Abstract
Leukocyte telomere length (LTL) is linked to cardiovascular disease (CVD); however, it is unclear if LTL has an etiologic role in CVD. To gain insight into the LTL and CVD relationship, a cohort study of CVD mortality and single nucleotide polymorphisms (SNPs) in OBFC1 and TERC, genes related to LTL, was conducted among 3271 Caucasian participants ages ≥65 years enrolled 1989–1990 in the Cardiovascular Health Study. Leukocyte DNA was genotyped for SNPs in OBFC1 (rs4387287 and rs9419958) and TERC (rs3772190) that were previously associated with LTL through genome-wide association studies. Cox regression was used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). The OBFC1 SNPs were in linkage disequilibrium (r2=0.99), and both SNPs were similarly associated with CVD mortality in women. For women, there was a decreased risk of CVD death associated with the minor allele (rs4387287), HR=0.7; 95% CI: 0.5–0.9 (CC vs. AC) and HR=0.5; 95% CI: 0.20–1.4 (CC vs. AA) (p-trend <0.01). For men there was no association, HR=1.0; 95% CI: 0.7–1.3 (CC vs. AC) and HR=1.7; 95% CI: 0.8–3.6 (CC vs. AA) (p-trend=0.64). These findings support the hypothesis that telomere biology and associated genes may play a role in CVD-related death, particularly among women.
1. Introduction
Telomeres are important to the structural integrity of chromosomes and help to maintain genomic stability (Chan and Blackburn, 2002). In replicating somatic cells without sufficient telomerase activity, telomeres undergo progressive shortening. After a finite number of cellular divisions, when telomere length becomes critically short, a signal is relayed to the replicative machinery to exit the cell cycle, a phenomenon referred to as replicative senescence (Harley et al., 1990).
At birth, telomere length is similar across tissue types; however, with increasing age a difference in telomere length is established between distinct tissue types, with highly proliferative tissues displaying longer telomeres than slowly replicating tissues (Gardner et al., 2007). Thus, it is not surprising that leukocytes, which represent the highly proliferative hematopoietic system, display relatively high rates of telomere shortening with advancing age (Sidorov et al., 2009). Average leukocyte telomere length (LTL) varies among individuals, with LTL tending to decrease with increasing age (Lindsey et al., 1991).
Several studies have linked a short LTL to increased risks of cardiovascular disease (CVD) (Aviv, 2002; Edo and Andres, 2005; Fitzpatrick et al., 2007; Fuster and Andres, 2006) and other age-related diseases (Jeanclos et al., 1998; Valdes et al., 2010; Yaffe et al., 2009). A short LTL has also been associated with risk factors for CVD, such as cigarette smoking, obesity, and inflammation (McGrath et al., 2007; Valdes et al., 2005). Moreover, a short LTL has been associated with a 1.6 to 2-fold increase in overall mortality and a 1.8 to 3-fold increase in mortality due to CVD (Cawthon et al., 2003; Fitzpatrick et al., 2011). However, it is unclear if a short LTL, which reflects telomere length in hematopoietic stem cells (Sidorov et al., 2009), is a determinant in CVD, or if CVD and LTL are merely influenced by the same risk factors.
LTL is a complex genetic trait (Aviv, 2011). Gene loci associated with LTL have been identified through genome wide association studies (GWAS), and these associations have been replicated in independent study populations. These include single nucleotide polymorphisms (SNPs) in oligonucleotide/oligosaccharide-binding folds containing one gene (OBFC1) (rs4387287 and rs9419958) and a locus of the telomerase RNA component gene (TERC) (rs3772190) (Levy et al., 2010). OBFC1 and TERC are both genes recognized for their importance in telomere maintenance (Blasco et al., 1997; Chiang et al., 2004; Palm and de Lange, 2008). For OBFC1 SNPs, rs4387287 and rs9419958, the minor allele of each SNP (AA for rs4387287 and TT for rs9419958) was associated with a longer LTL; for the TERC polymorphism, rs3772190, the minor allele (AA) was associated with a shorter LTL (Levy et al., 2010).
If hematopoietic telomere biology has an etiologic relationship with CVD-related mortality, then genotypes associated with a shorter LTL would likely increase the risk of cardiovascular death. Thus, we conducted a Mendelian randomization study among a cohort of 3,271 Caucasian men and women in the Cardiovascular Health Study (CHS) to evaluate the risk of CVD mortality in relation to the OBFC1 and TERC SNPs linked to LTL through prior GWAS.
2. Materials and methods
2.1 Study population
The CHS, which began recruitment in 1989, is a prospective cohort study aimed at identifying risk factors for CVD (Fried et al., 1991). Participants, ages 65 years and older at baseline, were recruited from Medicare eligibility lists at four sites: Forsyth County, North Carolina, Washington County, Maryland, Sacramento County, California, and Pittsburgh, Pennsylvania (Tell et al., 1993). In total, 5,888 men and women were eligible and agreed to participate (response rate of approximately 60%). All study participants completed written informed consent for participation in CVD research, and approximately 85% of these consented to genetic research using DNA. Institutional review boards at each study site approved all procedures and protocols for use with human subjects.
