Abstract
Objective
Data documenting the functional impairment associated with the diagnosis of bipolar disorder (BD) in children and adolescents highlight the need for greater understanding of its pathophysiology. Toward that end, we demonstrated previously that BD youth have behavioral deficits on reversal learning tasks. On such tasks, participants must first acquire a stimulus/response relationship through trial-and-error learning, and then discern when the stimulus/reward relationship reverses. Here, we use event-related functional magnetic resonance imaging (fMRI) to elucidate neural correlates of reversal learning deficits in euthymic BD youth compared to typically developing controls.
Method
We compared euthymic pediatric BD participants (n = 16) versus age-, sex-, and IQ-matched controls (n = 16). Our main outcome measure was blood oxygen level-dependent (BOLD) signal measured with fMRI during an event-related probabilistic reversal task.
Results
Pediatric BD participants had significantly greater neural activity than controls in fronto-parietal regions during the reversal phase, particularly in response to punished reversal errors (p < 0.05 corrected for multiple comparisons).
Conclusions
Our current study suggests that during reversal learning, BD youths inefficiently recruit regions associated with processing response conflict and implementing alternative responses, including subdivisions of the frontal cortex and the parietal cortex. Such deficits are present in euthymic BD youth. Further work is necessary to evaluate the specificity of such alterations.
Keywords: adolescent, bipolar disorder, child, magnetic resonance imaging, reversal learning
Pediatric bipolar disorder (BD) has become a growing health concern (1–4). Greater neurobiological understanding could aid the diagnostic process and the development of new treatments.
Functional impairments seen in pediatric BD include dysfunction in cognitive flexibility, defined as the ability to adapt one's thinking and behavior in response to changing environmental conditions, such as rewards (5, 6). The ability to alter one's response to changing reinforcement contingencies is one aspect of cognitive flexibility that can be measured in the laboratory using reversal learning tasks. In such tasks, two stimuli, A and B, are presented, and participants must use trial-and-error learning to determine that stimulus A but not B is rewarded. Then, without warning, the stimulus/response relationship is reversed, so that participants must learn that now B but not A is rewarded (7).
From an affective neuroscience perspective, many clinical symptoms of BD suggest impaired cognitive flexibility and adaptation to changing stimulus-response-reward contingencies, as occurs on reversal learning tasks. For example, mania can be viewed as a hyperhedonic state characterized by excessive involvement in pleasurable activities with high potential for painful consequences and abnormally inflated self-esteem or grandiosity. In contrast, depression may represent a hypohedonic state characterized by decreased interest in such activities, anhedonia, and feelings of worthlessness (8). Furthermore, reversal learning deficits may play a role in the irritability found during mania, depression, and euthymia in BD, since participants may become frustrated when they continue to anticipate but not receive rewards (9–12).
BD youths show significant impairment in reversal learning (13–17), a task that in healthy people engages neural regions previously implicated in BD. Specifically, successful reversal learning depends on engagement of the medial orbital frontal cortex (mOFC) for representation of reinforcement contingencies and prediction error signaling, allowing recoding of the reinforcement value associated with the response (18–20). Ventral striatal engagement further facilitates performance, allowing transformation of concrete stimulus exemplar information into motor responses (5, 21, 22). Additional areas of the frontal cortex that are involved in reversal learning include the dorsomedial frontal cortex (dmFC) and cingulate cortex. These regions enable mediation of response conflict between the executed incorrect response and the alternative response, as signaled by punishment (23, 24). In turn, the dmFC is hypothesized to recruit other regions implicated in top-down attentional control, including the lateral superior frontal cortex (lsFC) and parietal cortex (25, 26), as well as regions implicated in object/response selection, including the inferior frontal cortex (iFC) (27, 28), to orchestrate a change in response on the subsequent trial (5, 18, 24, 29).
While no prior imaging studies use reversal learning paradigms to study BD, prior work suggests that regions implicated in reversal learning may be dysfunctional in BD. For example, pediatric and adult BD structural magnetic resonance imaging (MRI) studies have shown volumetric alterations in components of the circuit engaged by reversal learning, including the dmFC/cingulate cortex, lsFC, iFC, striatum, and parietal cortex (30–41). Functional MRI (fMRI) studies of BD adults have demonstrated altered neural activation in these regions on tasks besides reversal learning paradigms. For example, BD adults have reduced OFC activity during an emotional go/no-go paradigm, reduced lsFC activity during affect discrimination and in response to emotional stimuli, and reduced iFC activity during decision making (42–45). Notably, a recent study reported reduced OFC, striatal, and anterior cingulate cortex (ACC) habituation-related activity in BD adults (46). Work with pediatric BD has also typically revealed reduced attention-related activity within iFC and lsFC in response to emotional provocation (47–49). Moreover, reduced striatal and iFC activity was seen in pediatric BD patients when failing to inhibit their responses on a motor inhibition paradigm (50, 51), whereas increased lsFC activity was necessary for successful performance on change trials on a motor control task for BD youths (52).
Based on these prior findings, we hypothesized that reversal deficits observed in youths with BD result from dysfunction either in regions putatively signaling reinforcement/prediction errors (i.e., mOFC) and/or in regions involved in related tasks, including processing response conflict, implementing alternative responses, and controlling attention (i.e., dmFC, lsFC, iFC). We tested this hypothesis in euthymic BD youth (n = 16) and typically developing controls (n = 16) using fMRI during performance of a probabilistic reversal paradigm. The paradigm we used was adapted from one on which we previously demonstrated behavioral impairments in pediatric populations, and whose mediating neural circuitry has been characterized in both adult and youth samples using fMRI (7, 15–18, 53). We examined euthymic patients with pediatric BD in this study to minimize the impact of mania or depression on any between-group differences.
Methods
Participants
All participants were enrolled in studies conducted at the National Institute of Mental Health's Division of Intramural Research Programs that were approved by the National Institute of Health's Combined Neuroscience Institutional Review Board in compliance with the Declaration of Helsinki. After the studies were explained and prior to participation, parents gave written informed consent, and children gave written assent. Participants were recruited through advertisements placed in local parenting magazines, on support groups' Web sites, and distributed to psychiatrists nationwide.
