/kg) group did not differ from the sham group (39.4±2.1%; p=0.4013), which is indicative of memory retention comparable to non-injured controls (Fig. 5). Aside from the TBI + BUS (0.3 mg/kg) group, the sham group was significantly better than all other TBI groups (range 25.6±2.4% to 31.1±3.2%; p≤0.0003). No other probe comparisons were significant.
FIG. 5.
Mean (±standard error of the mean) percentage of time spent in the target quadrant (i.e., where the platform was previously located) following a single probe trial 19 days after TBI or sham injury [*p=0.0013 versus TBI + VEH; **p<0.0003 versus all TBI groups, except TBI + BUS (0.3 mg/kg); p=0.4013]. The dotted line represents performance at the chance level (25%) (•=target quadrant; TBI, traumatic brain injury; BUS, buspirone; VEH, vehicle).
Cognitive: Visible platform and swim speed
A one-factor ANOVA performed on the visible platform data revealed a significant group effect (F6,87=8.655, p<0.0001), which was attributed to the sham group locating the platform quicker than all of the TBI groups. No significant differences in swim speed (range=27.1±1.4 cm/sec to 29.3±1.2 cm/sec) were observed among the groups during the last day of testing, suggesting that neither motor impairments nor visual disparities influenced the assessment of place learning.
Histology: Quantification of hippocampal neurons
A significant reduction in normal-appearing (i.e., morphologically intact) CA1 and CA3 neurons was observed in the hippocampus ipsilateral to the impact. Regarding the CA1 region, a one-factor ANOVA revealed a significant group effect (F6,28=19.243, p<0.0001). All TBI groups differed from the sham control group (p<0.0001; Table 1). No statistically significant differences were revealed among the TBI groups regardless of treatment or dose. Similarly to the CA1 data, all TBI groups differed from the sham control group in the CA3 region as well (F6,28=15.284, p<0.0001). While the TBI + BUS (0.3 mg/kg) group appeared to show a greater percentage of viable cells versus the TBI + VEH group and various doses of BUS, the difference was not significant (p>0.05).
Table 1.
Effects of Vehicle (VEH) or Buspirone (BUS) on Histological Outcome after TBI
| Groups | CA1 | CA3 | Lesion volume (mm3) |
|---|---|---|---|
| TBI+VEH (1.0 mL/kg) | 34.4±2.2 | 41.0±6.0 | 43.1±2.3 |
| TBI+BUS (0.01 mg/kg) | 35.9±4.6 | 41.7±3.4 | 42.0±2.4 |
| TBI+BUS (0.05 mg/kg) | 36.3±5.6 | 44.1±5.9 | 41.0±1.7 |
| TBI+BUS (0.1 mg/kg) | 42.1±4.6 | 48.3±4.4 | 38.3±2.3 |
| TBI+BUS (0.3 mg/kg) | 43.7±10.1 | 54.2±5.4 | 33.0±2.2* |
| TBI+BUS (0.5 mg/kg) | 36.9±3.4 | 42.1±8.7 | 43.2±1.6 |
| Sham | 99.6±2.5** | 100.6±1.1** |
p<0.0001 versus all TBI groups.
p<0.003 versus TBI+VEH, TBI+BUS (0.01 mg/kg), and TBI+BUS (0.5 mg/kg).
Mean (±standard error of the mean) number of normal-appearing hippocampal neurons (expressed as a percentage of the contralateral hippocampus), and cortical lesion volume quantified 3 weeks after TBI or sham injury.
TBI, traumatic brain injury; BUS, buspirone; VEH, vehicle.
Histology: Cortical lesion volume
Analysis of the lesion data showed a significant group effect (F5,24=15.284, p=0.01). The mean cortical lesion volume of the TBI + BUS (0.3 mg/kg) group was 33.0±2.2 mm3, which was significantly smaller than the TBI + VEH (43.1±2.3 mm3), TBI + BUS (0.01 mg/kg; 42.0±2.4 mm3), and TBI + BUS (0.5 mg/kg; 43.2±1.6 mm3) groups (p<0.003). No other group comparisons were significant (Table 1).
