Abstract
Indoor residual spraying (IRS) has become an increasingly popular method of insecticide use for malaria control, and many recent studies have reported on its effectiveness in reducing malaria burden in a single community or region. There is a need for systematic review and integration of the published literature on IRS and the contextual determining factors of its success in controlling malaria. This study reports the findings of a meta-regression analysis based on 13 published studies, which were chosen from more than 400 articles through a systematic search and selection process. The summary relative risk for reducing malaria prevalence was 0.38 (95% confidence interval = 0.31–0.46), which indicated a risk reduction of 62%. However, an excessive degree of heterogeneity was found between the studies. The meta-regression analysis indicates that IRS is more effective with high initial prevalence, multiple rounds of spraying, use of DDT, and in regions with a combination of Plasmodium falciparum and P. vivax malaria.
Introduction
Despite progress in reducing the malaria burden over the past half decade, malaria remains a leading cause of mortality and morbidity in the developing world.1 The global incidence of malaria was at a peak of 244 million cases in 2005, and it decreased to 225 million cases in 2009, below the 233 million incident cases in 2000.2,3 Mortality caused by malaria was 781,000 in 2009, compared with 860,000 in the previous year. This significant decrease is attributed to a scale-up of global malaria prevention efforts and funding. Despite these advances, the number of malaria cases could rebound significantly without continuing control efforts because areas with high malaria transmission also have rapid population growth, resulting in large at-risk populations.4 Malaria control has historically been difficult in less developed nations because of lack of quality health services and access to treatment and prevention methods. New factors such as increasing insecticide resistance, drug resistance, and war and civil disturbances add to these difficulties and impede the efforts of public health workers and government actions towards controlling malaria.5
Some of the most successful malaria prevention strategies rely on controlling the mosquito vectors that transmit the disease, such as the use of insecticides. Use of insecticides for malaria control has seen a revival in recent years, in part because of the technique of indoor residual spraying (IRS). Indoor residual spraying involves careful, controlled spraying of insecticides along the inside walls of a home or community building. In 2006, the World Health Organization encouraged a scale-up in IRS methods for vector-borne disease control and concurrently endorsed DDT for this technique.6 Of 108 malaria-burdened countries, 44 reported the use of IRS and 12 of these countries used DDT.2 There are 11 other chemicals currently recommended by the World Health Organization for use in IRS, including bendiocarb, malathion, lambda-cyhalothrin, and alphacypermethrin.7 In 2009, IRS conferred protection from malaria to 75 million persons, or 10% of the population of Africa,3 and contributed to the decrease in this disease.
A number of field studies have reported the effectiveness of IRS in reducing malaria prevalence, but it is difficult to generalize from any single study how effective IRS is at reducing malaria prevalence because various studies have shown conflicting results. Aspects of geography, entomology, human behavior, and community acceptance of the program could contribute to why IRS is more successful in one community than in another. Few researchers have attempted to quantify the effects of IRS, even on a small scale, and fewer have addressed what factors might be major versus minor contributors to the relative success or failure of IRS programs worldwide. In addition, although there are many reviews arguing that DDT is safe,8–11 toxicity data from animal studies and recent epidemiologic studies suggest that there may be previously unrecognized long-term negative health consequences for those exposed to insecticides, particularly DDT, even at the low levels seen with IRS.12–18 This finding highlights the need for a synthetic perspective on the effectiveness of IRS in reducing the malaria burden, which can be weighed against potential harmful effects of exposure to those insecticides.
The goal of this study was to determine the overall effectiveness of IRS in reducing malaria prevalence, and to gain information on the different factors that may contribute to the relative strength or weakness of the effect of IRS in different scenarios, such as the characteristics of the population being sampled and the spatial and temporal parameters of the study site and spraying program design. A systematic literature review was performed to synthesize information from a collection of published studies and identify a range of potential outcomes and key factors that are different among these studies, which enables greater generalizations to be made on the topic than a single study alone.
In 2010, the Cochrane Collaboration published a review on the effectiveness of IRS at preventing malaria.19 The criteria for inclusion resulted in only six studies that were individually analyzed, of an initial pool of 134 potentially relevant studies. We expand upon the findings of the Cochrane Collaboration literature review by widening the criteria for inclusion. We included more studies despite larger differences between these studies to obtain statistical power to better quantify the effectiveness of IRS. We then used meta-regression analysis techniques to synthesize and statistically analyze the results and controlled differences across the included studies in terms of the impacts of each covariate on IRS effectiveness. Unlike the Cochrane review, we limited our literature search to only papers published in 2000 or later to minimize a confounding effect by the papers that might represent IRS effectiveness before insecticide resistance was a concern.
Meta-analysis technique has been widely used to integrate the results of a group of empirical studies on the effectiveness of various malaria control programs, such as the effectiveness of insecticide-treated bed nets (ITNs) in reducing malaria cases,20 the effects of artesunate combinations for malaria treatment,21 and efficacy of ITNs and IRS on preventing malaria mortality in children.22 However, no published meta-analysis research that we are aware of has assessed the overall impact of IRS programs in reducing malaria cases, and little effort has been made to identify factors affecting program effectiveness. Thus, we performed a meta-regression analysis to provide insight on the overall impact of IRS interventions and the factors that may have a significant effect on malaria reduction through IRS. Meta-regression aims not only to statistically determine the extent of variation caused by systematic differences between the studies, such as type of study design, but also to relate the effect magnitude and variation to relevant characteristics, such as geographic region.23 Meta-regression analysis can drive future research towards better-designed studies that more accurately capture reality, and influence policy towards more science-based actions.
