Abstract
A nationwide epidemiologic investigation of domestic animal infections has been conducted in nine provinces and one city during 2007–2010. Serum samples from a total of 707 goats, 433 cattle, and 219 dogs were collected for detecting Anaplasma phagocytophilum IgG antibody by immunofluorescence assays and the average seroprevalences were 10.05% for dogs, 3.82% for goats, and 0.69% for cattle, respectively. A total of 472 goats, 201 cattle, 102 dog blood clots, and 1,580 ticks were collected for polymerase chain reaction (PCR) amplifying A. phagocytophilum 16S rRNA genes and the PCR-positive rates were 26.69% for goats, 23.38% for cattle, and 10.89% for dogs. Six species were identified and the average PCR-positive rates were 58.3% for Dermacentor silvarum, 43.9% for Haemaphysalis longicornis, 12.5% for Ixodes persulcatus, 7.5% (3 of 40) for Boophilus microplus, and 5.2% for Rhipicephalus sanguineus, respectively. The evidence in the study indicated the zoonotic Rickettsia is highly prevalent in China.
Introduction
Anaplasma phagocytophilum, an obligate intracellular bacterium, is the etiological agent of human granulocytic anaplasmosis (HGA).1 Very limited epidemiological evidence was documented in China, although A. phagocytophilum infection was distributed worldwide.2 The disease is often misdiagnosed in clinics, resulting in multi-organ involvement and even the death of patients.3 Several seroepidemiologic surveys of HGA showed this disease was widely distributed in China.4–6 Although domestic animals have been implicated as important hosts for transmission of HGA, little epidemiologic investigation of domestic animal infections in rural areas of China has been conducted. In this study, we investigated the prevalence of A. phagocytophilum infection among domestic animals in 10 provinces/cities in China.
Methods And Materials
Animal blood sampling.
Approval for the survey was obtained from the China Centers for Disease Control and Prevention (CDC) Institutional Review Board and blood samples collected from the animals were approved by local Provincial animal care associations. The surveillance was conducted from March to May during the peak season of tick activities between 2007 and 2010 in rural areas in Beijing City and nine provinces including Anhui, Henan, Jiangsu, Xinjiang, Yunnan, Zhejiang, Hainan, Shandong, and Shanxi. For each province, three or five rural countries were selected for investigation. The investigated sites were randomly chosen based on the geographic locations (East, South, West, North, and Central parts), although farmer families were selected based on residence registration (selecting a single or double residence registration number). The participants were asked whether they raised domestic animals and how many of each kind of animal, and if so, 1 of 10 goats, 1 of 10 cattle, or 1 of 3 dogs from each family were selected for sampling; a 5 mL non-anti-coagulation blood sample was taken from each animal. Sampling blood for goats and cattle were performed by jugular vein, whereas dog blood sampling was conducted by the anterior tibial vein with the help of a local veterinarian. Sera were separated for detecting immunoglobulin G (IgG) antibody of A. phagocytophilum and the remaining blood clots were used to extract DNA for amplifying the A. phagocytophilum 16S rRNA gene by nested polymerase chain reaction (PCR). Serum and blood clot samples were stored at −20 or −80°C at the local CDC temporarily until transferred to the Department of Rickettsiology, China National Institute of Communicable Disease Control and Prevention (ICDC) for testing.
Tick sampling.
Ticks were collected from the body surface of goats, cattle, dogs, and farmers' houses before sampling animal blood and the living ticks were stored in a clean and ventilated container at 4°C temporally at the local CDC and then transferred by highway transportation to the Department of Rickettsiology, China ICDC for classification and testing within 24 hours. After taxonomic identification, 5∼10 non-blood-sucking ticks or 2~∼3 blood-sucking ticks were divided into one group and each group was soaked in 75% alcohol in separated containers for 30 min, and then washed with sterile distilled water for 10 min, and repeated three times. Finally, the ticks were ground by using the Germany mill (Retsch MM400) and the tick slurry was extracted for amplifying A. phagocytophilum 16S rRNA gene by nested PCR.