Discussion
The purpose of this study was to determine whether the 5-HT1A-receptor agonist buspirone (BUS), a pharmacotherapy novel to TBI, would improve behavior and reduce histopathology in a pre-clinical model of TBI. The rationale for evaluating this pharmacotherapy was based on previous findings from our laboratory showing that the 5-HT1A receptor agonist 8-OH-DPAT produces significant benefits relative to vehicle controls after TBI (Cheng et al., 2007, 2008; Kline et al., 2001,2002b,2004a,2007b,2010). Despite the marked benefits observed with 8-OH-DPAT, BUS was evaluated because it, unlike 8-OH-DPAT, is a clinically-relevant drug that is used routinely to treat anxiety and depression in humans, and therefore if shown to provide benefits in the laboratory, its translation to the clinic could be expedited.
The data revealed that BUS, regardless of dose, did not affect motor performance relative to vehicle-treated controls. However, the 0.3 mg/kg dose of BUS conferred significant, but specific neuroprotection, as demonstrated by a decrease in cortical lesion volume, but no statistically significant increase in hippocampus neuron survival versus all other TBI groups. The lack of any significant hippocampal neuron survival with BUS differs from the findings seen with repinotan HCl and 8-OH-DPAT, which we have shown in several experiments to produce protection (Kline et al., 2001, 2002b, 2004a). Despite a lack of hippocampal protection, the BUS (0.3 mg/kg) group exhibited accelerated spatial learning in the MWM, as evidenced by less time, and shorter distances, to locate the escape platform versus all other TBI groups, regardless of treatment (BUS or VEH) or dose. The 0.3-mg/kg dose of BUS also exhibited significant memory retention, as evidenced by searching significantly longer in the target quadrant during a single probe trial. Specifically, the 0.3-mg/kg BUS group spent as much time in the correct quadrant of the pool as the sham group, which suggests memory ability comparable to non-injured controls. The enhancement in cognitive performance despite a lack of hippocampal protection underscores the tenuous link correlating histology and behavior (Kline et al., 2010; Lyeth et al., 1990).
It is possible that several potentially effective pharmacotherapies evaluated in pre-clinical TBI models have not shown benefits due to study designs that limit dosing, and thus we powered our study sufficiently by designing and implementing a thorough dose-response profile consisting of five different doses of BUS. The data revealed that BUS has a narrow effective dose range, as only the group administered 0.3 mg/kg exhibited significant improvement relative to the VEH-treated group. Moreover, the group receiving the slightly higher dose of 0.5 mg/kg performed worse on the beam-balance task than the 0.3-mg/kg group, and also performed worse than the lower BUS (0.01 mg/kg) dose in the water maze. A narrow dose-response range for 5-HT1A-receptor agonists has been shown in other studies. For example, an acute and single administration paradigm with 8-OH-DPAT after CCI injury showed that 0.5 mg/kg was beneficial, but 0.1 mg/kg was not (Kline et al., 2002b). In contrast, a chronic administration dose-response study revealed the opposite, with 0.1 mg/kg of 8-OH-DPAT showing a significant benefit, and 0.5 mg/kg producing a slight, albeit not statistically significant, worsening of outcomes (Cheng et al., 2008). Differences between relatively narrow dose ranges of 8-OH-DPAT (0.5 mg/kg versus 0.06 mg/kg) have also been reported in memory performance in non-TBI models of cognition (Meneses, 2007).
The differences in dose response in mediating behavioral performance after TBI may be due to alterations in the number of 5-HT1A receptors, receptor sensitivity (Kreiss and Lucki, 1992), or effects at pre-synaptic versus post-synaptic receptors (Barnes and Sharp, 1999; Lüttgen et al., 2005; Meneses, 2007). In addition to 5-HT1A-receptor agonist properties, BUS is also a partial D2 antagonist (Coop and McNaughton, 1991; McMillen et al., 1983), which could explain, in part, the worsening effect seen with the higher BUS dose, if it surpassed its threshold for 5-HT1A-receptor activation and instead antagonized D2 receptors. Support for this notion derives from previous work in our laboratory demonstrating that the D2-receptor antagonist haloperidol impedes the ability of rats to successfully navigate a water maze task to find the escape platform (Hoffman et al., 2008; Kline et al., 2007a,2008). Water maze performance is also impaired by haloperidol in a fluid percussion model of TBI (Wilson et al., 2003). Taken together, these TBI studies from independent laboratories indicate that D2-receptor antagonism impairs cognitive outcome. Moreover, several studies have shown that D2-receptor agonists improve motor, cognitive, and histological outcomes after TBI (Dixon et al., 1999; Garcia et al., 2011; Kline et al., 2002a,2004b).