2.2 Data collection
At the baseline clinic visit, investigators used standardized protocols to collect data on medical history, including prevalence of diabetes, hypertension, angina pectoris, previous myocardial infarction, and prior stroke (Psaty et al., 1995). Also, measures of subclinical CVD, such as resting blood pressure and ankle-arm index, were assessed, as were anthropometric measurements, health-related behaviors, physical function, and psychosocial factors (Fried et al., 1991). During the 10 years following their baseline exam, participants completed up to 10 annual clinic visits, at which changes in measurements were evaluated utilizing the same protocols as the baseline exam. Blood was collected at most clinic visits and used to measure circulating levels of specific biomarkers, including fasting levels of triglycerides, glucose, insulin, c-reactive protein (CRP) and interleukin-6; blood was also stored for future analyses (Cushman et al., 1995). Active surveillance for hospitalizations, mortality, and incident CVD began after the baseline exam and is ongoing (Ives et al., 1995).
2.3 Genotype data
We restricted the study population to Caucasian participants consenting to genetic research, resulting in 3,373 study participants eligible for genotype analyses. Genomic DNA was extracted from leukocytes and genotyped, using the Illumina 370CNV BeadChip system, as previously described by Levy, et al (Levy et al., 2010). Of the eligible participants, 3,271 (97%) were genotyped successfully. We selected the following candidate polymorphisms: rs4387287 (OBFC1), rs9419958 (OBFC1), and 3772190 (TERC), because all three were associated with LTL through GWAS and these associations replicated (Levy et al., 2010).
2.4 LTL data
LTL was measured in a subset of the study population (N=1,056) using Southern blot analysis of the mean length of terminal restriction fragments, as previously described (Benetos et al., 2001; Kimura et al., 2010).
2.5 Statistical Analyses
All analyses were conducted utilizing STATA version 10.1, StataCorp LP, College Station, TX. Descriptive statistics for the study population included reporting frequencies for baseline CVD risk factors and genotypes, stratified by sex. Chi-square P-value <0.05 was used to determine which factors were statistically significantly different between men and women. Linear regression models, adjusted for age and sex, were used to evaluate the association between LTL and genotype for each SNP. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the separate associations between genotype and baseline risk factors and biomarkers for CVD for each SNP. The following CVD-related biomarkers and risk factors were evaluated as dichotomous outcomes in separate models, adjusted for age and sex: hypertension, diabetes, ankle-arm index blood pressure, body mass index (kg/m2), triglycerides (mg/dL), glucose (mg/dL), insulin (μU/mL), CRP (mg/L), and interleukin-6 (pg/mL). Clinically relevant cutpoints were used for each measurement, aside from interleukin-6, for which no clinically relevant cutpoint was available. Participants were classified as hypertensive if they used antihypertensive medication or had systolic pressure ≥ 140 mmHg. Diabetes was assessed using the American Diabetes Association criteria (1997). The ankle-arm index blood pressure was used to detect peripheral arterial disease; a ratio of <0.9 was indicative of disease (Newman et al., 1993). Body mass index (BMI) was calculated as a function of weight and height (kg/m2); per National Institutes of Health guidelines, participants with BMI ≥30 kg/m2 were classified as obese. The cutpoint for triglycerides was ≥200 mg/dL (1993); for glucose it was >125 mg/dL (1997), insulin >15 μU/mL (Goren, 2005), CRP >3 mg/L (de Ferranti and Rifai, 2002), and the cutpoint for interleukin 6 was set at >3.7 pg/mL (above the 90th percentile in the study population). Each gene loci was modeled as a 3-level categorical variable, assuming a log-additive model, with the homozygous major allele as the referent group. The distribution of polymorphisms evaluated in this cohort did not deviate from Hardy-Weinberg equilibrium (P-value >0.05 for each SNP) (Rodriguez et al., 2009).
Cox proportional hazards regression, adjusting for baseline age and sex, was used to estimate hazard ratios (HRs) and 95% CIs for the association between genotype and overall mortality, as well as CVD-specific mortality. Regression models included age as the time axis and allowed for staggered entry at age at baseline clinic visit. For overall mortality, participants remaining alive were censored at the end of follow-up. For CVD-specific mortality, those with CVD at baseline and those with procedure-related CVD (n=390) were excluded; participants with non-CVD deaths were censored at the date of death, and those who remained alive throughout follow-up were censored at the end of follow-up. Separate models, with genotypes modeled as 3-level categorical variables, assuming a log-additive model, were used to assess each outcome.