Materials And Methods
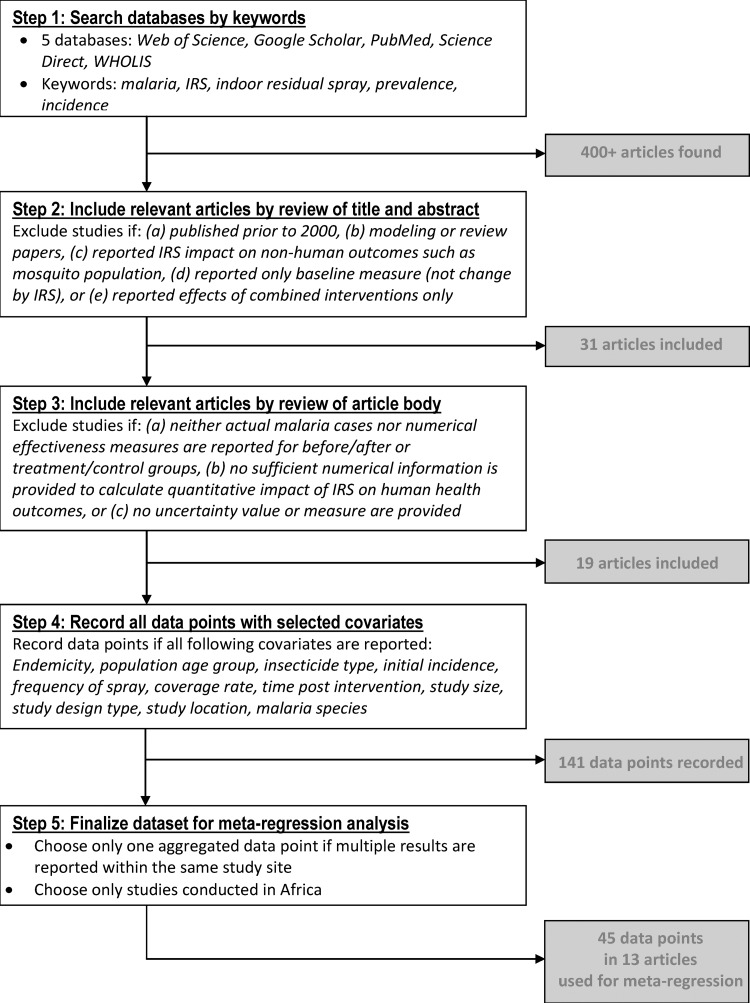
The process used to search for literature and compile the dataset before conducting the meta-regression analysis is shown in Figure 1. In the first step, the following five electronic databases were searched and finds were restricted to articles in peer-review journals within the last 10 years (publication years 2000–2010): Web of Science, Google Scholar, PubMed, Science Direct, and WHOLIS. Key terms for this initial search included malaria, indoor residual spray, IRS, prevalence, and incidence. More than 400 articles were found by this initial search. Limiting our search to papers published in the past decade enabled us to ensure that all resulting papers represented the effectiveness of IRS in a climate of similar insecticide resistance.
Figure 1.
Literature search and meta-regression analysis of reduction of malaria prevalence by indoor residual spraying.
In the second step, titles and abstracts were scanned for articles that fit three broad inclusion criteria: 1) observational (case–control, cross-sectional, cohort) or experimental (randomized control trial) study designs; 2) reported change in measurable human health outcome caused by IRS interventions; and 3) a study design that enabled measurement of effect of IRS alone (i.e., not in combination with other interventions). Within the bounds of the above criteria, we excluded papers that discussed predictive modeling of IRS effect or reported the effect of IRS on the mosquito population (rather than the human population) or established and reported a baseline measure of malarial outcomes in a region at a single point in time without providing any comparison population or time-lapse measures.
Studies from all malaria-risk regions (Africa, Southeast Asia, Central America, and South America) and all study populations (children, women, refugee populations, rural, and urban) were included if the above three criteria were met. Several different measurable health outcomes were initially included as appropriate, including anemia rates, malaria parasitemia prevalence rates, mortality rates, and clinical malaria incidence. However, all studies that met the other criteria coincidentally focused on some form of measurement of parasitemia (i.e., slide positivity rate, cases, prevalence or incidence as verified by positive blood test results). A total of 31 studies were identified as potentially relevant by this initial search of title and abstract.
In the third step, the 31 studies were retrieved and reviewed to confirm they actually met the criteria stated above. We collected and recorded detailed information on the study location, study population, the intervention, and the health measurements and effects. We also included some variables such as insecticide type, frequency of spray, and total time between initial spray and final monitoring. These factors are theoretically influential in the effectiveness of a campaign to reduce malaria, regardless of human behavior within the community towards malaria and health practices that might result in different levels of health outcomes. If malaria parasitemia rates were provided by the authors, this information was recorded. When not reported, malaria parasitemia rates were calculated on the basis of effects measurements and population sizes provided.