An alternative explanation for the poor performance of the 0.5-mg/kg BUS group in the water maze is that the group as a whole demonstrated pronounced thigmotaxis (i.e., wall hugging; Fig. 4). Thigmotaxic behavior may be associated with anxiety, in which rats fear open spaces and tend to stay near the edges, which precludes meaningful search strategies. In this case it is a bit ironic, as BUS is typically used as an anxiolytic (Chew and Zafonte, 2009). However, the fact that only the higher-dose group exhibited such behavior is consistent with the narrow BUS dose-response profile. However, it should be noted that thigmotaxis cannot be the only explanation for why rats are unable to learn in the water maze. As mentioned above, rats receiving the antipsychotics haloperidol and risperidone also did not learn, and indeed were significantly impeded versus vehicle controls, even though they engaged in search strategies. Thus while thigmotaxic behavior is a plausible explanation, it cannot be considered as the sole cause. Future studies will shed light on other potential mechanisms.
Possible explanations for how BUS may have contributed to the enhanced cognitive performance are varied, as the 5-HT1A-receptor subtype interacts with multiple neurotransmitter systems (Barnes and Sharp, 1999; Meneses, 2007). Like other 5-HT1A-receptor agonists (e.g., 8-OH-DPAT), BUS increases dopamine levels in the prefrontal cortex and hippocampus (Barnes and Sharp, 1999; Sakaue et al., 2000), which are important regions for cognitive processing. Moreover, as stated above, there are a plethora of studies supporting the notion that dopamine neurotransmission is important for spatial learning and memory (Bales et al., 2009; Garcia et al., 2011; Kokiko and Hamm, 2007; Wheaton et al., 2009). Recent data from our laboratory showed that the 5-HT1A-receptor agonist 8-OH-DPAT attenuated TBI-induced loss of choline acetyltransferase (ChAT)-positive medial septal cells, which correlated with improved cognitive performance (Kline et al., 2010), thus supporting the notion that neuroprotection of the cholinergic system may be another potential mechanism mediating the cognitive benefits observed in the present study. Furthermore, the 5-HT1A-receptor agonist repinotan hydrochloride (BAY X 3702) has been reported to increase ChAT activity (Harkany et al., 2001), which is correlated with cholinergic neurotransmission.
In summary, the significant enhancement in cognitive performance and selective histological protection observed with this clinically-relevant treatment paradigm suggests that BUS may have potential as a novel pharmacotherapeutic approach for human TBI. However, the benefits of this treatment must be replicated in other models of TBI before BUS can be legitimately considered for use in the clinical treatment of TBI. Additionally, future studies are needed to determine what mechanisms might be mediating the beneficial effects of BUS. To this end, studies investigating the effects of BUS on the cholinergic system are ongoing in our laboratory, as are studies assessing the potential additive effect of BUS combined with rehabilitation-relevant environmental enrichment paradigms.
Acknowledgments
Supported, in part, by National Institutes of Health grants HD043851, HD046700, and NS060005 (to A.E.K.).
Author Disclosure Statement
No competing financial interests exist.
