Abstract
We investigated serum total immunoglobulin E (IgE), IgG, and IgG1 levels in patients with and without echinococcosis-induced anaphylactic shock. This was a case-control study of 11 patients with echinococcosis-induced anaphylactic shock and 22 echinococcosis patients with cyst rupture but without anaphylactic shock. Blood was collected before surgery (T0), at the time of cyst rupture (T1), and shock (Tx), 1 h (T2), 1 day (T3), and 1 week (T4) after cyst rupture. Serum IgE, IgG, and IgG1 were determined by enzyme-linked immunosorbent assay. Serum IgE, IgG, and IgG1 levels were significantly higher in patients who developed anaphylactic shock at all time points. Increased pre-surgical IgG and IgG1 levels were identified to be a significant risk factors for developing anaphylactic shock. The results showed that a serum IgG concentration of 312.25 μg/mL could be used as a cut-off point to predict whether an echinococcosis patient would develop anaphylactic shock.
Introduction
Cystic echinococcosis (CE) infection remains very common in large parts of the world despite improved preventive and therapeutic measures.1 Anaphylactic shock that can result from echinococcosis cyst rupture either during surgery or caused by trauma or spontaneous cyst rupture is a severe complication with an incidence of about 4.6% of CE cases. Echinococcosis-induced anaphylactic shock usually develops rapidly and inappropriate treatment may lead to detrimental consequences and even death.2 Clinical observations indicate that echinococcosis patients who are susceptible to anaphylactic shock have a unique immune status.
The anaphylactic shock that results from cyst rupture is different from type I hypersensitivity in its clinical magnitude and immunological features. In addition, patients with echinococcosis-induced anaphylactic shock usually have poor responses to treatments for type I hypersensitivity.3 Indeed, these treatments may even result in a poor prognosis for these patients, which pose significant difficulties for its clinical prevention and treatment. Thus, a clinical method to determine which CE patients are at risk for developing anaphylactic shock subsequent to cyst rupture is critical.
Previous studies have examined the dynamic changes in echinococcus antigen-specific immunoglobulin G (IgG) subtypes during different clinical stages of echinococcosis, although these results were not useful for predicting the possibility of anaphylaxis.4 To clarify the pathogenesis of echinococcosis-induced anaphylactic shock, we analyzed the serum levels of total IgE, IgG, and IgG1 both during and after anaphylactic shock and compared these to echinococcosis patients without shock after cyst rupture.
Materials and Methods
Patients.
This was a case-control study that was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. Eleven consecutive patients with echinococcosis-induced anaphylactic shock in the peri-operative period were recruited from January 2008 to March 2010 (Shock group). We also included echinococcosis patients without anaphylactic shock after cyst rupture (N = 22) as the Control group. The patients in the Shock group were matched with those in the Control group at a ratio of 1:2 by cyst types and age. All patients provided written informed consent.
The Shock group included patients with echinococcosis of the liver or lung who developed anaphylactic shock after cyst rupture during surgery. The Control group included patients with echinococcosis of the liver or lung who did not develop anaphylactic shock after cyst rupture during surgery.
Patients with the following conditions were excluded from this study: 1) had concomitant infections; 2) developed anaphylactic shock caused by drugs, other parasites, or specific allergic diseases; 3) refused to participate in this study.
Criteria for the diagnosis of anaphylactic shock.
The following criteria were used to diagnose anaphylactic shock5: 1) Echinococcosis patients who previously had a negative skin prick test results to general antigens not related to echinococcus (patients were also screened for these antigens 1/2 hour before cyst surgery); 2) developed rapid decrease in blood pressure to < 80/50 mm of Hg (or systolic pressure was decreased by > 30%) at several minutes or hours after cyst rupture; and 3) before or concomitant with anaphylactic shock, patients usually had allergy-related symptoms, including high airway pressure, skin redness, itching, and subsequent massive urticaria and/or angioneurotic edema.
Methods used during surgical cyst removal.
Cystic echinococcosis type I and type II were classified according to the criteria of Caremani and others.6 This is an ultrasonographic classification. Type I, Simple CE: a) overall echo free; b) with fine echoes. Type II, Multiple CE: a) multiple contiguous cysts; b) multi-septated with rosette, honey-comb, or wheel-like pattern. Pulmonary echinococcosis does not have its own classification and is usually classified according to the criteria for hepatic echinococcosis. Pulmonary echinococcosis is often monocystic, and may also be ruptured or calcified.