As a result of comparing the articles in full to the selection criteria, 12 of the 31 studies were determined to be inappropriate for the analysis and were therefore omitted. Six of these studies reported the effect of integrated vector management (such as combined use of IRS and ITNs) or combined introduction of preventative and treatment methods (such as IRS and rapid diagnostic tests) in a way that it was not possible to attribute a proportion of the change to a single intervention.24–29 Three studies did not measure human health outcomes.30–32 Two studies did not measure a change caused by IRS but rather established a baseline prevalence rate33 or a change over time after termination of IRS.34 Two studies reported the same data but with a different focus of analysis; one was included35 and the other was excluded.36
In the fourth step, we recorded actual data points from the 19 selected studies associated with the numerical information for the following covariates: endemicity status, population age group, insecticide type, initial incidence, frequency of spray, coverage rate, time post intervention, study size, study design type, study location, and malaria species. This process produced 141 data points, but we found that more than half (77 of 141 data points) were from only three studies. To avoid any overwhelming influence from a few studies, we kept only one aggregated data point in cases in which multiple results were reported within the same study site. In addition, we eliminated data points from studies conducted outside Africa because more than 90% of the data points were from studies in Africa, and preliminary analysis confirmed that Africa versus a study site outside Africa was not a significant factor in determining IRS effectiveness. As a result, the final sample for the meta-regression model includes 45 data points from the 13 studies.
In the fifth step, several covariates were reclassified and new variables were included. This data manipulation was conducted in a way that enabled a more straightforward analysis and interpretation. Relative risk (RR; also referred to as risk ratio or incidence rate ratio) measures were recorded as a typical indicator of the IRS effectiveness, but if not reported in the papers, we calculated RR by using malaria incidence data before and after the IRS treatment (for cohort studies), or malaria cases from treatment and control groups (for randomized control trials and cross-sectional studies). The initial incidence variable was log-transformed to adjust its non-linear and non-normal aspects in association with RRs.
The World Health Organization Recommended Insecticides for IRS list was referenced to reduce the type of insecticide variable from eight chemical names to four chemical classes.37 This modification increased the sample size for each option and simplified analysis because some papers reported use of combinations of insecticides. For example, in the report by Kleinschmidt and others,38 different insecticides were used at different times, but those insecticides were all part of the same chemical class. Likewise, the age group category contained nine classifications among the 13 studies. To simplify this classification, the two dummy variables under 5 and under 15 were created to emphasize the higher-risk nature of children, but only the under 15 variable was included for the model because of limited data. A dummy variable was also created for malaria parasite type, where 1 represented only Plasmodium falciparum, and 0 represented combinations of P. falciparum and P. vivax. Another dummy variable was created for the studies with multiple rounds of IRS spraying. Lastly, two dummy variables were created to indicate the cohort-based studies and the randomized controlled trials compared with the cross-sectional studies as baseline. Data were organized by using Excel (Microsoft, Redmond, WA) and statistical analysis was performed by using STATA 10 software (StataCorp LP, College Station, TX).
Results
Descriptive statistics.
A summary of the final 13 publications included in the meta-regression analysis is shown in Table 1 . Among the 13 studies selected for meta-regression analysis, two studies were randomized control trials (RCTs),39,40 four were cross-sectional studies comparing malaria parasitemia rates in a population that lived in IRS-covered homes to another group that did not,35,41–43 and seven were cohort studies that assessed the parasitemia rate before IRS intervention and again at a certain time after IRS.38,44–49 This classification resulted in seven observations from RCTs (16%), 20 observations from cross-sectional studies (44%), and 18 observations from cohort studies (40%).
Table 1.
Summary of studies included in meta-regression of reduction of malaria prevalence by indoor residual spraying
| Variable | All responses (no.) |
|---|---|
| Study location | Equatorial Guinea (3), Kenya (2), Madagascar (2), Mozambique (2), Democratic Republic of Sao Tome and Principle (1), Eritrea (1), Sudan (1), Uganda (1), Zambia (1) |
| Study type | Cohort (7), cross-sectional (4), randomized control trials (2) |
| Malaria species | Plasmodium falciparum (10), P. falciparum/vivax (3) |
| Insecticide type, chemical class | Pyrethroid (6), organochlorine: DDT (4), carbamate–bendiocarb (2), organophosphate: malathion (1) |
Of the 13 studies, 10 addressed malaria caused only by P. falciparum (34 observations, 76%). Three studies identified malaria caused by P. falciparum and P. vivax. However, when reported, P. falciparum accounted for most (74–90%) of infections in these studies. Studies reported on interventions that used four different insecticide types by chemical class. Pyrethroids were the most common insecticide class, used in 6 of the 13 studies (18 observations, 40%). This class included lambda-cyhalothrin, deltamethrin, and alphacypermethrin. Organochlorines, which were represented only by DDT, were used in four studies and produced eight observations, and carbamates, which were represented only by bendiocarb, were used only in two studies and produced 16 observations. Organophosphates, which were represented only by malathion, were used in one study.
The average number of spray rounds per study was 2.8 (range = 1–12). The length of time an RCT or cohort study proceeded after the first insecticide spray, or the number of months post-spray that a cross-sectional survey was conducted, ranged from 2 to 72 months (6 years), but the middle 50% of data ranged from 8 to 36 months.