References
- Albert P.R. Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Alessandri B. Tsuchid E. Bullock R.M. The neuroprotective effect of a new serotonin receptor agonist, BAY x3702, upon focal ischemic brain damage caused by acute subdural hematoma in the rat. Brain Res. 1999;845:232–235. doi: 10.1016/s0006-8993(99)01948-4. [DOI] [PubMed] [Google Scholar]
- Bales J.W. Wagner A.K. Kline A.E. Dixon C.E. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baños J.H. Novack T.A. Brunner R. Renfroe S. Lin H.Y. Meythaler J. Impact of early administration of sertraline on cognitive and behavioral recovery in the first year after moderate to severe traumatic brain injury. J. Head Trauma Rehabil. 2010;25:357–361. doi: 10.1097/HTR.0b013e3181d6c715. [DOI] [PubMed] [Google Scholar]
- Barnes N.M. Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Binder L. Persistent symptoms after mild head injury: a review of the postconcussive syndrome. J. Clin. Exp. Neuropsychol. 1986;8:323–346. doi: 10.1080/01688638608401325. [DOI] [PubMed] [Google Scholar]
- Cheng J.P. Aslam H.A. Hoffman A.N. Zafonte R.D. Kline A.E. The neurobehavioral benefit conferred by a single systemic administration of 8-OH-DPAT after brain trauma is confined to a narrow therapeutic window. Neurosci. Lett. 2007;416:165–168. doi: 10.1016/j.neulet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.P. Hoffman A.N. Zafonte R.D. Kline A.E. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E. Zafonte R.D. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J. Rehabil Res. Dev. 2009;46:851–878. doi: 10.1682/jrrd.2008.09.0120. [DOI] [PubMed] [Google Scholar]
- Coop C.F. McNaughton N. Buspirone affects hippocampal rhythmical slow activity through serotonin1A rather than dopamine D2 receptors. Neuroscience. 1991;40:169–174. doi: 10.1016/0306-4522(91)90182-n. [DOI] [PubMed] [Google Scholar]
- De Vry J. Jentzsch K.R. Discriminative stimulus properties of the 5-HT1A receptor agonist BAY x 3702 in the rat. Eur. J. Pharmacol. 1998;357:1–8. doi: 10.1016/s0014-2999(98)00503-2. [DOI] [PubMed] [Google Scholar]
- De Vry J. Dietrich H. Glaser T. Heine H.-G. Horváth E. Jork R. Maertins T. Mauler F. Opitz W. Scherling D. Schohe-Loop R. Schwarz T. BAY x 3702. Drugs Future. 1997;22:341–349. [Google Scholar]
- De Vry J. Schohe-Loop R. Heine H.-G. Greuel J.M. Mauler F. Schmidt B. Sommermeyer H. Glaser T. Characterization of the aminomethylchroman derivative BAY x 3702 as a highly potent 5-hydroxytryptamine 1A receptor agonist. J. Pharmacol. Exp. Ther. 1998;284:1082–1094. [PubMed] [Google Scholar]
- Dixon C.E. Kraus M.F. Kline A.E. Ma X. Ya H.Q. Griffith R.G. Wolfson B.M. Marion D.W. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 1999;14:285–294. [PubMed] [Google Scholar]
- Dixon C.E. Ma X. Marion D.W. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J. Neurotrauma. 1997;14:161–169. doi: 10.1089/neu.1997.14.161. [DOI] [PubMed] [Google Scholar]
- Faul M. Xu L. Wald M.M. Coronado V.G. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- Feeney D.M. Gonzalez A. Law W.A. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Garcia A.N. Shah M.A. Dixon C.E. Wagner A.K. Kline A.E. Biologic and plastic effects of experimental traumatic brain injury treatment paradigms and their relevance to clinical rehabilitation. PMR. 2011;3:S18–S27. doi: 10.1016/j.pmrj.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. Traumatic brain injury: a silent epidemic. Ann. Neurol. 1990;27:327. doi: 10.1002/ana.410270315. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Dixon C.E. Gbadebo D.M. Singha A.K. Jenkins L.W. Lyeth B.G. Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Harkany T. Mulder J. Horvath K.M. Keijser J. Van Der Meeberg E.K. Nyakas C. Luiten P.G.M. Oral post-lesion administration of the 5-HT1A receptor agonist repinotan hydrochloride (BAY X 3702) attenuates NMDA-induced delayed neuronal death in rat magnocellular nucleus basalis. Neuroscience. 2001;108:629–642. doi: 10.1016/s0306-4522(01)00444-4. [DOI] [PubMed] [Google Scholar]
