Abstract
We evaluated the diagnostic accuracy of two broadly reactive rapid immunochromatographic tests (ICTs) for detection of IgM and IgG against Orientia tsutsugamushi by using archived acute-phase serum samples from 102 patients with laboratory-confirmed scrub typhus, and from 62 archived serum samples from patients with other causes of fever as a negative control. These ICTs were constructed by using a mixture of recombinant proteins: 1) C1, a chimeric protein containing epitopes of the 56-kD antigen from Karp and TA763 strains; 2) Ktr56; and 3) Gmr56. Sensitivities of the ICTs for detection of IgM and IgG were 90.2% (95% confidence interval [CI] = 84.4–96.0%) and 86.3% (95% CI = 80.9–93.8%), respectively. Specificities were 85.5% (95% CI = 73.9–92.2%) and 96.8% (95% CI = 90.3–100%), respectively. Both assays were more sensitive and specific than the standard immune immunofluorescence assay for the early diagnosis of scrub typhus.
Introduction
Scrub typhus is caused by Orientia tsutsugamushi, an obligate intracellular gram-negative bacterium that has a different cell wall structure and genetic makeup from those of the Rickettsiae species.1 It is transmitted to humans by the bite of the larval stage of trombiculid mites (chiggers). The endemic areas of scrub typhus include rural areas of Southeast Asia throughout the Asia Pacific rim and Northern Australia. More than one billion persons are at risk for infection and approximately one million cases occur annually.1,2 The reported incidence of scrub typhus has increased during the past decade.1,2 Clinical manifestations of scrub typhus vary widely from a mild and self-limiting febrile illness to a more severe illness that may be fatal.3,4 It has recently been recognized as an important cause of acute undifferentiated fever in indigenous populations and in travelers returning from the tropics.4,5
Despite the availability of low-cost and effective antibiotic treatment, scrub typhus causes significant morbidity and mortality in otherwise healthy adults and children. The greatest challenge to clinicians is diagnosing these infections early in their course, when antibiotic therapy is most effective. Serologic diagnosis using an indirect immunofluorescence assay (IFA) or an immunoperoxidase assay is the standard laboratory diagnosis for scrub typhus.6 These tests are not widely available in rural hospitals. There is a need for a simple, rapid diagnostic test for scrub typhus. In this study, we developed and evaluated a rapid colloidal gold immunochromatographic test (ICT) to detect IgM and IgG against O. tsutsugamushi in serum samples from patients. The antigens used for the antibody detection were a mixture of recombinant proteins C1, a chimeric protein containing epitopes of the 56-kD antigen from the Karp strain and the TA763 strain, along with the Ktr56 from the Kato strain and the Gmr56 from the Gilliam strain.
Materials and Methods
Patient samples.
All samples were stored at –70°C until testing.
Scrub typhus patient samples.
Five milliliters of blood was collected from 102 patients (64 males and 38 females) 15–84 years of age (mean age = 45 years) with early signs and symptoms of scrub typhus at six hospitals in Thailand during August 2000–January 2009. Three of these hospitals (Maharaj Nakhon Ratchasima Hospital, Nakhon Ratchasima Province; Loei Hospital, Loei Province; and Banmai Chaiyapod Hospital, Burirum Province) are located in northeastern Thailand, one hospital (Chumphon Hospital, Chumphon Province) is located in southern Thailand, and two hospitals (Ratchaburi Hospital, Ratchaburi Province; and Siriraj Hospital, Bangkok) are located in central Thailand. These samples were collected as part of studies investigating the causes of fever.4,7 This clinical study was approved by the Ethical Review Subcommittee of the Public Health Ministry of Thailand, and Siriraj Institutional Review Board Faculty of Medicine Siriraj Hospital, Mahidol University.
Patients provided informed written consent before sample were collected. For this study, the diagnosis of scrub typhus was confirmed by either 1) nested polymerase chain reaction (PCR) detection of the 16S ribosomal RNA gene or 56-kD protein gene8,9; or 2) IFA detection of an IgM O. tsutsugamushi titer ≥ 1:400 or a ≥ 4-fold increase in IFA IgM titer, or an IgG titer ≥ 1:800 or a ≥ 4-fold increase in IFA IgG titer.7,8
Non-scrub typhus patient samples.