BD (n = 16) inclusion criteria were: (i) meeting DSM-IV-TR criteria for type I or type II BD, including history of at least one episode meeting full duration criteria for hypomania (≥ 4 days) or mania (≥ 7 days) wherein the child exhibited abnormally elevated or expansive mood accompanied by at least three other DSM-IV-TR criterion `B' mania symptoms; (ii) involvement with ongoing mental health treatment; and (iii) presence of a primary caretaker to grant consent and participate in the research process. Children with irritable mania only, without elevated or expansive mood, were excluded from this group (54); thus, these participants fulfill Leibenluft et al.'s “narrow phenotype” pediatric BD criteria (55). BD exclusion criteria were: age <7 years or >18 years; I.Q. ≤ 70; autism or Asperger's disorder; ongoing medical illness that is unstable or could cause psychiatric symptoms; pregnancy; or substance abuse within two months. While youth in all mood states were scanned, to ensure greater sample homogeneity we here report data only from those who were euthymic at the time of scanning [i.e., Young Mania Rating Scale (YMRS) (56) score <12 and Children's Depression Rating Scale (CDRS) (57) score <40].
Typically developing child control (n = 16) inclusion criteria were: age 7–18 years and negative psychiatric history. Exclusion criteria were: age <7 years or >18 years; I.Q. ≤ 70; ongoing medical illness; pregnancy; past or present psychiatric disorder; or substance abuse.
All participants completed the Child Schedule for Affective Disorders–Present and Lifetime version (K-SADS-PL), (58) administered to parents and children separately by graduate-level clinicians with high inter-rater reliability (kappa ≥ 0.9). Diagnoses comorbid to BD were assessed with the K-SADS-PL by inquiring about symptoms during a time of relative euthymia to ensure that manic or depressive BD symptoms were not counted toward another diagnosis. BD participants completed the Children's Global Assessment of Scale (CGAS) (59) in addition to mood ratings (YMRS, CDRS). Additionally, participants and controls completed the full-scale intelligence quotient (FSIQ) from the Wechsler Abbreviated Scale of Intelligence (WASI) (60).
Probabilistic response reversal (PRR) event-related fMRI task and procedure
As described elsewhere (7), the PRR task consisted of six 6.5-minute runs (7, 18). Each run consisted of 135 trials plus 6 fixation trials at the beginning and end of each run (147 events/run), with 30% of total trials consisting of jittered fixation trials. Each trial lasted 2500 ms, including 1600 ms for stimulus presentation and selection and 900 ms for feedback. Stimuli consisted of a pair of colored Snodgrass line drawings of neutrally valenced objects—e.g., blue cat, red bear, yellow dog—appearing against a white background. Stimuli were randomized to appear in one of 16 regions of the screen, 8 each on the left and right side, with stimuli never appearing on the same side together. Participants pressed the left button on a stimulus/response box to select the object on the left side of the screen, or pressed the right button to select the object on the right side. Feedback consisted of `you win 100 points' or `you lose 100 points', accompanied by the running total score for that run. If participants failed to make a selection, they received feedback stating `please respond faster next time' and their running total score remained unchanged. Participants began each run with 0 points.
To increase task difficulty, each task run included two probabilities of reward, 100:0 and 80:20. In 100:0 trials (Fig. 1), the preferred stimulus was rewarded in 100% of trials (and punished 0%), and the nonpreferred stimulus was punished in 100% of trials (and rewarded 0%). In 80:20 trials, the preferred stimulus was rewarded in 80% of trials (and punished in 20%), and the nonpreferred stimulus was punished in 80% of trials (and rewarded in 20%). Thus, the entire task consisted of 12 unique stimulus pairs, including five 100:0 reversing, five 80:20 reversing, one 100:0 non-reversing, and one 80:20 nonreversing. For the two runs containing one nonreversing pair, the other pair reversed.
Fig. 1.
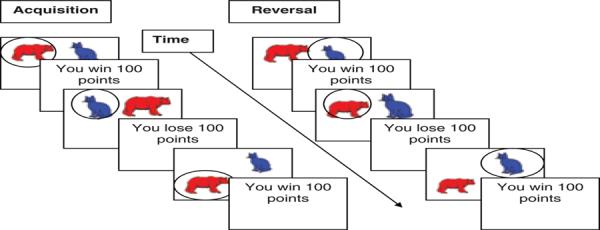
Probabilistic response reversal task. During initial acquisition trials (left), preferred stimulus (red bear) wins points and nonpreferred stimulus (blue cat) loses points. Participants receive feedback (win / lose 100 points) if their response is correct / incorrect, respectively. During subsequent reversal trials (right), stimulus / reward relationship is reversed, so that previously preferred stimulus (red bear) is now nonpreferred and loses points, and the previously nonpreferred stimulus (blue cat) is now preferred and wins points. This figure illustrates the 100:0 condition, when the preferred stimulus is rewarded 100% (and punished 0%). During the 80:20 condition, the preferred stimulus is initially rewarded 80% (and punished 20%), while the nonpreferred stimulus is rewarded 20% (and punished 80%). Then subsequent 80:20 reversal trials reverse this stimulus / reward relationship.
In each 100:0 reversing pair, stimuli were presented for 20 acquisition trials followed by 20 reversal trials, with 20 randomly jittered fixation trials. In the 100:0 nonreversing pair, stimuli were presented for 40 trials with 20 randomly jittered fixation trials. In each 80:20 reversing pair, stimuli were presented for 25 acquisition trials followed by 25 reversal trials, with 25 randomly jittered fixations. In each 80:20 nonreversing pair, stimuli were presented for 50 trials with 25 randomly jittered fixations. Two versions of the task were administered to counterbalance stimulus/pair reinforcement associations. The order of the runs and stimulus/pairs within each run was randomized for each participant.
Prior to entering the scanner, participants completed an out-of-scanner practice acquisition run using sample stimuli presented on a laptop computer with the following instructions: “Pairs of objects will appear on the screen. On each turn, you have to choose one of these objects and the computer will tell you if your choice was correct or wrong. If it is correct, you will win 100 points. If it is wrong you will lose 100 points. Each object will sometimes be correct and sometimes be wrong, but one of the objects will tend to be correct more often than the other one. Find out which object is usually correct, and choose that object every time. Stick with it even if it is occasionally wrong. At some point, it may change so that the other object is usually correct, in which case you should choose that one every time.”
