Abstract
Among other deficits, traumatic brain injury (TBI) causes impaired arousal and cognitive dysfunction. Hypothalamic orexin neuropeptides (also called hypocretins) regulate levels of arousal, and cerebrospinal fluid orexin levels are reportedly low in TBI patients. We hypothesized that TBI acutely impairs the dynamics of orexin release into brain interstitial fluid, and that these extracellular orexin levels correlate with wakefulness and motor activity. To test this in mice, we combined an electromagnetic controlled cortical impact (CCI) model of experimental TBI with dual intracerebral microdialysis using one catheter in the hypothalamus and one catheter in the hippocampus, plus electroencephalography/electromyography (EEG/EMG), and motor activity monitoring. Baseline data were continuously collected in tethered but relatively freely moving mice for 2 days. Then, ipsilateral CCI or sham surgery was performed, and data collection was continued for 3 additional days. At baseline, extracellular orexin levels in the hypothalamus showed a circadian rhythm, with peak levels during the dark (wake) phase, and a nadir during the light (rest) phase. Following CCI but not sham surgery, orexin levels were depressed in both the hypothalamus and hippocampus, and diurnal fluctuation amplitudes were blunted in the hypothalamus. At baseline, correlations of orexin with wakefulness and motor activity were positive and highly significant. Following CCI but not sham surgery, the mice exhibited reduced wakefulness and motor activity, and correlations between orexin and these measures were diminished. These abnormal orexin dynamics were associated with hypothalamic astrogliosis, but not acute loss of orexin neurons, as assessed by immunohistochemistry 3 days after injury. Future studies involving experimental manipulations of the orexin system will be required to determine its contribution to neurological outcomes following injury.
Key words: circadian rhythm, electroencephalography, electromyography, hypocretin, hypothalamus, microdialysis, orexin, sleep, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability, especially among young people (Okie 2005; Thurman et al., 1999). Intrinsic to the clinical diagnosis of TBI is acute loss of consciousness. Even after resumption of consciousness, however, abnormal patterns of arousal and cognitive difficulties remain. Recent studies show a high prevalence of chronic sleep disorders in the TBI population, including hypersomnia and narcolepsy-like symptoms, which can persist as long as 3 years after injury (Castriotta et al., 2007; Kempf et al., 2010; Watson et al., 2007). However, effective therapeutics are still lacking for acute and chronic effects of TBI, including post-traumatic hypersomnia (Castriotta et al., 2009).
The orexin (hypocretin) neuropeptide system is a critical regulator of behavioral arousal, including wakefulness and feeding behavior (Chemelli et al., 1999; Sakurai and Mieda, 2011; Willie et al., 2001,2003). Orexin-A and orexin-B (also called hypocretin-1 and hypocretin-2) peptides, produced in the lateral and posterior hypothalamus, are released together from axon terminals that project widely throughout the central nervous system (CNS) to modulate functions of the cortex and deep brain structures (Sakurai and Mieda 2011; Sakurai et al., 1998; Willie et al., 2001). Exogenous orexin administration to animals promotes cortical brain activity, wakefulness, and cognitive performance (Deadwyler et al., 2007; Mieda et al., 2004). Narcolepsy-cataplexy, a disorder characterized by disorganized sleep-wake states and inability to maintain wakefulness, is specifically caused by degeneration of orexin neurons, and deficient orexin levels in cerebrospinal fluid (CSF) may confirm the clinical diagnosis (Chemelli et al., 1999; Hara et al., 2001; Mignot et al., 2002; Nishino et al., 2000; Willie et al., 2003).
Severe TBI may be associated with injury to the hypothalamus and other deep brain structures known to interact with the orexin system (Crompton, 1971). Because of the fundamental role of the orexin system in coordinating activity of ascending arousal systems, orexinergic dysfunction is a candidate mechanism by which a TBI might result in signs and symptoms of disturbed arousal. Indeed, recent studies have investigated an association between orexin deficiency and human TBI. Orexin-A levels in CSF were found to be abnormally low in most patients during the acute phase following severe TBI (Baumann et al., 2005), and abnormally low orexin-A levels persisted 6 months after TBI in the subgroups suffering excessive sleepiness and poor clinical outcomes (Baumann et al., 2007). Furthermore, an autopsy study demonstrated significant reduction of orexin neurons in brains obtained following lethal TBI compared with control brains (Baumann et al., 2009). In animals, interstitial orexin dynamics have been correlated with circadian rhythms of wakefulness and locomotor activity (Kiyashchenko et al., 2002; Yoshida et al., 2001), but the effects of TBI upon these measures of arousal and interstitial orexin dynamics have not been explored.
We hypothesized that interstitial orexin-A levels correlate with arousal over time, and that TBI causes acutely depressed measures of arousal and impaired interstitial orexin-A release. To test these hypotheses, we combined a previously validated mouse model of experimental TBI (electromagnetic stereotactically-delivered controlled cortical impact [CCI]; Brody et al., 2007), with intracerebral microdialysis and continuous behavioral recording.
Methods
Experimental overview
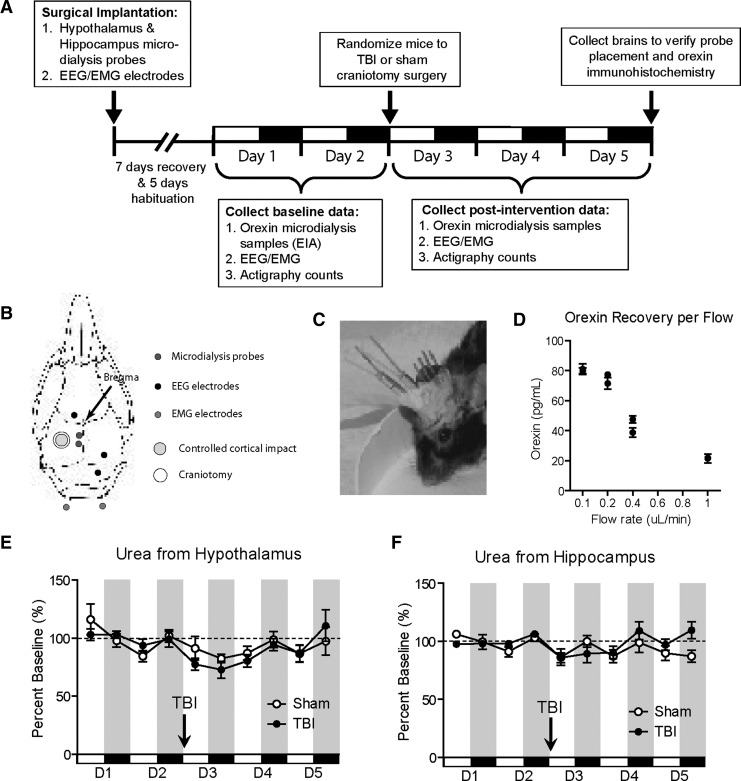
Experimental collection was performed according to the timeline detailed in Figure 1A. Surgical implantation of microdialysis probes and electroencephalography (EEG) and electromyography (EMG) wires was performed. Following a 2-week recovery and habituation period, baseline microdialysis fractions were collected for 48 h in these tethered, but otherwise relatively freely behaving, mice. Samples were analyzed for orexin and urea levels (probe control). On the morning of day 3, left craniotomy and CCI or left craniotomy only (sham) was performed ipsilateral to the implanted probes (n=5 mice per group). Microdialysis fraction collection resumed for 72 h following injury. On day 5, the animals were sacrificed and the brains were processed for histological verification of probe placement and immunohistochemistry.
FIG. 1.
