Abstract
Triatoma infestans and Panstrongylus megistus are relevant Chagas disease vectors. An apparent segregation among these triatomine species inside human households was suggested to rely on mutual repellence between them. However, P. megistus and T. infestans show aggregation responses to chemical signals emitted by the other species. These findings do not rule out the possibility that stimuli other than chemical signals could mediate repellence when these species exploit shelters simultaneously. In the present study, we investigated how P. megistus and T. infestans exploit shelters in controlled laboratory conditions and how insect density and environmental illumination modulate this behavior. We evaluated whether these species aggregate inside shelters or mutually repel each other. Panstrongylus megistus and T. infestans show specific patterns of shelter exploitation, which are differentially affected by insect density and environment illumination. In particular, P. megistus is more sensitive to insect density than T. infestans, whereas T. infestans shows higher sensitivity to illumination than P. megistus. Nevertheless, these species exploit shelters randomly without any apparent repellence.
Introduction
Triatoma infestans (Klug, 1834) is the most widespread domestic vector of Chagas disease in the southern cone of South America.1 It is also considered the main vector of Trypanosoma cruzi for humans.1 This species apparently originates from two areas: the Bolivian Andes, where sylvatic foci have been found consistently, and the Dry Chaco area of Argentina, Bolivia, and Paraguay.2–4 Triatoma infestans was apparently introduced in Brazil by human migration and it only occupies peridomiciliary and intradomiciliary ecotopes in this country.5 Because of high densities of domiciliary infestation and prevalence of Chagas disease associated with this species, T. infestans was considered the most important triatomine vector in Brazil, although it is not autochthonous. With the expansion of the Vectorial Control Program implemented by the Supervision of Health Campaigns, Ministry of Health, Brasilia, Brazil, in the early 1980s, this species was then eliminated from vast areas of the country.6
The current epidemiologic situation regarding Chagas disease indicates that Panstrongylus megistus (Burmeister, 1835) is one of the most important vector species in Brazil. Insects of this species can be found in sylvatic or domestic environments throughout their domain area, and they exhibit two types of behavior. In preserved forest areas, insects do not tend to invade artificial ecotopes.7,8 However, in areas where the sylvatic environment has been highly degraded, they are found mostly in domiciliary ecotopes.9,10 Studies on population diversity of P. megistus in different regions in Brazil reported distinct degrees of adaptation to domestic habitats.11–15 In northeastern states such as Bahia, P. megistus is well adapted to domestic habitats, but its presence in sylvatic foci has not been proven.11,12 However, in southeastern states, it can be found in sylvatic and domestic habitats. In southern states, P. megistus seemed to be predominantly sylvatic,11,12 but domestic infestation by this species has been reported.16,17
The distribution of T. infestans and P. megistus in Brazil overlapped to a large extent during the 1950s. Moreover, simultaneous colonization of human households by both species was reported during that time.18 However, this co-habitation was not observed by other authors, who suggested that T. infestans did not share domestic ecotopes with autochthonous triatomines.19 After the introduction of T. infestans into Brazil, domiciliary colonization by P. megistus was reduced, suggesting that T. infestans expelled P. megistus from domiciliary ecotopes.20 The elimination of T. infestans20 by insecticide spraying in southeastern states21–23 apparently facilitated the process of domiciliary colonization by sylvatic P. megistus. A drastic decrease in T. infestans detection inside households led to a concomitant increase in the number of P. megistus captured at these ecotopes.5 These epidemiologic data indicate that P. megistus competed at a disadvantage with T. infestans and suggest that T. infestans was better adapted to domiciliary ecotopes than P. megistus.
Triatoma infestans is more efficient than P. megistus in obtaining blood meals on non-anesthetized mice, possibly because it is more competent in avoiding host irritation.24,25 In addition, when T. sordida and T. infestans cohabitate under laboratory conditions, T. sordida become extinct after six months, whereas T. infestans are apparently not affected.26 Because T. infestans obtains blood meals more efficiently than T. sordida,26 a competitive process on the access to blood meals may help to explain why mixed populations of T. infestans and other species are not usually observed.
Species-specific chemical signals may also be responsible for the lack of simultaneous colonization by T. infestans and other species, but the literature on this subject is somewhat contradictory. Neves and Paulini27 suggested that odors and feces of T. infestans, T. sordida, and P. megistus mediate repellence between insects of these species. However, Lorenzo and others28 showed that aggregation signals emitted by dry excrement of T. infestans or T. sordida are capable of promoting cross-aggregation. Additionally, substances emitted by feces and cuticles of T. infestans and P. megistus promote interspecific aggregation responses.29
Despite this extensive literature on the cohabitation patterns of different Chagas disease vectors, no studies have investigated whether T. infestans and P. megistus actually aggregate inside shelters or tend to repel each other. The choice and subsequent exploitation of shelters by triatomines is mediated by diverse stimuli and behaviors, such as negative phototaxis, positive thigmotaxis, preference for low relative humidity, and distinct chemical signals.30–35 Although interspecific responses to fecal and cuticular chemical signals between T. infestans and P. megistus is well documented,29 whether other stimuli could be responsible for a mutual repellence process between cohabitating insects of these species is still unknown.
In the present study, we tested whether T. infestans and P. megistus repel each other when exploiting the same environment in which two shelters are available. We first compared specific patterns of shelter exploitation displayed by each species and analyzed how insect density and a light cycle affected them. We also evaluated whether insects of each species distribute homogeneously between two available shelters or tend to present exclusive aggregation in a single refuge. Finally, insects of both species were released together under the same conditions to test whether one species would induce a change in the pattern of shelter exploitation displayed by the other species.
Materials And Methods
Insects.
Panstrongylus megistus and T. infestans colonies originated from insects captured at domiciliary and peridomiciliary ecotopes in Minas Gerais State, Brazil. They were reared at the Laboratory of Triatomines and Chagas Disease Epidemiology (René Rachou Research Center, FIOCRUZ, Belo Horizonte, Brazil). Insects fed weekly on live chicken (Gallus gallus). Fifth instar larvae starved for seven days after ecdysis were used for all assays. Colonies were kept in a rearing chamber with controlled temperature and a 12:12 hour light:dark illumination regimen provided by artificial lights.
Effect of insect density.
In this experiment, we evaluated whether insect density affects the proportion of insects that choose to stay inside shelters. Assays were performed separately for each species. The number of insects found inside and outside a shelter was recorded when 20, 40, 60, 80, or 100 insects of one of the species were released in the experimental arena.