To test for trend, the ordinal categorical variable for each genotype was entered into the regression model, and the Wald-test P-values reported. Due to sex and age differences in the biology of LTL,(Aviv, 2002) we also tested for interactions between each genotype and sex, and between genotype and age, using the cross-product terms in each model and the associated Wald-test P-values. Analyses stratified by sex were conducted for the longitudinal analyses of mortality events, due to interaction P-values <0.05 for some associations.
3. Results
Table 1 displays baseline characteristics of the study cohort, stratified by sex. Men comprised about 40% of the study population. Compared to women study participants, men tended to be older, were more likely to have diabetes, blood glucose >125 mg/dL, and interleukin-6 ≥3.7 pg/mL, and were less likely to have BMI ≥30 kg/m2 and CRP >3 mg/L (P < 0.05 for each factor). Also, men had a higher prevalence than women of the homozygous major allele genotypes for both OBFC1 SNPs (P < 0.05).
Table 1.
Baseline characteristics of Caucasian participants genotyped in the Cardiovascular Health Study, stratified by sex
| Total (N=3271) | Men (N=1280) | Women (N=1991) | P-valueb | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | ||
| Age | |||||||
| 65–74 | 2298 | (70) | 857 | (67) | 1432 | (72) | |
| 75–84 | 882 | (27) | 367 | (29) | 515 | (26) | |
| 85+ | 100 | (3) | 56 | (4) | 44 | (2) | <0.001 |
| Cardiovascular diseasea | 278 | (9) | 106 | (8) | 172 | (9) | 0.72 |
| Ankle arm index | |||||||
| ≥0.9 | 2954 | (92) | 1166 | (92) | 1788 | (92) | |
| <0.9 | 259 | (8) | 98 | (8) | 161 | (8) | 0.60 |
| Hypertension | 1216 | (37) | 458 | (36) | 758 | (38) | 0.18 |
| Diabetes | 275 | (8) | 140 | (11) | 135 | (7) | <0.001 |
| Body mass index (kg/m2) | |||||||
| <30 | 2706 | (83) | 1089 | (85) | 1617 | (81) | |
| ≥30 | 556 | (17) | 186 | (15) | 370 | (19) | <0.01 |
| Triglycerides (mg/dL) | |||||||
| <200 | 2793 | (86) | 1098 | (86) | 1695 | (85) | |
| ≥200 | 471 | (14) | 182 | (14) | 289 | (15) | 0.78 |
| Glucose (mg/dL) | |||||||
| ≤ 125 | 2898 | (89) | 1101 | (86) | 1797 | (91) | |
| >125 | 361 | (11) | 178 | (14) | 183 | (9) | <0.001 |
| Insulin (μU/mL) | |||||||
| ≤ 15 | 2954 | (92) | 873 | (69) | 1386 | (71) | |
| > 15 | 259 | (8) | 394 | (31) | 579 | (29) | 0.32 |
| C-reactive protein (mg/L) | |||||||
| ≤3 | 2054 | (63) | 860 | (68) | 1194 | (60) | |
| >3 | 1191 | (37) | 406 | (32) | 785 | (40) | <0.001 |
| Interluekin-6 (pg/mL) | |||||||
| <3.7 | 2708 | (90) | 1004 | (87) | 1704 | (92) | |
| ≥3.7 | 301 | (10) | 148 | (13) | 153 | (8) | <0.001 |
| rs4387287 (OBFC1) | |||||||
| CC | 2440 | (74) | 986 | (77) | 1454 | (73) | |
| AC | 774 | (24) | 270 | (21) | 504 | (25) | |
| AA | 57 | (2) | 24 | (2) | 33 | (2) | 0.02 |
| rs9419958 (OBFC1) | |||||||
| CC | 2440 | (75) | 986 | (77) | 1454 | (73) | |
| CT | 762 | (23) | 266 | (21) | 496 | (25) | |
| TT | 69 | (2) | 28 | (2) | 41 | (2) | 0.02 |
| rs3772190 (TERC) | |||||||
| GG | 1941 | (59) | 756 | (59) | 1185 | (60) | |
| AG | 1144 | (35) | 445 | (35) | 699 | (35) | |
| AA | 186 | (6) | 79 | (6) | 107 | (5) | 0.63 |
Includes: self-reported angina, myocardial infarction, bypass surgery, stroke, transient ischemic attack, or carotid endarterectomy
Chi-square p-value comparing men and women
In this study population, there was linkage disequilibrium between the two OBFC1 SNPs (r2=0.99); thus, point estimates displayed in tables 2 and 3 are similar for the rs4387287 and the rs9419958 genotypes. There was no evidence for correlation between OBFC1 SNPs and the TERC SNP (r2=0.01).
Table 2.