Behavioral data analysis
Behavioral data acquired during the fMRI scan was analyzed with a 2 (diagnosis: BD, control) × 2 (phase: acquisition, reversal) × 2 (accuracy: correct win, incorrect loss) repeated-measures ANOVA implemented in Statistical Package for Social Sciences (SPSS) v. 14 (SPSS, Inc., Chicago, IL, USA).
MRI data acquisition
Scans were conducted on a 1.5 Tesla General Electric Signa Scanner (GE, Milwaukee, WI, USA). During each run, functional images were collected with a gradient echo-planar imaging (EPI) sequence (repetition time = 2500 ms, echo time = 30 ms, 64 × 64 matrix, flip angle = 90, field of view = 24 cm) in 29 axial slices (thickness = 4.0 mm, in plane resolution 3.75 × 3.75). High-resolution anatomical scans were also acquired during the scanning session using an axial three-dimensional spoiled gradient recalled echo in the steady state (SPGR) sequence (124 axial slices, thickness = 1.5 cm, echo time = 3.2 ms, repetition time = 8.1 ms, 256 × 256 matrix, flip angle = 20, field of view = 24 cm).
Data analysis
Event-related fMRI analysis
As has been done in two prior published studies using this same PRR task (7, 18), Analysis of Functional Neuroimages (AFNI) (http://afni.nimh.nih.gov/afni/) was used to preprocess and analyze the data. At the individual level, image preprocessing for the six EPI runs included: (i) discarding the first five volumes of each EPI series, prior to the magnetization equilibrium being reached, when no data were collected; (ii) registering each to a volume collected just prior to the high-resolution anatomical scan acquisition; (iii) de-spiking the data; (iv) concatenating the six EPI runs into a single time series; and (v) spatially smoothing the data with a 6-mm Gaussian kernel to reduce the impact of anatomical variability among individual maps.
For this event-related fMRI experiment, trials were divided according to (i) phase (acquisition/reversal), (ii) accuracy of the subject's response (correct/incorrect), and (iii) reinforcement received (reward/punishment). This resulted in 10 modeled events: (i) acquisition correct win; (ii) acquisition correct lose (due to the probabilistic nature of task); (iii) acquisition incorrect win (due to probabilistic nature of the task); (iv) acquisition incorrect lose; (v) reversal correct win; (vi) reversal correct lose (due to the probabilistic nature of the task); (vii) reversal incorrect win (due to the probabilistic nature of the task); (viii) reversal incorrect lose; (ix) trials on which participants did not respond at all, modeled as events of no interest; and (x) blank fixation trials.
Based on prior research, our primary interest was on 4 of the 10 event types, comprising rewarded correct and punished incorrect responses during acquisition and reversal—i.e., (i) acquisition correct win (ACW), (ii) acquisition incorrect lose (AIL), (iii) reversal correct win (RCW), and (iv) reversal incorrect lose (RIL) (7, 15, 18, 61). Contrasts of these four classes of events have been previously shown to engage fronto-striatal-parietal circuitry relevant to our hypotheses on the pathophysiology of pediatric BD (7, 15, 18, 61).
Linear regression modeling was performed using the above regressors plus six motion parameters (x, y, z, roll, pitch, yaw) to produce beta coefficients and associated t-statistics for each voxel and each regressor. Each subject's anatomical scan was registered to the standard coordinate space of Talairach and Tourneaux, and then their functional EPI data were registered to their Talairach transformed anatomical scan.
Group-level analysis was conducted using an ANOVA implemented in AFNI consisting of diagnosis (2 levels = BD and control) × phase (2 levels = acquisition and reversal) × accuracy (2 levels = correct responses where the participant was rewarded versus incorrect responses where he or she was punished). We focus specifically on the diagnosis × phase (acquisition versus reversal) interaction, though we report all significant interactions. The rationale for this focus is that prior out-of-scanner behavioral data indicate that BD youth have deficits specifically during the reversal phase of the task (15, 16, 53). Moreover, statistical power is limited on tests of the three-way diagnosis × phase × accuracy interactions, relative to two-way diagnosis × phase interactions. This is because significance tests for interaction terms rely on estimates derived from more event replicates in the two-way, relative to three-way, interaction.
As is the norm for fMRI studies using AFNI, the threshold for statistical significance was set at p < 0.05 corrected across the whole brain for multiple comparisons using AFNI's AlphaSim program. This was derived through an initial p < 0.005 threshold that was then corrected for multiple comparisons across the whole brain using AlphaSim to conduct 1,000 Monte Carlo simulations. These simulations were used to calculate the probability of false-positive detection, taking into account both individual voxel probability thresholding and cluster size; a minimum cluster volume of 200 mm3 was required to meet the statistical threshold. All results meeting the whole-brain corrected pcorrected< 0.05 threshold are presented with their actual p-value.
We also conducted exploratory secondary analyses to evaluate the potential effect of medication, comorbid psychopathology, subsyndromal mood symptoms, BD subtype, and age/pubertal development on our primary analysis of diagnosis-by-phase interactions. Using SPSS, we used separate repeated-measures ANOVAs to compare mean BOLD fMRI neural activation from significant clusters in the primary diagnosis × phase analysis, including secondary analyses to evaluate the effect of psychotropic medications, psychiatric comorbidity, and BD subtype. To examine the effect of mood, we conducted Spearman correlations in the BD group between CDRS and YMRS scores and significant clusters in our primary analysis. To examine the effect of age and Tanner pubertal development, we conducted Spearman correlations in the BD and control group separately.
