Abstract
Plasmodium falciparum resistance to artemisinins by delayed parasite clearance is present in Southeast Asia. Scant data on parasite clearance after artemisinins are available from Africa, where transmission is high, burden is greatest, and artemisinin use is being scaled up. Children 1–10 years of age with uncomplicated malaria were treated with 7 days of artesunate and followed for 28 days. Blood smears were done every 8 hours until negative by light microscopy. Results were compared with a similar study conducted in the same village in 2002–2004. The polymerase chain reaction-corrected cure rate was 100%, identical to 2002–2004. By 24 hours after treatment initiation, 37.0% of participants had cleared parasitemia, compared with 31.9% in 2002–2004 (P = 0.5). The median parasite clearance time was 32 hours. Only one participant still had parasites at 48 hours and no participant presented parasitemia at 72 hours. Artesunate was highly efficacious, with no evidence of delayed parasite clearance. We provide baseline surveillance data for the emergence or dissemination of P. falciparum resistance in sub-Saharan Africa.
Introduction
Artemisinin-based combination therapies (ACTs) are currently the first-line therapy for uncomplicated falciparum malaria worldwide.1,2 In recent years, Plasmodium falciparum resistance to artemisinins has developed and been confirmed along the Thai-Cambodian border.3,4 In the absence of a molecular marker for artemisinin resistance,5 and with no validated in vitro assay predictive of clinical measures of resistance, delayed parasite clearance has been identified as a useful indicator of artemisinin resistance.6 Periodic surveillance for a subtle delay in parasite clearance after treatment with artemisinins may provide early warning of reduced ACT efficacy before it is clinically relevant.
The possibility that artemisinin resistance might spread or emerge independently elsewhere necessitates careful surveillance through periodic therapeutic efficacy studies in regions most heavily affected by malaria. However, few studies have measured parasite clearance after ACT treatment in Africa, where malaria transmission is high, the burden of malaria is highest, and ACT use has been scaled up in recent years. Artesunate monotherapy studies that allow direct estimation of parasite responses without confounding by ACT partner drugs are particularly rare.7 We previously conducted one such study in southern Mali in 2002–2004.8
This study had two aims. Our primary objective was to evaluate the therapeutic efficacy of artesunate in curing uncomplicated malaria in Malian children ∼6 years after ACTs were introduced as the national policy. Our secondary objective was to identify any trends in the parasite clearance dynamics that might herald the emergence of a resistance phenotype, and absent any such early signs of resistance, to provide a zero benchmark for future artemisinin resistance surveillance efforts in the region.
Materials And Methods
Trial design and participants.
From December 2010 to February 2011, we conducted a prospective therapeutic efficacy study of clinical and parasitological responses9 after administration of 7 days of curative artesunate therapy to 100 children from Bougoula-Hameau, Mali with uncomplicated falciparum malaria. Bougoula-Hameau is a peri-urban village in southern Mali where malaria is holoendemic. Results were compared with data from the artesunate monotherapy arm of a randomized controled trial conducted in the same village by the same study team and during the same months in 2002–2004.8
Inclusion criteria included age between 12 and 132 months, an axillary temperature of ≥ 37.5°C or a history of self-reported fever in the previous 24 hours, and a positive malaria blood smear with between 2,000 and 200,000 P. falciparum asexual forms per microliter. Exclusion criteria included a hemoglobin level ≤ 8.0 g/dL, any signs of severe malaria as defined by the World Health Organization (WHO),10 any severe co-morbid condition, ACT administration in the previous 2 weeks, and known allergy to artemisinin derivatives. Written informed consent was obtained from the parent or guardian of each child before inclusion. An independent monitor not involved in the conduct of the trial monitored the study. The Ethics Committee of the Faculty of Medicine, Pharmacy, and Odonto-Stomatology, University of Bamako approved the protocol. Participants were hospitalized until all malaria symptoms resolved and at least three consecutive slides were negative. Thereafter, they were followed actively and passively for 28 days following a standard protocol.8
Thick and thin blood smears were examined for asexual and sexual parasites after staining with 5% Giemsa every 8 hours starting immediately before the first dose until three consecutive slides were negative for asexual parasites. Slides were also prepared on follow-up Days 2, 3, 7, 14, 21, and 28. Blood smears were read by two trained microscopists according to WHO standard procedures. Species discrepancies were resolved by a third microscopist, and parasite density discrepancies of > 50% were resolved by averaging of a third microscopist's result and the closer of the two original results. Parasite density was estimated by counting the number of asexual parasites per 200 leukocytes and multiplying by 40, assuming 8,000 leukocytes/μL.9 Gametocyte density was calculated by counting the number of sexual forms per 1,000 leukocytes and multiplying by eight.11 To differentiate reinfections from recrudescences, we used paired nested polymerase chain reaction (PCR) to genotype each infection using primers for the polymorphic genes msp1, msp2, and glurp and the microsatellites ta87, ta99, and cal.