Regression analyses of the association between mean LTL and polymorphisms in OBFC1 and TERC: The Cardiovascular Health Study (N=1056)
| N | Mean LTL (SD) | Coefficienta (SE) | |
|---|---|---|---|
| rs4387287 (OBFC1) | |||
| CC | 796 | 6.30 (0.62) | Ref |
| AC | 243 | 6.42 (0.58) | 0.11 (0.04) |
| AA | 17 | 6.56 (0.61) | 0.31 (0.15) |
| P-trend | <0.01 | ||
| rs9419958 (OBFC1) | |||
| CC | 796 | 6.30 (0.62) | Ref |
| CT | 239 | 6.42 (0.58) | 0.11 (0.04) |
| TT | 21 | 6.58 (0.62) | 0.29 (0.13) |
| P-trend | <0.01 | ||
| rs3772190 (TERC) | |||
| GG | 623 | 6.34 (0.61) | Ref |
| AG | 375 | 6.34 (0.63) | 0.01 (0.04) |
| AA | 58 | 6.21 (0.59) | −0.14 (0.08) |
| P-trend | 0.32 | ||
Adjusted for age and sex
Table 3.
Odds ratios and 95% confidence intervals for the association between baseline cardiovascular disease risk factors and genotypes for OBFC1 and TERC.
| Outcome | rs4387287 (OBFC1) ORa (95% CI) | rs9419958 (OBFC1) ORa (95% CI) | rs3772190 (TERC) ORa (95% CI) | |||
|---|---|---|---|---|---|---|
| Hypertension | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| AC | 0.89 (0.75–1.05) | CT | 0.88 (0.74–1.05) | AG | 0.99 (0.85–1.15) | |
| AA | 0.95 (0.55–1.65) | TT | 1.01 (0.61–1.66) | AA | 1.07 (0.79–1.46) | |
| P-trend | 0.22 | 0.25 | 0.86 | |||
| Ankle arm index | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| ≥ 0.9 | AC | 0.84 (0.61–1.15) | CT | 0.82 (0.60–1.14) | AG | 0.69 (0.52–0.93) |
| AA | 1.29 (0.54–3.11) | TT | 1.35 (0.60–3.06) | AA | 1.23 (0.74–2.05) | |
| P-trend | 0.52 | 0.56 | 0.29 | |||
| Body mass index | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| ≥ 30 kg/m2 | AC | 0.88 (0.71–1.10) | CT | 0.90 (0.72–1.12) | AG | 1.03 (0.85–1.25) |
| AA | 0.80 (0.37–1.71) | TT | 0.70 (0.34–1.43) | AA | 1.21 (0.82–1.78) | |
| P-trend | 0.23 | 0.19 | 0.43 | |||
| Triglycerides | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| ≥ 200 mg/dL | AC | 0.99 (0.79–1.25) | CT | 0.98 (0.77–1.24) | AG | 0.99 (0.81–1.23) |
| AA | 1.28 (0.63–2.55) | TT | 1.36 (0.74–2.52) | AA | 1.28 (0.85–1.91) | |
| P-trend | 0.78 | 0.70 | 0.47 | |||
| Diabetes | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| AC | 0.93 (0.69–1.25) | CT | 0.88 (0.65–1.19) | AG | 1.03 (0.79–1.34) | |
| AA | 0.38 (0.09–1.57) | TT | 1.00 (0.43–2.33) | AA | 0.95 (0.55–1.65) | |
| P-trend | 0.28 | 0.50 | 0.97 | |||
| Glucose | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| >125 mg/dL | AC | 0.91 (0.70–1.19) | CT | 0.87 (0.67–1.14) | AG | 1.00 (0.79–1.26) |
| AA | 0.14 (0.02–0.98) | TT | 0.60 (0.24–1.50) | AA | 0.84 (0.51–1.39) | |
| P-trend | 0.08 | 0.17 | 0.65 | |||
| Insulin | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| > 15 μU/mL | AC | 0.82 (0.69–0.99) | CT | 0.82 (0.68–0.99) | AG | 1.00 (0.85–1.17) |
| AA | 0.59 (0.31–1.12) | TT | 0.65 (0.37–1.15) | AA | 1.00 (0.71–1.39) | |
| P-trend | 0.01 | 0.01 | 0.99 | |||
| C-reactive protein | CC | 1.00 (ref) | CC | 1.00 (ref) | GG | 1.00 (ref) |
| > 3 mg/L | AC | 0.89 (0.75–1.05) | CT | 0.87 (0.73–1.03) | AG | 1.12 (0.96–1.31) |
| AA | 0.54 (0.30–1.01) | TT | 0.78 (0.46–1.30) | AA | 1.01 (0.73–1.38) | |
| P-trend | 0.04 | 0.07 | 0.31 | |||
| Interluekin-6 | CC | 1.00 (ref) | CC | 1.00 (ref) | CC | 1.00 (ref) |
| ≥ 3.7 pg/mL | AC | 0.92 (0.69–1.23) | CT | 0.91 (0.68–1.21) | AC | 1.36 (1.06–1.75) |
| AA | 0.16 (0.02–1.16) | TT | 0.42 (0.13–1.34) | AA | 1.44 (0.88–2.34) | |
| P-trend | 0.14 | 0.19 | 0.01 |
Adjusted for age and sex
The association between LTL and each genotype is presented in table 2. In the subset of the study population with LTL measurements (N=1,056), the minor allele for both OBFC1 SNPs were associated with statistically significantly longer telomeres (P-trend <0.01). However, there was not a significant association between LTL and genotype for the TERC SNP in this population (P-trend=0.32). There was no evidence for effect modification by sex or age (Wald test P-values > 0.05) (data not shown).