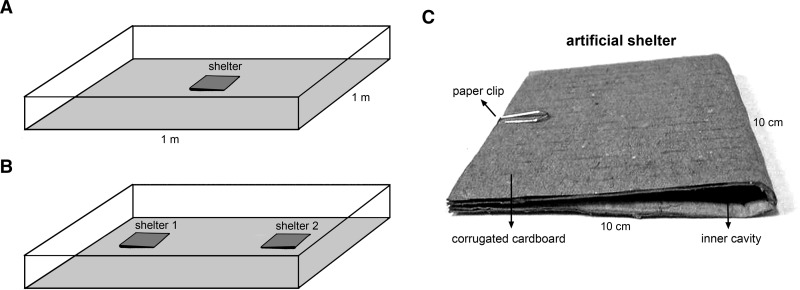
A square glass arena with an area of 1 meter2 was used for this experiment (Figure 1A). Its substratum was covered with Kraft paper and an artificial shelter was placed in the center of the arena (Figure 1A). The shelter (Figure 1C) consisted of a piece of corrugated cardboard (10 × 20 cm) folded in the middle to become a square (10 × 10 cm) with two lateral accesses and an inner cavity that was approximately 0.5 cm high.33,36 Assays were performed in a room at 25 ± 2°C and subjected to an artificial light cycle (12:12 hour light:dark) controlled by a timer clock. Each group of insects was released in the center of the arena two hours before the beginning of the dark phase. After four days, the shelter was carefully removed from the arena during the second hour of the light phase and the number of insects found inside and outside it was recorded.
Figure 1.
Experimental arena and artificial shelter. A, Scheme of the experimental arena used to analyze how insect density and environmental illumination modulate shelter exploitation. The square glass arena with an area of 1 meter2 had its substratum covered with Kraft paper and an artificial shelter placed in the center. B, Scheme of the experimental arena used to evaluate whether Panstrongylus megistus and Triatoma infestans aggregate inside shelters or repel each other. The same experimental arena was used, but two shelters were placed at opposite sides of the arena. C, Artificial shelter used for studies of shelter exploitation by triatomines.33,36 The shelter consisted of a piece of corrugated cardboard (10 × 20 cm) folded in the middle to become a square (10 × 10 cm) with two lateral accesses and an inner cavity that was approximately 0.5 cm high.
Three replicates were carried out for each insect density (20, 40, 60, 80, or 100 insects) and for each species. We applied two-way analysis of variance (ANOVA) (species versus density) to compare the mean proportion of insects outside the shelter. For each species, the mean proportion of insects found outside the shelter at different densities was compared by using one-way ANOVA. All variance analyses were followed by Tukey's multiple comparisons. Lastly, the mean proportion of insects outside the shelter was compared between both species for each density by using a t-test for independent samples.
Effect of illumination.
To test whether a light cycle affects the use of shelters by each species, a group of P. megistus or T. infestans was kept inside the same experimental arena with a central shelter (Figure 1A) for three days in complete darkness. Assays were performed in a room at 25 ± 2°C. A group of 40 insects was released in the center of the arena and remained in this environment for the duration of the experiment. After the first two hours of the fourth day, the number of insects found inside and outside the shelter (Figure 1C) was recorded. The results were then compared with those of the preceding experiment, when 40 larvae were released under a 12:12 hour light:dark cycle.
Three replicate assays were performed for each species (P. megistus or T. infestans) and each treatment (light cycle or constant darkness). We performed t-tests for independent samples to analyze whether there was a significant difference between 1) the mean number of T. infestans and P. megistus outside the shelter under the light cycle; 2) the mean number of T. infestans and P. megistus outside the shelter under constant darkness; and 3) the mean number of insects outside the shelter under the light cycle and under constant darkness for each species.
Aggregation or repellence between T. infestans and P. megistus.
Experiment 1.
This experiment determined whether P. megistus and T. infestans aggregate together and randomly inside shelters or if any repellence processes occur when insects of these species interact. The same arena with substratum covered with Kraft paper was used for these assays. For this experiment, we placed two shelters at opposite sides of the arena (Figure 1B). The assays were carried out in a room at 25 ± 2°C and an artificial illumination regimen (12:12 hours light:dark) controlled by a timer. At the beginning of each assay, 20 larvae of each species were released in the center of the arena. After seven days, the shelters (Figure 1C) were carefully removed and the number of insects of each species inside each shelter was recorded. Twelve assays were performed under these conditions.
Experiment 2.
This experiment tested whether previous presence of insects from one species inside a shelter would affect its suitability for insects of the other species. The same arena (Figure 1B) used in the previous experimental series was subjected to identical manipulation and environmental conditions. Twenty larvae of one of the species were first released in the center of the arena. After four days, 20 larvae of the other species were released in the center of arena and in the middle of the light phase. The experiment was then continued for three additional days. After the seventh day, shelters were carefully removed and the number of insects of each species found inside each shelter was recorded. Under these conditions, insects that were initially released could occupy both shelters, whereas insects from the second species would find these shelters already in use. Two experimental series with eight replicates each were performed: 1) with P. megistus or 2) with T. infestans released in the first place.
To better analyze the results obtained in this experiment, we developed a new experimental series based on the sequential release of two groups of P. megistus larvae. Our goal was to analyze whether the distribution pattern observed for P. megistus in this experimental series was similar to the one observed when it was released after T. infestans. We thus performed eight assays in which two groups of 20 P. megistus were released sequentially in the arena (Figure 1B) under the same conditions in which one species was released before or after the other species. First, 20 larvae of P. megistus were released. After three days, another group of 20 insects of the same species was released in the arena. The last group was discriminated by marking one of the back legs of the insects with yellow, non-toxic, acrylic ink (Azo Pigment; Alba, Buenos Aires, Argentina).
Control assays.
As a control for this experiment, we developed assays in which the distribution of insects of each of the species in both shelters was studied separately. For each assay, 20 larvae of one of the species were released in the arena (Figure 1B), thus keeping the same conditions of the preceding experiment. After seven days, the number of insects found inside each shelter was recorded. This series of assays was performed to compare the distribution of insects with that observed when larvae of both species were tested together. We performed eight replicates for each species.
Statistical analysis.
To compare the distribution of insects in shelters between the distinct experimental series, four treatments were considered for each species: 1) isolated in the arena (control); 2) simultaneously released with the other species (experiment 1); 3) released before the other species (experiment 2); and 4) released after the other species (experiment 2). For P. megistus, a fifth treatment was included in the analysis: 5) released in two phases (experiment 2). The distribution of insects in both shelters was considered for statistical analysis as the absolute difference between the number of insects in shelter 1 and the number of insects in shelter 2. For T. infestans, the mean difference between the four treatments was analyzed by using one-way ANOVA, followed by Tukey's multiple comparisons. Data for P. megistus did not meet the assumptions required for performing an ANOVA. Therefore, the Kruskal-Wallis test was first performed and followed by Dunn's multiple comparisons.
