Abstract
Group B streptococcus is the most common cause of bacterial infection in the newborn. Infection in many cases causes persistent pulmonary hypertension which impairs gas exchange in the lung. We purified the bacterial components causing pulmonary hypertension and showed that they are the phospholipids cardiolipin and phosphatidylglycerol. Synthetic cardiolipin or phosphatidylglycerol also induced pulmonary hypertension in lambs. The recognition that bacterial phospholipids may cause pulmonary hypertension in newborns with Group B streptococcal infection opens new avenues for therapeutic intervention and generates hypotheses concerning the etiology of respiratory distress in the newborn and the possible effect of antibiotic therapy.
Introduction
Group B Streptococcus (GBS) is the most frequent cause of sepsis and meningitis in the human newborn.1 Despite prompt treatment with antibiotics, neonates with early onset GBS infection are often quite ill and the fatality rate is 5%.2 Respiratory distress, a prominent sign in these babies, may be caused by persistent pulmonary hypertension of the newborn (PPHN) which is induced by the GBS infection.3 The pulmonary hypertension reflects an increase in pulmonary vascular resistance, which impairs exchange of oxygen and carbon dioxide. Infusion of live or heat-killed GBS into sheep promptly induces pulmonary hypertension, with little or no effect on systemic pressure.4 This response has been reproduced by many investigators, who also established that the pulmonary hypertension is not a consequence of simple embolization because infusion of latex beads of the same size as GBS caused no change in pulmonary physiology.5 The hypertension is mediated by an increase in thromboxane A2, so that treatment with cyclooxygenase inhibitors such as indomethacin or with thromboxane synthesis inhibitors prevents or reverses the increase in pulmonary arterial pressure.5,6 However, the component(s) of GBS which activate thromboxane synthesis had not been clearly defined. Guided by bioassays performed in neonatal lambs7, we undertook the purification and identification of these components, employing standard biochemical techniques. Our methodology and results have been reported.8 We review and discuss them in this paper.
Results
Purification and Identification of the Active Components
Although our initial purification scheme was lengthy with many steps, once the chemical nature of the bioactive material was elucidated we were able to develop a much simpler scheme (Fig. 1). Biologically active material was extracted from GBS by a mixture of chloroform and methanol, and two components were purified by anion exchange. The mass spectrum of the two active fractions established that they were phosphatidylglycerol and cardiolipin (Fig. 2A,B). Each caused pulmonary hypertension in the lamb assay.
Fig. 1.
Scheme for purification of the pulmonary hypertensive agents from GBS. Methodological details have been published.8
Fig. 2.
Mass spectra of the bioactive material purified from GBS. (A) Earlier eluting fraction from the reverse phase column. The peaks from 700–800 are singly-charged phosphatidylglycerol, free of cardiolipin. The structure is phosphatidylglycerol with fatty acids represented as FA. (B) Later eluting fraction from the reverse phase column. The peaks are singly and doubly-charged cardiolipin, free of phosphatidylglycerol. The structure is cardiolipin with fatty acids represented as FA. Both fractions were bioactive in the lamb assay.
The structure of the most prominent peak in the cardiolipin mass spectrum was established to be dipalmitoyl,dioleoyl cardiolipin by fragmentation in the mass spectrometer. The mass expected from the structure of this cardiolipin is 1,405 Da, equal to the experimentally measured value. The largest peak in the phosphatidylglycerol spectrum is palmitoyl, oleoyl phosphatidylglycerol, with the calculated and observed masses being 748.5 Da. The other peaks in the mass spectra are from phospholipids with fatty acids of different chain lengths. The observed distribution of fatty acids matches that expected for GBS.9
Synthetic Phospholipids Are Biologically Active
To assure that these phospholipids were the bioactive material rather than some other, minor component of the preparation, we tested pure, synthetic compounds using the dioleoyl species. Both phosphatidylglycerol and cardiolipin triggered an increase in pulmonary arterial pressure within minutes of infusion into the femoral vein of lambs or neonatal piglets, but diacylglyerol and phosphatiditic acid were not active (Table 1).