Serum samples (n = 62) from patients with laboratory-confirmed diagnosis of other tropical febrile illness were collected at the same study hospitals. All non-scrub typhus patient samples were negative for O. tsutsugamushi infection by IFA and PCR. The diagnosis in this control group included dengue infection in 17 patients; influenza A or influenza B infection in 10 patients; murine typhus in 11 patients; leptospirosis in 9 patients; bacteremia from other bacteria such as Burkholderia pseudomallei, Escherchia coli, and Salmonella spp. in 6 patients; Plasmodium falciparum malaria in 3 patients; suspected spotted fever group rickettsioses in 4 patients; and an unknown diagnosis in 2 patients.
Indirect immunofluorescent assay.
The IFA was performed on paired (acute-phase and convalescent-phase) serum samples at the Infectious Diseases and Tropical Medicine Laboratory, Siriraj Hospital, by using established methods.10 In brief, pooled antigens of O. tsutsugamushi strain Karp, Kato, and Gilliam were spotted on a glass slide. Patient serum samples were serially diluted two-fold from 1:50 to 1:6400 in phosphate-buffered saline (PBS) containing 2% (w/v) skim milk, incubated in a humidified chamber for 30 minutes at 37°C, and washed three times in PBS. Anti-human IgM and IgG fluorescein isothiocyanate conjugate (Jackson Immuno Research Laboratories, West Grove, PA), diluted in PBS–skim milk diluent containing 0.00125% (w/v) Evans Blue counter stain was applied to all wells, and wells were incubated in a humidified chamber for 30 minutes at 37°C. Slides were examined by fluorescence microscopy (BX50; Olympus, Tokyo, Japan) at a magnification of 400×. The endpoint titer was determined as the highest titer that showed fluorescence signal above the background. Known positive and negative control serum samples were included in each experiment.
PCR amplification of the O. tsutsugamushi DNA.
Chromosomal DNA extraction.
Chromosomal DNA of O. tsutsugamushi was extracted by using the minipreparation of bacterial genomic DNA method.8 In brief, EDTA-treated blood samples were centrifuged at 200 × g for 10 minutes. The buffy coat (leukocyte layer) was collected from the interface between the upper plasma layer and the lower erythrocyte layer. Erythrocytes contaminating the buffy coat were lysed by diluting the buffy coat sample with distilled water. Chromosomal DNA of intracellular bacteria was extracted from the leukocyte pellet and suspended with Tris-EDTA buffer, pH 8.0. The DNA was further purified by phenol-chloroform-isoamyl alcohol (25:24:1) extraction and precipitated with isopropanol. The precipitated DNA was washed with 70% ethanol. After aspiration of the 70% ethanol, the DNA pellet was air-dried for 10 minutes, resuspended in Tris-EDTA buffer, pH 8.0, and stored at –20°C until used.
PCR amplification of the 16S ribosomal RNA gene.
A described conventional PCR specific for the 16S ribosomal RNA gene was performed.8 The primers used in this procedure were OT1-F (5′-CGAATTAAT-GCTGAGTTTGCTTAG-3′) and OT1-R (5′-CTCTCAGA-CCAGCTACAGATCACA - 3′). Amplification was performed by using a thermal cycler (GeneAmp PCR System 9700; PE-Applied Biosystems, Inc., Foster City, CA, USA). The DNA template was amplified by using reagents supplied in a Taq polymerase kit (Promega, Madison, WI). Cycling conditions included an initial step at 95°C for 2 minutes; followed by 35 cycles at 95°C for 30 seconds, 57°C for 30 seconds, and 72°C for 1 minutes; and a final extension step at 72°C for 5 minutes. The PCR products were analyzed by agarose gel electrophoresis.
PCR amplification of the 56-kD protein-encoding gene.