Experimental design, methods, and controls. (A) Experimental timeline illustrating the sequence of surgical implantation, recovery and habituation periods, baseline data collection (days 1–2), sham or TBI surgery performed at the beginning of day 3, data collection post-intervention (days 3–5), and collection of brains on day 6 for histology. (B) Drawing of mouse skull illustrating the placement of microdialysis probes, EEG/EMG implants, and craniotomy site for sham or controlled cortical impact (CCI). (C) Photograph of a mouse with microdialysis and EEG/EMG implants sealed in bone cement. The craniectomy site underlies the scalp to the left of the cemented implants. (D) Orexin-A neuropeptide levels in intracerebral microdialysis samples from the hypothalamus as a function of dialysis flow rate. Slower rates increase recovery as expected. Fractions were collected in 90-min bins, and two independent fractions were collected and tested for each flow rate. An intermediate flow rate of 0.3 μL/mL was chosen for subsequent experimentation. (E and F) Microdialysis of urea, a stable non-circadian small-molecule metabolite, measured from the hypothalamus (E) and hippocampus (F) revealed no significant difference in probe function between the sham and CCI groups over 5 days of monitoring (p>0.05 by two-way analysis of variance; EEG, electroencephalography; EMG, electromyography; EIA, enzyme immunoassay; TBI, traumatic brain injury).
Animals
All experimental procedures involving animals were performed in accordance with guidelines established by the Animal Studies Committee at Washington University in St. Louis. Male C57BL6 mice (Jackson Laboratories, Bar Harbor, ME) 4–6 months of age were used for the experiments. All mice were housed under controlled laboratory conditions with temperature 23–25°C, humidity <10%, and a 12-h dark:light schedule (lights on at 07:00 and lights off at 19:00). The mice were initially raised in standard cages at 3–5 mice per cage under standard laboratory conditions prior to the experiment. They were individually housed during initial surgical recovery for 1–2 weeks, during which time all mice achieved at least 95% of their pre-surgical body weight when adjusted for the additional mass of the surgical implants (see below). The mice were then individually secured via a plastic collar to counterbalanced arms in electronic swivel cages with movement-responsive action to prevent tangling of inlet and outlet catheters and cables (Raturn® Caging System; Bioanalytical Systems, Inc., West Lafayette, IN) during experimental habituation (5 days), and data collection (an additional 5 days). The animals had access to food and water ad libitum throughout the experiments. Despite tethering, the mice were able to ambulate, rear, groom, feed, drink, and assume a normal sleep posture in a relatively unrestricted manner. Due to the sensitivity of sleep-wake studies, handling of animals was avoided during baseline and post-intervention recordings. Subjective health of individual animals was monitored by daily visual inspection, and verified retrospectively by inspection of circadian patterns of sleep-wake rhythms prior to intervention and following sham surgery. Likewise, urea levels in microdialysates also served as a retrospective monitor of overall health pre- and post-intervention. Severe cachexia and dehydration can cause uremia secondary to elevated protein catabolism and pre-renal failure (J.T. Willie, unpublished observations). Microdialysis evidence of uremia was not observed in the mice in this study.
Surgical implantation
Unilateral microdialysis probes were placed stereotactically into the left hypothalamus and left hippocampus of each animal. EEG/EMG electrodes were concurrently implanted for monitoring sleep-wake stages (Fig. 1B and C). Specifically, the mice were anesthetized under isoflurane, weighed, and placed prone on a warming pad at 37°C throughout the surgical procedure. Their heads were secured using a Kopf stereotactic frame with the bregma registered to 0.0 using standard rodent stereotactic techniques. Burr holes were placed full thickness through the skull using a stereotactic electric drill fitted with a 0.5 mm round dental drill bit. Two intracerebral guide cannulae, each 10 mm in length (MD-2256; Bioanalytical Systems, Inc.), were placed according to the following coordinates: (1) lateral hypothalamus (anterior/posterior [AP] −1.94 mm, medial/lateral [ML] 0.5 mm, dorsal/ventral [DV] 3.75 mm; directed vertically), and (2) hippocampus (AP −2.7 mm, ML 0.5 mm, DV 1.3 mm; directed medial to lateral at 38° from vertical). Stylets were left in place during the recovery and habituation periods. Tap sites for EEG screw electrodes were also drilled through the skull at the following coordinates: left frontal (AP +1.1 mm, ML 1.3 mm), right parietal (AP −3.4 mm, ML 2.2 mm), and right cerebellar ground (AP −5.6 mm, ML 1.1 mm). The ipsilateral skull was scored with a reference burr mark at the epicenter of the future craniotomy site for left cortical injury at AP −2.0 mm, ML 2.7 mm. Two EMG electrodes were placed bilaterally into the nuchal musculature. The EEG/EMG implant consisted of a custom-designed six-prong electrical mini-plug soldered with lengths of Teflon-insulated braided stainless-steel wire to three stainless-steel screws (EEG leads) and two exposed loops of wire (EMG electrodes), fixed and insulated with epoxy cement. All instrumentation was secured to the exposed skull using glass ionomer dental cement (ESPE Ketac Cem Applicaps and ESPE RotoMix capsule mixer; 3M, St. Paul, MN; Fig. 1C). The mice were weighed immediately following the procedure, providing an indirect determination mass of the final implant in each case. A custom-built head stage amplifier (Washington University Electronics Shop, St. Louis, MO), in series with a recording cable distal to the implant was used to eliminate external electrical noise. The animals were housed individually and recovered for 1–2 weeks prior to experimental habituation and data collection (see below).
Microdialysis
Mice were habituated to tethered recording conditions for 5 days in electronic swivel cages (described above) prior to data collection. Brain microdialysis probes with an exposed 2-mm membrane tip and 38-kDa molecular weight cutoff pore size (MD-2232; Bioanalytical Systems, Inc.) were flushed prior to implantation with CNS perfusion fluid (0.15% sterile human albumin, diluted from 25% human albumin) in sterile isotonic saline solution (147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 0.85 mM MgCl2). The mice were sedated briefly and lightly with isoflurane, intracranial cannula stylets were withdrawn, and microdialysis probes were inserted through implanted cannulae. Microdialysis was initiated 16 h prior to data collection in order to provide adequate behavioral habituation and perfusion equilibration.
Microdialysis was performed at varying perfusion rates to determine the optimal protocol for orexin recovery. Two separate trials of each individual flow rate utilizing an individual animal implanted with a microdialysis catheter were performed at perfusion rates selected from previous experience with microdialysis of small peptides (0.1, 0.2, 0.4, and 1.0 μL/min; Cirrito et al., 2003; Schwetye et al., 2010), with the order of trials pseudo-randomized. Adequate dead space volumes were discarded between trials. Orexin-A levels measured from samples collected at each microdialysis flow rate demonstrates that orexin recovery is a function of flow rate in vivo (Fig. 1D). An optimum perfusion rate of 0.3 μL/min was selected for all subsequent experiments, as it fell within the linear recovery range as a function of rate, while also yielding fraction volumes practical for subsequent assays at the desired temporal resolution (>50 μL/3 h). In subsequent experiments, fractions were collected every 90 min into chilled microcentrifuge tubes following equilibration. Samples were recombined into 3-h fractions for the orexin-A immunoassay.
Immunoassay for orexin-A
Microdialysate samples were analyzed for orexin-A (human, rat, mouse, porcine, bovine, and ovine) levels using a commercially available fluorescent enzyme immunoassay (EIA) kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA). All samples were assayed in duplicate, and the mean of the two values was reported. All immunoassays were performed within 1–2 weeks of fraction collection, with storage at 4°C until testing. All samples from an individual animal were assayed together to avoid inter-plate variability. The protocol exactly followed the manufacturer's instructions, and we obtained a linear range typically down to 10–15 pg/mL. This protocol has been successfully used for detection of orexin-A in previously reported microdialysis experiments (Yoshida et al., 2001).
Urea assay
After the volume required for orexin quantification was removed from the microcentrifuge tubes, urea concentration was measured on 2 μL of the remaining volume using a commercially-available kit (Quanti-Chrom Urea Assay Kit; BioAssay Systems, Hayward, CA) as previously described (Brody et al., 2008; Schwetye et al., 2010).