The absolute difference between the number of insects in shelter 1 and shelter 2 was also used to compare the distribution of T. infestans and P. megistus in both shelters when insects of these species cohabited in the arena (treatments 2, 3, and 4). For each treatment, the mean difference was compared for the two species by means of t-tests for independent samples. In those cases that did not meet the assumptions for a t-test, the Mann-Whitney test was performed. The mean number of insects outside shelters among different treatments was compared by using the Kruskal-Wallis test, followed by Dunn's multiple comparisons.
Results
Effect of insect density.
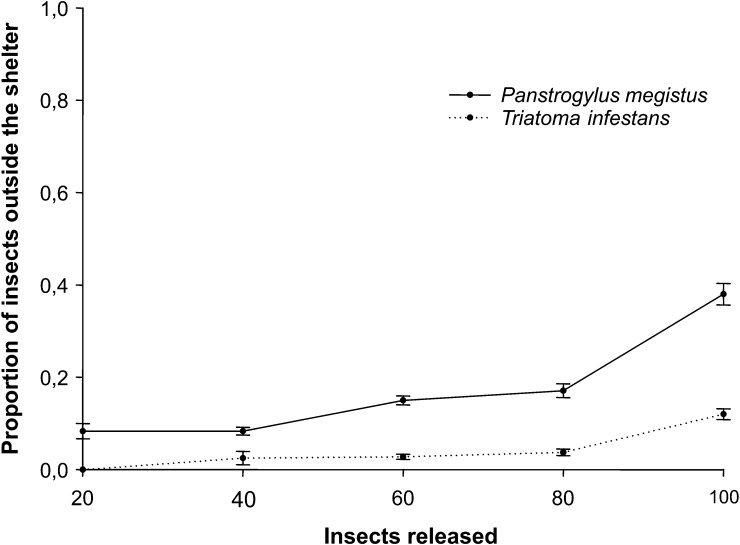
We analyzed how insect density affects the proportion of P. megistus or T. infestans that remain outside shelters. Variation in the proportion of insects found outside the shelter when increasing numbers of insects were released in the arena is shown in Figure 2. Panstrongylus megistus showed a global stronger tendency to remain outside the shelter than T. infestans (Figure 2). This finding was observed even at the lowest density, for which 9% of the insects of this species were found outside the shelter (Figure 2). The proportion of insects found outside the shelter was significantly higher for P. megistus (P < 0.001, by t-test) at all densities tested. Conversely, few T. infestans were found outside the shelter at most of the densities tested.
Figure 2.
Effect of the density of insects on the mean proportion of Triatoma infestans and Panstrongylus megistus larvae found outside shelters.
Density of insects had significant influence on the proportion of insects that remained outside shelters for T. infestans (ANOVA density effect, P < 0.001) and P. megistus (Kruskal-Wallis test density effect, P < 0.001). We observed that T. infestans (Figure 2) only showed a significant effect of density on its behavior when 80 or 100 insects were released in the arena (P < 0.001, by Tukey's multiple comparisons). Panstrongylus megistus (Figure 2) showed a significant increase in the proportion of insects found outside the shelter between the densities of 40 and 80 insects (P < 0.01, by Dunn's multiple comparisons) and between 80 insects and 100 insects (P < 0.001, by Dunn's multiple comparisons). Finally, the effect of density was significantly different between the two species (ANOVA species effect, P < 0.001), with P. megistus showing a larger propensity to respond to high-density environments by avoiding crowded shelters, even at lower density levels.
Effect of illumination.
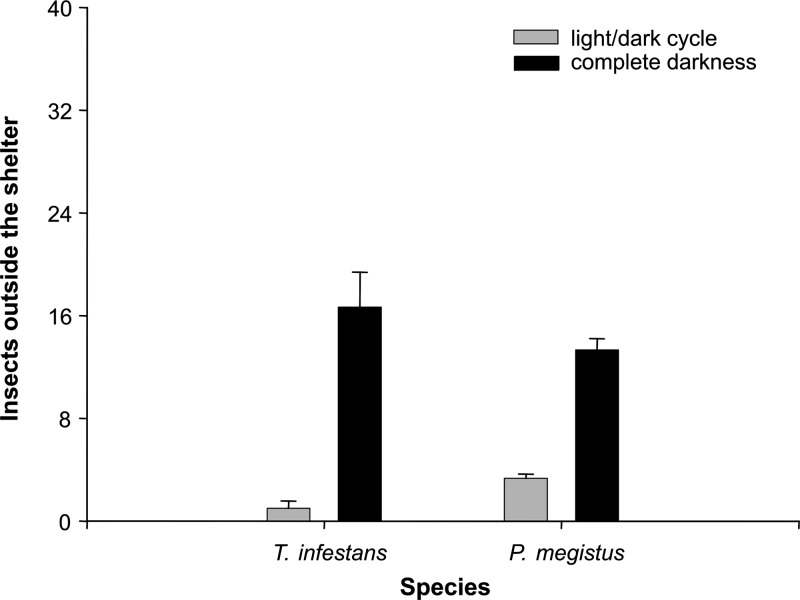
We analyzed how the presence or absence of a light cycle affects the use of shelters by P. megistus and T. infestans, respectively. The proportion of insects found outside the shelter when T. infestans and P. megistus were subjected to a light cycle or kept under constant darkness is shown in Figure 3. The mean number of insects found outside the shelter under a light cycle regimen (Figure 3) was significantly lower than that under constant darkness for P. megistus (P < 0.01, by t-test) and T. infestans (P < 0.0001, by t-test). No significant difference was observed in the behavior of P. megistus and T. infestans under constant darkness (Figure 3). However, the mean number of insects observed outside the shelter under a light cycle (Figure 3) was significantly higher for P. megistus than for T. infestans (P < 0.05, by t-test).
Figure 3.
Mean number of Triatoma infestans and Panstrongylus megistus larvae found outside shelters under a light cycle or constant darkness. Error bars indicate SE.
Aggregation or repellence between T. infestans and P. megistus.