At the time of surgery, the surgeon performing the procedure was blinded to all blood test results. Anesthesia was induced with midazolam (0.02–0.05 mg/kg), fentanyl (1–2 μg/kg), and vecuronium (0.1–0.15 mg/kg). Propofol (1–4 μg/mL) was used for anesthesia maintenance. Fentanyl was continuously administered (1–3 μg·kg-1·hr-1) and fentanyl (5–10 μg) and vecuronium were given intermittently. At 30 min before the end of surgery, fentanyl injection was discontinued. As a surgeon removed the cyst, sterile gauze was wrapped around the cyst to avoid the contents from spilling out. When there was high tension in the cyst, a syringe was used to remove part of the cyst fluid. Cyst rupture and fluid spillage were observed by naked eye. After surgery, patients were allowed to recover in the post-anesthesia care unit (PACU).
Based on the criteria for the diagnosis of anaphylactic shock, shock and anaphylactic shock because of other causes were excluded. Patients were grouped according to whether anaphylactic shock was present.5 Venous blood was obtained, kept at room temperature for 4 hr, and then centrifuged at 3,000 rpm for 10 min. The supernatant was added to an Eppendorf tube and stored at −80°C for later use.
Time points.
For both groups of patients, venous blood was obtained before surgery (T0), immediately after cyst rupture (T1), 1 hr after cyst rupture (T2), 1 day after cyst rupture (T3), and 1 week after cyst rupture (T4). Additionally for shock patients, venous blood was obtained immediately after the onset of shock (Tx).
Serum antibody determinations.
We used enzyme-linked immunosorbent assay (ELISA) kits to determine the levels of serum IgE, IgG, and IgG1. ELISA kits (No. 200909) were from R&D Systems, Inc. (Minneapolis, MN). One day before analysis, the frozen blood samples were warmed at 4°C. Determinations for serum antibody levels were done according to the assay kit manufacturer's instructions. Optical density was measured at 450 nm and the background optical density was subtracted. Standard curves were generated using standards provided with the ELISA kits and used to calculate serum concentrations of IgE, IgG, and IgG1.
Statistical analysis.
Patient's ages are given as medians (interquartile ranges), and group genders are given as numbers (n) and percentages (%). Patients with echinococcosis with or without anaphylactic shock were compared by Mann-Whitney U test for age and by Fisher's exact test for gender. Serum antibody levels were logarithmically transformed because of their skewed distributions. Results for log-transformed serum antibody levels are given as means ± SD. Serum antibody levels between groups before surgery were compared by an independent two sample t test. Repeated measures, linear mixed models were used to determine group effects for changes in antibody levels before and after surgery. Logistic regression analysis with stepwise selection was used to determine the odds ratios (ORs) for significant parameters associated with the development of anaphylactic shock caused by ruptured cysts during surgery. A receiver operating characteristic curve was generated and the area under the curve was used to determine sensitivity and specificity. All statistical assessments were two-sided and P < 0.05 was considered significant. Statistical analyses used SPSS 18.0 statistical software (SPSS Inc., Chicago, IL).
Results
A total of 11 consecutive patients with echinococcosis who developed anaphylactic shock caused by ruptured cysts during surgery were enrolled from January 2008 to March 2010. An age-matched and cyst type-matched control group composed of echinococcosis patients who did not develop anaphylactic shock after cyst rupture were also enrolled.
Table 1 shows the patients' demographic and clinical characteristics and serum IgE, IgG1, and IgG levels before surgery. The patients who did and did not develop anaphylactic shock were similar with regard to age, gender, and the types of echinococcosis. Patients who developed anaphylactic shock had significantly higher levels of IgE, IgG1, and IgG before surgery than those who did not develop anaphylactic shock (P < 0.05).
Table 1.