- Hoffman A.N. Cheng J.P. Zafonte R.D. Kline A.E. Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 2008;83:602–607. doi: 10.1016/j.lfs.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneman G. Emanuelson I. Cognitive outcome in children and young adults who sustained a severe and moderate traumatic brain injury 10 years earlier. Brain Inj. 2009;23:907–914. doi: 10.1080/02699050903283239. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Hoffman A.N. Cheng J.P. Zafonte R.D. Massucci J.L. Chronic administration of antipsychotics impede behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 2008;448:263–267. doi: 10.1016/j.neulet.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Dixon C.E. Zafonte R.D. Bolinger B.D. The therapeutic efficacy conferred by the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma. 2004a;21:175–185. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Marion D.W. Dixon C.E. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma. 2002a;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Ma X. Zafonte R.D. Dixon C.E. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J. Neurotrauma. 2004b;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Zafonte R.D. Dixon C.E. DeFeo J.R. Rogers E.H. Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental traumatic brain trauma. Crit. Care Med. 2007a;35:919–924. doi: 10.1097/01.CCM.0000256722.88854.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A.E. McAloon R.L. Henderson K.A. Bansal U.K. Ganti B.M. Ahmed R.H. Gibbs R.B. Sozda C.N. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A.E. Wagner A.K. Westergom B.P. Malena R.R. Zafonte R.D. Olsen A.S. Sozda C.N. Luthra P. Panda M. Cheng J.P. Aslam H.A. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 2007b;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A.E. Yu J. Horváth E. Marion D.W. Dixon C.E. The selective 5-HT1A receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–555. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Yu J. Massucci J.L. Zafonte R.D. Dixon C.E. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci. Lett. 2002b;333:179–182. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- Koen N. Stein D.J. Pharmacotherapy of anxiety disorders: a critical review. Dialogues Clin. Neurosci. 2011;13:423–437. doi: 10.31887/DCNS.2011.13.4/nkoen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokiko O.N. Hamm R.J. A review of pharmacological treatments used in experimental models of traumatic brain injury. Brain Inj. 2007;21:259–274. doi: 10.1080/02699050701209964. [DOI] [PubMed] [Google Scholar]
- Kreiss D.S. Lucki I. Desensitization of 5-HT1A autoreceptors by chronic administration of 8-OH-DPAT. Neuropharmacology. 1992;10:1073–1076. doi: 10.1016/0028-3908(92)90110-b. [DOI] [PubMed] [Google Scholar]
- Lüttgen M. Elvander E. Madjid N. Ögren S.O. Analysis of the role of 5-HT1A receptors in spatial and aversive learning in the rat. Neuropharmacology. 2005;48:830–852. doi: 10.1016/j.neuropharm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Lyeth B.G. Jenkins L.W. Hamm R.J. Dixon C.E. Phillips L.L. Clifton G.L. Young H.F. Hayes R.L. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Matter A.M. Folweiler K.A. Curatolo L.M. Kline A.E. Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair. 2011;25:558–564. doi: 10.1177/1545968310397206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max W. Mackenzie E.J. Rice D.P. Head injuries: costs and consequences. J. Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- McDowell S. Whyte J. D'Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121:1155–1164. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- McMillen B.A. Matthews R.T. Sanghera M.K. Shepard P.D. German D.C. Dopamine receptor antagonism by the novel anti-anxiety drug, buspirone. J. Neurosci. 1983;3:733–738. doi: 10.1523/JNEUROSCI.03-04-00733.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A. Perez-Garcia G. 5-HT(1A) receptors and memory. Neurosci. Biobehav. Rev. 2007;31:705–727. doi: 10.1016/j.neubiorev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Meneses A. 5-HT system and cognition. Neurosci. Biobehav. Rev. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behav. Brain Res. 2007;184:81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Millis S.R. Rosenthal M. Novack T.A. Sherer M. Nick T.G. Kreutzer J.S. High W.M., Jr. Ricker J.H. Long-term neuropsychological outcome after traumatic brain injury. J. Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Parton A. Coulthard E. Husain M. Neuropharmacological modulation of cognitive deficits after brain damage. Curr. Opin. Neurol. 2005;18:675–680. doi: 10.1097/01.wco.0000189872.54245.13. [DOI] [PubMed] [Google Scholar]