Table 1.
Effect of dioleolyl glycerol compounds on pulmonary arterial pressure.
| Compound | Change in pressure, mm |
|---|---|
| Dioleoyl glycerol | 0 |
| Dioleoyl phosphatidic acid | 0 |
| Dioleoyl phosphatidylglycerol | +14 |
| Dioleoyl cardiolipin | +21 |
Values are the average from two 5 kg lambs given 35 nmol/kg of each compound.
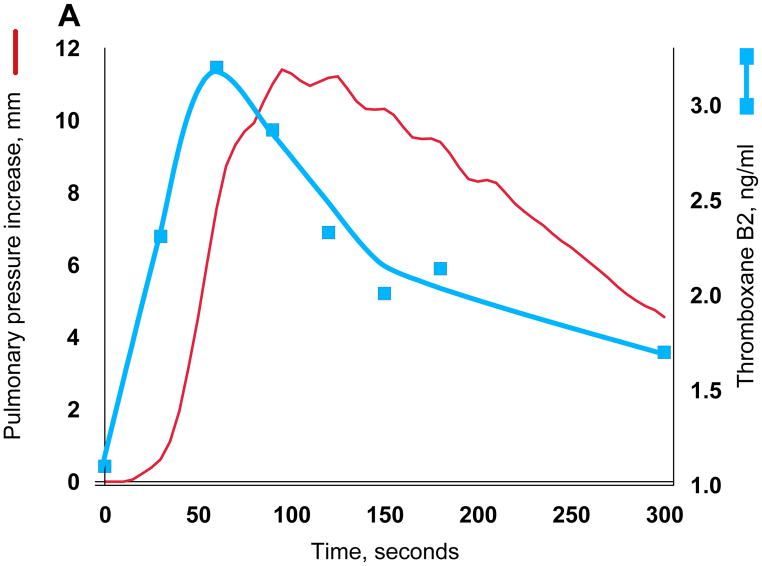
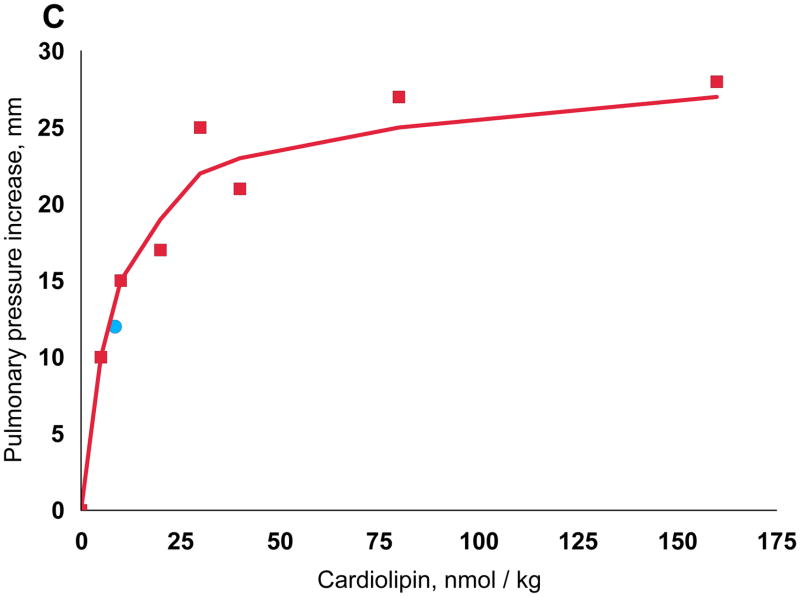
While both phosphatidylglycerol and cardiolipin were bioactive, the increase in pulmonary arterial blood pressure was more reproducible with cardiolipin, perhaps because of the ease of rehydrating the dried preparations to form liposomes. We therefore used cardiolipin for subsequent studies. As mentioned, GBS infusion is known to induce pulmonary hypertension by stimulating the production of thromboxane A2, and we found that the increase in pulmonary arterial pressure closely tracks that of the plasma thromboxane concentrations (Fig. 3A). We established that synthetic cardiolipin has the same activity (Fig. 3B). A dose response curve shows half-maximal response at ~10 nmol/kg (Fig. 3C).
