Abstract
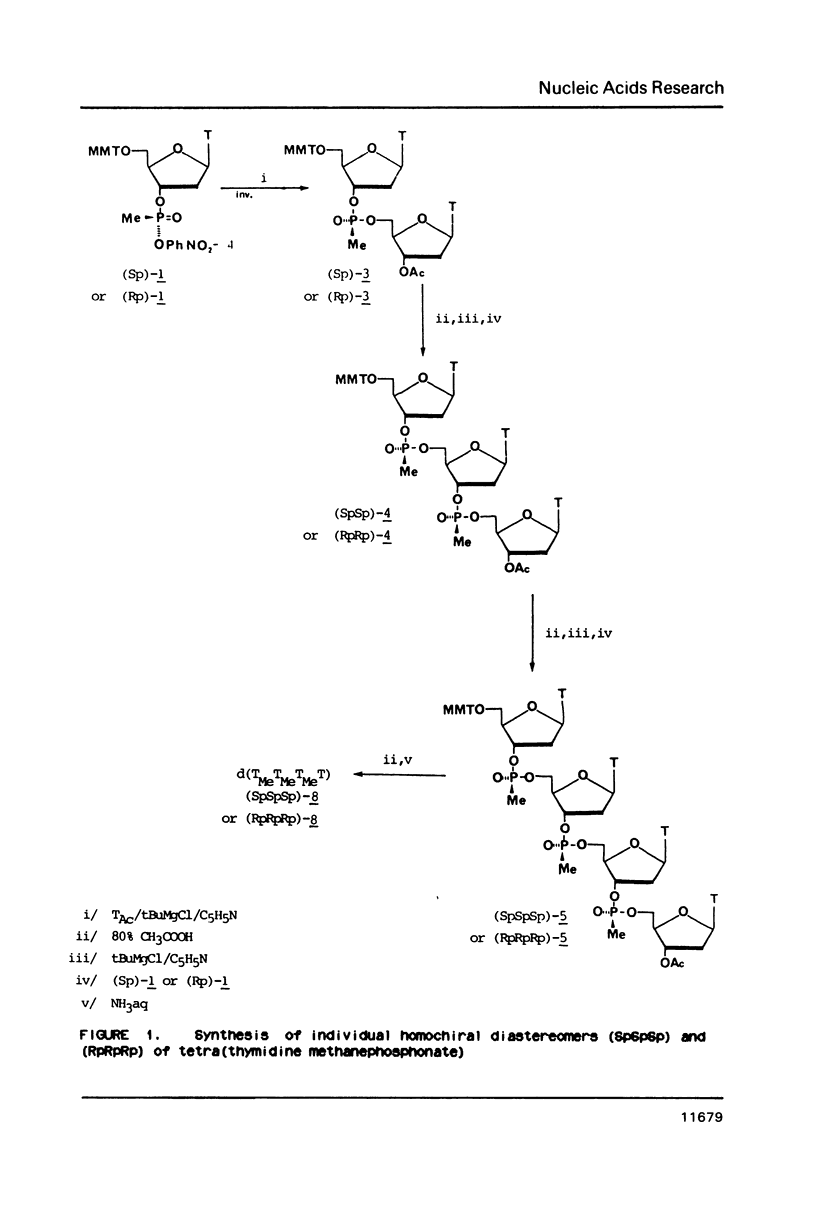
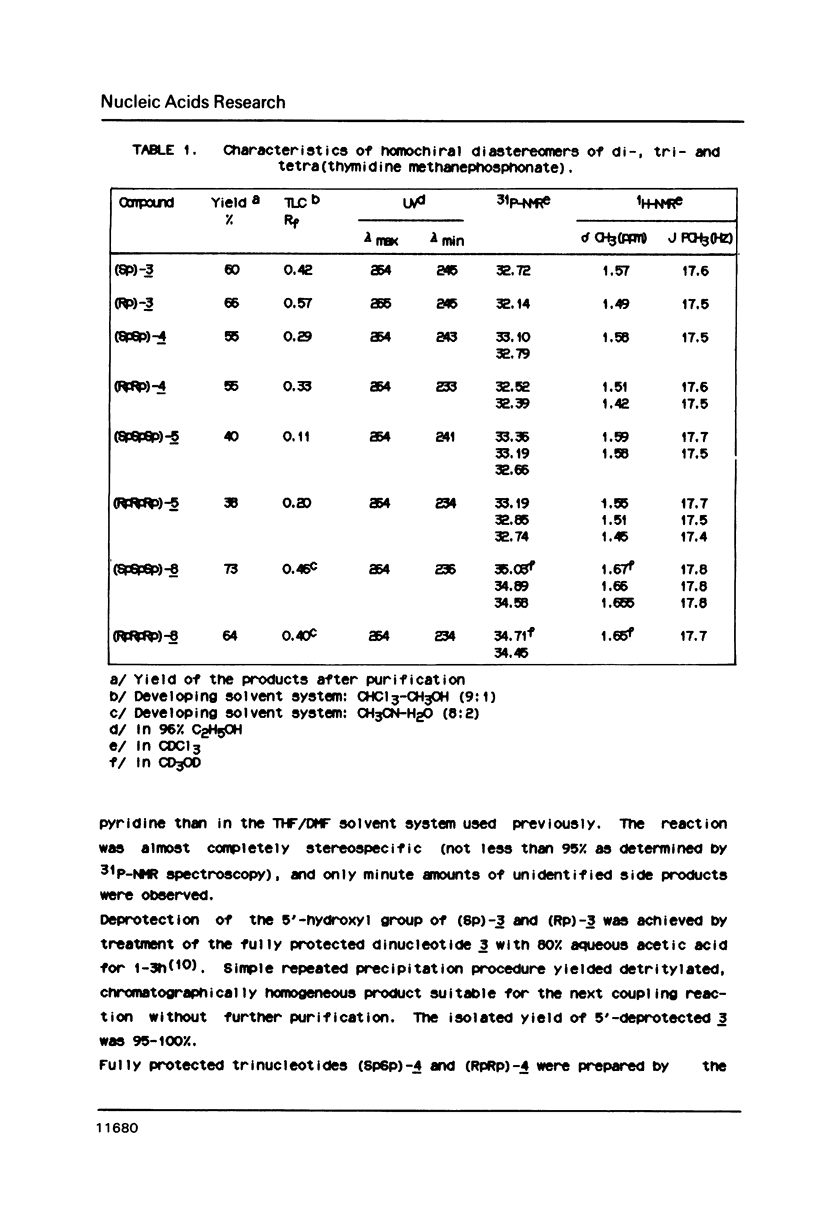
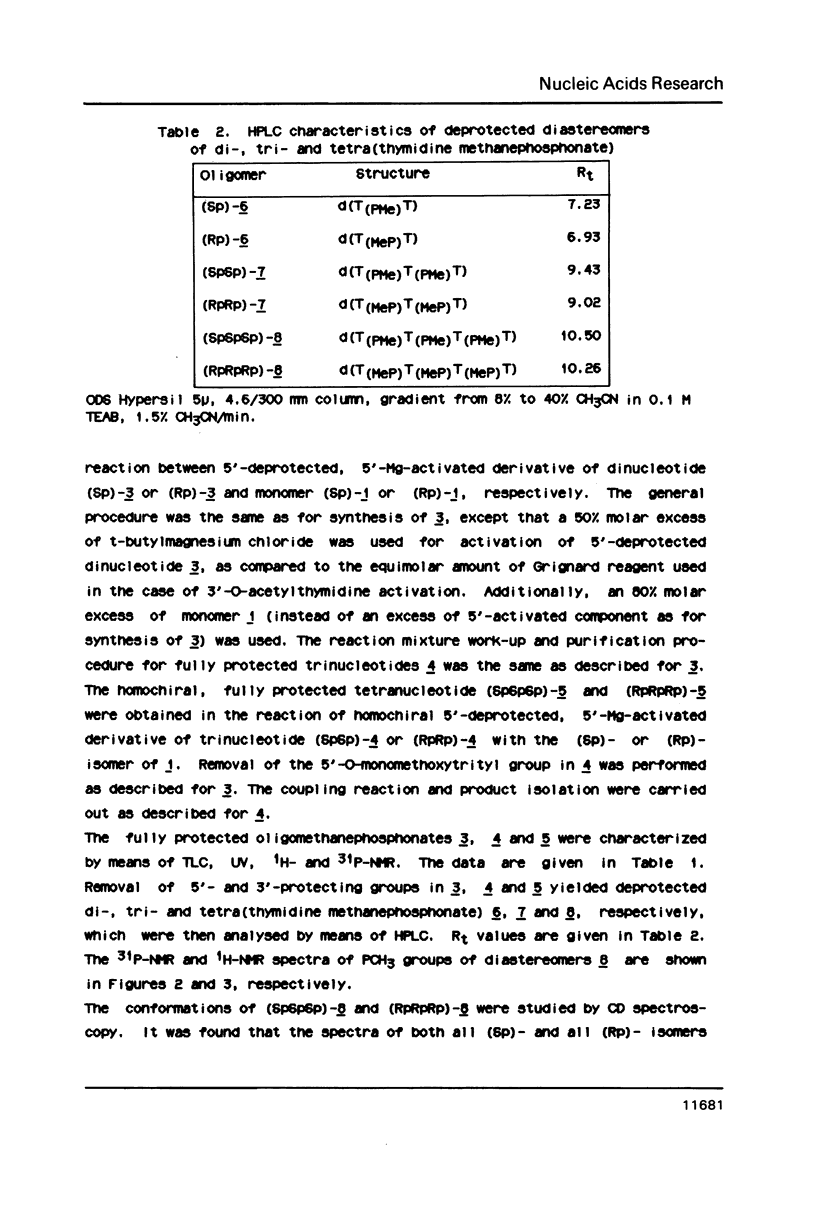
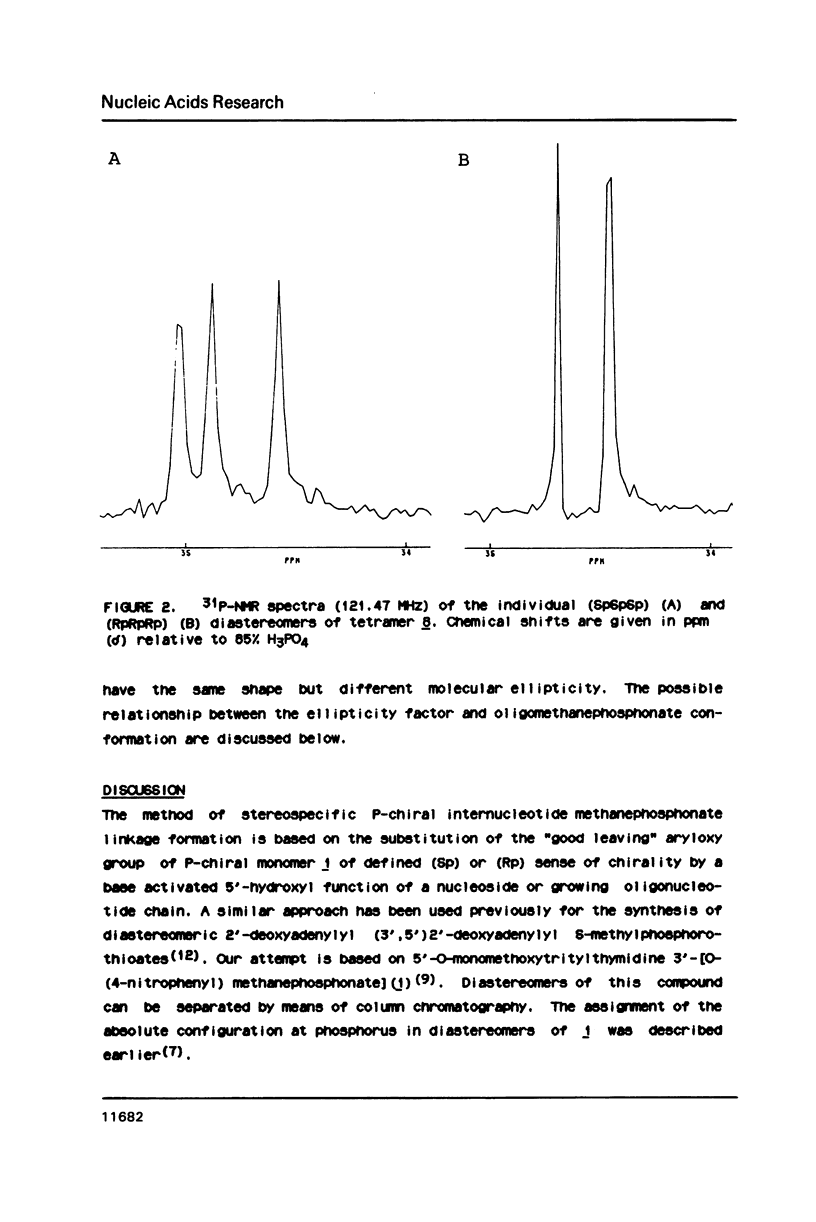
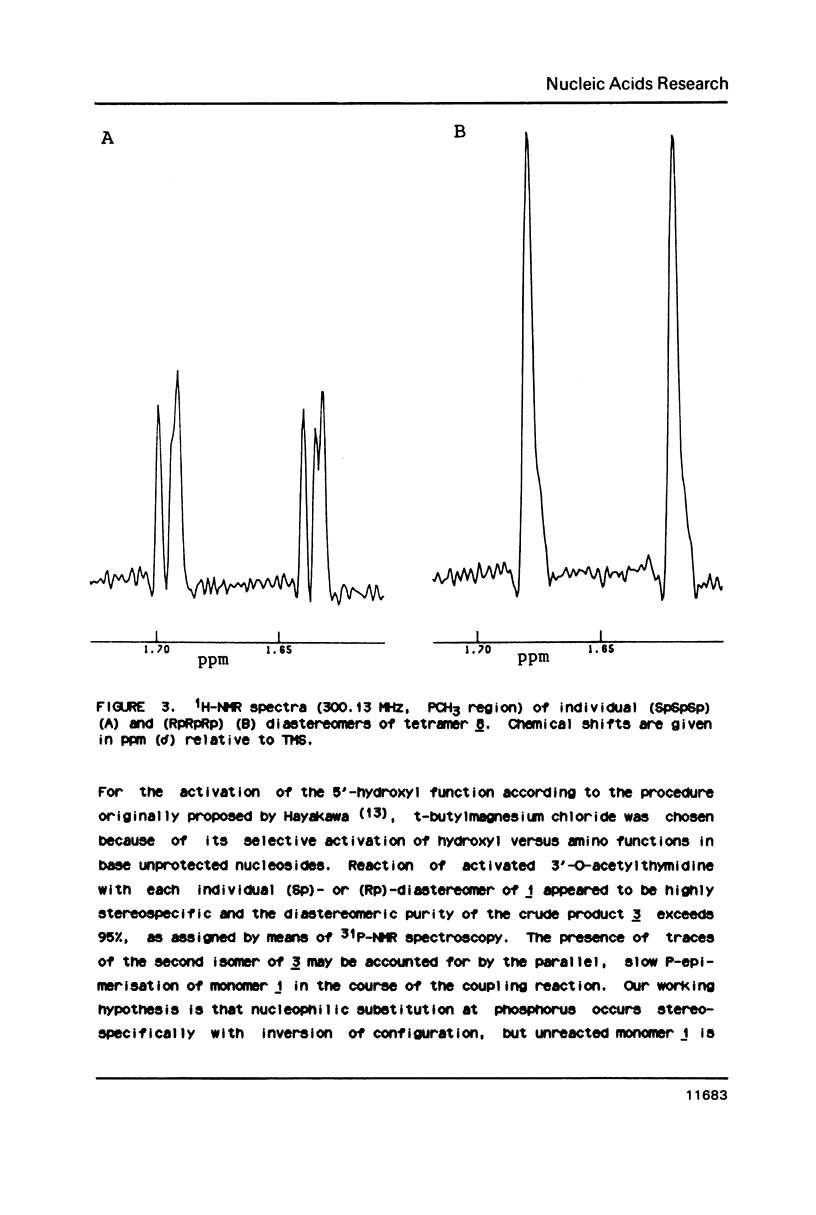
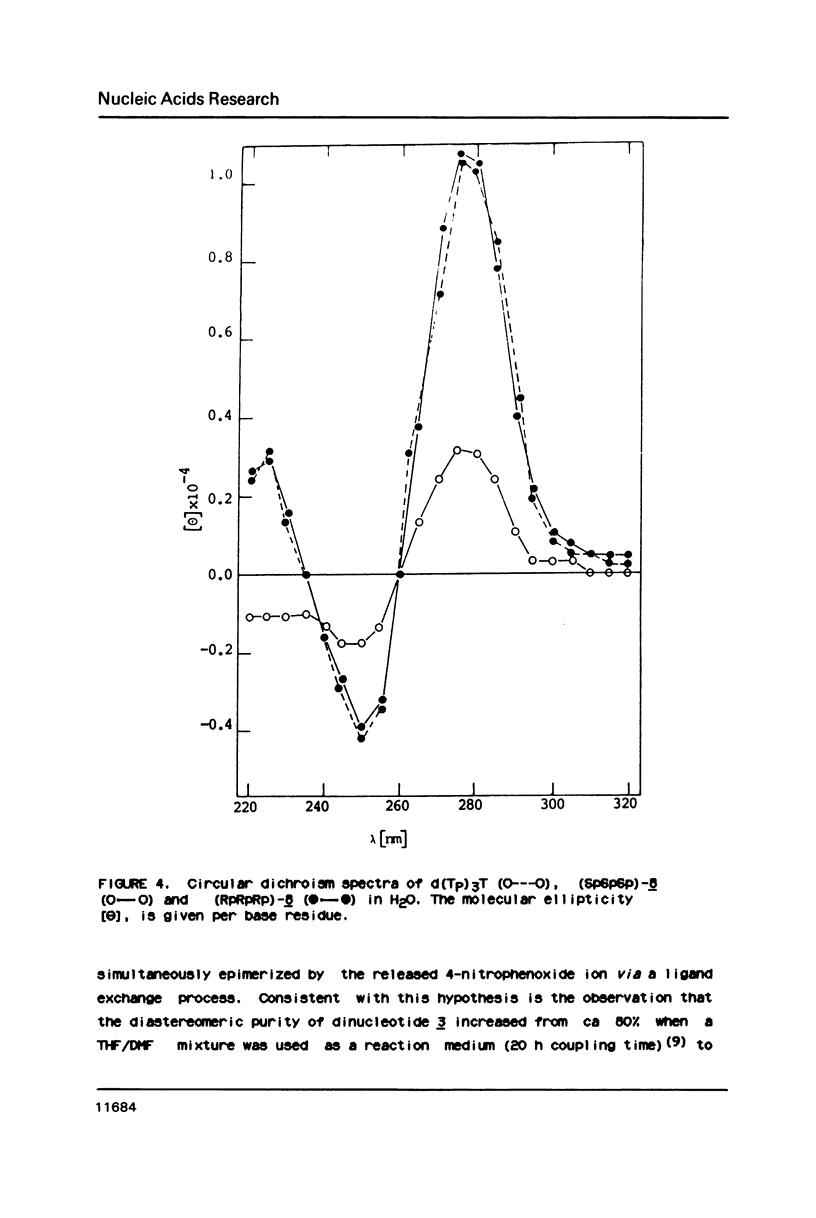
An approach to the stereoselective synthesis of P-homochiral oligo(thymidine methanephosphonates) is described. Fully protected (Rp)- and (Sp)-diastereomers of MMTrTPMeTAC (3) were prepared in the stereospecific reaction of P-chiral nucleotide component 5'-O-monomethoxytritylthymidine 3'-O-[O-(4-nitrophenyl)methanephosphonate] (1) and 3'-O-acetylthmydine (2) bearing activated 5'-hydroxyl function. Deprotection of the 5'-OH