The mean study size was 8,656 participants. However, study size ranged from 93 to 48,715, and the middle 50% ranged from 1,058 to 11,775 persons. A total of 87% of the observations were in children less than 15 years of age, but only 13% of all observations were exclusively in children less than five years of age. None of the 13 studies addressed a population of pregnant women. We found only one study focusing on malaria burden in pregnancy (Step 2 in Figure 1), but this study could not be included in the analysis because it did not separate the impact of IRS from the impact of other interventions.24
Meta-regression analysis.
In the 13 studies, we assessed the effectiveness of IRS spraying in reducing malaria prevalence in terms of RR. A forest plot that illustrates the contribution of each study to the random effects meta-regression analysis is shown in Figure 2. Relative weight of each study is shown by the area of a box whose midpoint represents the size of the RR estimated from each study.50 Unlike a fixed effect model, the size of weights appears well distributed across all included studies because we conducted random effects analysis. The plot shows not only the summary RRs for each of the 13 studies, but also the combined RR of 0.38, indicating a reduction in malaria prevalence of 62% caused by IRS implementation (95% confidence interval = 0.31–0.46).
Figure 2.
Forest plot of the meta-regression analysis of relative risk (pooled effect estimate is from a random-effects model) of reduction of malaria prevalence by indoor residual spraying.
There was significant heterogeneity in the magnitude of the effect of IRS (i.e., RR) between the 13 studies (heterogeneity χ2 = 1,488.12, P = 0.0001). The largest reported effect was found in the study by Romi and others,51 in which an RR for malaria prevalence between the groups with and without coverage by IRS was 0.01 (95% confidence interval = 0.01–0.02), which represented a reduction of 99%. Conversely, the RRs were only approximately 0.9 the study by Charlwood,52 which indicated a reduction of malaria of only 10%. Thus, we learned from this meta-regression analysis that the IRS effect in reducing malaria prevalence is strong and statistically significant (P < 0.001), but the excessive degree of heterogeneity raises concern over determining to which populations and situations this finding applies. Therefore, we performed a meta-regression analysis using 45 observations to explore factors to explain this heterogeneity by a systematic investigation of whether and how much the IRS effect was affected by study design, target group characteristics, or various spraying program factors.
Meta-regression.
Several meta-regression models were constructed. The best-fit model is shown in Table 2 . Among the potential predictor covariates described above, the following variables had positive influences on the RR: use of organophosphate class insecticide (binary), use of carbamate class insecticide (binary), P. falciparum-caused malaria only (binary), cross-sectional study design (binary), and cohort study design (binary). Significant negative predictors included log-transformed proportion of study population with malaria before intervention (continuous), use of multiple rounds of spraying (binary), and use of organochlorine insecticide (binary). Various age-related variables such as age under 15 years were used in the model but none were significant at the 10% significance level. We also found that sample size was not a significant predictor of the IRS impact on reduction of malaria prevalence.
Table 2.
Meta-regression results (n = 45) of reduction of malaria prevalence by indoor residual spraying*
| Covariate (dependent variable = log RR) | Coefficient (95% confidence interval) |
|---|---|
| Log of initial prevalence (proportion of study population with malaria before intervention) | −0.447 (−0.736 to −0.158)† |
| Multiple rounds of spraying (1 = yes, 0 = no) | −1.911 (−2.632 to −1.191)† |
| Total sample size | 0.000003 (−0.000037 to 0.000043) |
| Use of organochlorine class insecticide | −0.918 (−1.843 to 0.006)‡ |
| Use of organophosphate class insecticide | 2.610 (0.041 to 5.179)§ |
| Use of carbamate class insecticide | 1.426 (0.589 to 2.263)† |
| Child less than 15 years of age | −0.487 (–1.307 to 0.333) |
| Plasmodium falciparum only | 1.052 (0.412 to 1.691)† |
| Cross-sectional study design | 2.269 (1.275 to 3.264)† |
| Cohort study design | 1.404 (0.260 to 2.547)§ |
| Constant | −3.076 (−4.182 to −1.969)† |
Adjusted R2 = 0.786; τ2 = 0.2255. RR = relative risk.
P < 0.01.
P < 0.1.
P < 0.05.
Overall, the meta-regression results can be interpreted as follows. First, starting prevalence of malaria has a significant negative effect on RR, meaning that higher starting prevalence results in lower log-transformed RR. This finding indicates that IRS is more effective in communities with higher starting prevalence of malaria. Second, studies with a multiple spraying schedule also have a significantly negative effect on RR for studies with a one-time spray, meaning that IRS seems to be more effective when sprayed multiple times over a long period. Third, the organochlorine class, which is simply DDT, is more effective for IRS at reducing malaria than the pyrethroid class (omitted in the model as baseline) on the basis of a negative coefficient. However, the organophosphates and carbamate classes have a positive coefficient, meaning that they are less effective than pyrethroids. We can therefore assume a ranking of insecticide types from most to least effective as follows: DDT, pyrethroids, carbamate, and organophosphates. Fourth, studies addressing only P. falciparum malaria are positively associated with RR, meaning that IRS is less effective against P. falciparum malaria (or in regions where only P. falciparum is present) than in studies in which there was a combination of P. falciparum and P. vivax. This result seems consistent with the findings from the literature that P. falciparum malaria is associated with the greatest malaria mortality and intensity of transmission in Africa compared with P. vivax malaria, which is more prevalent in Southeast Asia and the Western Pacific.53 Finally and more importantly, we controlled for any effect of the study design in the meta-regression and found that variables indicating cross sectional studies and cohort studies (randomized control trials as baseline) have positive coefficients, suggesting that these study designs found IRS to be less effective than the randomized control trials, when other factors are controlled.