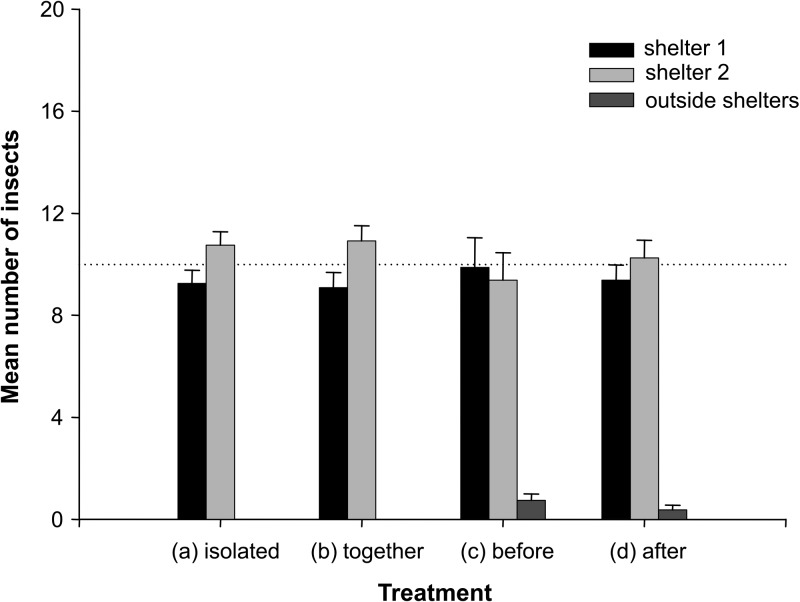
We evaluated whether P. megistus and T. infestans aggregate randomly inside shelters or if any repellence processes occur when insects of these species interact. Data from control assays, in which insects from both species were studied separately, showed that the distribution of T. infestans (Figure 4A ) or P. megistus (Figure 5A ) was homogeneous, given that approximately 50% of the insects were found in each shelter. Generally, approximately 10% of P. megistus larvae did not enter shelters (Figure 5A). For T. infestans, this tendency was not observed, and 100% of insects were found inside shelters in all control assays (Figure 4A).
Figure 4.
Mean number of Triatoma infestans larvae found inside shelter 1 and shelter 2, and outside shelters when T. infestans was released alone in the arena (A); T. infestans was released simultaneously with Panstrongylus megistus (B); T. infestans was released before P. megistus (C); and T. infestans was released after P. megistus (D). Horizontal line indicates expected random distribution (50%) of insects between the two shelters. Error bars indicate SE.
Figure 5.
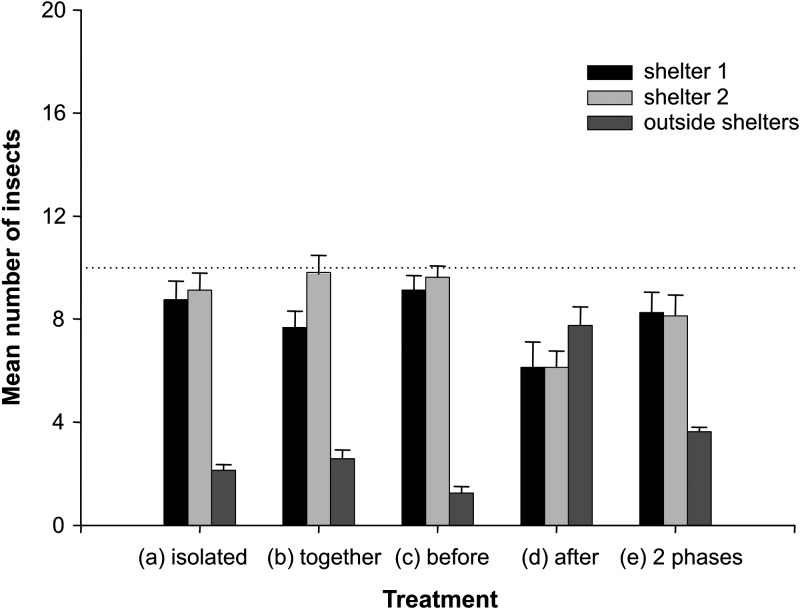
Mean number of Panstrongylus megistus larvae found inside shelter 1 and shelter 2, and outside shelters when P. megistus was released alone in the arena (A); P. megistus was released simultaneously with Triatoma infestans (B); P. megistus was released before T. infestans (C); P. megistus was released after T. infestans (D); and P. megistus was released in two phases into the arena (E). Horizontal line indicates expected random distribution (50%) of insects between the two shelters. Error bars indicate SE.
This distribution pattern was not modified when insects from both species were released together in the arena over a seven-day period. In this case, approximately 50% of insects of each species were found inside each shelter (Figures 4B and 5B). Under this treatment, approximately 10% of P. megistus larvae stayed outside shelters (Figure 5B), and no T. infestans was found outside shelters (Figure 4B).
When P. megistus larvae were released first (Figure 5C) and T. infestans larvae were released three days later (Figure 4D), both species distributed randomly and approximately 50% of the insects were found inside each shelter. Virtually no T. infestans larvae were found outside shelters (Figure 4D), and nearly 10% of P. megistus larvae remained outside shelters (Figure 5C) at the end of these assays.
When T. infestans larvae were released first (Figure 4C) and P. megistus larvae were released three days later (Figure 5D), both species distributed randomly between shelters (Figures 4C and 5D). However, the proportion of P. megistus larvae found outside shelters was higher and represented approximately 40% of the insects (Figure 5D).
There was no significant difference in the distribution of insects between shelter 1 and shelter 2 (Figure 1B) for the four treatments for T. infestans (Figure 4) (ANOVA treatment effect not significant) and P. megistus (Figure 5) (Kruskal-Wallis test treatment effect not significant).
Distribution of T. infestans (Figure 4B–D) or P. megistus (Figure 5B–D) in both shelters was not significantly different under any of the treatments in which insects of these species occupied the arena together: when simultaneously released (Figures 4B and 5B) (P not significant, by t-test), or P. megistus (Figures 4D and 5C) (P not significant, by t-test) or T. infestans larvae were released first (Figures 4C and 5D) (P not significant, by Mann-Whitney test).
Triatoma infestans (Figure 4) did not show significant differences in the mean number of insects found outside shelters for any of the treatments (Kruskal-Wallis test treatment effect, P not significant). Conversely, when P. megistus larvae were released in the arena second, the number of insects of this species found outside shelters was significantly higher (Figure 5D) (Dunn's multiple comparisons, P < 0.0001) than in the other three treatments (Figure 5A–C). The mean number of P. megistus larvae found outside shelters was not significantly different for treatments 1, 2, and 3 (Figure 5A–C) (Dunn's multiple comparisons, P not significant).
When two groups of 20 P. megistus were released sequentially in the arena, the mean number of insects found outside shelters that belonged to the group released later was not significantly different (Figure 5E) (Dunn's multiple comparisons, P not significant) from the one observed when P. megistus were released after T. infestans (Figure 5D). The mean number of P. megistus outside shelters when released in two phases into the arena was significantly different only from treatment 3 (Figure 5C and E) (Dunn's multiple comparisons, P < 0.01), but was not different for any other treatment.