Patient demographics and antibody levels before surgery
| With anaphylactic shock (N = 11) | Without anaphylactic shock (N = 22) | P value | |
|---|---|---|---|
| Age, years* | 26 (4, 72) | 34 (5, 77) | 0.819 |
| Gender, n (%)† | 0.805 | ||
| Male | 5 (45.5) | 11 (50.0) | |
| Female | 6 (54.5) | 11 (50.0) | |
| Type of echinococcosis, n (%)† | 1.000 | ||
| Cystic echinococcosis I | 1 (9.1) | 2 (9.1) | |
| Cystic echinococcosis II | 4 (36.4) | 8 (36.4) | |
| Pulmonary echinococcus | 6 (54.5) | 12 (54.5) | |
| Antibody levels before operation‡ | |||
| log (IgE), μg/mL§ | 2.78 ± 0.30 | 2.40 ± 0.29 | 0.002 |
| log (IgG1), μg/mL§ | 1.97 ± 0.22 | 1.68 ± 0.25 | 0.002 |
| log (IgG), μg/mL§ | 2.56 ± 0.35 | 1.97 ± 0.70 | 0.014 |
| log (IgE/IgG1)§ | 0.80 ± 0.21 | 0.72 ± 0.36 | 0.508 |
P values are from * Mann-Whitney U test, † Fisher's exact test, and ‡ independent two sample t test.
Results are * medians (interquartile ranges), † numbers (percentages), and ‡ means ± SD.
Values are logarithmic transformations.
Cystic echinococcosis I and II were classified using the criteria of Caremani and others6 (see Materials and Methods).
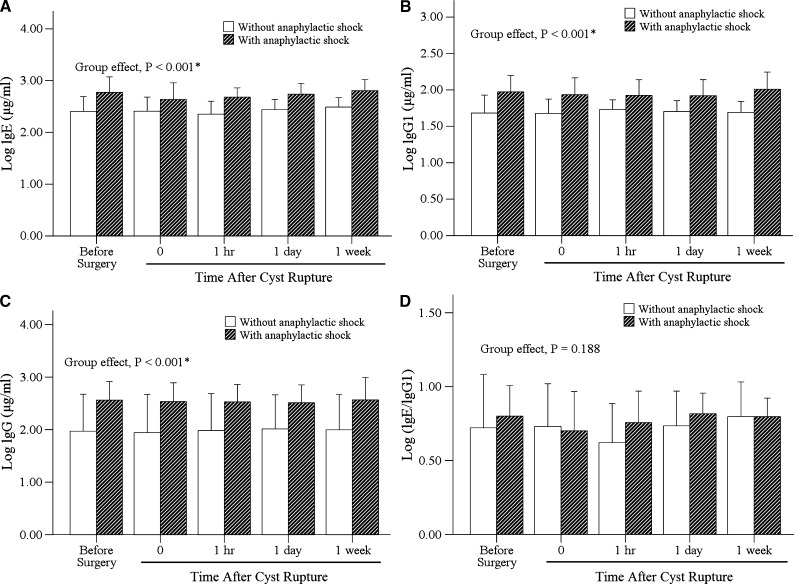
Figure 1 shows the changes in antibody levels for patients with and without anaphylactic shock. There were no significant changes in serum IgE, IgG1, IgG levels and the IgE/IgG1 ratio before and after surgery in both groups with and without anaphylactic shock (P > 0.05). However, there were significant differences in serum IgE, IgG1, and IgG levels between the two groups during the time course after surgery (all P < 0.001). Patients who developed anaphylactic shock had significantly higher levels of IgE, IgG1, and IgG than those who did not develop anaphylactic shock.
Figure 1.
Comparisons of antibody levels (logarithmically transformed) in echinococcosis patients with and without anaphylactic shock.
The significant risk factors associated with the development of anaphylactic shock caused by a ruptured cyst during surgery are shown in Table 2. After adjustments for age, gender, and the type of echinococcosis, serum levels of IgG1 (OR = 1.077, 95% confidence interval [CI]: 1.000–1.160; P = 0.049) and IgG (OR = 1.009, 95% CI: 1.001–1.017; P = 0.025) before surgery were significantly associated with the development of anaphylactic shock in patients with echinococcosis.
Table 2.