Discussion
Indoor residual spraying is considered to be one of the most promising technologies for achieving reductions in global malaria burden.7 The results of this meta-regression analysis imply that IRS is significantly effective in decreasing prevalence of malaria parasitemia in a community by approximately 62% on the basis of aggregation of data from studies with RRs, although an excessive degree of heterogeneity was found between the studies. Much work is needed in terms of experimental evaluations of IRS effectiveness and monitoring and evaluation of IRS programs to ensure widespread success with implementation of this approach to vector control.
Descriptive statistics of a set of the included studies identified areas where academic research and applied public health are misaligned. The World Health Organization reported that 38 of the 108 countries with malaria showed significant successes toward reducing malaria burden during 2000–2008,2 but only 5 of these 38 countries were represented in the literature review. It is important to determine how many other countries among these 38 have been using IRS as part of their prevention plan because we believe that the effectiveness of IRS needs to be discussed not just in terms of a single community impact, but also within a context of global control efforts. Moreover, the initial body of literature we found by key word search resulted in representation of only 13 countries, 11 in sub-Saharan Africa. There are 108 countries with malaria worldwide, 44 of which use IRS. Of those 44 countries, 19, less than half, are in Africa.2 Although approximately 85% of malaria prevalence occurs in Africa (approximately 208 million cases), there are significant percentages of the global burden in countries in Latin America, South America, and the eastern Mediterranean, and IRS is used in many of these countries.2 For example, Brazil has more than half of the malaria cases in the Western Hemisphere, more than 300,000 cases annually,54 and considers IRS a primary vector control intervention.55 Research on IRS efficacy needs to be expanded and locations need to be diversified. There are many untapped opportunities for significant advancements in research on the impact of IRS programs across varied communities and landscapes.
We also found that DDT was used in only 30% (4 of 13) of the studies in our analysis, which is consistent with the WHO report that only 12 of 44 malaria-endemic countries using IRS have used DDT for their spraying intervention.2 Although the controversy over the risks and benefits of DDT draws much public interest to the topic and thus to scientific interest in the use of DDT, our results further support the fact that public health practices have continued to emphasize insecticides other than DDT.
The results from the meta-regression analysis provide some interesting information that can help governments direct their strategic plans, and aid non-government organizations in managing existing or developing new projects. For instance, the relationship between IRS effectiveness and starting prevalence shows that programs in communities with higher initial malaria rates might benefit more from IRS than communities with lower initial malaria rates. Considering that one would expect to see a larger proportional reduction in prevalence when the initial prevalence is lower,56 this finding seems quite interesting but needs careful interpretation. Indoor residual spraying is an expensive method for malaria control, requiring major coordination efforts between the funding agencies, implementation agencies, and communities receiving treatment. In addition, precautionary steps must be taken when using insecticides to protect humans and the environment from adverse effects, which require major efforts in education of workers and the community. The controversial impacts of accidental environmental contamination also present potential national and international public policy concerns. Identifying regions that might benefit more from an IRS campaign, such as areas with a higher initial malaria prevalence, can serve to target a limited amount of resources towards a maximum impact.
Recently, many countries have indicated a desire to use DDT in their national malaria strategies, despite known environmental and suspected human health consequences (Mwita A, unpublished data). Our meta-regression results show that DDT is more effective at reducing malaria prevalence than pyrethroids or other insecticides. These findings could serve to change the cost-benefit analysis of DDT use on a local or regional scale, and should be considered in areas with severe malaria burden. In addition, we found from the meta-regression analysis that IRS is more effective when multiple rounds of spraying are administered in a community where P. falciparum and P. vivax malaria is more prevalent. These findings could also inform the malaria control program officials in malaria-endemic countries on the efficient allocation of limited resources for maximum impact.
Our meta-regression analysis attempts to integrate the results of different study designs, including randomized control trials, cohort studies (i.e., before–after studies), and cross-sectional studies. Although we realize that it is challenging to directly compare the results of these studies because of unique aspects in study design and outcome measurements, our meta-regression analysis includes binary variables, indicating that the magnitude of IRS effectiveness appears to be significantly different among study design types; RCT studies have the largest effects and cross-sectional studies have the smallest effects. This information can serve as a guide to health policy makers and malaria control program officials for a better interpretation and use of various results of IRS effectiveness across different study designs.