As described, nested PCR specific for the VD I-IV region of the 56-kD protein-encoding gene was performed.9 The outer and inner primer pairs were designed by nucleotide sequence alignment of the conserved regions of several strains of O. tsutsugamushi in the GenBank database including UT219 (EF213100), FPW2031 (EF213098), Karp (M33004), Kato (M63382), UT329 (EF213099), FPW2016 (EF213085), and UT196 (EF213079). The nucleotide sequence of the outer (E) and inner (I) primer pairs were E1: 5′-GCTAAAGTTGGAGTTGTTGGAGG-3′; E2: 5′-CCACATACACACCTTCAGCAGC-3′; I1: 5′-CCATTTGGTGGAACGTTGGCTGC-3′; and I2: 5′-GTCAGCATAGAGTTTAACTTGGC-3′. For the first round of PCR amplification, the sense (E1) and antisense (E2) of the outer primer set were added to the reaction mixture at the final concentration of 0.4 μM. The 50-μL reaction mixture contained 5 μL of 10× PCR buffer, 200 μM of each dNTP, 1 unit of TopTaq DNA polymerase (Qiagen, Hilden, Germany) and approximately 1 μg of DNA sample. DNase/RNase free sterile water was added to bring the total volume up to 50 μL. Cycling conditions included an initiation step at 95°C for 2 minutes; 35 cycles of DNA denaturation at 95°C for 1 minute, annealing at 50°C for 1 minute, and extension at 72°C for 2 minutes; followed by a final extension at 72°C for 5 minutes by using a GeneAmp PCR System 9700 (PE-Applied Biosystem Inc.). The nested PCR was performed by adding the internal primers at a final concentration of 0.4 μM to the same reaction mixture as used in the first round and 3 μL of the completed first round PCR reaction mixture was added as the template. The amplification cycle was the same as the first round of amplification. The amplicon size was 1,131 basepairs and the final reaction product was analyzed by agarose gel electrophoresis.
Immunochromatographic test for detection of IgM and IgG against O. tsutsugamushi.
The ICT was manufactured and donated by InBios International (Seattle, WA).
Construction of chimeric antigen.
Chimeric 1 r56 (C1) was designed manually by using the Karp strain 56-kDa protein sequence as the backbone. The sequence of the variable domain 1 was replaced by that in TA763 with modifications as described.11 The DNA was synthesized by Bioclone (San Diego, CA) and cloned into a pET29a vector (Novagen, Madison, WI) with built-in Nde I and Xho I restriction sites. The synthesized DNA sequences were confirmed. The construction of r56s from Gilliam and Kato strains was performed as described.12 The gene for C1 was further subcloned into a SUMO-containing vector.
Recombinants proteins applied to test membrane.
The three scrub typhus proteins were mixed at a concentration of 0.15–0.35 mg/mL for each antigen in Tris buffer. The mixture was sprayed onto nitrocellulose membranes. The membranes were dried and stored in a humidity- and temperature-controlled environment until ready to be assembled.
Preparation of gold particles.
Recombinant protein A or goat anti-human IgM was conjugated to 40-nm gold particles using a modification of a described protocol.13 The gold conjugates at different optical density values were applied onto glass fiber–based conjugate pads. The gold was dried in a controlled airflow incubator and then transferred to a humidity- and temperature-controlled environment.
Prototype assembly.
The antigen sprayed membrane and dried gold conjugate pad were assembled on a plastic membrane backing according to a manufacturing standard operation procedure). The assembled ICTs passed quality control standards at different steps and a final quality control procedure was performed before the product was released.
Quality control procedure.
A well-defined panel consisting of positive and negative samples was used to assess the quality of the assembled product. The panel was used to monitor consistency and stability of the scrub typhus prototypes.
Procedure for ICT.
Approximately 5 μL of serum was applied to the ICT sample application pad and the strip was then immersed in 3 drops (120 μL) of chase buffer in a microwell. The results were interpreted visually in 15 minutes. The test result was considered negative if only the control band was stained. If the test and control bands were stained, the test result was considered positive.
Data analysis.
Diagnostic performance was calculated by comparing the IgM and IgG ICT results with the results of the reference standard, PCR and/or IFA for each patient. Equivocal results were considered negative for the final analysis. A two-by-two table was constructed, in which the reference standard test results was cross-tabulated with the ICT results to define the rates of true-positive, false-positive, false-negative, and true-negative results. The standard diagnostic accuracy indices of sensitivity, specificity, positive predictive value, and negative predictive value with 95% confidence intervals (CIs) were calculated by using the SPSS18.0 software (SPSS Inc., Chicago, IL).
Results
The diagnosis of scrub typhus was confirmed in 102 patients by 1) PCR detection of either the 16S ribosomal RNA gene or 56-kD protein-encoding gene in 43 patients (42.2%); or 2) IFA detection of a four-fold increase in either IgM or IgG titer in 47 patients (46.1%); or 3) IFA detection of a high antibody titer (IgM > 400 or IgG > 800) in 12 patients (11.7%). The median duration of fever at the time of collection for testing was 6 days (range = 1–47 days), and the median interval between obtaining initial acute-phase samples and subsequent convalescence-phase samples was 13 days (range = 3–32 days).