Logistic regression results to determine risk factors for developing anaphylactic shock in patients with echinococcosis
| OR (95% CI) | Adjusted OR (95% CI)* | P value | |
|---|---|---|---|
| Age | 0.999 (0.963, 1.036) | ||
| Gender | |||
| Male | Reference | ||
| Female | 1.200 (0.281, 5.124) | ||
| Type of echinococcosis | |||
| Cystic echinococcosis I | Reference | ||
| Cystic echinococcosis II | 1.000 (0.068, 14.640) | ||
| Pulmonary echinococcosis | 1.000 (0.075, 13.367) | ||
| Antibody levels before operation | |||
| IgE | 1.007 (1.001, 1.012)† | 1.004 (0.995, 1.013) | 0.436 |
| IgG1 | 1.046 (1.011, 1.082)† | 1.077 (1.000, 1.160) | 0.049† |
| IgG | 1.007 (1.001, 1.013)† | 1.009 (1.001, 1.017) | 0.025† |
Adjustments were made for age, gender, and type of echinococcosis.
Significant risk factor, P < 0.05.
Cystic echinococcosis I and II were classified using the criteria of Caremani and others6 (see Materials and Methods). OR = odds ratio; CI = confidence interval.
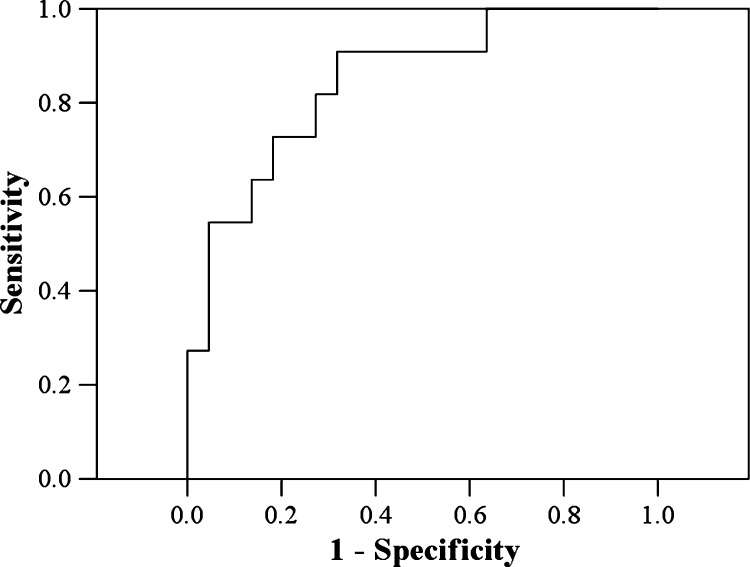
Because the IgG1 levels only showed borderline significance (P = 0.049), we chose IgG only to determine a cut-off point.Figure 2 shows the receiver operating characteristic curve from which the cut-off point for IgG was chosen. The area under the curve was 0.85 (P = 0.001; 95% CI: 0.71–0.99). The cut-off point for IgG was based on the maximum value of the Youden index, which was 312.25 μg/mL in this study. For IgG > 312.25 μg/mL, the development of anaphylactic shock was predicted with a sensitivity of 90.9% and a specificity of 68.2%.
Figure 2.
Receiver operating characteristic curve for determining if patients will develop anaphylactic shock caused by a ruptured cyst during surgery.
Discussion
Cystic echinococcosis is a parasitic disease caused by the larvae of Echinococcus granulosus that affects humans and other mammals. During E. granulosus infection, a host's immune response is quite complex due in large part to the numerous antigens that are present, particularly in cyst fluid.7 These antigens are often quite variable in nature and the immune response varies during different stages of E. granulosus infection, including host inflammatory responses to infection, the subsequent development of specific humoral and cellular immunity, allergic reactions, and immune evasion.
Thus, a host may present with anti-parasite immune responses and immune evasion, or immune protection and immunological injury may occur simultaneously.1 In the presence of host immune regulation, an E. granulosus infection usually becomes chronic. Echinococcosis granulosus can trigger a series of hypersensitive reactions, including urticaria, transient chills or fever, fatal bronchospasm, angioneurotic edema, and anaphylactic shock.8
It is considered that echinococcosis-induced anaphylactic shock is a type I immediate hypersensitivity. Miyajima and others9 have shown that systemic anaphylaxis is mediated in large part through IgG1 and FcγRII receptors. Other studies have confirmed that parasite antigen-induced shock or death depended not only on IgE, but also IgG, particularly IgG1.10 Related studies11 showed that IgG and IgG subtypes could induce complement activity. These were heterogeneous antibodies directed against the parasite antigen cyclophilin.