Results
Participants
There were no significant between-group differences in age (t = 0.25, p = 0.81), full-scale IQ (t = −0.15, p = 0.88), sex (7 female and 9 male participants in both BD and control groups; χ2= 0.00, p = 1.0), or Tanner pubertal stage (genitals: t = −0.08, p = 0.94; hair: t = 0.28, p = 0.78). Of note, although inclusion criteria allowed children as young as 7 years, no BD or control participants under 10 years old were actually involved in the present study. Only one BD participant had type II BD, whereas the remaining 15 had type I BD. All BD participants were euthymic and moderately impaired by ratings of mood (YMRS mean 7.1 ± 3.3, CDRS mean 23.7 ± 5.2) and of impairment (CGAS mean 56.6 ± 13.5). Of the BD group, 6/16 had at least one first-degree relative with a history of BD. Of the BD group, 13/16 were taking their usual outpatient medications (mean 2.3 ± 0.9), while 3/16 BD participants were medication free for four drug half-lives at time of scan (Table 1).
Table 1.
Participant demographics
| Bipolar disorder (n = 16) | Controls (n = 16) | |
|---|---|---|
| Age, years, mean (SD) | 14.1 (2.5) (range: 7.2, max: 17.7, min: 10.4) |
13.9 (2.4) (range: 7.8, max: 17.8, min: 10.0) |
| Full-scale IQ, mean (SD) | 108.1 (16.5) (range: 56, max: 138, min: 82) |
108.9 (13.5) (range: 48, max: 135, min: 87) |
| Sex (F:M) | 7:9 | 7:9 |
| Tanner pubertal stage, mean (SD)a | ||
| Genitals | 3.3 (1.5) | 3.3 (1.4) |
| Pubic hair | 3.5 (1.6) | 3.3 (1.4) |
| Young Mania Rating Scale score, mean (SD) | 7.1 (3.3) | |
| Child Depression Rating Scale score, mean (SD) | 23.7 (5.2) | |
| Children's Global Assessment Scale score, mean (SD) | 56.6 (13.5) | |
| Diagnosis, n | ||
| Bipolar I disorder | 15 | |
| Bipolar II disorder | 1 | |
| Current comorbid psychiatric diagnoses, n (%) | ||
| Attention-deficit hyperactivity disorder (ADHD) | 10 (63) | |
| Oppositional defiant disorder | 6 (38) | |
| Conduct disorder | 2 (13) | |
| Generalized anxiety disorder | 4 (25) | |
| Social phobia | 3 (19) | |
| Simple phobia | 3 (19) | |
| Separation anxiety disorder | 3 (19) | |
| Posttraumatic stress disorder | 0 (0) | |
| Obsessive-compulsive disorder | 1 (6) | |
| Medication free, n (%) | 3 (19) | |
| No. medications at scan, mean (SD) | 2.3 (0.9) | |
| Medication, n (%) | ||
| Lithium | 6 (38) | |
| Atypical neurolepticb | 8 (50) | |
| Antiepilepticc | 8 (50) | |
| Antidepressantd | 3 (19) | |
| Stimulante | 5 (31) | |
| Alpha agonist ADHD medicationf | 2 (12) |
Not provided by 2/16 controls.
4 quetiapine, 2 aripiprazole, 1 risperidone, 1 olanzapine.
3 valproate, 2 lamotrigine, 2 oxcarbazepine, 1 carbamazepine.
1 fluoxetine, 1 imipramine, 1 buspirone.
2 dextroamphetamine, 3 methyphenidate.
2 guanfacine.
Behavioral results
Table 2 displays the mean number of each of the four trial types of interest (ACW, AIL, RCW, RIL) for BD and healthy youths. There was a significant main effect of phase [F(1,30) = 24,180, p < 0.001], reflecting more acquisition versus reversal trials (149.2 ± 1.2 versus 84.8 ± 1.1). There also was a significant main effect of response [F(1,30) = 1,103, p = 0.000], reflecting more correct versus incorrect trials (198.5 ± 3.4 versus 35.4 ± 1.8), but no effect of diagnosis [F(1,30) = 2.34, p = 0.14]. Such effects did not impact our fMRI results given the rapid event-related design that separately examined neural response as a function of trial type and correct or incorrect responding. There were no significant interactions {diagnosis × phase × accuracy [F(1,30) = 1.18, p = 0.29]; diagnosis × phase [F(1,30) = 1.65, p = 0.21]; diagnosis × accuracy [F(1,30) = 1.51, p = 0.23]}.
Table 2.
Number of trials for each trial type
| Bipolar disorder Mean (SD) | Controls Mean (SD) | |
|---|---|---|
| Acquisition correct win | 261.2 (31.4) | 274.1 (12.8) |
| Acquisition incorrect lose | 33.1 (15.5) | 28.3 (12.0) |
| Reversal correct win | 126.4 (20.1) | 132.6 (12.6) |
| Reversal incorrect lose | 40.2 (10.3) | 39.9 (10.9) |
fMRI results
Separate effects were examined for phase (considering acquisition and reversal) and accuracy (separating correct responses where the participant was rewarded versus incorrect responses where he or she was punished). These separate effects were examined as a function of diagnosis. This was accomplished through a 2 (diagnosis: BD and controls) by 2 (phase: acquisition and reversal) by 2 (accuracy: correct-win and incorrect-loss) whole-brain ANOVA of the BOLD response data implemented in AFNI. This yielded significant (i) diagnosis-by-phase-by-accuracy, (ii) diagnosis-by-phase, and (iii) diagnosis-by-accuracy interactions, though, as noted above, results focus most deeply on two-way interactions, due to the small number of events used to test the three-way interaction. These are described below, with all p-values whole-brain corrected.
Diagnosis-by-phase-by-accuracy interaction
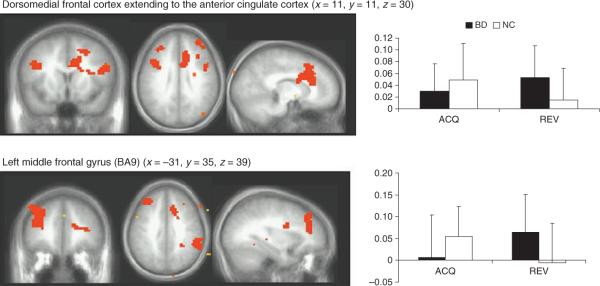
Significant diagnosis-by-phase-by-accuracy interactions occurred within the right inferior parietal lobule, with BD youths showing significantly greater BOLD responses than controls during punished reversal errors [Brodmann area (BA) 40, F(1,30) = 19, p = 0.004] (Table 3).