Fig. 3.
Thromboxane production and pulmonary hypertension induced by GBS and cardiolipin. Pulmonary arterial pressure is plotted in red using the left axis and thromboxane on the right. (A) GBS, 3 × 109 heat-killed cells (B) Cardiolipin, 75 nmol. The same quantitative responses in pulmonary arterial pressure and thromboxane were observed when we infused 75 nmol cardiolipin pre-incubated with 75 nmol purified human apolipoprotein H.10 (C) Cardiolipin dose response. Doses were injected in a randomized order into a 5 kg lamb, and the line was fit by linear regression of a double reciprocal plot. The blue circle was the response in a different lamb to cardiolipin purified from GBS. Cardiolipin liposomes prepared by simple rehydration are multilamellar, but because a liposome size effect has been observed by some investigators,35 we also prepared unilamellar liposomes with diameters ranging from 0.1 to 5.0 microns; all were active.
Effect of apolipoprotein H
Apolipoprotein H is the major phospholipid binding protein in plasma, with particular affinity for cardiolipin.10 The concentration of apolipoprotein H in adult serum is 170 mg/l11, and we found similar concentrations in the neonatal lamb and piglet as well as fetal calf serum. Infusion of apolipoprotein H should bind circulating bacterial phosphatidylglycerol or cardiolipin, but the rate of binding may not be fast enough to prevent unliganded phospholipid from reaching the pulmonary circulation.12 Binding could inhibit the hypertensive effect, or it could promote the hypertensive effect since apolipoprotein H binds to endothelial cells.13 We therefore tested the effect of cardiolipin which was mixed with a equimolar concentration of purified apolipoprotein H and allowed to stand overnight before testing, assuring that the binding reaction had gone to completion.12 The peak hypertensive effect and increase in plasma thromboxane were equivalent to that of the cardiolipin infused without apolipoprotein H (as in Fig. 3). Thus, circulating apoplipoprotein H appears sufficient to rapidly bind all of the infused cardiolipin, and the bound form is bioactive. We conclude that apolipoprotein H is unlikely to be useful in treating GBS-induced pulmonary hypertension.
Inhibition of Cyclooxygenase Prevents Pulmonary Hypertension
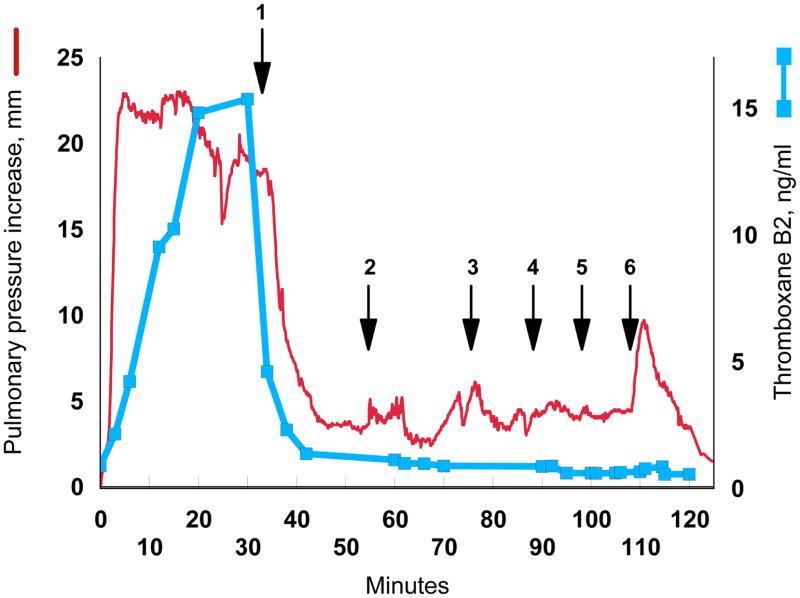
Cyclooxygenase inhibitors such as indomethacin consistently block thromboxane synthesis and the pulmonary hypertensive effect of infused GBS in animals.6,14–17 If the bacterial phospholipid is the bioactive molecule of GBS, then indomethacin should also prevent the pulmonary hypertensive response to cardiolipin. Administration of a single dose of 0.2 mg/kg indomethacin prevented the increase in pulmonary arterial blood pressure caused by either GBS or cardiolipin (Fig. 4). We then determined whether indomethacin could reverse persistent pulmonary hypertension caused by cardiolipin. A continuous infusion of cardiolipin clamped the pulmonary arterial blood pressure at an increased level (Fig. 5). Treatment with indomethacin lowered the pressure, bringing it close to the control basal level even though the cardiolipin infusion was continued. Increasing the infusion rate or even administering a bolus of more than 100 nmol/kg cardiolipin did not increase the pulmonary arterial pressure.