Immunofluorescence assays.
Serological assay were performed by using the immunofluorescence assays as the World Health Organization (WHO) proposed.7 Anaplasma phagocytophilum (RA2682, lot 03-0403N) antigen were provided by Dr. Robert Massung from the U.S. CDC Atlanta, GA). The positive control serum was prepared by immuring rabbit with A. phagocytophilum strain Webster, which was provided by Dr. J. S. Dumler at the Johns Hopkins University School of Medicine (Baltimore, MD), in our laboratory. Mixed fetal bovine sera, newborn goat sera, newborn dog sera, and 3% nonfat powdered milk and phosphate buffered saline (PBS) were used as a negative control, respectively. Briefly, sera were diluted 1:80 in PBS with 3% nonfat powdered milk and 25 μL of the diluted serum was placed in appropriate wells of the antigen slides and incubated for 60 min in a moist chamber at 37°C. After washing to remove unbound antibody, slides were reacted with fluorescein isothiocyanate-conjugated rabbit anti-goat, anti-cattle, anti-dog IgG (Sigma Co.), respectively. The slides were rinsed again and counterstained with Evan's blue before examination using a fluorescent microscope (Nikon, Tokyo, Japan). Samples were interpreted as reactive when clear fluorescent bacterial morphology was observed. Samples reactive at 1:80 dilutions were considered as positive.
Nested PCR.
For amplifying and analyzing the A. phagocytophilum 16S rRNA gene for animal blood and tick samples, DNA was extracted using a QIAamp blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Blood from healthy people, cattle, horses, goats, dogs, mice as well as sterile deionized water was used as negative controls and A. phagocytophilum DNA was used as positive control. The nested PCR for A. phagocytophilum 16S rRNA genes was performed as described previously.8 Briefly, Anaplasma genus-specific primers of outer-1 (5′-TTG AGA GTT TGA TCC TGG CTC AGA ACG-3′)/outer-2 (5′-CAC CTC TAC ACT AGG AAT TCC GCT ATC-3′) were used in the first round of amplification, and species-specific primers of HGA1 (5′-GTC GAA CGG ATT ATT CTT TAT AGC TTG -3′)/HGA2 (5′-TAT AGG TAC CGT CAT TAT CTT CCC TAC-3′) were used for nested PCR. A final 389-bp DNA fragment was produced. In the first round, 10 μL DNA was used for templates and 0.5 μL first round PCR products were used as templates for the nested PCR. Bidirectional sequencing of positive PCR products were commercially conducted by Shanghai Shengong Biotechnology Co. (Shanghai, China).
Data analysis.
Comparison of the total prevalence among different animals and prevalence of different animals among different provinces/cities were performed using the χ2 test and Fisher's exact test, using SAS software (version 9.1; SAS Institute, Inc., Cary, NC). Significance for these analyses was defined at a P level of 0.05. Sequence analysis was conducted by MEGA4.0 software and the phylogenetic tree was constructed by the neighbor-joining method.
Results
Investigated sites and animal blood sampling.
The study was conducted from 2007 to 2009 and samples were obtained during the peak of tick activity from March to May. This active surveillance covered nine Provinces including Anhui, Henan, Jiangsu, Xinjiang, Yunnan, Zhejiang, Shanxi, Hainan, Shandong, and Beijing City. A total of 62 natural villages belonging to 21 rural counties nationwide were investigated. The major economy of the investigated regions was agriculture and the breeding of domestic animals. The special breeding outside of the cages of goats, cattle, and dogs in the wild fields provided animals with a lot of ticks on their bodies. The worse problem was that the animals lived with people in the same yard in which only a wall separated them. At the same time, each family kept two or three dogs to guard belongings and all domestic animals could stroll anywhere in the countryside during daylight and live with the farmers during the night. A total of 707 serum from goats, 433 from cattle, and 219 from domestic dogs were obtained for serological detection and 472 blood clots from goats, 201 from cattle, and 102 from dogs were collected for PCR detection.