The sensitivities of the ICTs for the detection of IgM and IgG against O. tsutsugamushi were 90.2% (95% CI = 84.4–96.0%) and 86.3% (95% CI = 80.9–93.8%), respectively (Table 1 ). The specificities for the detection of IgM and IgG against O. tsutsugamushi were 85.5% (95% CI = 73.9–92.2%) and 96.8% (95% CI = 90.3–100%), respectively. Among patients with reference standard test–confirmed scrub typhus, the results of the ICT were as follows: 1) 85 (83.3%) patients were positive for IgG and IgM; 7 (6.9%) patients were positive for IgM only; 3 (2.9%) patients were positive for IgG only; and 7 (6.9%) patients were negative for IgG and IgM. Among patients with positive PCR results, ICT IgM and IgG were positive in 95.3% and 86.0%, respectively. Among patients whom the diagnosis of scrub typhus was positive by IFA antibody detection, ICT IgM and IgG were positive in 86.4% and 86.4%, respectively.
Table 1.
Overall diagnostic accuracy and sensitivity of the immunochromatographic test for detection of IgM and IgG against scrub typhus compared with results of the gold standard assays (PCR and IFA)*
| Antibody | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| IgM | 90.2 (84.4–96.0) | 85.5 (76.7–94.3) | 91.1 (85.5–96.7) | 84.1 (75.1–93.2) |
| IgG | 86.3 (79.6–93.0) | 96.8 (92.4–100) | 88.0 (81.6–94.4) | 81.1 (72.2–90.0) |
PCR = polymerase chain reaction; IFA = immunofluorescence assay; PPV = positive predictive value; NPV = negative predictive value. Values in parentheses are 95% confidence intervals.
Positive ICT IgM antibody detection was found for 9 patients with other infections (5 patients with dengue infection; 2 patients with P. falciparum malaria; 1 patient with influenza A infection; 1 patient with B. pseudomallei bacteremia; and 1 patient with E. coli bacteremia.) Positive ICT IgG antibody detection was found for 2 patients with dengue infection, both of whom also had positive IgM detection by the ICT.
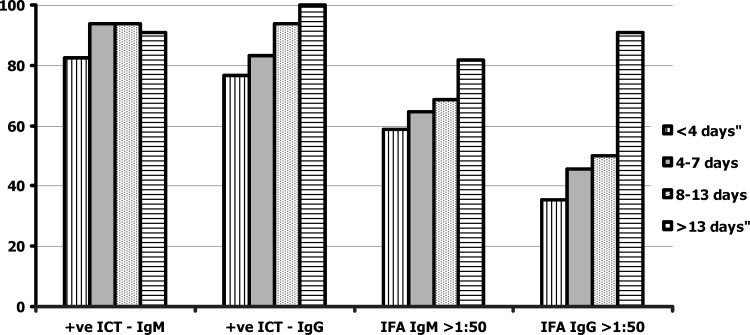
In patients with standard reference test–confirmed scrub typhus, 29 (78.3%) of 37 patients with negative IgM detection by IFA (titer ≤ 1: 50) showed positive results by the IgM ICT, and all patients except one with an IgM titer ≥ 1: 100 by IFA were positive by IgM ICT. Of the 56 patients with negative results for IgG by ICT (titer ≤ 1: 50), 46 (82.1%) were ICT IgG positive, and all patients with an IgG antibody titer ≥ 1:100 by IFA were positive by IgG ICT. The positive rates for IgM and IgG early after infection (< 4 days of fever) varied between 65% and 70%, but increased to 90% after 8 days of fever. The assay sensitivity for ICT IgG was 100% for samples collected two weeks after onset of fever. The proportions of positive results from ICTs for IgM and IgG among patients who had different durations of fever were compared with results from IFA (Figure 1).
Figure 1.
Proportion (%) of positive immunochromatographic test (ICT) results for detection of IgM and IgG against scrub typhus compared with those of immunofluorescence assays against days of fever before study.