The results of the meta-regression analysis are limited by the available literature. With only 13 studies published in the past 10 years, an obvious conclusion is that there simply is not enough information available to confidently determine the current nature and magnitude of the effect of programmatic variables on malaria prevalence. The meta-regression model is suggestive but cannot capture the full variation within the data set. This finding indicates that there may be additional important programmatic factors we should consider, which would require more extensive monitoring and record keeping during IRS effectiveness studies. For example, the amount of rainfall within the months before and after spraying could result in changes to the size of the mosquito population and would likely affect residual insecticide action. Differences in rainfall and temperature could also indicate human behavioral changes, such as the relative amount of time spent inside the residence. This finding could alter the personal contact with the mosquito vector. Such variables would have effects at such a micro scale, both spatially and temporally, that one cannot merge information from other databases on annual or monthly averages to get a valid proxy. The information should be collected at the time of the study to truly model what is occurring.
In addition, most of the existing IRS studies failed to consider the time spent outside the protected home or building. Different study populations, and even different persons within the same study, could have different levels of exposure to mosquito vectors outside the home. Assumptions that persons are adequately and equally protected because they live in a sprayed home or community may prove incorrect when more is known about their movements throughout a community or region. Analysis of mosquito exposure on an individual level might result in changes that alter calculations of odds ratios.
Reduction of malaria prevalence by ITNs was reported as 24% according to a meta-analysis paper published in 1995.20 An updated meta-analysis study for ITNs using the recent literature would enable direct comparison with findings from 1995, and demonstrate how much the effectiveness of the ITNs has changed. However, our finding for the overall IRS effectiveness of 62% implies that the effectiveness of vector control methods has improved substantially during the past decade, despite increasing insecticide resistance. Furthermore, it would be useful to conduct a meta-regression analysis for the combined effectiveness of IRS and ITNs on malaria prevalence reduction, in view of recent interest in determining whether there is any additional benefit of combining the use IRS and ITNs in the same households.
In conclusion, to translate the promise of IRS technology into wider application, we need a better understanding of the situational contexts behind different success levels of IRS campaigns. The literature review and meta-regression analysis reported in this paper provide a start toward better understanding. To better inform future malaria control policy and actions, more monitoring and evaluation of IRS campaigns is necessary to provide a full picture of how much IRS can impact malaria burdens and what factors are determining IRS effectiveness.
ACKNOWLEDGMENTS
We thank Adriane Lesser, Zachary Brown, and Christopher Paul for providing useful comments.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases, the National Institutes of Health, or the World Health Organization.
Footnotes
Financial support: This study was supported by Award no. R01AI088009 (Implementation Science to Optimize Malaria Vector Control and Disease Management) from the National Institute of Allergy And Infectious Diseases, and the World Health Organization under the project Malaria Decision Analysis Support Tool: Evaluating Health, Social and Environmental Impacts and Policy Tradeoffs.
Authors' addresses: Dohyeong Kim, North Carolina Central University, Durham, NC, E-mail: dkim@nccu.edu. Kristen Fedak, ICF International, Durham, NC, E-mail: kfedak@icfi.com. Randall Kramer, Nicholas School of the Environment, Duke University, Durham, NC, E-mail: kramer@duke.edu.
References
- 1.Guerra CA, Gikandi PW, Tatem AJ, Noor AM, Smith DL, Hay SI, Snow RW. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2008;5:300–311. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Malaria Report 2009. Geneva: World Health Organization; 2009. [Google Scholar]
- 3.World Health Organization . World Malaria Report 2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood B, Mutabingwa TK. Malaria in 2002. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 6.Kolaczinski K, Kolaczinski J, Kilian A, Meek S. Extension of indoor residual spraying for malaria control into high transmission settings in Africa. Trans R Soc Trop Med Hyg. 2007;101:852–853. doi: 10.1016/j.trstmh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Indoor Residual Spraying: Use of Indoor Residual Spraying for Scaling Up Global Malaria Control and Elimination. Geneva: World Health Organization; 2006. [Google Scholar]
- 8.Beard J. DDT and human health. Sci Total Environ. 2006;355:78–89. doi: 10.1016/j.scitotenv.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Curtis C. Should the use of DDT be revived for malaria vector control? Biomedica. 2002;22:455–461. [PubMed] [Google Scholar]
- 10.Smith A. How toxic is DDT? Lancet. 2000;356:267–268. doi: 10.1016/s0140-6736(00)02497-1. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . The Use of DDT in Malaria Vector Control. WHO Position Statement. Geneva: World Health Organization; 2007. [Google Scholar]