Sleep-wake staging
EEG and EMG electrodes soldered to a mini-connector for polysomnographic recordings were implanted as described above. The implant was connected to a head-stage amplifier via flexible recording cables supported by a counterbalanced arm. Signals were electronically saved to a file for the offline analysis of sleep states. EEG and EMG activity was assessed using a P511K AC pre-amplifier (Grass-Telefactor Instruments, Braintree, MA), digitized with a DigiData 1440A Data Acquisition System (Molecular Devices, Sunnyvale, CA), and recorded using pClamp 10.2 (Molecular Devices). EEG and EMG signals were filtered (EEG: high pass 1 Hz; low pass 30 Hz and EMG: high pass 10 Hz; low pass 100 Hz) and used to identify sleep-wake states. EEG/EMG records were scored automatically using sleep scoring software (SleepSign; Kissei Comtec Co., Ltd., Nagano, Japan) into 10-sec epochs as wake, REM, and non-REM on the basis of standard criteria of rodent sleep (Renger et al., 2004), and then over-scored manually by visual inspection and corrected when appropriate by a single investigator blinded to intervention (M.M. Lim).
Actigraphy
Individual accelerometric actigraphy devices (ActiGraph Sleep Monitoring Solution, Pensacola, FL) were set to maximal sensitivity and secured to counterbalanced arms of the caging system in which mice were tethered. Actigraphy data (vertical plus horizontal motor activity counts collected in 60-sec epochs) were uploaded and compiled using ActiGraph software.
Controlled cortical impact
We utilized a well-characterized electromagnetic stereotactically-mounted CCI for producing experimental TBI via left frontoparietal craniotomy in mice. Following 48 h of data collection under baseline conditions, injuries were performed during the first hour of light phase under anesthesia as previously described (Brody et al., 2007; Schwetye et al., 2010). Briefly, microdialysis probes were removed from intracranial cannulae and the tips maintained in artificial CSF. Mice were anesthetized with isoflurane and replaced in a stereotactic frame on a warming pad maintained at 37°C. The previous skin incision was retracted open over the left parietal hemicranium lateral to the implants. A circular extradural craniotomy (centered at bregma −2 mm, left 2.7 mm; marked during prior surgery) was performed using a stereotactic drill and 3.8-mm diameter trephine bit and the bone was elevated with a micro-cup curette. This method generally did not breach the dura. The mice were then subjected to CCI with a 3-mm-diameter flat metal tip impounder driven at a velocity of 5 m/sec by an electromagnetic device, directed 20° from dorsal-lateral to ventral-medial to a depth of 2.5 mm into the cortex, producing a moderately severe contusion to the cortex (Brody et al., 2007). Sham procedures consisted of identical anesthesia and craniotomy, but without impact. Following CCI or sham injury, the craniotomy was covered by a low-profile cranioplasty implant (5-mm-diameter plastic sheet punched out from standard plastic weighing boats), which was circumferentially cemented to the skull with tissue adhesive (Vetbond; 3M). The scalp flap was closed over the cranioplasty with sutures. Microdialysis probes were carefully reinserted intracranially within approximately 3 min of withdrawal, and the mice were returned to behavioral recording cages. They recovered briefly on warming pads until resumption of the righting reflex, at which time the warming pads were removed.
Immunohistochemistry and verification of probe placement
After day 5 (3 days after injury or sham injury), the mice were deeply anesthetized with isoflurane and transcardially perfused by a peristaltic pump with 15 mL chilled phosphate-buffered saline containing 1% heparin over approximately 5 min to achieve liver blanching, followed by approximately 15 mL chilled 4% phosphate-buffered paraformaldehyde over 5 min. The brains were removed and post-fixed in 4% phosphate-buffered paraformaldehyde for 24 h at 4°C, followed by equilibration in a 30% sucrose phosphate-buffered saline solution until sectioning and staining.
The brains were sectioned on a freezing microtome at 50-μm thickness. Floating brain sections at a 1:6 series were processed for anti-orexin-A, anti-melanin-concentrating hormone (MCH), and anti-glial fibrillary acidic protein (GFAP) immunohistochemistry as follows. The tissue was washed in Tris-buffered saline (TBS), then quenched in 3% hydrogen peroxide solution for 10 min, washed again in TBS, then incubated in 0.25% Triton-X solution plus 5% normal goat serum for 30 min. Finally the slides were incubated in primary antibody plus 5% normal goat serum, anti-orexin-A at 1:10,000 overnight (rabbit anti-mouse orexin-A, catalog no. H-003-30; Phoenix Pharmaceuticals, Inc.), anti-MCH at 1:20,000 (rabbit anti-mouse MCH, catalog no. H-070-47; Phoenix Pharmaceuticals Inc.), or anti-GFAP at 1:1000 (polyclonal chicken anti-GFAP; Chemicon, Temecula, CA). The GFAP staining protocol has been previously described by our laboratory (Brody et al., 2007; Mac Donald et al., 2007). The next day, the sections were incubated in a solution containing goat anti-rabbit IgG biotinylated secondary antibody (for orexin and MCH), or donkey anti-chicken secondary antibody (for GFAP) at 1:1000 dilution, and signal was then amplified using the Vectastain ABC kit at 1:400 (Vector Laboratories, Burlingame, CA), followed by visualization with DAB-nickel. The sections were carefully mounted onto glass microscope slides, air-dried, then dehydrated in ascending ethanols and cover-slips were applied.
Orexin and MCH neurons were quantified on a Nikon Eclipse E800 microscope (Tokyo, Japan) fitted with StereoInvestigator 6.0 (MicroBrightField, Williston, VT) by counting positively-staining cells within the ipsilateral hypothalamus across four 50-μm-thick sections. Counting began at the level of the anterior end of the hippocampus where the dentate gyrus is first visible (bregma −1.06 mm; Franklin and Paxinos, 2004), and included the next four stained sections posterior to this point (a total of 1.2 mm). Few orexin- or MCH-positive neurons were observed anterior to the bregma −1.06 mm, or posterior to the bregma −2.26 mm. All positive-staining neurons were clearly visible, and were outlined as the region of interest (ROI) under a 4×objective. Neurons were then counted under the 10×objective using the optical fractionator technique. In order to count all positive-staining neurons the grid size and the counting frame were set to the same size (250×250 microns). This was performed blinded to intervention.
Microdialysis probe placement was verified by review of stained slides under low magnification and comparison to a mouse stereotactic atlas (Franklin and Paxinos, 2004).
Statistical analysis
Data in the figures represent mean±standard error of the mean (SEM) unless otherwise noted. Statistical analyses were performed using GraphPad Prism version 5 for Windows, and Statistica 6.0. The statistical distribution of orexin-A levels and measurements of wake parameters did not differ significantly from the normal distribution (Shapiro-Wilk W tests; p>0.05). Therefore, statistical analyses for orexin-A levels and measurements of wake parameters were performed using two-way analyses of variance (ANOVAs), followed by post-hoc Bonferroni tests. Values were noted as significant if p<0.05. Relationships between orexin-A levels and amounts of wakefulness, and between orexin-A levels and motor activity, were performed using Spearman's non-parametric correlation tests with significance set at p<0.05. Statistical comparisons between two correlations was performed using the Fisher r-to-z transformation, which assesses the significance (set at p<0.05) of the difference between two correlation coefficients found in two independent samples.
Results
Experimental data collection was performed according to the timeline detailed in Figure 1A. Mice were implanted with microdialysis catheters targeting the hypothalamus and hippocampus, and EEG/EMG electrodes (Fig. 1B and C). This preparation provided the ability to continuously collect microdialysis samples and behavioral data in mice able to move with relative freedom.
Urea microdialysis to indicate stability of probe function over time
Urea is a stable endogenous compound that evenly distributes throughout body fluid compartments, including the brain, with levels that minimally fluctuate in healthy animals (Ronne-Engstrom et al., 2001). Urea may therefore serve as an endogenous reference to verify microdialysis probe function over time, as previously described (Brody et al., 2008; Schwetye et al., 2010). Following our experimental timeline (Fig. 1A), microdialysis samples were tested for urea. As expected, averaged absolute hypothalamic and hippocampal urea levels were similar (p>0.05 by Student's t-test). Likewise, hypothalamic (Fig. 1E) and hippocampal (Fig. 1F) urea levels did not significantly differ between sham and TBI mice (p>0.05 by Student's t-test). This indicated (1) comparable probe function independent of placement in the hypothalamus or hippocampus, (2) comparable probe function following both TBI and sham surgery, (3) stable probe function without evidence of drift over the experimental time frame, and (4) absence of severe uremia following interventions.