Discussion
Early diagnosis of scrub typhus is important to guide appropriate antimicrobial therapy. Empirical therapy with doxycycline is the most cost effective strategy for the management of patients with clinically suspected scrub typhus in disease-endemic areas.14 This empiric treatment is necessary because a rapid, sensitive, affordable diagnostic test for scrub typhus is not available.14 However, the empiric therapy could lead to the treatment of many patients who do not have scrub typhus. Standard laboratory diagnosis for scrub typhus is usually based on the detection of scrub typhus antibodies by using IFA. This assay is impractical for routine application in rural settings and has low sensitivity for the diagnosis of scrub typhus in acute-phase specimens.1,6 Conventional and real-time PCR for detecting O. tsutsugamushi have been developed, but these tests require special equipment and skillful personnel to perform the test. This study has demonstrated high sensitivity and moderate specificity for the IgM and IgG ICTs. Samples used in this study were collected during the acute phase of infection, which is the real world situation for early diagnosis and treatment. Both of these assays are easy to perform in point-of-care settings, and results can be obtained within 15 minutes for proper patient management.
False-positive IgG and IgM ICT results for patients with other infections in this study may be caused by a number of factors, including cross-reactive antibodies between the infecting pathogen and O. tsutsugamushi or persistence of antibody after recovery from previous scrub typhus infections. Reinfection of O. tsutsugamushi is not uncommon in areas to which scrub typhus is endemic.15 In this study, 17 (16.7%) of 102 patients with scrub typhus were diagnosed by a four-fold increase in IgG titer or by a high initial IgG titer. The kinetic pattern of antibody response of Orientia re-infection mimics those who had re-infection of dengue virus. The initial antibody response after re-infection is mainly the result of an increase of IgG level. Therefore, detection of IgM and IgG at the same time should increase the overall diagnosis sensitivity by the results of IgG and IgM ICTS in combination for primary infection and secondary infection.
In addition to the three prototype strains (Karp, Kato, and Gilliam strains of O. tsutsugamushi), more than 30 antigenically distinct strains are present in areas to which scrub typhus is endemic.16 Previous serologic studies suggested that TA763 (isolated in Thailand) is the prevalent infecting strain in Malaysia where peak IgG and IgM titers were most commonly observed against Karp, Gilliam, and TA763 strains.17,18 Anti-TA763 mouse serum recognizes antigens of many heterologous strains, indicating that it has broad reactivity. On the basis of this information, we believe that TA763 r56 should be included in the development of an improved antibody-capture test. A mixture of recombinant proteins C1, a chimeric protein containing epitopes of Karp and TA763, along with Ktr56 and Gmr56 antigens used in this study, should enable the ICTs to have broad reactivity and are appropriate for use in Southeast Asia.
In conclusion, we have evaluated the newly developed rapid point-of-care tests for the detection of O. tsutsugamushi IgM and IgG. Results from this study using serum samples from 164 patients with a variety of acute tropical fever diagnoses suggest that the IgM and IgG ICTs for diagnosis of scrub typhus were more sensitive than the standard IFA. Additional studies are required to determine the true diagnostic utility of the ICT because discrepancies between archived samples and prospectively acquired samples may exist.
ACKNOWLEDGMENTS
We thank the doctors, nurses, and medical technologists of Chumphon Hospital, Maharaj Nakhon Ratchasima Hospital, Loei Hospital, Ratchaburi Hospital, and Ban Mai Chaiyapod Hospital, for their cooperation and help during the study period.
Disclaimer: This study was prepared as part of official duties. The opinions and assertions contained herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy, the Naval service at large, the Department of Defense, or the U.S. Government.
Footnotes
Disclosure: Chien-Chung Chao and Wei-Mei Ching are employees of the U.S. Government.
Authors' addresses: Saowaluk Silpasakorn, Department of Medicine, Mahidol University, Bangkok, Thailand, E-mail: aowaluk_8@yahoo.com. Nujorn Srisamut and Pattama Ekpo, Department of Immunology, Mahidol University, Bangkok Thailand, E-mails: nc-immuno@hotmail.com and sipep@mahidol.ac.th. Zhiwen Zhang, Chien-Chung Chao, and Wei-Mei Ching, Viral and Rickettsial Diseases Department, Naval Medical Research Center, Silver Spring, MD, E-mails:zhiwen.zhang@med.navy.mil, chien-chung.chao@med.navy.mil, and weimei.ching@med.navy.mil. Yupin Suputtamongkol, Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand, E-mails: siysp@mahidol.ac.th or ysuputtamongkol@gmail.com.