Fig. 4.
Cyclooxygenase inhibition blocks the effect of bolus injection of GBS (3 × 109 heat-killed cells) or of cardiolipin (100 nmol). After demonstrating responsiveness to GBS and cardiolipin, 0.2 mg/kg indomethacin was administered over 10 min. The lamb was then challenged again with GBS followed by cardiolipin. Pulmonary artery pressure at time 0 was 24 mm.
Fig. 5.
Cyclooxygenase inhibition blocks the effect of a continuous infusion of cardiolipin. The red line plots the increase in mean pulmonary artery pressure while the blue squares and line show the plasma thromboxane B2 concentration. Beginning at time 0, 13 nmol/kg/min cardiolipin was infused through the femoral vein. A 5 mg/kg dose of indomethacin was then administered over 10 min (arrow 1). Next, the cardiolipin was increased to 27 nmol/kg/min, and then to 40 nmol/kg/min (arrows 2,3). Finally boluses of 67 nmol/kg, 133 nmol/kg, and 667 nmol/kg were infused (arrows 4,5,6). Pulmonary artery pressure at time 0 was 22 mm.
Discussion
The key finding in our study is that bacterial phospholipids cause pulmonary hypertension. Phosphatidylglycerol and cardiolipin are the dominant phospholipids of GBS18,19, located mainly in the cell wall, although their topography has not been established. Since infusion of intact, heat-killed GBS cells induces the pulmonary hypertensive effect, some of the molecules are presumably surface exposed and available for binding to pulmonary endothelial cell receptors.
In bacteria, both phosphatidylglycerol and cardiolipin synthesis require phosphatidylglycerol synthase. Mutants lacking this enzyme will be useful in identifying any other GBS components capable of inducing pulmonary hypertension.20 One such known molecule is CM101, an extracellular polysaccharide produced by GBS which is capable of inducing pulmonary hypertension. However, it was present only in the culture media and not in the GBS cells.21
Indomethacin is available for intravenous administration in the newborn to induce closure of a patent ductus arteriosus. It has not yet been studied as a treatment for pulmonary hypertension. Given that it prevents the acute pulmonary hypertensive effect of cardiolipin, one should consider further studying its efficacy in animal models. Attention to the dosage regimen may be useful because Nögel and colleagues reported that a single dose of indomethacin administered 2 hr after infusion of GBS in piglets did not prevent death of the animals at 12–16 h, although it did lower the pulmonary artery pressure in the 2 h following administration.22 Identification of the receptors and pathways which are activated by phosphatidylglycerol and cardiolipin may identify specific targets for therapeutic interventions.