Analyses of baseline factors and each genotype are summarized in table 3. Compared to the homozygous major allele, the minor allele in both OBFC1 polymorphisms was inversely associated with the odds of having fasting blood insulin >15 μU/mL (P-trend =0.01 for both) and CRP protein > 3 mg/L (P-trend=0.04 and 0.07). The minor allele for the TERC polymorphism was associated with an increased odds of having interluekin-6 levels ≥3.7 pg/mL (P-trend=0.01). There was no evidence for effect modification by sex or age (Wald test P-values > 0.05) (data not shown).
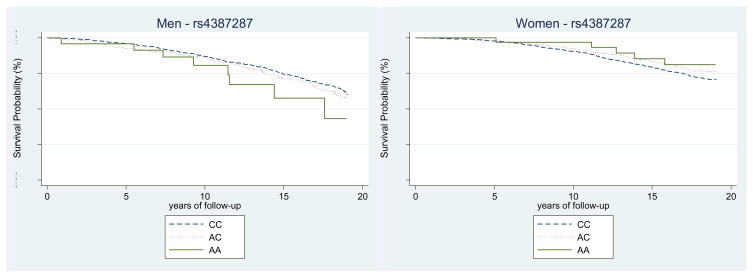
During a median follow-up of 14.8 years, there were 2,122 deaths overall; 628 were due to CVD, 138 were cerebrovascular disease deaths, and 457 were atherosclerotic disease deaths. In survival analyses (table 4), associations between OBFC1 genotypes and mortality appeared to be modified by sex (for overall mortality, P-interaction <0.01; for CVD-specific morality, P-interaction = 0.02). For women, there was a decreased risk of overall mortality (P-trend <0.01), and CVD-specific mortality (P-trend <0.01), associated with the minor allele in the OBFC1 polymorphism (rs4387287). In contrast, for men, there was no association between OBFC1 genotype and overall mortality (P-trend=0.11), nor CVD specific mortality (P-trend=0.64). Kaplan-Meier survival curves for cardiovascular mortality by genotype, and stratified by sex, for rs4387287 are displayed in figure 1. Similar associations were observed for the other OBFC1 SNP (rs9419958). There was no association between TERC genotype and mortality overall, nor CVD specific mortality for either men or women (table 4). Age did not appear to modify the association between genotype and mortality for either OBFC1 or TERC polymorphisms (Wald test P-values > 0.05 for each genotype) (data not shown).
Table 4.
Hazard Ratios and 95% confidence intervals for the association between OBFC1 and TERC genotypes and incident disease events, stratified by sex.
| # of Events | rs4387287 (OBFC1) | rs9419958 (OBFC1) | rs3772190 (TERC) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All HRa (95% CI) | Men HRb (95% CI) | Women HRb (95% CI) | All HRa (95% CI) | Men HRb (95% CI) | Women HRb (95 % CI) | All HRa (95 % CI) | Men HRb (95% CI) | Women HRb (95% CI) | |||||
| Overall | 2122 | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | GG | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Mortality | AC | 0.93 (0.83–1.02) | 1.05 (0.90–1.23) | 0.85 (0.74–0.98) | CT | 0.92 (0.83–1.02) | 1.05 (0.89–1.22) | 0.83 (0.73–0.96) | AG | 0.94 (0.86–1.03) | 0.90 (0.78–1.03) | 0.98 (0.87–1.10) | |
| AA | 1.01 (0.73–1.40) | 1.65 (1.07–2.56) | 0.65 (0.40–1.07) | TT | 1.09 (0.81–1.47) | 1.67 (1.10–2.49) | 0.77 (0.50–1.18) | AA | 0.96 (0.86–1.16) | 1.21 (0.92–1.58) | 0.78 (0.59–1.02) | ||
| P-trend | 0.22 | 0.11 | <0.01 | 0.29 | 0.10 | 0.01 | 0.24 | 0.81 | 0.17 | ||||
| P-interactionc | <0.01 | <0.01 | 0.48 | ||||||||||
| Fatal | 628 | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | GG | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Cardiovascular | AC | 0.80 (0.66–0.97) | 0.97 (0.72–1.29) | 0.70 (0.54–0.91) | CT | 0.79 (0.64–0.96) | 0.94 (0.70–1.27) | 0.69 (0.53–0.90) | AG | 0.90 (0.77–1.07) | 0.96 (0.74–1.23) | 0.87 (0.69–1.09) | |
| Diseased | AA | 0.96 (0.53–1.75) | 1.71 (0.80–3.63) | 0.54 (0.20–1.44) | TT | 1.11 (0.65–1.88) | 1.91 (0.98–3.73) | 0.62 (0.25–1.50) | AA | 0.85 (0.59–1.22) | 1.06 (0.63–1.76) | 0.69 (0.41–1.14) | |
| P-trend | 0.05 | 0.64 | <0.01 | 0.08 | 0.53 | 0.01 | 0.17 | 0.94 | 0.08 | ||||