Effect of TBI on orexin dynamics
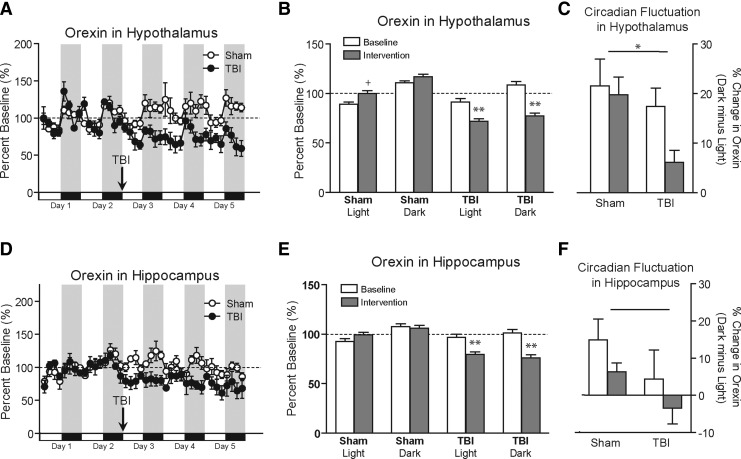
Baseline microdialysis of orexin in the hypothalamus over the first 48 h of collection showed diurnal rhythmicity with peaks and nadirs during the awake and rest phases, respectively (Fig. 2A). Baseline measured hypothalamic microdialysate orexin levels were 56±33 pg/mL (mean±standard deviation), not corrected for relative recovery. After TBI, hypothalamic microdialysate orexin levels were lower compared to baseline values (Fig. 2A and B). The effects of TBI were analyzed separately for the light and dark phases. For the light phase, a repeated-measures ANOVA revealed a significant main effect of group (F=5.9, p=0.04), no main effect of time, and a significant group×time interaction (F=10.3, p=0.01). Prespecified post-hoc testing indicated that in the light phase there was a reduction in hypothalamic orexin levels in the TBI group (p=0.03) compared to baseline, no difference compared to baseline in the sham group (p=0.1), and lower orexin levels in the TBI group post-intervention compared to the sham group post-intervention (p=0.02).
FIG. 2.
Effect of TBI on orexin dynamics as assessed by microdialysis. (A) Baseline orexin levels at 3-h intervals from the hypothalamus show predictable diurnal variations. After controlled cortical impact TBI, orexin levels were significantly suppressed compared to sham controls. (B) Data from panel A summarized by circadian phase reveal significantly reduced orexin levels in the hypothalamus during both the light and dark phases following TBI but not sham surgery (+indicates a non-significant trend towards increased orexin in the sham post-intervention group compared to baseline; ** indicates significantly lower orexin levels in the TBI group compared with baseline and compared with shams). (C) Circadian fluctuations of orexin (percent change in orexin over the entire dark minus light phases) is significantly reduced following TBI, but not sham surgery in the hypothalamus (* indicates reduced circadian fluctuation after TBI; p=0.03). (D) Baseline orexin levels at 3-h intervals from the hippocampus also show a diurnal rhythm that is significantly suppressed after TBI. (E) Data from panel D summarized by phase reveal significantly reduced orexin levels during both light and dark phases following TBI, but not sham surgery in the hippocampus (** indicates significantly lower orexin levels compared with shams). (F) Circadian fluctuations of orexin (percent change in orexin over the entire dark minus light phases) appeared more reduced following TBI than following sham surgery in the hippocampus, but these effects did not reach statistical significance (p=0.0506; TBI, traumatic brain injury).
In the dark phase the results were similar, but even more statistically significant. A repeated-measures ANOVA revealed a significant main effect of group (F=14.7, p=0.005), no main effect of time, and a significant group×time interaction (F=11.4, p=0.009). Prespecified post-hoc testing indicated that in the dark phase there was a reduction in hypothalamic orexin levels in the TBI group (p=0.008) compared to baseline, no difference compared to baseline in the sham group (p=0.22), and lower orexin levels in the TBI group post-intervention compared to the sham group post-intervention (p=0.006).
In addition, the amplitude of circadian fluctuation, as measured by average hypothalamic orexin levels in dark minus light, was significantly decreased after TBI (p=0.03 by one-sided t-test; Fig. 2C).
Orexin levels were also measured simultaneously from the hippocampus in all mice (Fig. 2D and E). Hippocampal microdialysis absolute orexin levels were lower on average (27±10 pg/mL, mean±standard deviation), and also appeared to have somewhat blunted rhythmicity compared to hypothalamic levels. For the light phase, a repeated-measures ANOVA revealed no main effects of group or time, but a significant group×time interaction (F=7.6, p=0.03). Prespecified post-hoc testing indicated that in the light phase there was a reduction in orexin levels in the TBI group (p=0.02) compared to baseline, no difference compared to baseline in the sham group (p=0.31), and a non-significant trend towards lower orexin levels in the TBI group post-intervention, compared to the sham group post-intervention (p=0.09). In the dark phase, repeated-measures ANOVA revealed a significant main effect of group (F=9.1, p=0.02), no main effect of time, and no significant group×time interaction. Similarly to the effects observed in the hypothalamus, the amplitude of circadian fluctuations of orexin in the hippocampus showed a trend toward a decrease after TBI (Fig. 2F).
Effects of TBI on wakefulness
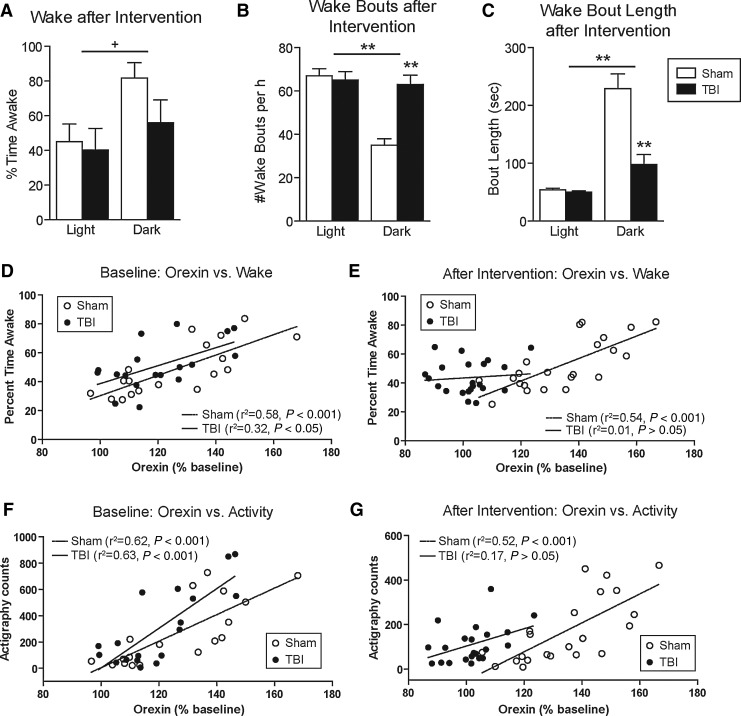
Mice typically sleep during the day and exhibit prolonged periods of wakefulness at night. EEG/EMG analysis for sleep-wake staging suggested that brain-injured mice appeared to be less awake during the dark phase than sham mice; however, this did not reach statistical significance. Two-way ANOVA revealed a main effect of phase (F=5.3, p=0.03), but no significant effect of injury or phase×injury (Fig. 3A). However, assessment of sleep fragmentation proved to be more sensitive to the effects of injury than total wake time. The number of wake bouts during the dark phase was significantly higher after brain injury compared to sham controls. Both groups had similar numbers of wake bouts during the light phase (two-way ANOVA for the main effect of phase: F=20.97, p=0.0003; main effect of injury: F=12.26, p=0.003; phase×injury interaction: F=16.3, p=0.0009; Fig. 3B). The average length of each wake bout was significantly lower in brain-injured mice compared to sham controls during the dark phase, but again no different during the light phase (two-way ANOVA for the main effect of phase: F=51.6, p<0.0001; main effect of injury: F=18.9, p=0.0005; phase×injury interaction: F=16.2, p=0.0008; Fig. 3C). There was no significant difference in the relative fraction of rapid eye movement (REM) sleep versus non-REM sleep between groups in either phase of the circadian cycle (Table 1).