In this study, significant differences were found in the IgG levels between the two groups of patients at all time points. In addition, significant differences in the IgG1 levels were noted at all time points. Our logistic regression results for the likelihood of developing anaphylactic shock caused by cyst rupture showed that increased pre-surgical levels of both IgG and IgG1 were significant risk factors (Table 2). In this analysis, increased pre-surgical IgE levels were not useful for predicting susceptibility to anaphylactic shock caused by cyst rupture, possibly because IgE is not associated with complement activation. Although, IgE is associated with mast cell and basophil degranulation and the release of significant amounts of histamine.
Increased levels of IgG and IgG1 predicted the susceptibility to anaphylactic shock caused by cyst rupture in our CE patients. The production of antibodies by B cells or plasma cells is a function of T-helper (Th) lymphocytes of the Th2 subset.12 The particular antibody type or subtype that is produced by B cells is mediated by specific cytokines produced by Th2 cells during the process of immunoglobulin isotype class switching.13 However, the particular isotype that is produced is not necessarily a function of some disease process, because it has been shown that it is equally likely for people with and without allergic sensitivities to particular allergens to produce IgG1 antibodies specific for a particular allergen.14 However, what does differ is that people with an allergic sensitivity to a particular allergen produce greater amounts of allergen-specific IgG1 than those who are not allergically sensitized,15 which suggests that IgG1 is an antibody related to hypersensitivity.
The IgE/IgG1 ratio may be associated with allergic manifestations. In our shock group, the IgE/IgG1 ratio was comparable to that in the control group before surgery, after shock occurred, and at 1 week after surgery. Moreover, the IgE/IgG1 ratio was not different at different time points in both groups. These findings indicate the IgE/IgG1 ratio has no association with the occurrence, development, or prognosis of echinococcosis-induced anaphylactic shock.
This study had several limitations. Although our two groups of patients were age and gender matched, the total numbers were relatively small. The small number of patients and the rather large statistical variances (standard deviations) of the measured antibody levels may have contributed to the relatively low OR values shown in Table 2. Furthermore, we did not determine other biochemical variables possibly associated with anaphylactic shock. Most importantly, although we did find significant differences in the serum levels of total IgG and IgG1 between our CE patients who did and did not develop anaphylactic shock, these are only general indicators of immune responses. To identify the underlying mechanistic aspects will require determinations of antigen-specific immunoglobulin levels.
The diagnostic specificity of cystic echinococcosis is generally low. In this study, we diagnosed the occurrence of anaphylactic shock by total antibody concentrations instead of pathogen-specific antibodies. The low specificity of this diagnostic strategy is obvious. However, our aim was to be able to predict and prevent the occurrence of anaphylactic shock using a simple, easy, and economical strategy. The costs for determining total antibody concentrations are considerably less than for determining specific antibodies.
Taken together, increased levels of serum IgE, IgG, and IgG1 could predict the susceptibility to anaphylactic shock caused by cyst rupture in echinococcosis patients. IgG1 may be a hypersensitivity specific antibody and the IgG1 level may have value in predicting the prognosis of echinococcosis-induced anaphylactic shock. Furthermore, the IgE/IgG1 ratio had no association with the occurrence, development, or prognosis of echinococcosis-induced anaphylactic shock.
Footnotes
Financial support:This study was supported by the National Natural Science Foundation ofChina (no. 30960367, 2009) and theNatural Science Foundation of Xinjiang Uighur AutonomousRegion (no. 200821141, 2008).
Authors' addresses: Hong Zheng, Department of Anesthesiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: hanlihanyun@sina.com. Yimei Li, Department of Pain Medicine, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: hanyuner1969@163.com. Hong Zheng, Department of Anesthesiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: hanlihanyun@sina.com. Hao Wen, Hydatid Liver and gallbladder Surgery Department, Digestive Vascular Surgery Center, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: wenhao2002@hotmail.com. Meilin Gu, Department of Anesthesiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: drgumeiling@sina.com. Xinghua Cao, Department of Anesthesiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: drcaoxinghua@yahoo.com.cn. Zaoling Liu, College of Public Health, Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: yiliuzhimei@sohu.com. Tao Liu, Xinjiang Key Laboratory of Basic Medicine Research on Echinococcosis, Medical Research Center, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People's Republic of China, E-mail: LT02-16@163.com.