| P-interactionc | 0.02 | 0.02 | 0.28 | ||||||||||
| Fatal | 138 | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | GG | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Cerebrovascular | AC | 0.71 (0.46–1.09) | 1.12 (0.55–2.27) | 0.57 (0.33–0.97) | CT | 0.72 (0.47–1.10) | 1.13 (0.56–2.29) | 0.57 (0.33–0.98) | AG | 0.98 (0.69–1.39) | 1.08 (0.60–1.98) | 0.94 (0.61–1.44) | |
| Disease | AA | 1.49 (0.55–4.05) | 2.76 (0.66–11.54) | 0.97 (0.24–3.95) | TT | 1.35 (0.50–3.67) | 2.46 (0.59–10.26) | 0.89 (0.22–3.62) | AA | 0.36 (0.11–1.14) | 0.42 (0.06–3.11) | 0.33 (0.08–1.35) | |
| P-trend | 0.36 | 0.34 | 0.08 | 0.34 | 0.92 | 0.08 | 0.23 | 0.74 | 0.22 | ||||
| P-interactionc | 0.06 | 0.07 | 0.70 | ||||||||||
| Fatal | 457 | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | CC | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | GG | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Atherosclerotic | AC | 0.92 (0.74–1.15) | 1.12 (0.82–1.54) | 0.77 (0.57–1.05) | CT | 1.90 (0.72–1.13) | 1.24 (0.87–1.77) | 0.76 (0.56–1.03) | AG | 0.88 (0.72–1.07) | 0.95 (0.72–1.25) | 0.82 (0.62–1.08) | |
| Disease | AA | 0.89 (0.42–1.88) | 1.59 (0.65–3.87) | 0.41 (0.10–1.65) | TT | 1.13 (0.60–2.13) | 2.14 (0.79–5.78) | 0.56 (0.18–1.76) | AA | 0.93 (0.62–1.40) | 1.08 (0.61–1.91) | 0.80 (0.43–1.40) | |
| P-trend | 0.44 | 0.28 | 0.04 | 0.57 | 0.20 | 0.05 | 0.27 | 0.93 | 0.14 | ||||
| P-interactionc | 0.03 | 0.03 | 0.34 | ||||||||||
Adjusted for age at baseline and sex
Adjusted for age at baseline
p-value for the Wald test of an interaction between sex and genotype
Includes 138 with cerebrovascular disease, 457 with atherosclerotic disease, and 33 with other cardiovascular disease (valvular heart disease, pulmonary embolism, and cardiomyopathy)
Figure 1.
Kaplan-Meier curves for cardiovascular disease mortality by each genotype for the OBFC1, rs4387287, stratified by sex.
Due to the potential for the low prevalence of homozygous minor allele genotypes to limit study power, we conducted exploratory analyses collapsing the heterozygous genotype with the homozygous genotype to determine associations with at least one copy of the minor allele (data not shown). These analyses did not change interpretation of study results. Additional exploratory analyses were conducted to evaluate the potential mode of inheritance using the Akaike information criterion (AIC). For each genotype, we calculated the AIC to determine relative goodness of fit for the dominant, recessive, and log-additive models. There was little variation in AIC between models (data not shown).
4. Discussion
We found that individuals with OBFC1 genotypes associated with longer LTL had better overall, and CVD specific, survival. This finding is complementary with the previously reported inverse associations between CVD mortality and longer LTL (Cawthon et al., 2003; Fitzpatrick et al., 2011). Moreover, genotypes linked to longer LTL were associated with reduced risks of atherosclerotic CVD-related biomarkers, including fasting insulin and CRP levels for OBFC1 polymorphisms and interleukin-6 for the TERC polymorphism. However, we found no association between TERC genotype and CVD mortality.
Of note, in contrast to the larger GWAS study (Levy et al., 2010), which included a subset of CHS participants and 3 other study populations, genotypes for the TERC SNP were not statistically significantly associated with LTL in this study population. However, similar to the prior GWAS results, the minor allele for the OBFC1 SNP was statistically significantly associated with a longer LTL in this study population.