FIG. 3.
Effect of TBI upon wakefulness and motor activity: Correlations with orexin. (A) Wake time during light and dark phases in sham versus TBI groups. There was a non-significant trend, suggesting that the TBI group exhibited less wakefulness during the dark (active) phase compared to shams. (B) Number of wake bouts during the light and dark phases in sham versus TBI groups. The frequency of wake bouts at night was increased in the TBI group, indicating fragmented states (**indicates significant increase in the TBI group compared to shams and compared to baseline). (C) Wake bout length in the dark is reduced following TBI, indicating a reduced ability to maintain wakefulness (**indicates significant decrease in the TBI group compared to shams and compared to baseline). (D) Correlations between time spent awake and orexin at baseline. Mean times spent awake for each sham group (white circles) and TBI group (black circles) by 3-h intervals are graphed against mean orexin levels during the corresponding time period with linear trend lines. At baseline, wake and orexin correlations are positive and parallel in both groups prior to intervention. Spearman correlations (r2) for each group did not significantly differ from each other (p>0.05 by one-tailed Fisher's r-to-z transformation test). (E) Correlation between time spent awake and orexin following intervention. The Spearman correlations following intervention were significantly different from one another (p<0.05 by one-tailed Fisher's r-to-z transformation test), indicating a loss of correlation after TBI, with preservation of correlation following sham injury. (F) Correlation between actigraphy assessments of motor activity and orexin at baseline. Note the significant parallel positive correlations between activity and orexin in each group prior to any intervention (baseline). The two correlations do not significantly differ from each other (p>0.05 by one-tailed Fisher's r-to-z transformation test). (G) Correlation between motor activity and orexin following intervention. Again, the Spearman correlations following intervention were significantly different from one another (p<0.05 by one-tailed Fisher's r-to-z transformation test), indicating loss of correlation after TBI with preservation of correlation following sham injury (TBI, traumatic brain injury).
Table 1.
Sleep Staging across Study Groups
| Wake | REM | Non-REM | |
|---|---|---|---|
| Overall total time (%) | |||
| Sham, baseline | 54±9 | 5±3 | 41±9 |
| Sham, post-intervention | 57±8 | 3±2 | 40±8 |
| TBI, baseline | 53±7 | 4±2 | 42±7 |
| TBI, post-intervention | 47±10 | 4±3 | 50±9 |
| Overall bout duration (sec) | |||
| Sham, baseline | 147±31 | 17±3 | 75±8 |
| Sham, post-intervention | 98±16 | 16±3 | 56±6 |
| TBI, baseline | 73±8 | 20±2 | 48±5 |
| TBI, post-intervention | 69±9 | 17±2 | 62±6 |
| Light period total time (%) | |||
| Sham, baseline | 38±7 | 8±4 | 54±7 |
| Sham, post-intervention | 45±7 | 4±3 | 52±8 |
| TBI, baseline | 44±6 | 5±3 | 50±6 |
| TBI, post-intervention | 39±9 | 6±4 | 56±8 |
| Light period bout duration (sec) | |||
| Sham, baseline | 63±9 | 18±6 | 83±13 |
| Sham, post-intervention | 55±4 | 16±5 | 69±9 |
| TBI, baseline | 51±2 | 22±2 | 53±10 |
| TBI, post-intervention | 49±4 | 19±4 | 68±10 |
| Dark period total time (%) | |||
| Sham, baseline | 69±11 | 2±2 | 29±10 |
| Sham, post-intervention | 70±8 | 2±1 | 28±9 |
| TBI, baseline | 62±8 | 3±2 | 34±7 |
| TBI, post-intervention | 54±10* | 2±1 | 44±10 |
| Dark period bout duration (sec) | |||
| Sham, baseline | 224±47 | 16±4 | 67±10 |
| Sham, post-intervention | 147±28 | 15±5 | 42±9 |
| TBI, baseline | 96±12 | 18±2 | 43±5 |
| TBI, post-intervention | 89±15* | 16±3 | 55±5 |
Indicates statistically significant difference between TBI post-intervention versus sham post-intervention by two-way ANOVA followed by Bonferroni post-hoc testing.
Data expressed as mean±standard error of the mean (n=5 per group).
ANOVA, analysis of variance; TBI, traumatic brain injury.
Correlations of orexin with measures of arousal
On average, orexin levels showed a significant positive correlation with percent time spent awake across all interventions and phases of day (r2=0.67, p<0.05 by Spearman's correlation). However, when the data were segregated by time (baseline versus post-intervention), and group (sham versus TBI), significant differences in relationships emerged. During the baseline portion, orexin levels significantly positively correlated with percent time spent awake in both the sham and CCI groups (r2=0.58, p<0.001 and r2=0.32, p<0.05, respectively, by Spearman's correlation; Fig. 3D). After sham surgery, orexin levels continued to significantly correlate with wakefulness (r2=0.54, p<0.001; Fig. 3E). However, after CCI, the correlation between orexin levels and wakefulness deteriorated (r2=0.01, p>0.05; Fig. 3E). These two correlations were significantly different (p<0.05 by one-tailed Fisher's r-to-z transformation test).
Motor activity was independently quantified using accelerometer actigraphy counts. During the baseline portion, orexin levels significantly positively correlated with motor activity in both the CCI and sham groups (r2=0.62, p<0.001 and r2=0.63, p<0.001, respectively, by Spearman's correlation; Fig. 3F). Animals that underwent sham surgery continued to show a strong correlation between orexin levels and motor activity (r2=0.52, p<0.001). However, similarly to the correlation between orexin and wakefulness, after CCI, the correlation between orexin levels and motor activity was no longer apparent (r2=0.17, p>0.05; Fig. 3G).
Histological verification of probe placement
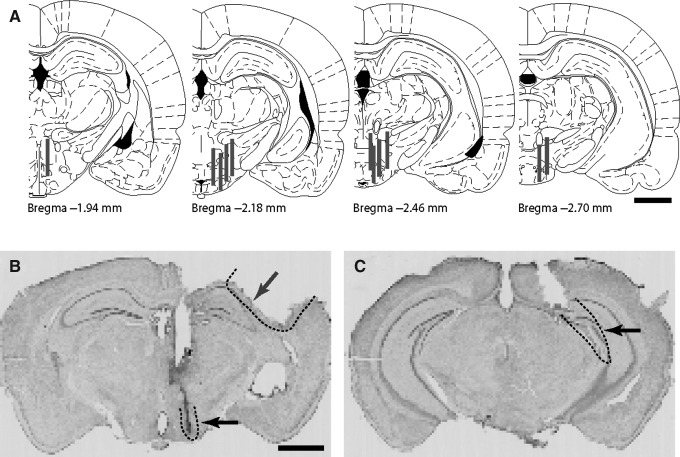
On the fourth day following TBI or sham surgery, all animals were sacrificed and the brains were removed for verification of cortical impact sites and probe placement. Hypothalamic probe placements spanning the anteroposterior distance from bregma −1.94 to −2.70 mm are illustrated for all 11 mice in Figure 4A. Microdialysis probes were placed in the lateral hypothalamus in 4 animals, more anterior in 1 animal, and in the posterior hypothalamus in 6 animals (bregma −2.46 mm, or slightly posterior; Franklin and Paxinos, 2004). The vast majority of orexin neurons and the greatest density of orexin projections are located in the lateral and posterior hypothalamus (Peyron et al., 1998). A representative cresyl violet-stained section following 2.5-mm electromagnetic CCI is shown in Figure 4B, which resembles those previously described for this depth of injury (Brody et al., 2007). We have previously verified reliable hippocampal localization of microdialysis catheters using similar coordinates in mice following sham surgery and 2.0-mm electromagnetic CCI (Schwetye et al., 2010). As expected, sham animals in the present study showed reliable probe placement in the hippocampus, as shown in a representative photomicrograph in Figure 4C. Structural damage to the hippocampus and overlying cortex inherent to the 2.5-mm electromagnetic CCI in the present study made histological verification of hippocampal probe placement following brain sectioning in the TBI group unreliable.