Sampling and identification of ticks.
A total of 1,580 ticks were collected and they were divided into 654 sample pools for nested PCR amplifying the A. phagocytophilum 16S rRNA gene. Classification of ticks showed there are at least six species including 570 Haemaphysalis longicornis (363 from goats, 120 from cattle, and 87 from dogs) from Shandong Province, 694 Dermacentor silvarum (360 from goats, 240 from cattle, and 94 from dogs) from Shanxi Province, 80 Haemaphysalis concinna (50 from goats and 30 from cattle) from Henan Province, 80 Ixodes persulcatus (42 from goat, 18 from cattle, and 20 from horse) from Xinjiang Provine, 80 Boophilus microplus (from dogs), and 76 Rhipicephalus sanguineus (40 from dogs and 36 from farmers' houses from Hainan Province).
Seroprevalence.
The nationwide average seroprevalences of A. phagocytophilum were 10.05% for dogs, 3.82% for goats, and 0.69% for cattle, respectively (Table 1). The seroprevalence of dogs was significantly higher than goats (P = 0.0005) and cattle (P = 0.0001), respectively. Similarly, the seroprevalence of goats was higher than cattle (P = 0.0017). A comparison of goat infections in the seven provinces/cities showed that the highest rate was found in Xinjiang (18.18%, [P < 0.001]). No antibodies to A. phagocytophilum were found among the cattle samples from surveyed areas, except for Henan Province (3.49%). Antibodies were found more often in dogs than in other animals with higher seroprevalence rates at 66.67% in Henan, 27.27% in Anhui, and 9.84% in Jiangsu, respectively (P < 0.001).
Table 1.
Seropositive rates among different animals*
| Areas | Goat | Cattle | Dog | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of tested sera | No. of positive | Seropositive rates (%) | No. of tested sera | No. of positive | Seropositive rates (%) | No. of tested sera | No. of positive | Seropositive rates (%) | |
| Anhui | 131 | 0 | 0.00 | – | – | – | 11 | 27.27 | |
| Beijing | 90 | 0 | 0.00 | 72 | 0 | 0.00 | 2 | 0 | 0.00 |
| Henan | 95 | 1 | 1.05 | 86 | 3 | 3.49 | 6 | 4 | 66.67 |
| Jiangsu | 150 | 0 | 0.00 | 162 | 0 | 0.00 | 122 | 12 | 9.84 |
| Yunnan | 58 | 2 | 3.45 | – | – | – | – | – | – |
| Xinjiang | 132 | 24 | 18.18 | 60 | 0 | 0.00 | 78 | 3 | 3.85 |
| Zhejiang | 51 | 0 | 0.00 | 53 | 0 | 0.00 | – | – | – |
| Total | 707 | 27 | 3.82 | 433 | 3 | 0.69 | 219 | 22 | 10.05 |
– = no samples were obtained.
Nested PCR amplifying.
The average PCR-positive rates were 26.69% (126 of 472) for goats, 23.38% (47 of 201) for cattle, and 10.89% (11 of 101) for dogs, respectively. The PCR results showed that a total of 184 samples were positive for 16S rRNA sequences of A. phagocytophilum including 126 goat samples (44 from Anhui, 44 from Beijing, 4 from Xinjiang, 16 from Yunnan, 12 from Zhejiang, 6 from Shandong), 47 cattle samples (17 from Beijing, 30 from Zhejiang), and 11 dog samples (3 from Anhui, 8 from Shandong). The sequences were deposited into GenBank with 44 sequences from Anhui (GQ499885–907; GQ499909–18; GQ499921–28; GQ499930–32) for goats; 3 sequences (GQ49919–20, GQ499929) for dogs; 44 sequences from Beijing (GQ499956; GQ4999770–GQ500012; GQ500022–24; GQ500034–37) for goats; 17 sequences (GQ500013–20; GQ 500025–33) for cattle; 4 sequences from Xinjiang (GQ900621–24) for goats; 16 sequences from Yunnan (GQ500079–94) for goats; 12 sequences from Zhejiang (GQ500038; GQ500068–78) for goats; 30 sequences (GQ499976; GQ500039–67) for cattle, and 6 sequences from Shandong (EU982704–09) for goats.