Other human studies of the relationship between genes associated with LTL and CVD have been limited in scope and number. Similar to our results for TERC, a recent cohort study of over 23,000 women identified no association between TERC polymorphisms and CVD risk (Zee et al., 2011). To date, there are no prior studies that specifically evaluate genotypic variation in OBFC1 in relation to CVD. However, a GWAS of Framingham Heart Study participants linked OBFC1 to brachial artery endothelial function, which is an index of atherosclerotic risk (Vasan et al., 2007).
Specifically, we observed differences in the association between CVD mortality and OBFC1 genotype according to sex. For women, the minor allele was inversely associated with the risk of death due to CVD, but for men, there was no association between OBFC1 genotypes and CVD mortality. Sex differences for the associations between LTL and cognition and LTL and CRP have recently been reported (Harris et al., 2010). However, further investigation is needed to determine if our results, and results from studies reporting sex differences in associations with LTL, can be replicated in separate study populations. We note that men and women differ with respect to CVD, with men having higher incidence and women having higher case-fatality rates (Miller, 2010). However, the mechanism by which LTL regulating genes influence risk of death due to CVD only in women is not clear. Several studies have demonstrated the importance of sex hormones in regulating telomerase (Liu et al., 2010), an enzyme responsible for telomere lengthening (Chan and Blackburn, 2004). Estrogens increase telomerase activity (Liu and Li); whereas androgens may down-regulate or up-regulate the activity of the enzyme (Calado et al., 2009; Moehren et al., 2008). Potentially, estrogen and androgen levels might modify the relationship between OBFC1 genotype and CVD mortality. Further research is needed to explore this hypothesis.
When our CVD death data were split according to atherosclerotic disease and cerebrovascular disease, only the association between OBFC1 genotype and atherosclerotic disease was statistically significant. This may be due to limited power based on the low number of cerebrovascular disease deaths. However, increasing evidence points towards the importance of telomere biology in the hematopoietic system specifically for the pathogenesis of atherosclerotic CVD (Aviv, 2011). As previously noted, LTL reflects telomere length in hematopoietic stem cells, and is hence a marker for both their replicative history and replicative potential (Sidorov et al., 2009). Hematopoietic stem cells also share their embryonic origin with the vascular endothelium (Adamo et al., 2009; Yoshimoto and Yoder, 2009) and are the source of endothelial progenitor cells, which are engaged in repairing damaged vascular endothelial tissue (Urbich and Dimmeler, 2004). Clearly, the elucidation of genes that explain the inter-individual variation in LTL might provide insight into mechanisms that link telomere dynamics in the hematopoietic system to CVD and CVD mortality.
The results of this study should be interpreted in light of several limitations. Power was limited due to the low prevalence of the homozygous minor allele genotypes. As previously presented, we conducted exploratory analyses collapsing the heterozygous genotype with the homozygous genotype, and these analyses did not change interpretation of study results. Also, the low prevalence of the homozygous minor allele genotypes limited analyses of mode of inheritance. Our analyses using AIC suggested that there was little variation between dominant, recessive, and log-additive models, but this lack of variation may be attributed to the low frequency of the homozygous minor allele genotype. Another potential limitation is that although OBFC1 has been linked to LTL in genomic studies, the specific mechanisms of action for OBFC1 have not been fully elucidated. Therefore, we cannot rule out the possibility that OBFC1 may be associated with CVD mortality independent of the gene’s potential role in LTL regulation. Also, results of this investigation may not be generalizable to CVD mortality in those under the age of 65.
5. Conclusions
In summary, our results support the hypothesis that LTL regulating genes play a role in the biology of CVD and may impact the risk of CVD death, particularly in women. Future investigations into the genetics of LTL and its associations with CVD risk will hopefully shed light on the etiologic role of the hematopoietic system telomere biology in the pathogenesis of atherosclerotic CVD.
Highlights.
OBFC1 and TERC are genes associated with leukocyte telomere length (LTL).
Polymorphisms in OBFC1 were significantly associated with CVD mortality.
The association between polymorphisms in OBFC1 and CVD mortality varied by sex.
Telomere biology may impact the risk of CVD death, particularly among women.