group in 3 and subsequent stepwise reactions of activated 5'-OH oligonucleotide components with (Rp)- or (Sp)- isomers of 1 gave the trinucleotide MMTrTPMeTPMeTAC (4) and, subsequently, the tetranucleotide MMTrTPMeTPMeTPMeTAC (5) possessing all (Rp)- or all (Sp)- configurations at their internucleotide methanephosphonate P-atoms.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake K. R., Murakami A., Spitz S. A., Glave S. A., Reddy M. P., Ts'o P. O., Miller P. S. Hybridization arrest of globin synthesis in rabbit reticulocyte lysates and cells by oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1985 Oct 22;24(22):6139–6145. doi: 10.1021/bi00343a016. [DOI] [PubMed] [Google Scholar]
- Callahan L., Han F. S., Watt W., Duchamp D., Kézdy F. J., Agarwal K. B- to Z-DNA transition probed by oligonucleotides containing methylphosphonates. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1617–1621. doi: 10.1073/pnas.83.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerman N., Fawcett J. K., Cameran A. Molecular structure of a deoxyribose-dinucleotide, sodium thymidylyl-(5' yields to 3')-thymidylate-(5') hydrate (pTpT), and a possible structural model for polythymidylate. J Mol Biol. 1976 Nov 15;107(4):601–621. doi: 10.1016/s0022-2836(76)80086-1. [DOI] [PubMed] [Google Scholar]