References
- 1.Siracusanao A, Teggi A, Ortona E. Human cystic echinococcosis: old problems and new perspectives. Interdiscip Perspect Infect Dis. 2009 doi: 10.1155/2009/474368. doi:10.1155/2009/474368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pu XM. Cysticercus induced severe allergy: a report of 27 cases. Chinese J Parasitol Parasitic Dis. 1989;7:170–173. [Google Scholar]
- 3.Li Y, Zheng H, Cao X, Lui Z, Chen L. Demographic and clinical characteristics of patients with anaphylactic shock after surgery for cystic echinococcosis. Am J Trop Med Hyg. 2011;85:452–455. doi: 10.4269/ajtmh.2011.10-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tappe D, Sako Y, Itoh S, Frosch M, Grüner B, Kern P, Ito A. Immunoglobulin G subclass responses to recombinant Em18 in the follow-up of patients with alveolar echinococcosis in different clinical stages. Clin Vaccine Immunol. 2010;17:944–948. doi: 10.1128/CVI.00026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson HA, Munoz-Furlong A, Bock SA, Schmitt C, Brass R, Chowdhury BA, Decker WW, Furlong TJ, Galli SJ, Golden DB, Gruchalla RS, Harlor AD, Jr, Hepner DL, Howarth M, Daplan AP, Levy JH, Lewis LM, Lieberman PL, Metcalfe DD, Murphy R, Pollart SM, Pumphrey RS, Rosenwasser LJ, Simons FE, Wood JP, Camargo CA., Jr Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol. 2005;115:584–591. doi: 10.1016/j.jaci.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Caremani M, Benci A, Maestrini R, Accorsi A, Caremani D, Lapini L. Ultrasound imaging in cystic echinococcosis. Proposal of a new sonographic classification. Acta Trop. 1997;67:91–105. doi: 10.1016/s0001-706x(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 7.Annen JM, Kohler P, Ecker J. Cytotoxicity of Echinococcus granulosus cyst fluid in vitro. Zeitschrift fur Parasitenkunde. 1981;65:79–88. doi: 10.1007/BF00926556. [DOI] [PubMed] [Google Scholar]
- 8.Pawlowski ZS. Critical points in the clinical management of cystic echinococcosis: a revised review. In: Andersen FL, Ouhelli H, Kachani M, editors. Compendium on Cystic Echinococcosis. Provo, UT: Brigham Young University Print Services; 1997. pp. 199–235. [Google Scholar]
- 9.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Gallli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gamma R III. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE-or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng H, Xu ZX, Yang GX, Wen H. Study on the level of specific IgG, IgG1 and IgE during anaphylactic shock in sheep induced by Echinococcus granulosus. Chinese J Parasitol Parasitic Dis. 2003;21:42–45. [PubMed] [Google Scholar]
- 11.Ortona E, Vaccari S, Margutti P, Delunardo F, Rigano R, Profumo E, Buttari B, Rasool O, Teggi A, Siracusano A. Immunological characterization of Echinococcus granulosus cyclophilin, an allergen reactive with IgE and IgG4 from patients with cystic echinococcosis. Clin Exp Immunol. 2002;128:124–130. doi: 10.1046/j.1365-2249.2002.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy KM, Ouyany W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 13.Manis JP, Tian M, Alt FW. Mechanism and control of class-switch recombination. Trends Immunol. 2002;23:31–39. doi: 10.1016/s1471-4906(01)02111-1. [DOI] [PubMed] [Google Scholar]
- 14.Jackola DR, Pierson-Mullany LK, Liebeler CL, Blumenthal MN, Rosenberg A. Variable binding affinities for allergen suggests a ‘selective competition’ among immunoglobulins in atopic and non-atopic humans. Mol Immunol. 2002;39:367–377. doi: 10.1016/s0161-5890(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 15.Liebeler CL, Basu S, Jackola DR. Allergen-specific IgG1 provides parsimonious heritability estimates for atopy-associated immune responses to allergens. Hum Immunol. 2007;68:113–121. doi: 10.1016/j.humimm.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]