References
- 1.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Silpapojakul K. Scrub typhus in the Western Pacific region. Ann Acad Med Singapore. 1997;26:794–800. [PubMed] [Google Scholar]
- 3.Thap LC, Supanaranond W, Treepresertsuk S, Kitvatanachai S, Chinprasatsak S, Phonrat B. Septic shock secondary to scrub typhus: characteristics and complications. S E Asian J Trop Med Pub Health. 2002;33:780–786. [PubMed] [Google Scholar]
- 4.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Suwancharoen D, Panlar P, Saisongkorh W, Rolain JM, Raoult D, Suputtamongkol Y. Causes of acute undifferentiated febrile illness in rural Thailand: a prospective observational study. Ann Trop Med Hyg. 2006;100:363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 5.Rolain JM, Jensenius M, Raoult D. Rickettsial infections: a threat to travelers? Curr Opin Infect Dis. 2004;17:433–437. doi: 10.1097/00001432-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 6.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–2727. doi: 10.1128/jcm.35.11.2715-2727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suputtamongkol Y, Niwattayakul K, Suttinont C, Losuwanaluk K, Limpaiboon R, Chierakul W, Wuthiekanun V, Triengrim S, Chenchittikul M, White NJ. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39:1417–1424. doi: 10.1086/425001. [DOI] [PubMed] [Google Scholar]
- 8.Sonthayanon P, Chierakul W, Wuthiekanun V, Blacksell SD, Pimda K, Suputtamongkol Y, Pukrittayakamee S, White NJ, Day NP, Peacock SJ. Rapid diagnosis of scrub typhus in rural Thailand using polymerase chain reaction. Am J Trop Med Hyg. 2006;75:1099–1102. [PubMed] [Google Scholar]
- 9.Wongprompitak P, Anukool W, Wongsawat E, Silpasakorn S, Dyong V, Buchy P, Morand S, Frutos R, Ekpo P, Suputtamongkol Y. Broad-coverage molecular epidemiology of Orientia tsutsugamushi in Thailand. Infect Genet Evol. 2011;25 doi: 10.1016/j.meegid.2011.06.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Bozeman FM, Elisberg BL. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- 11.Chao CC, Huber ES, Porter TB, Zhang Z, Ching WM. Analysis of the cross-reactivity of various 56 kDa recombinant protein antigens with serum samples collected after Orientia tsutsugamushi infection by ELISA. Am J Trop Med. 2011;84:967–972. doi: 10.4269/ajtmh.2011.10-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Ching WM, Jiang J, Lousteau L, Richards AL. An improved method for the purification and refolding of r56-kDa proteins from Gilliam and Kato strains of Orientia tsutsugamushi. Ann N Y Acad Sci. 2003;990:375–385. doi: 10.1111/j.1749-6632.2003.tb07395.x. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri B, Raychaudhuri S. Manufacturing High-Quality Gold Sol: Understanding Key Engineering Aspects of the Production of Colloidal Gold Can Optimize the Quality and Stability of Gold Labeling Components. IVT Technology; 2001. www.ivdtechnology.com Available at. [Google Scholar]
- 14.Suputtamongkol Y, Pongtavornpinyo W, Lubell Y, Suttinont C, Hoontrakul S, Phimda K, Losuwanaluk K, Suwancharoen D, Silpasakorn S, Chierakul W, Day N. Strategies for diagnosis and treatment of suspected leptospirosis: a cost-benefit analysis. Plos Nleg Trop Med. 2010;4:e610. doi: 10.1371/journal.pntd.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgeois AL, Olson JG, Fang RCY, Huang J, Wang CL, Chow L. Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am J Trop Med. 1982;31:532–540. doi: 10.4269/ajtmh.1982.31.532. [DOI] [PubMed] [Google Scholar]
- 16.Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48:S203–S230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- 17.Shirai A, Robinson DM, Brown GW, Gan E, Huxsoll DL. Antigenic analysis by direct immunofluorescence of 114 isolates of Rickettsia tsutsugamushi recovered from febrile patients in rural Malaysia. Jpn J Med Sci Biol. 1979;32:337–344. doi: 10.7883/yoken1952.32.337. [DOI] [PubMed] [Google Scholar]
- 18.Tay ST, Rohani MY, Ho TM, Devi S. Antigenic types of Orientia tsutsugamushi in Malaysia. Southeast Asian J Trop Med Public Health. 2002;33:557–564. [PubMed] [Google Scholar]