- Chacko K. K., Lindner K., Saenger W., Miller P. S. Molecular structure of deoxyadenylyl-3'-methylphosphonate-5'-thymidine dihydrate, (d-ApT x 2H2O), a dinucleoside monophosphate with neutral phosphodiester backbone. An X-ray crystal study. Nucleic Acids Res. 1983 May 11;11(9):2801–2814. doi: 10.1093/nar/11.9.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D. M., Sarma R. H. Intimate details of the conformational characteristics of deoxyribodinucleoside monophosphates in aqueous solution. J Am Chem Soc. 1977 Oct 26;99(22):7333–7348. doi: 10.1021/ja00464a038. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Cheng D. M., Miller P. S., Yano J., Ts'o P. O. Proton nuclear magnetic resonance studies on dideoxyribonucleoside methylphosphonates. Biochemistry. 1980 May 13;19(10):2122–2132. doi: 10.1021/bi00551a020. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Bach S. A., Eadie J. S. Effects of pendant groups at phosphorus on binding properties of d-ApA analogues. Nucleic Acids Res. 1986 Apr 25;14(8):3487–3499. doi: 10.1093/nar/14.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. G., Edington B. V., Schendel P. F. Inducible repair of phosphotriesters in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7380–7384. doi: 10.1073/pnas.80.24.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Dreon N., Pulford S. M., McParland K. B. Oligothymidylate analogues having stereoregular, alternating methylphosphonate/phosphodiester backbones. Synthesis and physical studies. J Biol Chem. 1980 Oct 25;255(20):9659–9665. [PubMed] [Google Scholar]
- Miller P. S., Fang K. N., Kondo N. S., Ts'o P. O. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J Am Chem Soc. 1971 Dec;93(24):6657–6665. doi: 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- Pless R. C., Ts'o P. O. Duplex formation of a nonionic oligo(deoxythymidylate) analogue (heptadeoxythymidylyl-(3'-5')-deoxythymidine heptaethyl ester (d-(Tp(Et))7T)) with poly(deoxyadenylate). Evaluation of the electrostatic interaction. Biochemistry. 1977 Mar 22;16(6):1239–1250. doi: 10.1021/bi00625a033. [DOI] [PubMed] [Google Scholar]