FIG. 4.
Histological verification of probe placement and injury. (A) Estimated microdialysis probe placements in the hypothalamus of all 11 animals utilized in this study shows probe placements from bregma −1.94 through −2.70 mm (Franklin and Paxinos, 2004). (B) Cresyl violet staining of mouse brain section illustrating the tract of a microdialysis catheter (lower arrow with dotted outline) in the hypothalamus, and an overlying cortical impact injury (upper arrow with dotted outline). (C) Cresyl violet staining of a brain section of a mouse that underwent sham surgery illustrating the tract of a microdialysis catheter through the hippocampus (arrow with dotted outline; scale bars=1 mm).
Immunohistochemistry for orexin
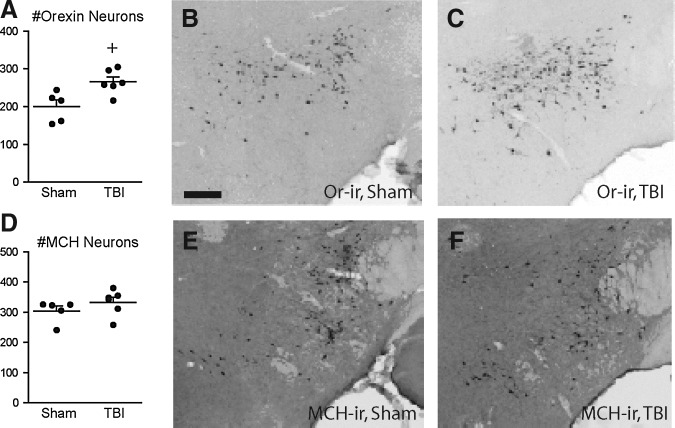
Quantitative immunohistochemistry was performed for orexin-A and melanin-concentrating hormone (MCH)-positive neurons. MCH is a neuropeptide also abundant in the hypothalamus that may play a role in mood and vigilance states (Willie et al., 2008), providing a histological control for this brain region (Baumann et al., 2009). Surprisingly, immunostaining for intracellular orexin-A peptide with stereological cell counting revealed a small but significant apparent increase in the number of orexin-positive cells in the lateral hypothalamus of mice after brain injury compared to sham controls (p<0.05 by Student's t-test; Fig. 5A). Representative photomicrographs for orexin-A-immunoreactivity in the lateral hypothalamus of sham versus CCI mice are shown in Figure 5B and C, respectively. There was no difference between the numbers of MCH-immunoreactive cells in this region (p>0.05 by Student's t-test; Fig. 5D). Representative photomicrographs for MCH-immunoreactivity in the lateral hypothalamus of sham versus CCI mice are shown in Figure 5E and F, respectively. Thus, orexin and MCH neurons are preserved during the experimental time frame in which extracellular orexin release is dysregulated following CCI.
FIG. 5.
Orexin- and melanin-concentrating hormone (MCH)-positive neurons in the hypothalamus are preserved following injury. (A) There was a small but significant elevation in the number of orexin-positive neurons after TBI compared to sham surgery (+indicates p=0.014 by two-sided Student's t-test). (B) Orexin immunoreactivity in the hypothalamus of a sham control animal. (C) Orexin immunoreactivity after TBI. (D) There was no significant difference in the number of MCH-positive neurons between groups (p>0.05, by Student's t-test). (E) MCH immunoreactivity in the hypothalamus of a sham control animal. (F) MCH immunoreactivity after TBI (scale bar=200 μm; TBI, traumatic brain injury).
Immunohistochemistry for GFAP
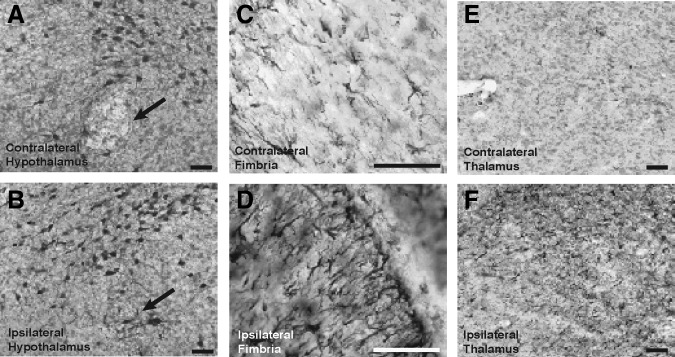
Because orexin neurons appeared to be generally preserved, we sought other histological evidence of hypothalamic injury after CCI. Immunohistochemistry was performed for glial fibrillary acidic protein (GFAP), a robust marker of reactive astrogliosis. Brain sections that included the lateral and posterior hypothalamus with overlying areas of CCI were reviewed. Qualitative immunostaining for GFAP revealed more obvious astrogliosis in the hypothalamus ipsilateral to CCI, especially in the ipsilateral fornix (fibers of passage), compared to the relatively uninjured contralateral side (Fig. 6A and B). Similarly, the ipsilateral hippocampal fimbria and ipsilateral thalamus, regions in closer proximity to the site of CCI, both showed marked GFAP immunoreactivity in comparison to their contralateral counterparts (Fig. 6C and F). These areas were closer in proximity to the epicenter of CCI (the parietal cortex overlying the hippocampus), and together with the hypothalamus, demonstrate evidence of injury deep into the ipsilateral hemisphere. This provided histological evidence for injury to deep regions of the ipsilateral hemisphere, including the hypothalamus, following this experimental injury.
FIG. 6.
Glial fibrillary acidic protein (GFAP) staining reveals the astrogliosis following injury is widespread, including in the ipsilateral hypothalamus. (A and B) GFAP immunohistochemistry in the hypothalamus reveals mild astrogliosis, especially in the fornix (black arrow), on the side ipsilateral to injury compared to the contralateral side. (C and D) Astrogliosis of ipsilateral hippocampal fimbria compared with the contralateral side. (E and F) Astrogliosis of ipsilateral thalamus compared with the contralateral side (scale bar=50 μm).
Discussion
Traumatic brain injury causes cognitive dysfunction and impaired arousal, often manifested as disabling hypersomnia and fatigue (Thurman et al., 1999; Watson et al., 2007). The hypothalamic orexin system normally orchestrates arousal, including wakefulness, and orexinergic dysfunction following brain injury might lead to an impaired level of arousal and persistent neuropsychiatric deficits. We combined a mouse model of TBI with intracranial microdialysis and behavioral monitoring to test the hypotheses that TBI impairs the dynamics of interstitial brain orexin, and that orexin levels correlate with wakefulness and overall motor activity. We found that extracellular brain orexin levels fluctuate dynamically and correlate with diurnal patterns of wakefulness and motor activity under normal (i.e., uninjured) conditions. CCI-induced TBI disturbs extracellular orexin release and impairs these measures of arousal. Interestingly, the correlations between orexin levels with wakefulness and motor activity were lost following TBI. These extracellular neurochemical and behavioral changes following TBI were not associated acutely with the loss of orexin neurons, as quantified from intracellular orexin staining, but likely were due to more subtle injury as evidenced by astrogliosis.
Technically, this study demonstrates that it is possible to successfully combine CCI with dual hypothalamic and hippocampal orexin intracerebral microdialysis, EEG/EMG recording, and motor actigraphy in conscious, relatively mobile mice. We reliably detected orexin in brain microdialysates using an ultra-sensitive fluorescent EIA. To our knowledge, this is the first report of the direct effects of TBI upon brain extracellular orexin dynamics; it is also the first detailed report of sleep-wake abnormalities in this animal model of TBI. While our study focused on acute neurochemical and behavioral changes following acute TBI, our experimental preparation is amenable to studying longer-term effects upon orexin, behavioral arousal, and other outcomes.