A single dose of 4 mg/kg Artesunate (Asunate Denk, Denk Pharma, Munich, Germany) was directly administered on the first day and a single dose of 2 mg/kg/day on each day thereafter. Doses were rounded up to the nearest quarter tablet. A full dose was re-administered in the case of rejection or vomiting in the first 30 minutes. Participants in the 2002–2004 study were treated for 5 days with Arsumax (Sanofi-Aventis, Paris, France), 4 mg/kg on the first day and 2 mg/kg/day thereafter.8 Cases of treatment failure were treated according to national guidelines.
Our primary outcome was the 28-day per-protocol cure rate, defined as the number of participants who completed follow-up (±1 day) minus the number of early treatment failures, late clinical failures, and late parasitological failures, divided by the total number of participants who completed follow-up, multiplied by 100.7 Secondary outcomes were parasite clearance time, gametocyte clearance time, and fever clearance time. Parasite clearance time was defined as the time from the first dose to the first negative blood smear followed by two additional negative blood smears. Parasite clearance was also evaluated as a categorical variable (proportion having cleared 100% of parasites by Day 1). Given the limited sensitivity of microscopy at very low parasitemias,12 we also calculated the time to clear 95% of initial parasites and compared the proportion of participants clearing 95% of initial parasites by Day 1. We also used the WorldWide Antimalarial Resistance Network (WWARN) parasite clearance estimator, a new approach to modeling clearance of malaria parasites,13 to calculate the clearance rate constant and slope half-life for the 2010–2011 study. Fever clearance time was defined as the time from the first dose to the first time that the axillary temperature declined < 37.5°C for at least 48 hours.
Sample calculations were done as suggested in the WHO guidelines for a single therapy efficacy assessment; in the absence of up-to-date artesunate monotherapy efficacy data, we used the suggested 50% conservative treatment failure rate.14 For a 95% confidence interval (CI) and 10% precision, at least 96 participants were required, or 100 for predicted losses to follow-up.
Statistical analysis.
Descriptive statistics were expressed as means, medians, or proportions. Categorical variables were compared using the χ2 test and two-sided Fisher's exact test, and continuous variables were compared using the Student's t test or Mann-Whitney test, as appropriate. Multivariable models were constructed for the primary and secondary outcomes. The two most powerful predictors of parasite clearance time were age (proxy for acquired immunity in this population) and asexual parasitemia at the time of enrollment.15–18 Based on previous studies,16–19 the following variables were also included in the initial models as potential confounders of parasite clearance: sex, mixed parasite infection, and hemoglobin, gametocyte density, gametocyte carriage, and fever at the time of enrollment. All statistical analyses were done with Stata version 11.0 (StataCorp., College Station, TX). P values < 0.05 were considered significant. Distribution of parasite clearance rate constants and slope half-lives were generated by the new WWARN Parasite Clearance Estimator (http://www.wwarn.org/research/parasite-clearance-estimator).13 Furthermore, when we used the WWARN estimator, all positive parasitemia (by light microscopy) were included in the calculations.
Role of the funding source.
The funding sources had no role in study design; data collection, analysis, or interpretation; or the writing of this report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Results
Of 500 children screened between December 2010 and February 2011, 244 had parasitemias in the inclusion range and, of those, 100 were enrolled. The most common reason for not enrolling a child was that the daily capacity for inclusion had been reached; a small proportion were not enrolled because of low hemoglobin < 8.0 g/dL (N = 15) or another inclusion or exclusion criterion (N = 9). Of the 100 children initially enrolled, because of an inadvertent protocol violation, six were given an additional dose of 2 mg/kg 8 hours after the first dose, for a total 6 mg/kg/day on Day 0 rather than the 4 mg/kg/day intended; all six completed the full 28-day follow-up, and no adverse clinical or laboratory effects were recorded (Figure 1) .