Table 3.
Significant interactions in euthymic bipolar disorder (n = 16) versus typically developing control (n = 16) youtha
| Contrast | Side | Region | BA | Volume (mm3) | F(df) | p-value | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis × phase × accuracy | Right | Inferior parietal lobule | 40 | 1566 | 19(1,30) | 0.0004 | 50 | −41 | 60 |
| Diagnosis × phase | Right | Dorsomedial frontal cortex extending to the anterior cingulate cortex | 24/32 | 20061 | 27(1,30) | 0.00006 | 11 | 11 | 30 |
| Left | Middle and superior frontal gyri | 9/8 | 5022 | 19(1,30) | 0.0004 | −31 | 35 | 39 | |
| Right | Middle and superior frontal gyri | 10 | 2970 | 23(1,30) | 0.0001 | 32 | 50 | 15 | |
| Right | Inferior parietal lobule, supramarginal gyrus | 40 | 2403 | 21(1,30) | 0.0002 | 59 | −47 | 36 | |
| Left | Middle and inferior frontal gyri | 9 | 2079 | 21(1,30) | 0.0002 | −38 | 11 | 24 | |
| Diagnosis × accuracy | Right | Precuneus and posterior cingulate cortex | 7 | 2592 | 15(1,30) | 0.001 | 5 | −59 | 66 |
BA = Brodmann area.
All comparisons thresholded at p < 0.05 whole-brain corrected for multiple comparisons using AlphaSim implemented in Analysis of Functional Neurolmages software (AFNI).
Diagnosis-by-phase interaction
Significant diagnosis-by-phase interactions were found within the dmFC, bilateral regions of the lsFC, and the right inferior parietal cortex. Within all regions, the BD youths showed significantly greater BOLD responses than controls during the reversal phase (p < 0.05 in all cases) (Fig. 2).
Fig. 2.
Probabilistic response reversal significant diagnosis × phase interactions. All comparisons thresholded at p < 0.05 whole-brain corrected for multiple comparisons using AlphaSim implemented in Analysis of Functional NeuroImages software (AFNI). Left: neural clusters (orange) meeting this threshold. Right: mean blood oxygen level-dependent signal for selected contrasts with black = pediatric bipolar disorder (BD) and white = normal controls (NC); error bars = SD. ACQ = acquisition phase (sum of acquisition correct win and acquisition incorrect lose); REV = reversal phase (sum of reversal correct win and reversal incorrect lose); BA = Brodmann area.
Diagnosis-by-accuracy interaction
Significant diagnosis-by-accuracy interactions were found within the right superior frontal gyrus and bilateral precuneus extending on the right into the posterior cingulate cortex with BD youths showed greater BOLD responses (i.e., failed to demonstrate decreased BOLD responses when losing) than controls [BA7, F(1,30) = 15, p = 0.001].
Secondary analyses: exploration of medication, comorbidity, and mood effects
Although exploratory, we sought to evaluate potential medication effects on our primary analysis of diagnosis-by-phase interactions. Comparing acquisition versus reversal neural activation from significant clusters identified by our primary diagnosis-by-phase interaction analysis, we did not find any significant differences among BD participants currently taking atypical antipsychotic medication (n = 8) versus those not (n = 8), or among BD participants currently taking anticonvulsant medications (n = 8) versus those not (n = 8).
To explore potential comorbidity effects on our primary analysis of diagnosis-by-phase interactions, we compared acquisition versus reversal neural activation in those BD participants without comorbid attention-deficit hyperactivity disorder (ADHD) (n = 6) versus the entire control sample (n = 16). In accord with our primary analysis, BD participants without ADHD had significantly greater neural activation than controls during the reversal phase in the bilateral middle and superior frontal gyri [left F(1,20) = 4.99, p = 0.04; right F(1,20) = 8.48, p = 0.009] and right inferior parietal lobule/supramarginal gyrus [F(1,20) = 6.45, p = 0.02].
Similarly, we compared BD participants without any anxiety disorder (n = 10) to the entire control sample (n = 16). BD participants without anxiety had significantly greater neural activation than controls during the reversal phase in the bilateral middle and superior frontal gyri [left F(1,24) = 5.59, p = 0.03; right F(1,24) = 10.75, p = 0.003], right inferior parietal lobule/supramarginal gyrus [F(1,24) = 8.76, p = 0.007], and right inferior parietal lobule [F(1,24) = 10.80, p = 0.003].
To explore the potential effect of BD subtype on our primary analysis of diagnosis-by-phase interactions, we excluded the only BD participant with type II BD and then compared acquisition versus reversal neural activation in BD type I (15/16) participants versus the entire control sample (n = 16). Consistent with our results with the BD II participant included, the analysis including only BD type I participants found that patients had significantly greater neural activation than controls in the right dorsomedial prefrontal cortex (dmPFC) [F(1,29) = 6.55, p = 0.02], left middle/superior frontal gyri [F(1,29) = 9.27, p = 0.005], right middle/superior frontal gyri [F(1,29) = 12.34, p = 0.001], right inferior parietal lobule [F(1,29) = 12.42, p = 0.001], and left middle/inferior frontal gyri [F(1,29) = 4.19, p = 0.05].
To explore the potential effect of subsyndromal mood symptoms on our data, we evaluated Spearman correlations between mood measures (CDRS, YMRS) and significant neural clusters from our primary analysis in the BD sample. No region had a significant correlation with YMRS or CDRS scores.
To evaluate the effect of age and pubertal development, we evaluated Spearman correlations between age and Tanner pubertal development and significant neural clusters from our primary analysis. Neither BD nor control participants had significant correlations with age or pubertal development.