Discussion
The present study demonstrates that P. megistus and T. infestans present species-specific profiles of shelter exploitation, which are differentially affected by insect density and environmental illumination. Despite these differentiated patterns of shelter exploitation, we found that these species aggregate randomly inside shelters, without any apparent repellence or segregation process.
The effect of insect density in the behavior of P. megistus and T. infestans.
Our data indicate that insect density influences the proportion of insects of both species that enter and remain inside a shelter. The comparison between them indicates that a significantly higher proportion of P. megistus was found outside shelters than T. infestans at all densities tested. Furthermore, the effect of an increase in density was significantly stronger on P. megistus (Figure 2) than on T. infestans.
The proportion of larvae of T. infestans that remained outside shelters was null or extremely low at almost all densities tested (Figure 2). This proportion was significantly higher only at the highest density tested (Figure 2), suggesting that T. infestans expressed a change in their behavior only when the maximum capacity of the shelter was attained. Consequently, the maximum number of larvae of T. infestans that apparently fit inside a shelter under our experimental paradigm would vary between 80 and 100 insects (Figure 2).
Some P. megistus larvae remained outside shelters even at the lowest densities (Figure 2), suggesting that this behavioral pattern is characteristic of the species. The density range between 80 and 100 insects also seems to be critical for P. megistus because the most abrupt alteration in the proportion of P. megistus that remained outside shelters was observed in that density interval (Figure 2). This variation is probably related to the same critical reason suggested for T. infestans: this appears to be the maximum number of insects that fit inside these shelters. However, P. megistus also showed a significant alteration of their behavior between the densities of 40 and 80 insects (Figure 2). Thus, the influence of density on the behavior of P. megistus can be perceived at a lower interval than of T. infestans (Figure 2).
The difference observed between the two species might be caused by a lower tolerance to high insect density in P. megistus. An alternative explanation would be that the maximum number of P. megistus that fit inside a shelter is lower than that of T. infestans. This explanation could be caused by a size difference, a variation in the intensity of thigmotaxis between these species, or both. In the second instance, T. infestans would show stronger thigmotaxis and consequently, it would tend to aggregate more tightly than P. megistus inside shelters. A small proportion of P. megistus always remained outside shelters, suggesting that this species has a weaker motivation to seek refuge. Therefore, any feature that can make a shelter less attractive, as the increase of density, might promote rejection of that shelter by insects of this species.
A previous study24 investigated the connection between blood meal size obtained under different insect density conditions on non-anesthetized hosts for P. megistus and T. infestans. The authors observed a negative relationship between insect density and increase in body weight. Consequently, they proposed that higher insect densities promote lower weight gains through feeding. However, this effect was more evident for P. megistus than for T. infestans. These data and our results about the relationship between insect density and the use of shelters suggest that P. megistus is more sensitive to high insect densities than T. infestans. Therefore, T. infestans may tolerate development of larger colonies than P. megistus. This hypothesis could help to explain why intra-domestic colonies of P. megistus never reached the high density of insects observed for T. infestans, which can reach > 3,000 insects in a single home.37
The effect of illumination and darkness on the behavior of P. megistus and T. infestans.
The proportion of insects that remained outside shelters under a light cycle was significantly lower than under permanent darkness for P. megistus and T. infestans (Figure 3). This result confirms that the negative phototaxis of these insects has a strong influence on their motivation for seeking shelter. The intense photonegative sensitivity of T. infestans has already been demonstrated in several studies.30,31,38 Under natural conditions of illumination, T. infestans shows a negative phototactic response to white light that seems to be mainly caused by the green component of that light.38 The negative phototactic response of T. infestans increases as light intensity increases.30 Moreover, this response changes along the daily cycle because insects show a more intense light avoidance during the scotophase.30 This variation in photonegative sensitivity is under circadian control, suggesting an important adaptive role of this behavior.30,31,38
Our results showed an apparent difference in the intensity of the negative phototactic response of P. megistus and T. infestans, suggesting that the sensitivity of P. megistus is lower. A significantly higher number of P. megistus than T. infestans were found outside shelters under a light cycle (Figure 3). However, when exposed to constant darkness, the proportion of insects of both species found outside shelters did not vary significantly. This finding suggests that negative phototaxis may be a factor responsible for the difference observed when both species were exposed to a light cycle (Figure 3). The circadian control of the phototactic response of T. infestans is modulated by the migration of visual pigments inside the retinulla of these insects.32 Therefore, the study of the morphology and physiology of the visual organs of P. megistus may shed some light on our understanding of the mechanisms underlying the different phototactic responses of these species.
The pattern of distribution of P. megistus and T. infestans among shelters.
Beyond the comparative evaluation of how density and illumination modulate the use of shelters by each species, an essential feature evaluated was the pattern of distribution of each species in arenas that offer two alternative shelters. By determining these intra-specific distribution patterns (Figures 4A and 5A), we could subsequently evaluate whether a change was induced when both species were released together. We found that both species tend to distribute homogeneously between the two available shelters, i.e., approximately 50% of the insects remained in each shelter when both species were released alone (Figures 4A and 5A). This experiment demonstrated that triatomines do not gather in unique assemblies, but aggregate in evenly split groups when possible. Because triatomines show a typical aggregation behavior characterized by a tendency to remain grouped inside shelters,33,39 it would not be surprising if even in the presence of two available shelters insects only occupied one of them. However, we observed exactly the opposite for both species studied (Figures 4A and 5A). Additionally, our findings suggest that this tendency to distribute evenly between available shelters is independent of density. Insects occupied shelters in equal proportions, even at the lowest density of 20 insects. Therefore, this behavior might have relevant epidemiologic consequences because we can expect that an infested household may present sub-colonies occupying diverse refuges, making their elimination more difficult. This observation confirms the relevance of spraying every part of infested domiciliary units with insecticide, even if a single insect is detected.
We found approximately 10% of P. megistus outside shelters when tested alone in the arena (Figure 5A). Conversely, all T. infestans were found inside shelters when only insects of this species were released (Figure 4A). These results are consistent with those obtained studying the effect of density on the use of shelters (Figure 2) and the effect of illumination (Figure 3). Differences in the intensity of the negative phototaxis of P. megistus and T. infestans (Figure 3) help to explain the higher proportion of P. megistus that remained outside shelters. This pattern of shelter use might facilitate detection of P. megistus either by house residents or by control guards. However, because T. infestans hardly leaves shelters during daylight hours, it hides better, impairing detection. Moreover, our results confirm that detection would be density-dependent in both cases, with higher densities yielding a larger number of insects roaming outside shelters.
The interaction between P. megistus and T. infestans inside shelters.