- Prehn J.H. Backhauss C. Karkoutly C. Nuglisch J. Peruche B. Rossberg C. Krieglstein J. Neuroprotective properties of 5-HT1A receptor agonists in rodent models of focal and global cerebral ischemia. Eur. J. Pharmacol. 1991;203:213–222. doi: 10.1016/0014-2999(91)90717-5. [DOI] [PubMed] [Google Scholar]
- Prehn J.H. Welsch M. Backhauss C. Nuglisch J. Ausmeier F. Karkoutly C. Krieglstein J. Effects of serotonergic drugs in experimental brain ischemia: evidence for a protective role of serotonin in cerebral ischemia. Brain Res. 1993;630:10–20. doi: 10.1016/0006-8993(93)90636-2. [DOI] [PubMed] [Google Scholar]
- Sakaue M. Somboonthum P. Nishihara B. Koyama Y. Hashimoto H. Baba A. Matsuda T. Postsynaptic 5-hydroxytryptamine (1A) receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br. J. Pharmacol. 2000;129:1029–1034. doi: 10.1038/sj.bjp.0703139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S.W. Baldwin S.A. Brown R.W. Kraemer P.J. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Selassie A.W. Zaloshnja E. Langlois J.A. Miller T. Jones P. Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Semkova I. Wolz P. Krieglstein J. Neuroprotective effect of 5-HT1A receptor agonist, Bay x 3702, demonstrated in vitro and in vivo. Eur. J. Pharmacol. 1998;359:251–260. doi: 10.1016/s0014-2999(98)00634-7. [DOI] [PubMed] [Google Scholar]
- Sosin D.M. Sniezek J.E. Waxweiler R.J. Trends in death associated with traumatic brain injury, 1979 through 1992. JAMA. 1995;273:1778–1780. [PubMed] [Google Scholar]
- Sozda C.N. Hoffman A.N. Olsen A.S. Cheng J.P. Zafonte R.D. Kline A.E. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C.R. Ivins B. Schwab K.A. Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 2009;76:105–110. doi: 10.1002/msj.20100. [DOI] [PubMed] [Google Scholar]
- Sutton R.L. Feeney D.M. α-Noradrenergic agonists and antagonists affect recovery and maintenance of beam-walking ability after sensorimotor cortex ablation in the rat. Restor. Neurol. Neurosci. 1992;4:1–11. doi: 10.3233/RNN-1992-4101. [DOI] [PubMed] [Google Scholar]
- Temple M.D. Hamm R.J. Chronic, post-injury administration of D-cycloserine, an NMDA partial agonist, enhances cognitive performance following experimental brain injury. Brain Res. 1996;741:246–251. doi: 10.1016/s0006-8993(96)00940-7. [DOI] [PubMed] [Google Scholar]
- Verbois S.L. Hopkins D.M. Scheff S.W. Pauly J.R. Chronic intermittent nicotine administration attenuates traumatic brain injury-induced cognitive dysfunction. Neuroscience. 2003;119:1199–1208. doi: 10.1016/s0306-4522(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Wang Y. Neumann M. Hansen K. Hong S.M. Kim S. Noble-Haeusslein L.J. Liu J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J. Neurotrauma. 2011;28:259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton P. Mathias J.L. Vink R. Impact of early pharmacotherapy treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J. Clin. Psychopharmacol. 2009;29:468–477. doi: 10.1097/JCP.0b013e3181b66f04. [DOI] [PubMed] [Google Scholar]
- Wilson M.S. Gibson C.J. Hamm R.J. Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am. J. Phys. Med. Rehabil. 2003;82:871–879. doi: 10.1097/01.PHM.0000091982.33232.CB. [DOI] [PubMed] [Google Scholar]
- Wilson M.S. Hamm R.J. Effects of fluoxetine on the 5-HT1A receptor and recovery of cognitive function after traumatic brain injury in rats. Am. J. Phys. Med. Rehabil. 2002;81:364–372. doi: 10.1097/00002060-200205000-00009. [DOI] [PubMed] [Google Scholar]