Discussion
We tested two hypotheses in our present study, the first to evaluate the neural basis of reversal learning deficits in pediatric BD. Specifically, we predicted BD youths would demonstrate dysfunction in regions associated with processing response conflict, implementing alternative responses, and controlling attention (i.e., dmFC, iFC, lsFC, parietal cortex, and caudate), and/or that BD youths would show dysfunction in regions of mOFC associated with reinforcement processing. Consistent with the former hypothesis, we found that pediatric BD participants had significantly greater neural activity than controls in the dmFC, lsFC, and parietal cortex during the reversal phase, particularly in response to punished reversal errors. We did not find support for the second hypothesis, as we observed no between-group differences in mOFC activation for which our fronto-parietal findings would be compensating. Nevertheless, we cannot make strong claims in this regard because the study does not have sufficient statistical power to rule out a type II error if anything less than a large effect size occurs within this region. Moreover, it should be noted that BD youths showed less deactivation than did controls in other regions previously seen to show deactivation in the context of punishments during reversal learning tasks, including the posterior cingulate cortex and precuneus (7, 18). Given that our analysis only included euthymic BD youths, these between-group differences are unlikely to be artifacts of mood state.
Our work is the first to begin to elucidate the neural underpinnings of cognitive flexibility using a reversal learning task in pediatric BD. Compared to controls, BD youths had greater activation during the reversal phase, particularly during reversal errors, in the dmFC, iFC, lsFC, and parietal cortex. It has been hypothesized that the dmFC is engaged during the response conflict following punished reversal errors, and that this region recruits regions implicated in top-down attentional control and object/response selection to orchestrate a change in response on the subsequent trial (18, 23). We found increased activity in BD versus controls during punished reversal errors in the dmFC, lateral regions of the superior and middle frontal cortex, and the parietal cortex. This finding suggests two possibilities. First, since task performance did not differ between groups, this increased activity may reflect inefficient recruitment of these regions to achieve satisfactory task performance. Second, this increased activity may reflect compensatory activity in response to dysfunction elsewhere.
The available literature provides more support for the first possibility. In previous work with euthymic adults with BD, there have been reports of enhanced activity within these regions during successful performance on other tasks that engage psychological processes involved in reversal learning. Thus, for example, euthymic BD adults show greater iFC activation than controls during an emotional go/no-go task when inhibiting emotional versus neutral stimuli (62). Euthymic BD adults also show greater neural activation than controls in the parietal cortex during a two-back working memory task (63). In addition, euthymic BD adults show greater PFC activation than controls during an affective face-matching task (64). Moreover, pediatric BD participants demonstrated increased lsFC activity when successfully performing change trials on a motor control task (52). In contrast, we did not find support for the second possibility (compensation) because we did not detect neural alterations in other areas, such as the mOFC, for which these fronto-parietal alterations would be compensating. However, caution is urged so as not to commit a type II error by overinterpreting a negative finding, and thus the current data cannot fully distinguish between these two possibilities.
In addition to showing increased activity versus controls in dmPFC and parietal regions during punished reversal errors, on these trials BD youths also showed less deactivation than controls in the posterior cingulate cortex and precuneus. Previous work is consistent with ours in finding that, during punished reversal errors, healthy individuals show decreased activation to punishment versus reward within these regions (7, 18). Decreased neural responding following punishment has been assumed to relate to recoding of the reinforcement value associated with the response (7, 18). Thus, our data suggest the possibility that a reduced ability to recode reinforcement values requires a compensatory increased recruitment of the dmFC, lsFC, and parietal cortex in order to achieve successful response change.
Importantly, we did not find between-group differences in the mOFC. It is possible that this represents a type II error, and that OFC dysfunction would be detected with either a larger sample of BD and controls, or a more homogeneous sample of BD youths. For example, gender effects have been demonstrated in BD youths in several brain regions, including the OFC (40, 65). While our study is underpowered to examine such possibilities, another possibility is that reversal learning impairments do not involve the OFC in pediatric BD. Support for the latter possibility comes from another study using this PRR paradigm, which found that both typically developing control children and those with ADHD had the expected decrease in BOLD signal in the OFC to reversal errors, but those with psychopathic tendencies did not (7). That study, by Finger et al. (7), involved three groups of 14 children, none of whose data are included in our present study. Additional support comes from a recent study of BD adults (66) that did not find differences in OFC volume or that of its subregions (including the mOFC) in BD adults compared to controls, although differences were found between depressed and euthymic BD adults. Thus, it is possible that there are diagnosis-specific effects on the brain / behavior interactions underlying reversal learning in child psychiatric disorders. Our future work is geared toward addressing both possibilities.
Although highly speculative, there are several potential clinical implications of our present study. Our data potentially suggest inefficient functioning of a series of regions implicated in attentional control and response selection. In turn, this suggests the possibility that BD youths may have difficulty maximally utilizing psychotherapies relying on such cognitive capacities. However, studies have demonstrated that when such therapies are modified to address BD-specific deficits in cognitive flexibility—e.g., when they include skills training to address impaired problem solving and affect regulation—they have great promise in the treatment of BD. Such therapies include cognitive behavioral therapy [studied in BD adults and children (67)], family-focused therapy [studied in BD children (68)], and interpersonal social rhythm therapy [studied in BD adults (69)]. In this context, it is also interesting to consider whether the brain / behavior interactions underlying cognitive flexibility and reversal learning impairments in pediatric BD may respond to training, such as the use of specialized computer games for `cognitive remediation' (70, 71).
There are several caveats and limitations of our study. First, while we previously found impaired performance on reversal learning paradigms in children with BD (15–17, 53), we observed no significant group differences in behavior here. This contrast with previous behavioral studies likely reflects task differences between the current fMRI study and previous behavioral work. Importantly, the failure to find between-group behavioral differences eliminates the possibility that our fMRI results are epiphenomena of, or are confounded by, group differences in task performance. Along these lines, it is common for fMRI studies in psychiatric patients to find between-group differences in neural activity without between-group behavioral differences; an example is the recent study in children with psychopathy versus controls that used the same reversal learning paradigm as here (7). Indeed, some have argued that fMRI is more sensitive than behavior in detecting important between-group differences (72). On the other hand, others suggest that the absence of between-group behavioral differences complicates attempts to link the observed differences in brain activity to symptoms (73). Although from this perspective, the lack of between-group behavioral differences might call into question whether reversal tasks engage neural circuitry relevant to the pathophysiology of BD, ample evidence, including our own work in pediatric BD and that of others in BD adults, documents that reversal learning and cognitive flexibility deficits are present in BD (74–76).