Many bacteria, especially the Streptococci, normally secrete some of the phospholipids which they synthesize. In log phase this is 15% of the total synthesized, increasing to 30% during stationary phase.23 Thus, patients infected with GBS24 may receive an endogenous infusion of the phospholipids capable of inducing pulmonary hypertension.
Because GBS is sensitive to the penicillin class of antibiotics, neonates suspected of having sepsis usually receive such antibiotics. However exposure of certain Streptococci to cell-wall synthesis inhibitors such as penicillin induces a prompt secretion of phospholipids, without lysis of the cells (Fig. 6).23,25–27 Then over the next several hours the antibiotic actually induces an increase in lipid synthesis, all of which is excreted.28 This might also occur with other Gram positive and Gram negative infections in patients of any age because excretion of phospholipids, including cardiolipin, is a common response of bacteria to certain antibiotics.23,24,26,27,29–33 Lipid excretion by Streptococci species may increase 15 fold with over half of the material excreted being phosphatidylglycerol and cardiolipin.27 Thus, antibiotic treatment effects an increase in production and excretion of the molecules which we showed cause pulmonary hypertension. Whether this actually occurs in a laboring patient given peripartum antibiotics or in an infected neonate treated with antibiotics is at present a matter of speculation. Similarly, given that the phospholipids of GBS can cause pulmonary hypertension, one might wonder whether milder forms of respiratory distress in the newborn are mediated by this mechanism. These issues are being investigated in ongoing studies being conducted by investigators in the Section on Bacterial Disease, Pathogenesis & Immunity of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Protocol 08-CH-N069).34
Fig. 6.
Excretion of lipids by S. mutans in response to penicillin. Penicillin (0.2 μg/ml) was added to exponentially growing bacteria at time 0 and total lipid content was measured with radioactively labeled glycerol. The intracellular content of lipid was unchanged in the penicillin-exposed culture compared to a control culture, but as shown in the graph, extracellular lipid content increased. Lipid content was normalized to the value at the time of addition of penicillin. The data are from Fig. 2 of Brissette and colleagues.27
Acknowledgments
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute and by grants to JC from the National Naval Medical Center and The Chief, Navy Bureau of Medicine and Surgery, Washington, District of Columbia, Clinical Investigation Program (#B00-022). The views expressed are those of the author (JC) and do not reflect the official policy or position of the USUHS, the Department of Defense, or the United States Government.
Footnotes
Dedicated to Philip Sunshine, MD, physician and teacher extraordinaire.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Schuchat A. Group B streptococcus. Lancet. 1999;353(9146):51–56. doi: 10.1016/S0140-6736(98)07128-1. [DOI] [PubMed] [Google Scholar]
- 2.Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342(1):15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 3.Rojas J, Stahlman M. The effects of group B streptococcus and other organisms on the pulmonary vasculature. Clin Perinatol. 1984;11(3):591–599. [PubMed] [Google Scholar]