Figure 1.
Trial profile. PCT = parasite clearance time.
Two participants were excluded from the study because of treatment change. One participant's parasitemia had only declined to 50% of the initial parasitemia 48 hours after inclusion, and we strongly suspected he had not swallowed the first artesunate doses; he was switched to oral quinine and was cured without complications. Another child's parasitemia immediately before treatment increased to > 250,000 parasites/μL, and persisted at the 8-hour smear; she was given intramuscular artemether and recovered without incident. Of the remaining 92 children, there were two late treatment failures, at Days 27 and 28, and one case of mixed Plasmodium ovale and Plasmodium malariae reinfection at Day 21. The latter was initially considered a late treatment failure pending PCR confirmation that the P. ovale and P. malariae were not present at the time of inclusion at sub-microscopic levels. All three were cured with oral artemether-lumefantrine without complications. There were no losses to follow-up in this study.
The median age of participants was 5.8 years, and 51% were males (Table 1). The median falciparum parasitemia immediately before artesunate treatment was 27,070 parasites/μL, and 5.4% had mixed infections (i.e., P. falciparum plus P. ovale or P. malariae). At inclusion, 22.8% were gametocyte carriers, with a mean gametocyte density of 11.8/μL. Compared with participants in the 2002–2004 study, our participants were older, had higher initial parasite densities, and were less likely to be febrile on inclusion (all P < 0.001). Gametocyte clearance dynamics will be reported separately.
Table 1.
Baseline demographic characteristics*
| Characteristic | 2010–2011 (N = 92†) | 2002–20048 (N = 72) | P value |
|---|---|---|---|
| Age, median in years [IQR] | 5.8 [3.6–8.4] | 3.0 [2.0–5.0] | < 0.001‡ |
| Sex, n (%) male | 51 (55.4%) | 45 (62.5%) | 0.425§ |
| Pf. parasitemia/μL, median [IQR] | 27,070 [15,390–57,050] | 12,638 [7,688–24,188] | < 0.001‡ |
| Pf. gametocyte carriers, n (%) | 21 (22.8%) | 8 (11.1%) | 0.064§ |
| Pf. gametocyte density/μL, mean [SD] | 11.8 [41.8] | 7.6 [30.7] | 0.072‡ |
| Mixed infection (P. malariae or P. ovale), n (%) | 5 (5.4%) | 3 (4.2%) | 1.000§ |
| Hemoglobin, mean [SD] | 10.2 [1.7] | 10.4 [1.9] | 0.522¶ |
| Anemia (< 10 g/dL), n (%) | 45 (48.9%) | 29 (40.3%) | 0.343§ |
| Febrile on inclusion, n (%) | 74 (80.4%) | 71 (98.6%) | < 0.001§ |
IQR = interquartile range; SD = standard deviation.
Includes the 91 evaluable at Day 28 and the case of reinfection on Day 21.
Mann-Whitney test.
Two-sided Fisher's exact test.
Student's t test.
The 28-day uncorrected cure rate was 97.8% (N = 91; 95% CI 92.3–99.7) in 2010–2011 and 98.6% (N = 70; 95% CI 92.3–99.96) in 2002–2004. The PCR-corrected cure rate was 100% in both studies. The intention-to-treat analysis showed a cure rate of 97.9% (N = 94, 95% CI = 92.5–99.7).
The proportion of participants who completely cleared their parasitemia by 24 hours after treatment initiation were 37.0% (N = 92; 95% CI = 27.1–47.7) in 2010–2011 and 31.9% (N = 72; 95% CI = 21.4–44.0) in 2002–2004 (adjusted odds ratio [AOR] 1.9; 95% CI = 0.9–3.9; P = 0.074 after adjusting for initial parasitemia). There was also no difference in the proportions of participants having cleared 95% of their initial parasitemia by 24 hours, 98.9% (95% CI = 94.1–99.97) in 2010–2011 and 88.9% (95% CI = 79.3–95.1) in 2002–2004 (AOR 6.2; 95% CI = 0.9–51.9; P = 0.076 after adjusting for initial parasitemia).