Other potential limitations of our study include potential heterogeneity in the BD sample, including that due to psychiatric comorbidities, psychotropic medications, BD subtypes, and possible subsyndromal mood symptoms, as well as the wide age range of our participants. All of our BD youth had one or more co-occurring psychiatric disorders, most commonly ADHD or anxiety disorders. This is consistent with prior research showing that both pediatric and adult BD are characterized by high rates of comorbidity (77–80). However, our exploratory secondary analyses showed that our primary findings between patients and controls remained significant even when restricting the BD sample to those without ADHD (n = 6) or to those without an anxiety disorder (n = 10), suggesting that our results are not confounded by comorbid ADHD or anxiety. A study of adolescents with ADHD found no atypical neural response associated with acquisition correct versus reversal incorrect responses when performing this same reversal learning task (7). Hence, our results here add to an emerging body of literature suggesting that ADHD presenting in the context of BD may be a phenocopy of ADHD presenting alone (14, 81). Nonetheless, further work is necessary to directly evaluate the specificity of brain / behavior interactions underlying reversal learning in pediatric BD. For example, such work might compare directly youth with BD to those with either primary ADHD or primary anxiety disorders, as well as comparing BD youth with versus without comorbid ADHD and BD youth with versus without comorbid anxiety (82, 83). Similarly, our post-hoc analyses excluding the 1 / 16 BD participants with type II BD (rather than type I), confirmed our primary result. However, further work to explore how BD subtypes differ in the brain / behavior interactions underlying reversal learning is warranted. In addition, while all of our participants were euthymic by the definition of having CDRS scores<40 and YMRS scores <12, we cannot rule out the possibility that subsyndromal mood symptoms were nonetheless present and impacted on our findings (84, 85). However, the lack of significant correlations between neural activation during reversal and YMRS or CDRS scores suggests that subsyndromal mood effects are less likely. Moreover, since the K-SADS was administered at enrollment and not at scan day, we cannot say for certain that BD participants did or did not meet K-SADS mania or depressive episode criteria on the scan day; however, it is highly unlikely given their mood (YMRS, CDRS) scores.
With respect to medications, most of our BD participants (13/16) were taking a combination of psychotropic medications, with no single agent predominating. To begin addressing the potential confound of medications on our results, we conducted exploratory analyses of those BD youth taking either atypical antipsychotic or anticonvulsant medications compared to those not, since 50% of the BD participants were taking either medication. Such analyses failed to show any difference in group-by-phase neural activation among BD youth based on either medication category. Recent evidence suggests that medication may bias toward type II, rather than type I, error (86). Also, given the variety of our patients' medication regimens, it is unlikely that medications would account for the consistent and relatively specific findings that we observed. Nevertheless, additional studies that pair treatment and neuroimaging in their design are needed to examine whether pharmacotherapy can reverse the cognitive flexibility deficits present in BD and, if so, the neural mechanisms that might account for this effect.
A final potential limitation is the age range of our participants. Specifically, we recruited pediatric BD and control participants across a broad age range during which a considerable amount of neural development occurs. For example, longitudinal structural neuroimaging studies have demonstrated the dynamic changes in PFC as children develop into adolescents and young adults (87). Additionally, comparing cross-sectional and longitudinal fMRI data using a target detection task has shown a developmental shift from focal to diffuse PFC activation (88). In the present study, in which BD and control groups were matched for age, secondary analyses do not show developmental effects as evidenced by no significant age or Tanner pubertal stage correlations. Nevertheless, the sample is too small to fully examine developmental effects, and it is likely that development may impact the brain / behavior interactions underlying reversal learning. Larger studies, with sufficient power to examine age effects, would thus likely be of interest from both clinical and research perspectives.
Conclusion
Our current study suggests that, during reversal learning, BD youths inefficiently recruit regions associated with processing response conflict and implementing alternative responses, including several subdivisions of the frontal cortex (dmFC, iFC, lsFC) and parietal cortex. Further work is necessary to determine the impact of treatment, psychiatric comorbidity, and development on reversal learning.
Acknowledgements
This research was supported by the NIMH Division of Intramural Research Programs, K22 MH74945 (PI: DPD, sponsor: EL), and a NARSAD Young Investigator Award (PI: DPD, sponsor: EL). We gratefully thank all of the participants and their families, without whom this study would not be possible. We also truly appreciate the work of the NIMH DIRP's Section on Bipolar Spectrum Disorders and NIH's fMRI facility.