For Dermacentor silvarum collected from Ningwu County, Shanxi Province, 55% (66 of 120) of sample pools was positive for goats, 62.5% (50 of 80) for cattle, and 57.5% (27 of /47) for dogs. For Haemaphysalis longicornis obtained from Yiyuan County and Laizhou Bay areas, Shandong Province, 41.2 (49 of 119) of sample pools was positive for goats, 46.3% (19 of 41) for cattle, and 51.2 (15 of 29) for dogs. No PCR-positive results were observed on Haemaphysalis concinna from goats (30 sample pools) and from cattle (28 sample pools), respectively, collected from Xinyang areas, Henan Province. For I. persulcatus from Yili areas, Xinjiang Province, 14.28% (2 of 14) was positive for goats, 16.7% (1 of 6) for cattle, and 0% (0 of 4) for horse. For Boophilus microplus and Rhipicephalus sanguineus from Chengmai County, Hainan Province, 7.5% (3 of 40) of B. microplus and 7.5% (3 of 40) of R. sanguineus from dogs was positive, 3.6% (2 of 56) of R. sanguineus from farmers' houses was positive.
Phylogenetic analysis.
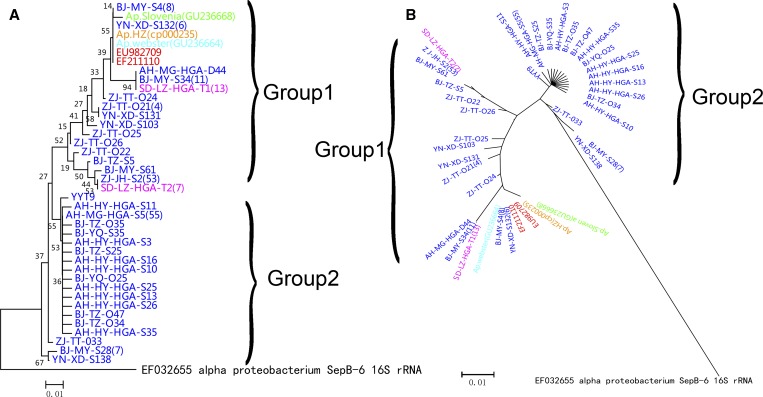
Phylogenetic analyses of partial A. phagocytophilum 16S rRNA genes (228 bp) detected in animals and ticks indicated that there are two dominated groups of A. phagocytophilum (Figure 1A and B). Group A, represented by ZJ-JH -S2 identified in goats from Zhejiang Province, consisted of 59.16% of detected sequences in the study and identified broadly in China, from Beijing in the north to Anhui and Zhejiang in the central and southeast, and Yunnan Province in southwest China. Another two sequences from Haemaphysalis longicornis (SD-LZ-HGA-T1 and SD-LZ-HGA-T2) were also classified in the same group. Moreover, this group was closely related with another two sequences including the patient (EF211110) from Anhui nosocomial HGA infection in 2006 and the patient (EU982709) from Yiyuan County, Shandong Province in 2007, respectively. Moreover, these Chinese strain sequences mentioned previously and the corresponding sequence of A. phagocytophilum strain Webster (United States), strain HZ (United States), and strain Slovenia (Slovenia) were also classified in the same group (Figure 1A and B). Group B, represented by AH-MG-HGA-S5 from Anhui Province, consisted of 40.84% of identified sequences and was mainly distributed in the north and central parts of China.
Figure 1.
Phylogenetic analyses of partial 16S rRNA genes of Anaplasma phagocytophilum identified in domestic animals from surveyed areas in China (A, rectangular tree; and B, radiation tree). Blue indicates sequences determined from animals in the study, red indicates sequences obtained from patients from Anhui Province in 2006 and Shandong Province in 2007, purple indicates sequences obtained from ticks from Shandong Province in 2007. Green is A. phagocytophilum strain Slovenia, orange is A. phagocytophilum strain HZ, and sky blue is A. phagocytophilum strain Webster.