The central objective of the present work was to clarify whether P. megistus and T. infestans aggregate randomly inside shelters or actively repel each other. Our results indicated that insects of both species promptly use the same shelter simultaneously, even when two shelters are available. When released together, insects of both species distributed homogeneously among the shelters (Figures 4B and 5B). This distribution pattern is basically the same observed when each species is released alone, evincing no apparent modification of insect behavior in either case (Figures 4A and 5A). Therefore, no mutual repellence27 seems to exist between these species, given that the presence of one species had no apparent effect on the distribution of the other. An indication of inter-specific repellence would exist if insects of the two different species occupied different shelters, or if one species occupied both shelters while the other remained outside. Our findings directly contradict previous reports of repellence between P. megistus and T. infestans.27
In agreement with the low specificity reported for aggregation signals of triatomines, which has been demonstrated for different triatomine species by diverse authors, our results suggest that these two species tend to cross-aggregate.29,40–42 It was previously demonstrated that P. megistus and T. infestans show cross-aggregation responses to chemical signals from feces and cuticle of the other species.29 In other taxa of Hemiptera, intra-specific and inter-specific aggregation responses were also demonstrated. Six different species of pentatomids (Hemiptera: Pentatomidae) showed a similar behavioral pattern in the presence of aggregation signals.43 When first instar larvae were placed together in a circular arena, inter-specific aggregation responses apparently mediated by common chemical compounds occurred.43
Interestingly, T. infestans did not have its distribution pattern modified after being released in an arena previously occupied by P. megistus (Figures 4D and 5C), suggesting that the previous presence of P. megistus does not affect the behavior of T. infestans. Coincidently, when T. infestans was released initially, the behavior of P. megistus was similar (Figure 4C). In spite of this finding we observed an abrupt increase in the number of P. megistus that remained outside shelters (Figure 5D).
To understand these results, we evaluated whether the increase of P. megistus found outside shelters was caused by T. infestans inside shelters or by previous colonization of shelters (Figure 5E). When two groups of P. megistus were released sequentially in the arena, an equivalent increase of insects remained outside shelters (Figure 5E). Therefore, P. megistus seems to prefer unexploited shelters than shelters previously colonized by other insects of the same species. The corresponding results lead us to suggest that P. megistus larvae change their distribution pattern because of an increase in density inside shelters and not because of interspecific repellence.
Similar density-dependent behavioral patterns were demonstrated in Blattella germanica (Dictyoptera: Blattellidae), which show a remarkable evolutionary convergence with triatomines concerning the use of chemical aggregation signals inside shelters.44–48 As for triatomines, aggregation signals of B. germanica induce aggregation responses. However, when high densities of insects are used for impregnating filter papers used as aggregation stimuli, B. germanica tend to avoid them and disperse after contacting the papers.44
Our results, together with inter-specific aggregation mediated by chemical signals,29 support the hypothesis that the spatial isolation between P. megistus and T. infestans is not mediated by visual and/or chemical signals. We further conclude that there is no repellence mediating interactions between these species. Additionally, simultaneous colonization of human households by P. megistus and T. infestans has been reported.18 It would be highly improbable if they repelled each other, as suggested.27 Dias18 reported that during 1943–1953, 877 domiciles with mixed infestations of P. megistus and T. infestans were identified in Brazil. Thus, the spatial isolation observed for these species5,7,20–23,49 is probably caused by competition processes.5,24,26,50
Differences in dispersal rates may represent an additional dimension that could interfere in the house infestation capacity of these species. Although T. infestans and P. megistus are known to promptly initiate flight,51,52 further studies would be necessary to analyze whether differences in flight capacity between them could be related to distinct dispersal rates and spatial distribution. It has also been found that P. megistus is more sensible to temperature shocks than T. infestans, and shows stronger effects after abrupt variations of temperature on ecdysis and survival53,54 and that P. megistus is much more susceptible than T. infestans to deltamethrin, a piretroid insecticide.51 Deltamethrin, even at sub-lethal doses, may inhibit re-colonization of households by P. megistus from sylvatic ecotopes.51 Our results, and those of other reports,5,24,37,51 reinforce the notion that T. infestans is better pre-adapted for exploiting domiciliary ecotopes than P. megistus.
The study of vector behavior can provide innovative strategies for epidemiologic surveillance and control of tropical infections such as Chagas disease. In this framework, T. infestans and P. megistus stand out because of their high potential as Chagas disease vectors in Latin America. In regard to shelter exploitation, we found that P. megistus is more sensitive to insect density than T. infestans, whereas T. infestans shows higher sensitivity to illumination than P. megistus. When placed together in a same artificial environment, we found no apparent repellence between these two species, as suggested.27 These findings do not rule out the possibility that competition processes24–26,37,51 between these species may be responsible for their segregation in nature.
For many decades, control of Chagas disease has been based on detecting vectors in human households and spraying insecticides thereafter. However, little is known about how different species of Chagas disease vectors search and exploit potential shelters and how they interact when cohabiting the same domestic environment. The present study provides an important contribution to our understanding of the joint and distinct shelter exploitation patterns of T. infestans and P. megistus. Therefore, our findings might aid development of control strategies better adapted to each species.
ACKNOWLEDGMENTS
We thank Mariana Horta and Christian Vair for kindly revising the manuscript.
Footnotes
Financial support: This study was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais and FIOCRUZ.
Authors' addresses: Theo Mota and Marcelo Gustavo Lorenzo, Laboratório de Triatomíneos e Epidemiologia da Doença de Chagas, Centro de Pesquisas René Rachou, FIOCRUZ; Av. Augusto de Lima 1715, Barro Preto, Belo Horizonte, Minas Gerais, Brazil, E-mails: mota@cict.fr and marcelo@cpqrr.fiocruz.br.