The median parasite clearance time in 2010–2011 was 32 hours. Parasite clearance time was significantly correlated with both age and initial parasite density (Figure 2A and B). Although the parasite density increased for some participants at 8 hours post-treatment initiation, the proportion of parasites remaining was already quite low by 16–24 hours after the first dose of artesunate (Figure 3B). The median and mean time to clear 95% of initial parasites were 16 and 16.9 hours, respectively. Only one participant still had parasites at 48 hours (data not shown), and the longest parasite clearance time (that individual) was 56 hours. Of note, the mean clearance time for participants erroneously given 6 mg/kg/day on Day 0 was 21.3 hours, compared with 31.9 for those receiving the correct dose (P = 0.007 after adjusting for inclusion parasitemia and gametocyte density), suggesting a possible dose-related reduction in clearance time. No parasite clearance time could be calculated with the 2002–2004 data because blood smears were only made on follow-up Days 1 and 3, and all participants had cleared by Day 3.
Figure 2.
(A and B) Correlation of parasite clearance time with age (A, left panel) and initial parasite density (B, right panel). Both correlations were statistically significant, P = 0.0116 and P < 0.0001 for age and initial parasitemia, respectively (Pearson's coefficient). Individual data points are depicted from all participants with the exception of the two excluded because of treatment change (N = 98). ASB = artesunate Bougoula 2010–2011 study.
Figure 3.
(A and B) Evolution of parasitemia over time. Panel A (left) shows the evolution of log parasitemia, and panel B (right) shows the evolution of fold initial parasitemia. Individual data points are depicted from all participants who received the correct artesunate dosing with the exception of the two excluded because of treatment change (N = 92). ASB = artesunate Bougoula 2010–2011 study.
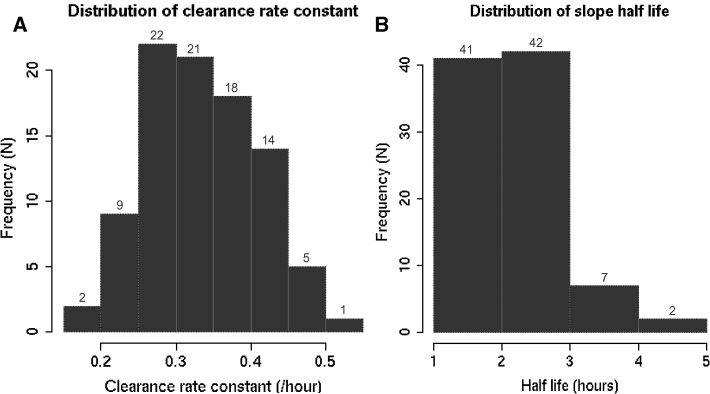
The median parasite clearance rate constant was 0.33 (interquartile range [IQR], 0.28–0.39) and the median slope half-life was 2.07 hours (IQR, 1.77–2.44) (Figure 4A and B). In contrast, the median clearance rate and slope half-life in the six participants who received 6 mg/kg on Day 0 were 0.36 (IQR, 0.32–0.41) and 1.94 (IQR, 1.69–2.17), respectively.
Figure 4.
(A and B) Distribution of parasite clearance rate constants (A, left panel) and slope half-lives (B, right panel). Data are depicted from all participants receiving 4 mg/kg on Day 0 with the exception of the two excluded because of treatment change (N = 92). Data generated by the WorldWide Antimalarial Resistance Network (WWARN) Parasite Clearance Estimator (http://www.wwarn.org/research/parasite-clearance-estimator).13
Among those febrile on inclusion, the median fever clearance time was 24 hours (IQR, 24–24 hours). There was no difference in the median fever clearance time from 2002–2004 to 2010–2011 (P = 0.3279).
Discussion
In this population of children 1–10 years of age in a setting of high-intensity seasonal malaria transmission, we found that the clinical efficacy of oral artesunate remained high for the treatment of uncomplicated malaria, with a PCR-corrected 28-day cure rate of 100% and an intention-to-treat cure rate of nearly 98%. Both the cure rate and the proportion of participants clearing parasites by 24 hours after treatment initiation remained unchanged from 2002–2004 to 2010–2011. We also identified no difference in the fever clearance time between the two time periods.