Discussion
It has been reported that the natural reservoirs for A. phagocytophilum were wild rodents and domestic animals including goats, sheep, red deer, and cattle in the United States and European countries.9 This large surveillance effort for A. phagocytophilum showed the frequent presence of A. phagocytophilum in domestic animals in China. The IgG antibody positive rates of A. phagocytophilum were 10.05% (22 of 219) for dogs, 3.82% (27 of 707) for goats, and 0.69% (3 of 433) for cattle, respectively. Here, we noticed that the PCR-positive rates of goats and cattle were significantly higher than seropositive in this study. The probable reason is that we chose a broad range rrs gene PCR to detection infection. Although the msp2 or AnkA was the best choice for detecting the zoonotic A. phagocytophilum agent, we did not know the diversity of the species-specific gene of our Chinese A. phagocytophilum isolates. Therefore, the PCR products in the study might include related Anaplasmataceae such as Anaplasma marginale and Anaplasma bovis because a pair of conserved primer (HGA1-HGA2) was used in the study and limited size sequences could not differentiate them. Perhaps serological cross-reaction assays among A. phagocytophilum and related A. marginale and A. bovis could help to explain the previous results. However, we do not have A. marginale and A. bovis antigens to fulfill these assays now. Although the highest seroprevalence rates of A. phagocytophilum were found in dogs (10.05%), it is significantly lower than that reported from Germany (43%)10 and Portugal (54.5%).11 The probable reasons that caused this situation could be that the A. phagocytophilum antigen used in the study were antigenically different from the local endemic strains of A. phagocytophilum in China. Another reason is that collecting samples from dogs is more difficult than goats and cattle and a small sample size might cause statistical deviation. However, the total average PCR-positive rates for three kinds of animals is up to 23.77%, which was higher than that of previous domestic surveys in China.12 The PCR-positive rates for goats were 26.69%, higher than that from Swaziland (9.30%).13 For cattle, PCR-positive rates were significantly higher than that of a farm in Japan (23.38% versus 3.4%).14 Similarly, the PCR-positive rates for dogs in the study were higher than that from Germany (10.89% versus 6.3%).15 It is reported that the tick was the major transmission vector of anaplasmosis. Recent investigations showed that many kinds of ticks except I. persulcatus could carry A. phagocytophilum.16,17 In this study, the 16S rRNA gene of A. phagocytophilum was nearly identified in all six species of ticks. The highest average PCR-positive rates are 58.3% for D. silvarum from Shanxi Province and the next is 43.9% for H. longicornis from Shandong Province. No differences are observed for the same species of ticks collected from different animal species.
In this study, two groups of A. phagocytophilum were detected in domestic animals, one identified in a broad area in China, which has been particularly associated with the outbreak of nosocomial human-to-human transmission of HGA in Anhui Province in 2006 and patients with HGA identified in Shandong Province in 2007.2,18 Partial sequence analyses of 16S rRNA genes of A. phagocytophilum of the representative- ZJ-JH- S2 of group A showed this pattern was the most common group in China but no 100% similarity sequence was observed in other locations in the world. Another group mainly identified in Beijing and Anhui Province, and also detected from goats in the southeast in another investigation (FJ389574),19 however it has not been detected in a human case until now. This genotype is 100% identical with that from Haemaphysalis longicornis collected from Korea (GU064903) and Sambar in Japan (AB454075).
Here, we concluded that the emerging tick-borne A. phagocytophilum infection is already prevalent in rural areas of China and domestic animals, especially dogs might be the important hosts for the transmission of the A. phagocytophilum infection. The documented data in the study suggest that human infections by these bacterial zoonoses are frequent and largely unrecognized.
ACKNOWLEDGMENTS
We appreciate the epidemiologists, technicians, and administrative personal from 21 Counties/districts in 10 Provinces/Cities who participated in the study.