References
- 1.Coura J, Dias J. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz. 2009;104:31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- 2.Noireau F, Cortez MG, Monteiro FA, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends Parasitol. 2005;21:7–10. doi: 10.1016/j.pt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Bargues M, Klisiowicz D, Panzera F, Noireau F, Marcilla A, Perez R, Rojas M, O'Connor J, Gonzalez-Candelas F, Galvão C. Origin and phylogeography of the Chagas disease main vector Triatoma infestans based on nuclear rDNA sequences and genome size. Infect Genet Evol. 2006;6:46–62. doi: 10.1016/j.meegid.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Ceballos L, Berkunsky I, Kitron U, Gürtler R. First finding of melanic sylvatic Triatoma infestans (Hemiptera: Reduviidae) colonies in the Argentine Chaco. J Med Entomol. 2009;46:1195. doi: 10.1603/033.046.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragão M. Sobre a dispersão do Triatoma infestans. Rev Soc Bras Med Trop. 1971;5:183–191. [Google Scholar]
- 6.Schofield C, Dias J. The southern cone initiative against Chagas disease. Adv Parasitol. 1999;42:1–27. doi: 10.1016/s0065-308x(08)60147-5. [DOI] [PubMed] [Google Scholar]
- 7.Forattini OP, Ferreira OA, Silva EO, Rabello EX. Aspectos ecológicos da tripanossomíase Americana: XII-Variação regional da tendência de Panstrongylus megistus à domiciliação. Rev Saude Publica. 1978;12:209–233. [PubMed] [Google Scholar]
- 8.Forattini OP, Santos JL, Ferreira OA, Rocha e Silva EO, Rabello EX. Aspectos ecológicos da tripanossomíase Americana: XVI-Dispersão e ciclos anuais de colônias de Triatoma sordida e de Panstrongylus megistus espontaneamente desenvolvidas em ecótopos artificiais. Rev Saude Publica. 1979;13:299–313. doi: 10.1590/s0034-89101979000400005. [DOI] [PubMed] [Google Scholar]
- 9.Forattini OP. São Paulo: Edgar Blücher/Ed. USP; 1972. Introdução à Geografia Médica do Brasil; pp. 191–212. [Google Scholar]
- 10.Forattini OP, Barata JM, Santos JL, Silveira AC. Hábitos alimentares, infecção natural e distribuição de triatomíneos domiciliados na região central do Brasil. Rev Saude Publica. 1982;16:171–204. doi: 10.1590/s0034-89101982000400001. [DOI] [PubMed] [Google Scholar]
- 11.Forattini OP. Biogeografia, origem e distribuição da domiciliação de triatomíneos no Brasil. Rev Saude Publica. 1980;14:265–299. doi: 10.1590/s0034-89101980000300002. [DOI] [PubMed] [Google Scholar]
- 12.Schofield CJ. Triatominae: Biologia y Control. United Kingdom: Eurocommunica Publications; 1994. [Google Scholar]
- 13.Barbosa SE, Soares RP, Pires HH, Diotaiuti L. Experimental evidence for a demographic cline in Panstrongylus megistus populations. Mem Inst Oswaldo Cruz. 2001;96:773–775. doi: 10.1590/s0074-02762001000600005. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa SE, Dujardin JP, Soares RP, Pires HH, Margonari C, Romanha AJ, Panzera F, Linardi PM, Duque-de-Melo M, Pimenta PF, Pereira MH, Diotaiuti L. Interpopulation variability among Panstrongylus megistus (Hemiptera: Reduviidae) from Brazil. J Med Entomol. 2003;40:144–420. doi: 10.1603/0022-2585-40.4.411. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa SE, Belisário CJ, Souza RC, Paula AS, Linardi PM, Romanha AJ, Diotaiuti L. Biogeography of Brazilian populations of Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) based on molecular marker and paleo-vegetational data. Acta Trop. 2006;99:144–154. doi: 10.1016/j.actatropica.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Falavigna-Guilherme AL, Lorosa ES, Costa AL, Pavanelli GC, Araújo SM. Panstrongylus megistus em ecótopos artificiais de ilhas do Alto Rio Paraná. Rev Soc Bras Med Trop. 2001;34:491–494. doi: 10.1590/s0037-86822001000500015. [DOI] [PubMed] [Google Scholar]
- 17.Ramos CJ, Tavares KC, Komati LK, Miletti LC. Colonization by Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) inside homes in São José do Cerrito, SC: first report. Rev Soc Bras Med Trop. 2008;41:421–423. doi: 10.1590/s0037-86822008000400021. [DOI] [PubMed] [Google Scholar]
- 18.Dias E. Variações mensais da incidência das formas evolutivas do Triatoma infestans e do Panstrongylus megistus no município de Bambuí, Estado de Minas Gerais. Mem Inst Oswaldo Cruz. 1955;53:457–472. [PubMed] [Google Scholar]
- 19.Pellegrino J, Brener Z. Profilaxia de um foco da doença de Chagas nas proximidades de Belo Horizonte (cidade industrial) Rev Assoc Med Minas Gerais. 1951;2:233–250. [Google Scholar]
- 20.Forattini OP, Ferreira OA, Silva EOR, Rabello EX. Aspectos ecológicos da tripanossomíase Americana: VIII-Domiciliação de Panstrongylus megistus e sua presença extradomiciliar. Rev Saude Publica. 1977;11:73–86. doi: 10.1590/s0034-89101977000100007. [DOI] [PubMed] [Google Scholar]
- 21.Dias JC. Reinfestação do Município de Bambuí por triatomíneos transmissores da doença de Chagas. Mem Inst Oswaldo Cruz. 1965;63:107–119. [PubMed] [Google Scholar]
- 22.Dias JC. Reinfestação do município de Bambuí por triatomíneos transmissores da doença de Chagas (Segunda Nota) Mem Inst Oswaldo Cruz. 1968;66:197–208. doi: 10.1590/s0074-02761968000200006. [DOI] [PubMed] [Google Scholar]
- 23.Rocha ES, Maluf J, Corrêa RR. La enfermedad de Chagas. Vigilância entomológica en el Estado de São Paulo, Brasil. Bol Oficina Sanit Panam. 1971;71:387–401. [PubMed] [Google Scholar]
- 24.Pereira M, Penido C, Martins M, Diotaiuti L. Triatoma infestans is more efficient than Panstrongylus megistus in obtaining blood meals on non anaesthetized mice. Mem Inst Oswaldo Cruz. 1995;90:765–767. doi: 10.1590/s0074-02761995000600019. [DOI] [PubMed] [Google Scholar]
- 25.Pereira MH, Gontijo NF, Guarneri AA, Sant'Anna MR, Diotaiuti L. Competitive displacement in Triatominae: the Triatoma infestans success. Trends Parasitol. 2006;22:516–520. doi: 10.1016/j.pt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Oscherov E, Damborsky M, Bar M, Gorla D. Competition between vectors of Chagas disease, Triatoma infestans and Triatoma sordida: effects on fecundity and mortality. Med Vet Entomol. 2004;18:323–328. doi: 10.1111/j.0269-283X.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 27.Neves D, Paulini E. Repelencia entre Panstrongylus megistus, Triatoma infestans e Triatoma sordida (Hemiptera: Reduviidae), por ação de feromonio. Rev Bras Entomol. 1982;26:349–354. [Google Scholar]