Acknowledgments
We thank the participants and investigators in the Cardiovascular Health Study. A full list of participating investigators and institutions can be found at http://www.chs-nhlbi.org. This research was supported by the National Heart, Lung, and Blood Institute (NHLBI) contract numbers N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant numbers U01 HL080295 and R01 HL80698-01, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). DNA handling and genotyping was supported in part by National Center for Research Resources grant M01-RR00425 to the Cedars-Sinai General Clinical Research Center Genotyping core and National Institute of Diabetes and Digestive and Kidney Diseases grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) Jama. 269:3015–3023. [PubMed] [Google Scholar]
- 2.American Diabetes Association: clinical practice recommendations. Diabetes Care. 1997;20(Suppl 1):S1–70. [PubMed] [Google Scholar]
- 3.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, Garcia-Cardena G, Daley GQ. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aviv A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med. 2002;80:689–695. doi: 10.1007/s00109-002-0377-8. [DOI] [PubMed] [Google Scholar]
- 5.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutation research. 2011 doi: 10.1016/j.mrfmmm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 7.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 8.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 10.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. doi: 10.1038/sj.onc.1205082. [DOI] [PubMed] [Google Scholar]
- 12.Chiang YJ, Hemann MT, Hathcock KS, Tessarollo L, Feigenbaum L, Hahn WC, Hodes RJ. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Mol Cell Biol. 2004;24:7024–7031. doi: 10.1128/MCB.24.16.7024-7031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 14.de Ferranti S, Rifai N. C-reactive protein and cardiovascular disease: a review of risk prediction and interventions. Clin Chim Acta. 2002;317:1–15. doi: 10.1016/s0009-8981(01)00797-5. [DOI] [PubMed] [Google Scholar]
- 15.Edo MD, Andres V. Aging, telomeres, and atherosclerosis. Cardiovasc Res. 2005;66:213–221. doi: 10.1016/j.cardiores.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66:421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 19.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- 20.Gardner JP, Kimura M, Chai W, Durrani JF, Tchakmakjian L, Cao X, Lu X, Li G, Peppas AP, Skurnick J, Wright WE, Shay JW, Aviv A. Telomere dynamics in macaques and humans. J Gerontol A Biol Sci Med Sci. 2007;62:367–374. doi: 10.1093/gerona/62.4.367. [DOI] [PubMed] [Google Scholar]
- 21.Goren HJ. Role of insulin in glucose-stimulated insulin secretion in beta cells. Curr Diabetes Rev. 2005;1:309–330. doi: 10.2174/157339905774574301. [DOI] [PubMed] [Google Scholar]
- 22.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 23.Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, Deary IJ. Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 25.Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram JH, Aviv A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47:482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- 26.Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, Harley CB, Aviv A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 27.Levy D, Neuhausen SL, Hunt SC, Kimura M, Hwang SJ, Chen W, Bis JC, Fitzpatrick AL, Smith E, Johnson AD, Gardner JP, Srinivasan SR, Schork N, Rotter JI, Herbig U, Psaty BM, Sastrasinh M, Murray SS, Vasan RS, Province MA, Glazer NL, Lu X, Cao X, Kronmal R, Mangino M, Soranzo N, Spector TD, Berenson GS, Aviv A. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107:9293–9298. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 29.Liu JP, Chen SM, Cong YS, Nicholls C, Zhou SF, Tao ZZ, Li H. Regulation of telomerase activity by apparently opposing elements. Ageing Res Rev. 2010;9:245–256. doi: 10.1016/j.arr.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu JP, Li H. Telomerase in the ovary. Reproduction. 2010;140:215–222. doi: 10.1530/REP-10-0008. [DOI] [PubMed] [Google Scholar]
- 31.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 32.Miller VM. Sex-based differences in vascular function. Womens Health (Lond Engl) 2010;6:737–752. doi: 10.2217/whe.10.53. [DOI] [PubMed] [Google Scholar]
- 33.Moehren U, Papaioannou M, Reeb CA, Grasselli A, Nanni S, Asim M, Roell D, Prade I, Farsetti A, Baniahmad A. Wild-type but not mutant androgen receptor inhibits expression of the hTERT telomerase subunit: a novel role of AR mutation for prostate cancer development. Faseb J. 2008;22:1258–1267. doi: 10.1096/fj.07-9360com. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 35.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 36.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. American journal of epidemiology. 2009;169:505–514. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidorov I, Kimura M, Yashin A, Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009;37:514–524. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 40.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circulation research. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 41.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 42.Valdes AM, Deary IJ, Gardner J, Kimura M, Lu X, Spector TD, Aviv A, Cherkas LF. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol Aging. 2010;31:986–992. doi: 10.1016/j.neurobiolaging.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasan RS, Larson MG, Aragam J, Wang TJ, Mitchell GF, Kathiresan S, Newton-Cheh C, Vita JA, Keyes MJ, O’Donnell CJ, Levy D, Benjamin EJ. Genome-wide association of echocardiographic dimensions, brachial artery endothelial function and treadmill exercise responses in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S2. doi: 10.1186/1471-2350-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, Simonsick EM, Kuller L, Li R, Ayonayon HN, Rubin SM, Cummings SR. Telomere length and cognitive function in community-dwelling elders: Findings from the Health ABC Study. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimoto M, Yoder MC. Developmental biology: Birth of the blood cell. Nature. 2009;457:801–803. doi: 10.1038/457801a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zee RY, Ridker PM, Chasman DI. Genetic variants in eleven telomere-associated genes and the risk of incident cardio/cerebrovascular disease: The Women’s Genome Health Study. Clin Chim Acta. 2011;412:199–202. doi: 10.1016/j.cca.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]