- 12.Bornman R, de Jager C, Worku Z, Farias P, Reif S. DDT and urogenital malformations in newborn boys in a malarial area. BJU Int. 2009;106:405–411. doi: 10.1111/j.1464-410X.2009.09003.x. [DOI] [PubMed] [Google Scholar]
- 13.Dalvie MA, Myers JE, Thompson ML, Robins TG, Dyer S, Riebow J. The long-term effects of DDT exposure on semen, fertility, and sexual function of malaria vector-control workers in Limpopo Province, South Africa. Environ Res. 2004;96:1–8. doi: 10.1016/j.envres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 14.de Jager C, Farias P, Barraza-Villarreal A, Avila MH, Ayotte P, Dewailly E. Reduced seminal parameters associated with environmental DDT exposure and p,p′-DDE concentrations in men in Chiapas, Mexico: a cross-sectional study. J Androl. 2006;27:16–27. doi: 10.2164/jandrol.05121. [DOI] [PubMed] [Google Scholar]
- 15.Longnecker MP, Gladen BC, Cupul-Uicab LA, Romano-Riquer SP, Weber JP, Chapin RE, Hernandez-Avila M. In utero exposure to the antiandrogen 1,1,-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) in relation to anogenital distance in male newborns from Chiapas, Mexico. Am J Epidemiol. 2007;165:1015–1022. doi: 10.1093/aje/kwk109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Rhouma K, Tebourbi O, Krichah R, Sakly M. Reproductive toxicity of DDT in adult male rats. Hum Exp Toxicol. 2001;20:393–397. doi: 10.1191/096032701682692946. [DOI] [PubMed] [Google Scholar]
- 17.ASTDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for DDT, DDE, and DDD. 2002. www.atsdr.cdc.gov/toxprofiles/tp35.pdf Available at. Accessed March 2011. [PubMed]
- 18.ASTDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Pyrethrins and Pyrethroids. 2003. www.atsdr.cdc.gov/toxprofiles/tp155.pdf Available at. Accessed March 2011. [PubMed]
- 19.Pleus B, Tanser FC, Lengeler C, Sharp B. Indoor Residual Spraying for Preventing Malaria (Review) Issue 4. New York; John Wiley & Sons, Ltd: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi HW, Breman JG, Teutsch SM, Liu S, Hightower AW, Sexton JD. The effectiveness of insecticide-impregnated bednets in reducing cases of malaria infection: a meta-analysis of published results. Am J Trop Med Hyg. 1995;52:377–382. doi: 10.4269/ajtmh.1995.52.377. [DOI] [PubMed] [Google Scholar]
- 21.International Artemisinin Study Group Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 22.Eisele TP, Larsen D, Steketee RW. Protective efficacy of interventions for preventing malaria mortality in children in Plasmodium falciparum endemic areas. Int J Epidemiol. 2010;39:88–101. doi: 10.1093/ije/dyq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 24.Hamer DH, Singh MP, Wylie BJ, Yaboah-Antwi K, Tuchman J, Desai M. Burden of malaria in pregnancy in Jharkhand State, India. Malar J. 2009;8:210. doi: 10.1186/1475-2875-8-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John CC, Riedesel MA, Magak NG, Lindblade KA, Menge DM, Hodges JS. Possible interruption of malaria transmission in highland Kenya, 2007–2008. Emerg Infect Dis. 2009;15:1917–1924. doi: 10.3201/eid1512.090627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinschmidt I, Schwabe C, Benavente L, Torrez M, Segura JL, Ehmer P. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80:882–888. [PMC free article] [PubMed] [Google Scholar]
- 27.Maharaj R, Mthembu DJ, Sharp BL. Impact of DDT reintroduction on malaria transmission in KwaZulu-Natal. S Afr Med J. 2005;95:871–874. [PubMed] [Google Scholar]
- 28.Nyarango PM, Gebremeskel T, Mebrahtu G, Mufunda J, Abdulmumini U, Ogbamariam A. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mnzava AE, Sharp BL, Mthembu DJ, Diamini SS, Gumede JK, Kleinschmidt I, Goodman C. Use of Insecticide-Treated Bednets by Communities Reduces Malaria Transmission in Comparison to House Spraying in Kwazulu-Natal. Policy Brief. Johannesburg, South Africa: South African Medical Research Council; 2000. [Google Scholar]
- 30.Djenontin A, Chandre F, Dabire KR, Chabi J, N'guessan R, Daldet T. Indoor use of plastic sheeting impregnated with carbamate combined with long-lasting insecticidal mosquito nets for the control of pyrethroid-resistant malaria vectors. Am J Trop Med Hyg. 2010;83:266–270. doi: 10.4269/ajtmh.2010.10-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pemba DF, Bandason E, Namangale J. Comparison of deltamethrin as indoor residual spray or on insecticide treated nets for mosquito control in Lake Chilwa. Malawi Med J. 2008;20:86–89. doi: 10.4314/mmj.v20i3.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Protopopoff N, Bortel VW, Marcotty T, Herp VM, Maes P, Baza D. Spatial targeted vector control is able to reduce malaria prevalence in the Brundian highlands. Am J Trop Med Hyg. 2008;79:12–18. [PubMed] [Google Scholar]
- 33.Ansari MA, Razdan RK. Follow-up studies after withdrawal of deltamethrin spraying against Anopheles culicifacies and malaria incidence. J Am Mosq Control Assoc. 2004;20:424–428. [PubMed] [Google Scholar]
- 34.Dhiman RC, Shahi B, Sharma SN, Nanda N, Khargiwarkar VN, Subbarao SK. Persistence of malaria transmission in a tribal area in Maharashtra, India. Curr Sci. 2005;88:475–478. [Google Scholar]
- 35.Kleinschmidt I, Schwabe C, Shiva M, Segura JS, Sima V, Mabunda SJ. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81:519–524. [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinschmidt I, Torrez M, Schwabe C, Benavente L, Seocharan I, Jituboh D. Factors influencing the effectivenss of malaria control in Bioko Island, equatorial Guinea. Am J Trop Med Hyg. 2007;76:1027–1032. [PMC free article] [PubMed] [Google Scholar]
- 37.WHOPES World Health Organization Pesticide Evaluation Program. 2010. www.who.int/whopes/en Available at. Accessed November 2010.