Footnotes
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Rucklidge JJ. Psychosocial functioning of adolescents with and without paediatric bipolar disorder. J Affect Disord. 2006;91:181–188. doi: 10.1016/j.jad.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 5.Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stemme A, Deco G, Busch A, Schneider WX. Neurons and the synaptic basis of the FMRI signal associated with cognitive flexibility. Neuroimage. 2005;26:454–470. doi: 10.1016/j.neuroimage.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Finger EC, Marsh AA, Mitchell DG, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision (DSM-IV-TR) American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- 9.Blair RJ, Cipolotti L. Impaired social response reversal. A case of `acquired sociopathy'. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 10.Leibenluft E, Blair RJ, Charney DS, Pine DS. Irritability in pediatric mania and other childhood psychopathology. Ann NY Acad Sci. 2003;1008:201–218. doi: 10.1196/annals.1301.022. [DOI] [PubMed] [Google Scholar]
- 11.Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 12.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 13.Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry. 2001;158:1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- 14.Dickstein DP, Garvey M, Pradella AG, et al. Neurologic examination abnormalities in children with bipolar disorder or attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;58:517–524. doi: 10.1016/j.biopsych.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- 16.Dickstein DP, Nelson EE, McClure EB, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- 17.Dickstein DP, Finger EC, Brotman MA, et al. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010;40:1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34:1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 19.O'Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards and choices. Ann NY Acad Sci. 2007;1121:254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- 20.Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neuroscience. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 22.Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci. 2003;23:9833–9841. doi: 10.1523/JNEUROSCI.23-30-09833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell DG, Luo Q, Avny SB, et al. Adapting to dynamic stimulus-response values: differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. J Neuroscience. 2009;29:10827–10834. doi: 10.1523/JNEUROSCI.0963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulus MP, Hozack N, Zauscher B, et al. Prefrontal, parietal, and temporal cortex networks underlie decision making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- 26.Huettel SA. Behavioral, but not reward, risk modulates activation of prefrontal, parietal, and insular cortices. Cogn Affect Behav Neurosci. 2006;6:141–151. doi: 10.3758/cabn.6.2.141. [DOI] [PubMed] [Google Scholar]
- 27.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while medial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 29.Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26:609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Strakowski SM, Delbello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 31.Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Sem Clin Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- 32.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Sassi RB, Brambilla P, Hatch JP, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Lyoo IK, Kim MJ, Stoll AL, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 36.Frazier JA, Breeze JL, Makris N, et al. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005;7:555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur S, Sassi RB, Axelson D, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- 38.Ahn MS, Breeze JL, Makris N, et al. Anatomic brain magnetic resonance imaging of the basal ganglia in pediatric bipolar disorder. J Affect Disord. 2007;104:147–154. doi: 10.1016/j.jad.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Gogtay N, Ordonez A, Herman DH, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 40.Najt P, Nicoletti M, Chen HH, et al. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 2007;413:183–186. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 42.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 43.Rubinsztein JS, Fletcher PC, Rogers RD, et al. Decision making in mania: a PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- 44.Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Hassel S, Almeida JR, Kerr N, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killgore WD, Gruber SA, Yurgelun-Todd DA. Abnormal corticostriatal activity during fear perception in bipolar disorder. Neuroreport. 2008;19:1523–1527. doi: 10.1097/WNR.0b013e328310af58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 48.Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 51.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson EE, Vinton DT, Berghorst L, et al. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disord. 2007;9:810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 53.Dickstein DP, Treland JE, Snow J, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 54.Geller B, Williams M, Zimerman B, Frazier J, Beringer L, Warner KL. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord. 1998;51:81–91. doi: 10.1016/s0165-0327(98)00175-x. [DOI] [PubMed] [Google Scholar]
- 55.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 56.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 57.Emslie GJ, Weinberg WA, Rush AJ, Adams RM, Rintelmann JW. Depressive symptoms by self-report in adolescence: phase I of the development of a questionnaire for depression by self-report. J Child Neurol. 1990;5:114–121. doi: 10.1177/088307389000500208. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman J, Birmaher B, Brent D, Rao U. Schedule for affective disorders and schizophrenia for school aged children (6–18 years)—present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 59.Shaffer D, Gould MS, Brasic J, et al. Children's Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 60.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- 61.Mitchell DG, Rhodes RA, Pine DS, Blair RJ. The contribution of ventrolateral and dorsolateral prefrontal cortex to response reversal. Behav Brain Res. 2008;187:80–87. doi: 10.1016/j.bbr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wessa M, Houenou J, Paillere-Martinot ML, et al. Frontostriatal overactivation in euthymic bipolar patients during an emotional go / nogo task. Am J Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- 63.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 64.Robinson JL, Monkul ES, Tordesillas-Gutierrez D, et al. Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Res. 2008;164:106–113. doi: 10.1016/j.pscychresns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Frazier JA, Hodge SM, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34:37–46. doi: 10.1093/schbul/sbm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nery FG, Chen HH, Hatch JP, et al. Orbitofrontal cortex gray matter volumes in bipolar disorder patients: a region-of-interest MRI study. Bipolar Disord. 2009;11:145–153. doi: 10.1111/j.1399-5618.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 67.Pavuluri MN, Graczyk PA, Henry DB, Carbray JA, Heidenreich J, Miklowitz DJ. Child- and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: development and preliminary results. J Am Acad Child Adolesc Psychiatry. 2004;43:528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL. A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch Gen Psychiatry. 2003;60:904–912. doi: 10.1001/archpsyc.60.9.904. [DOI] [PubMed] [Google Scholar]
- 69.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 70.Elgamal S, McKinnon MC, Ramakrishnan K, Joffe RT, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: a proof of principle study. Psychol Med. 2007;37:1229–1238. doi: 10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- 71.Tchanturia K, Davies H, Campbell IC. Cognitive remediation therapy for patients with anorexia nervosa: preliminary findings. Ann Gen Psychiatry. 2007;6:14. doi: 10.1186/1744-859X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- 74.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 75.Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med. 2000;30:1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- 76.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 77.Harpold TL, Wozniak J, Kwon A, et al. Examining the association between pediatric bipolar disorder and anxiety disorders in psychiatrically referred children and adolescents. J Affect Disord. 2005;88:19–26. doi: 10.1016/j.jad.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 78.Kessler RC, Rubinow DR, Holmes C, Abelson JM, Zhao S. The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychol Me. 1997;27:1079–1089. doi: 10.1017/s0033291797005333. [DOI] [PubMed] [Google Scholar]
- 79.Reich W, Neuman RJ, Volk HE, Joyner CA, Todd RD. Comorbidity between ADHD and symptoms of bipolar disorder in a community sample of children and adolescents. Twin Res Hum Genet. 2005;8:459–466. doi: 10.1375/183242705774310105. [DOI] [PubMed] [Google Scholar]
- 80.Sachs GS, Baldassano CF, Truman CJ, Guille C. Comorbidity of attention deficit hyperactivity disorder with early-and late-onset bipolar disorder. Am J Psychiatry. 2000;157:466–468. doi: 10.1176/appi.ajp.157.3.466. [DOI] [PubMed] [Google Scholar]
- 81.Lopez-Larson M, Michael ES, Terry JE, et al. Subcortical differences among youths with attention-deficit / hyperactivity disorder compared to those with bipolar disorder with and without attention-deficit / hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:31–39. doi: 10.1089/cap.2008.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adler CM, DelBello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord. 2005;7:577–588. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 83.Moore CM, Biederman J, Wozniak J, et al. Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: a proton magnetic resonance spectroscopy study. Am J Psychiatry. 2006;163:316–318. doi: 10.1176/appi.ajp.163.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clark L, Goodwin GM. State- and trait-related deficits in sustained attention in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:61–68. doi: 10.1007/s00406-004-0460-y. [DOI] [PubMed] [Google Scholar]
- 85.Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 86.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durston S, Davidson MC, Tottenham N, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]