- 4.Rojas J, Green RS, Hellerqvist CG, et al. Studies on group B beta-hemolytic Streptococcus. II. Effects on pulmonary hemodynamics and vascular permeability in unanesthetized sheep. Pediatr Res. 1981;15(6):899–904. doi: 10.1203/00006450-198106000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gibson RL, Redding GJ, Truog WE, Henderson WR, Rubens CE. Isogenic group B streptococci devoid of capsular polysaccharide or beta-hemolysin: pulmonary hemodynamic and gas exchange effects during bacteremia in piglets. Pediatr Res. 1989;26(3):241–245. doi: 10.1203/00006450-198909000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Philips JB, III, Lyrene RK, Godoy G, et al. Hemodynamic responses of chronically instrumented piglets to bolus injections of group B streptococci. Pediatr Res. 1988;23(1):81–85. doi: 10.1203/00006450-198801000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter D, Larkin H, Chang A, et al. Superoxide dismutase and catalase do not affect the pulmonary hypertensive response to group B streptococcus in the lamb. Pediatr Res. 2001;49(2):181–188. doi: 10.1203/00006450-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Curtis J, Kim G, Wehr NB, Levine RL. Group B streptococcal phospholipid causes pulmonary hypertension. Proc Natl Acad Sci USA. 2003;100(9):5087–5090. doi: 10.1073/pnas.0931493100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer W. The polar lipids of group B Streptococci. II. Composition and positional distribution of fatty acids. Biochim Biophys Acta. 1977;487(1):89–104. doi: 10.1016/0005-2760(77)90046-7. [DOI] [PubMed] [Google Scholar]
- 10.Steinkasserer A, Barlow PN, Willis AC, et al. Activity, disulphide mapping and structural modelling of the fifth domain of human beta 2-glycoprotein I. FEBS Lett. 1992;313(2):193–197. doi: 10.1016/0014-5793(92)81442-o. [DOI] [PubMed] [Google Scholar]
- 11.Hoeg JM, Segal P, Gregg RE, et al. Characterization of plasma lipids and lipoproteins in patients with beta 2-glycoprotein I (apolipoprotein H) deficiency. Atherosclerosis. 1985;55(1):25–34. doi: 10.1016/0021-9150(85)90163-7. [DOI] [PubMed] [Google Scholar]
- 12.Schousboe I. Binding of beta 2-glycoprotein I to platelets: effect of adenylate cyclase activity. Thromb Res. 1980;19(1–2):225–237. doi: 10.1016/0049-3848(80)90421-1. [DOI] [PubMed] [Google Scholar]
- 13.Ma K, Simantov R, Zhang JC, et al. High affinity binding of beta 2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem. 2000;275(20):15541–15548. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 14.Rojas J, Larsson LE, Ogletree ML, Brigham KL, Stahlman MT. Effects of cyclooxygenase inhibition on the response to group B streptococcal toxin in sheep. Pediatr Res. 1983;17(2):107–110. doi: 10.1203/00006450-198302000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gibson RL, Truog WE, Henderson WR, Jr, Redding GJ. Group B streptococcal sepsis in piglets: effect of combined pentoxifylline and indomethacin pretreatment. Pediatr Res. 1992;31(3):222–227. doi: 10.1203/00006450-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hemming VG, O'Brien WF, Fischer GW, Golden SM, Noble SF. Studies of short-term pulmonary and peripheral vascular responses induced in oophorectomized sheep by the infusion of a group B streptococcal extract. Pediatr Res. 1984;18(3):266–269. doi: 10.1203/00006450-198403000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Huddleston KW, Lyrene RK, Dew A, Gray BM, Philips JB., III Influence of prostaglandin D2 on hemodynamic effects of group B streptococcus in neonatal lambs. Dev Pharmacol Ther. 1986;9(4):260–265. doi: 10.1159/000457101. [DOI] [PubMed] [Google Scholar]
- 18.Fischer W. The polar lipids of group B Streptococci. I. Glucosylated diphosphatidylglycerol, a novel glycopholipid. Biochim Biophys Acta. 1977;487(1):74–88. doi: 10.1016/0005-2760(77)90045-5. [DOI] [PubMed] [Google Scholar]