- 28.Lorenzo Figueiras AN, Lazzari CR. Aggregation behaviour and interspecific responses in three species of Triatominae. Mem Inst Oswaldo Cruz. 1998;93:133–137. doi: 10.1590/s0074-02761998000100025. [DOI] [PubMed] [Google Scholar]
- 29.Pires HH, Lorenzo MG, Diotaiuti L, Lazzari CR, Figueiras AN. Aggregation behaviour in Panstrongylus megistus and Triatoma infestans: inter and intraspecific responses. Acta Trop. 2002;81:47–52. doi: 10.1016/s0001-706x(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 30.Reisenman CE, Lazzari CR, Giurfa M. Circadian control of photonegative sensitivity in the haematophagous bug Triatoma infestans. J Comp Physiol Acta Trop. 1998;183:533–541. [Google Scholar]
- 31.Reisenman CE, Lorenzo Figueiras AN, Giurfa M, Lazzari CR. Interaction of visual and olfactory cues in the aggregation behaviour of the haematophagous bug Triatoma infestans. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2000;186:961–968. doi: 10.1007/s003590000149. [DOI] [PubMed] [Google Scholar]
- 32.Reisenman CE, Insausti TC, Lazzari CR. Light-induced and circadian changes in the compound eye of the haematophagous bug Triatoma infestans (Hemiptera: Reduviidae) J Exp Biol. 2002;205:201–210. doi: 10.1242/jeb.205.2.201. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzo MG, Lazzari CR. The spatial pattern of defaecation in Triatoma infestans and the role of faeces as a chemical mark of the refuge. J Insect Physiol. 1996;42:903–907. [Google Scholar]
- 34.Lorenzo MG, Lazzari CR. Temperature and relative humidity affect the selection of shelters by Triatoma infestans, vector of Chagas disease. Acta Trop. 1999;72:241–249. doi: 10.1016/s0001-706x(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzo Figueiras AN, Lazzari CR. Aggregation in the haematophagous bug Triatoma infestans: a novel assembling factor. Physiol Entomol. 1998;23:33–37. [Google Scholar]
- 36.Lorenzo MG, Lazzari CR. Activity pattern in relation to refuge exploitation and feeding in Triatoma infestans (Hemiptera: Reduviidae) Acta Trop. 1998;70:163–170. doi: 10.1016/s0001-706x(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 37.Marsden P. Doença de Chagas: ecologia domiciliar dos principais vetores no Brasil. J Bras Med. 1980;340:17–22. [Google Scholar]
- 38.Ward J, Finlayson L. Behavioural responses of the haematophagous bug Triatoma infestans (Klug) (Hemiptera: Reduviidae) to visual stimuli. Bull Entomol Res. 1982;72:357–366. [Google Scholar]
- 39.Figueiras AN, Kenigsten A, Lazzari CR. Aggregation in the hematophagous bug Triatoma infestans: chemical signals and temporal pattern. J Insect Physiol. 1994;40:311–316. [Google Scholar]
- 40.Cruz-Lopez L, Malo EA, Rojas JC. Aggregation pheromone in five species of Triatominae (Hemiptera: Reduviidae) Mem Inst Oswaldo Cruz. 1993;88:535–539. doi: 10.1590/s0074-02761993000200005. [DOI] [PubMed] [Google Scholar]
- 41.Figueiras AN, Lazzari CR. Aggregation behaviour and interspecific responses in three species of triatominae. Mem Inst Oswaldo Cruz. 1998;93:133–137. doi: 10.1590/s0074-02761998000100025. [DOI] [PubMed] [Google Scholar]
- 42.Figueiras AN, Lazzari CR. Aggregation behaviour and interspecific responses in Rhodnius prolixus Stal. Mem Inst Oswaldo Cruz. 2002;97:569–571. doi: 10.1590/s0074-02762002000400022. [DOI] [PubMed] [Google Scholar]
- 43.Fucarino A, Millar JG, McElfresh JS, Colazza S. Chemical and physical signals mediating conspecific and heterospecific aggregation behavior of first instar stink bugs. J Chem Ecol. 2004;30:1257–1269. doi: 10.1023/b:joec.0000030276.32665.cb. [DOI] [PubMed] [Google Scholar]
- 44.Suto C, Kumada N. Secretion of dispersion-inducing substance by the German cockroach, Blattella germanica L. (Orthoptera: Blattellidae) Appl Entomol Zool (Jpn) 1981;16:113–120. [Google Scholar]
- 45.Denzer D, Fuchs M, Stein G. Zum Verhalten von Blattella germanica L.: Aktionsradius und Refugientreue1. J Appl Entomol. 1988;105:330–343. [Google Scholar]
- 46.Sakuma M, Fukami H. The aggregation pheromone of the German cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae): isolation and identification of the attractant components of the pheromone. Appl Entomol Zool (Jpn) 1990;25:355–368. [Google Scholar]
- 47.Sakuma M, Fukami H. Aggregation arrestant pheromone of the German cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae): isolation and structure elucidation of Blattellastanoside-A and-B. J Chem Ecol. 1993;19:2521–2541. doi: 10.1007/BF00980688. [DOI] [PubMed] [Google Scholar]
- 48.Stejskal V. Distribution of faeces of the German cockroach, Blattella germanica, in a new refuge. Entomol Exp Appl. 1997;84:201–205. [Google Scholar]
- 49.Forattini OP. Effects of Control Measures on Vector Population Dynamics. Washington, DC: Pan American Health Organization; 1976. [Google Scholar]
- 50.Dan A, Amino R, Pereira M, Schenkman S, Pesquero J, Diotaiuti L, Lacerda Beirao P. Action of the saliva of Triatoma infestans (Heteroptera: Reduviidae) on sodium channels. Mem Inst Oswaldo Cruz. 2000;95:57–58. [Google Scholar]
- 51.Diotaiuti L, Penido CM, Araújo HS, Schofield CJ, Pinto CT. Excito-repellency effect of deltamethrin on triatomines under laboratory conditions. Rev Soc Bras Med Trop. 2000;33:247–252. doi: 10.1590/s0037-86822000000300002. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez-Prokopec GM, Ceballos LA, Marcet PL, Cecere MC, Cardinal MV, Kitron U, Gürtler RE. Seasonal variations in active dispersal of natural populations of Triatoma infestans in rural north-western Argentina. Med Vet Entomol. 2006;20:273–279. doi: 10.1111/j.1365-2915.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodrigues VL, Mello ML, Ferraz Filho AN, Dantas MM. Sobrevivência e ocorrência de muda em Triatoma infestans Klug (Hemiptera, Reduviidae) após choque de temperatura. Rev Saude Publica. 1991;25:461–467. doi: 10.1590/s0034-89101991000600007. [DOI] [PubMed] [Google Scholar]
- 54.Garcia SL, Rodrigues VL, Garcia NL, Ferraz Filho AN, Mello ML. Survival and molting incidence after heat and cold shocks in Panstrongylus megistus Burmeister. Mem Inst Oswaldo Cruz. 1999;94:131–137. doi: 10.1590/s0074-02761999000100026. [DOI] [PubMed] [Google Scholar]