Footnotes
Financial support: This study was supported by the China-U.S. Collaborative Program on Emerging and Re-emerging Infectious Diseases (no. 1U2GGH000018-01); the National Basic Research Program of China (973 Program) 2010CB530200 (2010CB530206); and the grants from the National Key Science and Technology Projects of China (no. 2009ZX10004-203 and 2008ZX10004-008).
Authors' addresses: Lijuan Zhang, Department of Rickettsiology, National Institute of Communicable Disease Control and Prevention, China CDC, Changping, Beijing 102206, People's Republic of China, E-mail: zhanglijuan@icdc.cn. Hong Liu, Department of Epidemiology, Centers for Disease Control and Prevention of Anhui Province, Hefei 650022, China, E-mail: lh@ahcdc.com.cn. Bianli Xu, Centers for Disease Control and Prevention of Henan Province, Zhengzhou 450016, China, E-mail: xubl@hncdc.com.cn. Qunying Lu, The Institute of Microbiology, Centers for Disease Control and Prevention of Zhejiang Province, Hangzhou 310051, China, E-mail: lqyzjcdc@yahoo.com.cn. Liang Li, Department of Epidemiology, Centers for Disease Control and Prevention of Jiangsu Province, Nanjing 210009, China, E-mail: lljscdc@163.com. Litao Chang, Department of Epidemiology, Centers for Disease Control and Prevention of Yunnan Province, Kunming 650022, China, E-mail: changlitao@yncdc.cn. Xiuchun Zhang, Department of Epidemiology, Beijing Center for Disease Control and Prevention, Beijing 100013, China, E-mail: xchzhang@163.com. Desheng Fan, YiLi Prefecture Center for Disease Control and Prevention, YiLi 835000, China, E-mail: fds8043092@163.com. Guohua Li, Department of Epidemiology, Centers for Disease Control and Prevention of Shanxi Province, Taiyuan 030012, China, E-mail: guohuasx@yahoo.com.cn. Yuming Jin, National Institute of Communicable Disease Control and Prevention, Centers for Disease Control and Prevention of Hainan Province, Haikou 570203, China, E-mail: jym1030@126.com. Feng Cui, The Institute of Communicable Disease Control and Prevention Zibo CDC, Zibo 255400, Shandong Province, China, E-mail: zbcdc@tom.com. Yonglin Shi, Department of Laboratory, Centers for Disease Control and Prevention of Anhui Province, Hefei 650022, China, E-mail: sylin540@126.com. Weihong Li, Beijing Center for Disease Control and Prevention, Beijing 100013, China, E-mail: liweihong@yahoo.com.cn. Jianguo Xu, National Institute of Communicable Disease Control and Prevention, China CDC, Beijing 102206, China, E-mail: xujianguo@icdc.cn. XueJie Yu, Departments of Pathology, Center for Biodefence and Emerging Infectious Diseases, Sealy Center for Vaccing Development, University of Texas Medical Branch, Galveston Island, TX, E-mail: xuyu@utmb.edu.