- 19.Filgueiras MH, Op den Kamp JA. Cardiolipin, a major phospholipid of Gram-positive bacteria that is not readily extractable. Biochim Biophys Acta. 1980;620(2):332–337. doi: 10.1016/0005-2760(80)90215-5. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi S, Shibuya I, Matsumoto K. Viability of an Escherichia coli pgsA null mutant lacking detectable phosphatidylglycerol and cardiolipin. J Bacteriol. 2000;182(2):371–376. doi: 10.1128/jb.182.2.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellerqvist CG, Rojas J, Green RS, et al. Studies on group B beta-hemolytic Streptococcus. I. Isolation and partial characterization of an extracellular toxin. Pediatr Res. 1981;15(6):892–898. doi: 10.1203/00006450-198106000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Nogel SC, Chada M, Schmidt AM, et al. Parecoxib does not suppress thromboxane synthesis in newborn piglets with group B streptococcal sepsis. Prostaglandins Other Lipid Mediat. 2009;90(1–2):7–12. doi: 10.1016/j.prostaglandins.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Cabacungan E, Pieringer RA. Excretion of extracellular lipids by Streptococcus mutans BHT and FA-1. Infect Immun. 1980;27(2):556–562. doi: 10.1128/iai.27.2.556-562.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowfoot PD, Esfahani M, Wakil SJ. Relation between protein synthesis and phospholipid synthesis and turnover in Escherichia coli. J Bacteriol. 1972;112(3):1408–1415. doi: 10.1128/jb.112.3.1408-1415.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brissette JL, Pieringer RA. The effect of penicillin on fatty acid synthesis and excretion in Streptococcus mutans BHT. Lipids. 1985;20(3):173–179. doi: 10.1007/BF02534250. [DOI] [PubMed] [Google Scholar]
- 26.Horne D, Hakenbeck R, Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brissette JL, Shockman GD, Pieringer RA. Effects of penicillin on synthesis and excretion of lipid and lipoteichoic acid from Streptococcus mutans BHT. J Bacteriol. 1982;151(2):838–844. doi: 10.1128/jb.151.2.838-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brissette JL, Pieringer RA. The effect of penicillin on fatty acid synthesis and excretion in Streptococcus mutans BHT. Lipids. 1985;20(3):173–179. doi: 10.1007/BF02534250. [DOI] [PubMed] [Google Scholar]
- 29.Nakao Y, Kanamaru T, Kikuchi M, Yamatoda S. Action of Penicillin on Membrane-Permeability Barrier to L-Glutamic Acid .1. Extracellular Accumulation of Phospholipids, Udp-N-Acetylhexosamine Derivatives and L-Glutamic Acid by Penicillin-Treated Corynebacterium-Alkanolyticum. Agricultural and Biological Chemistry. 1973;37(10):2399–2404. [Google Scholar]
- 30.Kikuchi M, Kanamaru T, Nakao Y. Action of Penicillin on Membrane-Permeability Barrier to L-Glutamic Acid .2. Relation Between Extracellular Accumulation of L-Glutamic Acid and Excretion of Phospholipids by Penicillin-Treated Corynebacterium-Alkanolyticum. Agricultural and Biological Chemistry. 1973;37(10):2405–2408. [Google Scholar]
- 31.Molenkamp GC, Veerkamp JH. Effects of Antibiotics on Metabolism of Peptidoglycan, Protein, and Lipids in Bifidobacterium-Bifidum Subsp Pennsylvanicus. Antimicrobial Agents and Chemotherapy. 1976;10(5):786–794. doi: 10.1128/aac.10.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veerkamp JH. Biochemical Changes in Bifidobacterium-Bifidum Var Pennsylvanicus After Cell-Wall Inhibition .9. Metabolism and Release of Cellular Lipids in Presence of Antibiotics. Biochimica et Biophysica Acta. 1976;450(3):277–287. doi: 10.1016/0005-2760(76)90001-1. [DOI] [PubMed] [Google Scholar]
- 33.Horne D, Tomasz A. Tolerant Response of Streptococcus-Sanguis to Beta-Lactams and Other Cell-Wall Inhibitors. Antimicrobial Agents and Chemotherapy. 1977;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin FY, Troendle JF. Hypothesis: Neonatal respiratory distress may be related to asymptomatic colonization with group B streptococci. Pediatr Infect Dis J. 2006;25(10):884–888. doi: 10.1097/01.inf.0000239322.58890.94. [DOI] [PubMed] [Google Scholar]
- 35.Szebeni J, Baranyi L, Savay S, et al. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am J Physiol Heart Circ Physiol. 2000;279(3):H1319–H1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]