Several observations support the physiological relevance of our extracellular orexin measurements. Commensurate with other animal studies, orexin levels followed a predictable diurnal rhythm under normal (i.e., uninjured) conditions, and orexin levels strongly correlated with observed levels of arousal as determined independently by EEG/EMG and actimetry (Fenzl et al., 2009; Yoshida et al., 2001). Fasting is a known behavioral stimulus that promotes orexin mRNA expression in rodents (Sakurai et al., 1998), and we have likewise also observed dynamic increases in orexin microdialysate levels, wakefulness, and motor activity associated with imposed fasting in uninjured control animals (J.T. Willie, unpublished observations). Also, an anatomic gradient of brain orexin concentration was observed in that measured extracellular orexin levels were approximately twofold higher in the hypothalamus, where orexin cell bodies and projections are concentrated, compared to the hippocampus, a region without orexin cell bodies and one less densely innervated with orexinergic projections (Peyron et al., 1998; Sakurai et al., 1998; Yoshida et al., 2006). Nonetheless, it remains to be determined whether microdialysis sampling fully captures the physiologically relevant orexin dynamics.
Are brain-injured mice narcoleptic?
Our experimental model of TBI (CCI) induces some, but not all, behavioral features of narcolepsy-cataplexy (Chemelli et al., 1999; Hara et al., 2001; Mignot et al., 2002; Nishino et al., 2000; Willie et al., 2003). Notably, brain-injured mice exhibit less ability to maintain prolonged wakefulness (as evidenced by shortened wake bouts), with fragmentation of sleep-wake states (as evidenced by an increased number of sleep-wake transitions). These are cardinal signs of disrupted sleep-wake organization in narcolepsy, but also in other sleep-wake disorders not known to be related to the orexin system. The pathognomonic feature of narcolepsy-cataplexy in clinical and experimental diagnosis, setting it apart from other sleep-wake disorders, is direct transitions from wakefulness to REM sleep. Importantly, we never observed direct transitions from active wakefulness to REM sleep in the EEG/EMG records, nor did we observe their behavioral correlate cataplexy following TBI in our study. Furthermore, the pathological correlate of both clinical and experimental narcolepsy-cataplexy is a profound loss to complete absence of orexin neurons in the hypothalamus (Chemelli et al., 1999; Hara et al., 2001; Mignot et al., 2002; Nishino et al., 2000; Peyron et al., 2000; Thannickal et al., 2000; Willie et al., 2003). Our immunohistochemical studies revealed that orexin neurons appeared intact in all mice after acute brain injury. Thus, despite abnormal brain orexin dynamics, brain-injured mice in this report did not fulfill physiological, behavioral, or pathological criteria for narcolepsy. Consistent with this interpretation, other models of partial loss of orexin signaling, such as experimental genetic ablations (knockouts) of either orexin receptor-1 or −2, result in incomplete narcolepsy syndromes. Mice lacking the orexin receptor-2 in particular express an inability to maintain wakefulness with relatively mild abnormalities of REM sleep (Chemelli et al., 1999; Hara et al., 2001; Mignot et al., 2002; Nishino et al., 2000; Willie et al., 2003). We speculate that if the degree of abnormal orexin dynamics following brain injury were more severe, and the injury resulted in orexin neuronal cell death, we might observe features of secondary or post-traumatic narcolepsy, as has been reported in some patients after TBI (Castriotta and Lai, 2001; Lankford et al., 1994).
Effects of injury on orexin-immunoreactive cell counts
Unexpectedly, we found a small but significant increase in the number of orexin-immunoreactive cells in the lateral hypothalamus after injury. While the critical finding is that orexin cells were not selectively destroyed under the conditions of our experiment, the explanation for this increase is not clear. It is possible that physiological or metabolic stresses (such as mild cachexia) after injury increased orexin expression, as has been previously reported for fasting rodents (Sakurai et al., 1998; Willie et al., 2008). Alternatively, injury or transection of orexinergic axons could have resulted in an intracellular build-up of unreleased orexin in cell bodies. Whether the causes were physiological or mechanical, increased orexin peptide staining may have altered the signal-to-noise ratio for threshold-dependent counting, giving the appearance of increased cellular numbers in the TBI group.
Notably, a recent study revealed persistently increased orexin-A peptide immunostaining within axons in the peri-contusional penumbra at day 1 and day 7 after CCI in mice (Mihara et al., 2011). These results are consistent with our own observations. Mihara and associates also reported that increased orexin-1 receptor (OX1R)-like immunoreactivity in the post-CCI penumbra peaked at day 1, but subsequently decreased. In addition to the expected neuronal co-localization, this OX1R-like immunoreactivity co-localized with reactive microglia, but not astrocytes following injury. The significance of this is unclear, but in situ mRNA hybridization studies would be useful in order to determine whether OX1R is truly expressed by reactive microglia, or whether this immunoreactivity results from phagocytosis of neuronal debris following injury. These studies suggest that many components of the orexin signaling system may be affected by TBI.
Limitations and future investigations
The present study has several limitations to be addressed in future investigations. While CCI is an accepted model producing cortical contusion and peri-contusional injuries, it clearly does not reflect the entire scope of mechanisms of human TBI (Marklund and Hillered, 2011; Morales et al., 2005). The acute experimental time frame of our experiment prevents direct comparisons to the findings reported in humans with regard to loss of orexin neurons in post-mortem human brains in which time of death was later and more variable (Baumann et al., 2009). Clearly, future investigations will be required in order to determine the long-term effects of TBI on the orexin system. In some human subjects after TBI, CSF orexin levels were normalized by 6 months following injury, despite persistence of sleep-wake disturbances (Baumann et al., 2005). Our study did not explore chronic behavioral and neurochemical changes following injury, nor did it involve CSF collection. Likewise, future studies could utilize other mechanisms of injury (e.g., blast injury), explore more severe or lethal injuries, and study orexin dynamics and neuron counts following chronic injuries.
Another limitation of this study is that it does not address causality in the association we observed between deficient orexin and features of arousal. Reduced wakefulness and motor activity could be the direct result of local orexin deficiency, abnormal orexin dynamics, or alternations in other aspects of the orexin signaling system. Alternatively, reduced wakefulness, activity, and orexin levels may all be due to unmeasured neurochemical or neurophysiological abnormalities. This limitation could be addressed by examining the effects of injury in the setting of genetic or pharmacological manipulations of orexin signaling. Probing the effects of exogenous orexin administration or orexin overexpression (Deadwyler et al., 2007; Mieda et al., 2004) upon measures of arousal following CCI would be of great interest. Finally, the question of whether human extracellular orexin dynamics are similarly affected by TBI could also be addressed in a parallel human microdialysis study. Such studies will be critical for investigating the orexin system as a potential therapeutic target for brain injury.
Therapeutic implications
Overall mortality following severe TBI has improved due to advances in supportive care. However, effective therapeutics are still lacking for post-traumatic hypersomnia and other sleep-wake disorders (Castriotta et al., 2009). Strategies that target both short-term and long-term cognitive impairments are urgently needed. Continuous monitoring of orexin release in the brain at frequent (i.e., hourly) time points could be used in future studies to examine whether orexin release predicts changes in neurological status and cognitive outcomes in animal models as well as human patients. Orexin-based therapeutics should be considered to treat excessive sleepiness and cognitive dysfunction following TBI. Orexin-A is able to enter the CNS when administered intravenously or transnasally, and may itself be utilized therapeutically, as it has been shown to promote cognitive performance in nonhuman primates (Deadwyler et al., 2007). Similarly, intraventricular administration of orexin-A reverses signs of narcolepsy-cataplexy in a rodent model (Mieda et al., 2004). Administration of orexin-based therapeutics in conjunction with microdialysis would provide a readily translatable method for altering clinical outcomes, including level of arousal, following TBI. Similar methods could be applied to other CNS conditions in which disordered sleep-wakefulness is a prominent clinical feature.