- 38.Kleinschmidt I, Sharp B, Benavente LE, Schwabe C, Torrez M, Kuklinski J, Morris N, Raman J, Carter J. Reduction in infection with Plasmodium falciparum one year after the introduction of malaria control interventions on Bioko Island, Equatorial Guinea. Am J Trop Med Hyg. 2006;74:972–978. [PubMed] [Google Scholar]
- 39.Charlwood JD, Qassim M, Elnsur EI, Donnelly M, Petrarca V, Billingsley PF. The impact of indoor residual spraying with malathion on malaria in refugee camps in eastern Sudan. Acta Trop. 2001;80:1–8. doi: 10.1016/s0001-706x(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 40.Guyatt HL, Corlett SK, Robinson TP, Ochola SA, Snow RW. Malaria prevention in highland Kenya: indoor residual house-spraying vs. insecticide-treated bednets. Trop Med Int Health. 2002;7:298–303. doi: 10.1046/j.1365-3156.2002.00874.x. [DOI] [PubMed] [Google Scholar]
- 41.Jambou R, Ranaivo L, Raharimalala L, Randrianaivo L, Rakotomanana F, Modiano D. Malaria in the highlands of Madagascar after five years of indoor house spraying of DDT. Trans R Soc Trop Med Hyg. 2001;95:14–18. doi: 10.1016/s0035-9203(01)90317-7. [DOI] [PubMed] [Google Scholar]
- 42.Sintasath DM, Ghebremeskel T, Lynch M, Kleinau E, Bretas G, Shililu J. Malaria prevalence and associated risk factors in Eritrea. Am J Trop Med Hyg. 2005;72:682–687. [PubMed] [Google Scholar]
- 43.Zhou G, Githeko AK, Noboru M, Yan G. Community-wide benefits of targeted indoor redisual spray for malaria control in the western Kenya highland. Malar J. 2010;9:67. doi: 10.1186/1475-2875-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bukirwa H, Yau V, Kigozi R, Filler S, Quick L, Lugemwa M. Short Report: assessing the impact of indoor residual spraying on malaria morbidity using a sentinel site surveillance system in western Uganda. Am J Trop Med Hyg. 2009;81:611–614. doi: 10.4269/ajtmh.2009.09-0126. [DOI] [PubMed] [Google Scholar]
- 45.Pardo G, Descalzo MA, Molina L, Custodio E, Lwanga M, Mangue C. Impact of different strategies to control Plasmodium infection and anaemia on the island of Bioko (Equatorial Guinea) Malar J. 2006;5:10. doi: 10.1186/1475-2875-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romi R, Razaiarimanga MC, Raharimalala R, Rakotondraibe EM, Ranaivo LH, Pietra V. Impact of the malaria control campaign (1993–1998) in the highlands of Madagascar: parasitological and entomological data. Am J Trop Med Hyg. 2002;66:2–6. doi: 10.4269/ajtmh.2002.66.2. [DOI] [PubMed] [Google Scholar]
- 47.Sharp B, van Wyk P, Sikasote JB, Banda P, Kleinschmidt I. Malaria control by residual insecticide spraying in Chingola and Chililabombwe, Copperbelt Province, Zambia. Trop Med Int Health. 2002;7:732–736. doi: 10.1046/j.1365-3156.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 48.Sharp BL, Kleinschmidt I, Streat E, Maharaj R, Barnes KI, Durrheim DN, Ridl FC, Morris N, Seocharan I, Kunene S, La Grange JJ, Mthembu JD, Maartens F, Martin CL, Barreto A. Seven years of regional malaria control collaboration–Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg. 2007;76:42–47. [PMC free article] [PubMed] [Google Scholar]
- 49.Tseng LF, Chang WC, Ferreira MC, Wu CH, Rampao HS, Lien JC. Rapid control of malaria by means of indoor residual spraying of alphacypermethrin in the Democratic Republic of Sao Tome and Principe. Am J Trop Med Hyg. 2008;78:248–250. [PubMed] [Google Scholar]
- 50.Egger M, Smith GD, Altman D. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Publishing Group; 2001. [Google Scholar]
- 51.Romi R, Razaiarimanga MC, Raharimanga R, Rakotondraibe EM, Ranaivo LH, Pietra V. Impact of the malaria control campaign (1993–1998) in the highlands of Madagascar: parasitological and entomological data. Am J Trop Med Hyg. 2002;66:2–6. doi: 10.4269/ajtmh.2002.66.2. [DOI] [PubMed] [Google Scholar]
- 52.Charlwood JD. The impact of indoor residual spraying with malathion on malaria in refugee camps in eastern Sudan. Acta Trop. 2001;80:1–8. doi: 10.1016/s0001-706x(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 53.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77((Suppl 6)):79–87. [PMC free article] [PubMed] [Google Scholar]
- 54.Pan American Health Organization . Report on the Situation of the Americas; Working Document. Washington, DC: Pan American Health Organization; 2008. [Google Scholar]
- 55.World Health Organization . Profiles: 31 High Burden Countries. World Malaria Report Appendix. Geneva: World Health Organization; 2009. [Google Scholar]
- 56.Smith D, Dushoff J, Snow R, Hay S. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]