References
- 1.Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler SJ, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis–United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 2.Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am. 2008;92:1345–1361. doi: 10.1016/j.mcna.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther. 2009;7:709–722. doi: 10.1586/eri.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Shan A, Mathew B, Yin J, Fu X, Zhang J, Lu J, Xu J, Dumler JS. Rickettsial seroepidemiology among farm workers, Tianjin, People's Republic of China. Emerg Infect Dis. 2008;14:938–940. doi: 10.3201/eid1406.071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin GY, Zhang SY, Xie KJ. A serum-epidemiological study on Anaplasma phagocytophila in the Wuyishan forest area in Fujian. Zhonghua Liu Xing Bing Xue Za Zhi. 2008;29:1160–1161. [PubMed] [Google Scholar]
- 6.Zhang S, Hai R, Li W, Li G, Lin G, He J, Fu X, Zhang J, Cai H, Ma F, Yu D, Yu XJ. Seroprevalence of human granulocytotropic anaplasmosis in central and southeastern China. Am J Trop Med Hyg. 2009;81:293–295. [PubMed] [Google Scholar]
- 7.Eremeeva ME, Balayeva NM, Raoult D. Serological response of patients suffering from primary and recrudescent typhus: comparison of complement fixation reaction, Weil-Felix test, microimmunofluorescence, and immunoblotting. Clin Diagn Lab Immunol. 1994;1:318–324. doi: 10.1128/cdli.1.3.318-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen B, Jian R, Zhang Y, Chen R. Simultaneous detection of Anaplasma marginale and a new Ehrlichia species closely related to Ehrlichia chaffeensis by sequence analyses of 16S ribosomal DNA in Boophilus microplus ticks from Tibet. J Clin Microbiol. 2002;40:3286–3290. doi: 10.1128/JCM.40.9.3286-3290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci. 2011;91:71–76. doi: 10.1016/j.rvsc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Santos AS, Alexandre N, Sousa R, Nuncio MS, Bacellar F, Dumler JS. Serological and molecular survey of Anaplasma species infection in dogs with suspected tickborne disease in Portugal. Vet Rec. 2009;164:168–171. doi: 10.1136/vr.164.6.168. [DOI] [PubMed] [Google Scholar]
- 12.Zhan L, Cao WC, Jiang JF, Zhang XA, Wu XM, Zhang WY, Liu W, Zuo SQ, Cao ZW, Yang H, Richardus JH, Habbema JD. Anaplasma phagocytophilum in livestock and small rodents. Vet Microbiol. 2010;144:405–408. doi: 10.1016/j.vetmic.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Silaghi C, Scheuerle MC, Friche Passos LM, Thiel C, Pfister K. PCR detection of Anaplasma phagocytophilum in goat flocks in an area endemic for tick-borne fever in Switzerland. Parasite. 2011;18:57–62. doi: 10.1051/parasite/2011181057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murase Y, Konnai S, Hidano A, Githaka NW, Ito T, Takano A, Kawabata H, Ato M, Tajima T, Tajima M, Onuma M, Murata S, Ohashi K. Molecular detection of Anaplasma phagocytophilum in cattle and Ixodes persulcatus ticks. Vet Microbiol. 2011;149:504–507. doi: 10.1016/j.vetmic.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Jensen J, Simon D, Murua Escobar H, Soller JT, Bullerdiek J, Beelitz P, Pfister K, Nolte I. Anaplasma phagocytophilum in dogs in Germany. Zoonoses Public Health. 2007;54:94–101. doi: 10.1111/j.1863-2378.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim CM, Yi YH, Yu DH, Lee MJ, Cho MR, Desai AR, Shringi S, Klein TA, Kim HC, Song JW, Baek LJ, Chong ST, O'guinn ML, Lee JS, Lee IY, Park JH, Foley J, Chae JS. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol. 2006;72:5766–5776. doi: 10.1128/AEM.00431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao WC, Zhan L, He J, Foley JE, DE Vlas SJ, Wu XM, Yang H, Richardus JH, Habbema JD. Natural Anaplasma phagocytophilum infection of ticks and rodents from a forest area of Jilin Province, China. Am J Trop Med Hyg. 2006;5:664–668. [PubMed] [Google Scholar]
- 18.Zhang LJ, Cui F, Wang L, Zhang L, Zhang JS, Yang SX, Han J. Anaplasma phagocytophilum and Ehrlichia chaffeensis in Yiyuan County, Shandong. Infect Dis Informat. 2009;22:21–25. [Google Scholar]
- 19.Zhou Z, Nie K, Tang C, Wang Z, Zhou R, Hu S, Zhang Z. Phylogenetic analysis of the genus Anaplasma in southwestern China based on 16S rRNA sequence. Res Vet Sci. 2010;89:262–265. doi: 10.1016/j.rvsc.2010.02.009. [DOI] [PubMed] [Google Scholar]