Acknowledgments
Funding for this study was provided by a grant from the Neurosurgery Research Education Fund and Porex Corporation to J.T.W., and the National Institutes of Health R01 NS065069 to D.L.B. We thank Dr. David M. Holtzman and members of the Holtzman Laboratory for allowing us to use their controlled light/dark mouse room and electrophysiological monitoring equipment.
Author Disclosure Statement
No competing financial interests exist.
References
- Baumann C.R. Bassetti C.L. Valko P.O. Haybaeck J. Keller M. Clark E. Stocker R. Tolnay M. Scammell T.E. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann. Neurol. 2009;66:555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C.R. Stocker R. Imhof H.G. Trentz O. Hersberger M. Mignot E. Bassetti C.L. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- Baumann C.R. Werth E. Stocker R. Ludwig S. Bassetti C.L. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- Brody D.L. Mac Donald C. Kessens C.C. Yuede C. Parsadanian M. Spinner M. Kim E. Schwetye K.E. Holtzman D.M. Bayly P.V. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody D.L. Magnoni S. Schwetye K.E. Spinner M.L. Esparza T.J. Stocchetti N. Zipfel G.J. Holtzman D.M. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castriotta R.J. Lai J.M. Sleep disorders associated with traumatic brain injury. Arch. Phys. Med. Rehabil. 2001;82:1403–1406. doi: 10.1053/apmr.2001.26081. [DOI] [PubMed] [Google Scholar]
- Castriotta R.J. Atanasov S. Wilde M.C. Masel B.E. Lai J.M. Kuna S.T. Treatment of sleep disorders after traumatic brain injury. J. Clin. Sleep Med. 2009;5:137–144. [PMC free article] [PubMed] [Google Scholar]
- Castriotta R.J. Wilde M.C. Lai J.M. Atanasov S. Masel B.E. Kuna S.T. Prevalence and consequences of sleep disorders in traumatic brain injury. J. Clin. Sleep Med. 2007;3:349–356. [PMC free article] [PubMed] [Google Scholar]
- Chemelli R.M. Willie J.T. Sinton C.M. Elmquist J.K. Scammell T. Lee C. Richardson J.A. Williams S.C. Xiong Y. Kisanuki Y. Fitch T.E. Nakazato M. Hammer R.E. Saper C.B. Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cirrito J.R. May P.C. O'Dell M.A. Taylor J.W. Parsadanian M. Cramer J.W. Audia J.E. Nissen J.S. Bales K.R. Paul S.M. DeMattos R.B. Holtzman D.M. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M.R. Hypothalamic lesions following closed head injury. Brain. 1971;94:165–172. doi: 10.1093/brain/94.1.165. [DOI] [PubMed] [Google Scholar]
- Deadwyler S.A. Porrino L. Siegel J.M. Hampson R.E. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J. Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenzl T. Flachskamm C. Rossbauer M. Deussing J.M. Kimura M. Circadian rhythms of basal orexin levels in the hypothalamus are not influenced by an impaired corticotropin-releasing hormone receptor type 1 system. Behav. Brain Res. 2009;203:143–145. doi: 10.1016/j.bbr.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Franklin K.B. Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2004. [Google Scholar]
- Hara J. Beuckmann C.T. Nambu T. Willie J.T. Chemelli R.M. Sinton C.M. Sugiyama F. Yagami K. Goto K. Yanagisawa M. Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Kempf J. Werth E. Kaiser P.R. Bassetti C.L. Baumann C.R. Sleep-wake disturbances 3 years after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2010;81:1402–1405. doi: 10.1136/jnnp.2009.201913. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko L.I. Mileykovskiy B.Y. Maidment N. Lam H.A. Wu M.F. John J. Peever J. Siegel J.M. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford D.A. Wellman J.J. O'Hara C. Posttraumatic narcolepsy in mild to moderate closed head injury. Sleep. 1994;17:S25–S28. doi: 10.1093/sleep/17.suppl_8.s25. [DOI] [PubMed] [Google Scholar]
- Mac Donald C.L. Dikranian K. Bayly P. Holtzman D. Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N. Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M. Willie J.T. Hara J. Sinton C.M. Sakurai T. Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc. Natl. Acad. Sci. USA. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E. Lammers G.J. Ripley B. Okun M. Nevsimalova S. Overeem S. Vankova J. Black J. Harsh J. Bassetti C. Schrader H. Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- Mihara Y. Dohi K. Yofu S. Nakamachi T. Ohtaki H. Shioda S. Aruga T. Expression and localization of the orexin-1 receptor (OX1R) after traumatic brain injury in mice. J. Molec. Neurosci. 2011;43:162–168. doi: 10.1007/s12031-010-9438-6. [DOI] [PubMed] [Google Scholar]
- Morales D.M. Marklund N. Lebold D. Thompson H.J. Pitkanen A. Maxwell W.L. Longhi L. Laurer H. Maegele M. Neugebauer E. Graham D.I. Stocchetti N. McIntosh T.K. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Nishino S. Ripley B. Overeem S. Lammers G.J. Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Okie S. Traumatic brain injury in the war zone. N. Engl. J. Med. 2005;352:2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- Peyron C. Faraco J. Rogers W. Ripley B. Overeem S. Charnay Y. Nevsimalova S. Aldrich M. Reynolds D. Albin R. Li R. Hungs M. Pedrazzoli M. Padigaru M. Kucherlapati M. Fan J. Maki R. Lammers G.J. Bouras C. Kucherlapati R. Nishino S. Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C. Tighe D.K. van den Pol A.N. de Lecea L. Heller H.C. Sutcliffe J.G. Kilduff T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger J.J. Dunn S.L. Motzel S.L. Johnson C. Koblan K.S. Sub-chronic administration of zolpidem affects modifications to rat sleep architecture. Brain Res. 2004;1010:45–54. doi: 10.1016/j.brainres.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Ronne-Engstrom E. Cesarini K.G. Enblad P. Hesselager G. Marklund N. Nilsson P. Salci K. Persson L. Hillered L. Intracerebral microdialysis in neurointensive care: the use of urea as an endogenous reference compound. J. Neurosurg. 2001;94:397–402. doi: 10.3171/jns.2001.94.3.0397. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Amemiya A. Ishii M. Matsuzaki I. Chemelli R.M. Tanaka H. Williams S.C. Richarson J.A. Kozlowski G.P. Wilson S. Arch J.R. Buckingham R.E. Haynes A.C. Carr S.A. Annan R.S. McNulty D.E. Liu W.S. Terrett J.A. Elshourbagy N.A. Bergsma D.J. Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1–696. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol. Sci. 2011;32:451–462. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Schwetye K.E. Cirrito J.R. Esparza T.J. Mac Donald C.L. Holtzman D.M. Brody D.L. Traumatic brain injury reduces soluble extracellular amyloid-beta in mice: a methodologically novel combined microdialysis-controlled cortical impact study. Neurobiol. Dis. 2010;40:555–564. doi: 10.1016/j.nbd.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal T.C. Moore R.Y. Nienhuis R. Ramanathan L. Gulyani S. Aldrich M. Cornford M. Siegel J.M. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Watson N.F. Dikmen S. Machamer J. Doherty M. Temkin N. Hypersomnia following traumatic brain injury. J. Clin. Sleep Med. 2007;3:363–368. [PMC free article] [PubMed] [Google Scholar]
- Willie J.T. Chemelli R.M. Sinton C.M. Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu. Rev. Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Willie J.T. Chemelli R.M. Sinton C.M. Tokita S. Williams S.C. Kisanuki Y.Y. Marcus J.N. Lee C. Elmquist J.K. Kohlmeier K.A. Leonard C.S. Richardson J.A. Hammer R.E. Yanagisawa M. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Willie J.T. Sinton C.M. Maratos-Flier E. Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156:819–829. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K. McCormack S. Espana R.A. Crocker A. Scammell T.E. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y. Fujiki N. Nakajima T. Ripley B. Matsumura H. Yoneda H. Mignot E. Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur. J. Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]