The median parasite clearance time in this study was 32 hours, much lower than the 84 hours reported in western Cambodia that was linked to artemisinin resistance.6 Similar to a study of two ACTs in similarly-aged Nigerian children, some participants had an initial increase in their parasite density after treatment initiation.20 However, parasitemia thereafter rapidly declined, and participants eliminated 95% of their initial parasites by a mean 16 hours after the first dose of artesunate. No participants in our study had a positive blood smear at 72 hours, an in vivo predictor of subsequent treatment failure with ACTs,15 and indeed only one participant had parasites 48 hours after starting treatment. Our incidental finding that the parasite clearance time of participants who received 6 mg/kg on Day 0 was significantly shorter than that of participants receiving the standard 4 mg/kg dose contrast with a recent study from Cambodia that found no change in clearance time or rate with higher artemisinin doses.21 This observation, although based on small numbers, provides potentially important empirical evidence for the ability of higher artemisinin doses to hasten the parasite clearance of African isolates in the current stage of artemisinin usage on the continent.
Three points may help to explain the continued high susceptibility of falciparum parasites to artemisinin therapy in this region. First, artemisinins have only been widely used in Mali since 2004, compared with more than 30 years in Cambodia, and thus there has been relatively little drug pressure favoring resistance.2,6 Second, because artemisinins are mainly available as co-formulated ACTs through subsidized programs, artemisinin monotherapy use in Mali is likely proportionally much lower than the 78% documented in western Cambodia in 2002.22 Third, given the high intensity of falciparum malaria transmission in Mali, even in this pediatric population, host immunity and the large transmission reservoir of asymptomatic untreated individuals likely serve as powerful obstacles to the emergence of artemisinin resistance.23
Two patients were switched to alternative therapy after enrollment. In both cases, there was an increase in parasitemia during the first hours after artesunate treatment. This increase in parasitemia, which was also seen in a number of other patients, is likely to be the result of schizonts bursting and contributing more ring stages into the blood stream. In most cases, the parasitemia came back down with no further consequences. However, based on clinical judgment, alternative parenteral treatment was provided to these two patients.
To our knowledge, this is one of the first estimates of parasite clearance after curative artesunate therapy in an area of high-intensity falciparum malaria transmission. Thanh and others24 recently reported stable levels of P. falciparum sensitivity to artemisinins between 1998 and 2008–2009 in Vietnam, with a mean parasite clearance time ranging from 1.8 to 2.3 days. We used three different measures of parasite clearance: time to clearance of 100%, time to clearance of 95%, and slope half-life. Currently, there is not good consensus around using one parasite clearance metric. Slope half-life, which was calculated using the WWARN parasite clearance estimator tool is derived from the linear part of the parasite clearance curve only and thus does not take into account the lag and tail phases of clearance.25 This model attempts to better standardize the measurement of parasite clearance after artemisinin treatment and to improve the comparability of data from various origins. Although attractive mathematically because of its independence from initial parasitemia,13 using only slope half-lives to compare parasite clearance across space and time may miss important biological phenotypes represented by these differing lag and tail phases that could contribute to slowed elimination and eventually reduced efficacy of artemisinins.
It is critically important that surveillance for the emergence or dissemination of artemisinin resistance in Africa take into account the well-known effects of intense malaria transmission and elevated natural immunity on treatment efficacy and parasite clearance dynamics. At our study site in Mali, artesunate was highly efficacious, and we have therefore provided baseline parasite clearance metrics appropriate for the surveillance of artemisinin efficacy against P. falciparum isolates from similar settings of intense seasonal transmission in sub-Saharan Africa. Further studies are necessary to establish the relevance of a reduced parasite clearance rate as a surrogate marker for artemisinin resistance in high-transmission, high-immunity settings.
Our study has some limitations. Because the precise time of artesunate administration and blood smears were not recorded for the 2002–2004 study, the Day 1 blood smear corresponds to 24 ± 4 hours. We sought to model this study as closely as possible on the artesunate monotherapy arm of the 2002–2004 study, but there were two important differences. We were obliged to select a different artesunate manufacturer for this study, as Sanofi-Aventis no longer produces Arsucam, and we treated for 7 days rather than 5, in line with more recent recommendations.26 Another limitation of our study is the possibility of a type II error. Because no parasite clearance time or rate could be calculated with the 2002–2004 data as slides were read only at 24-hour intervals, we may have missed a subtle difference in the parasite clearance dynamics over time.
The primary strength of our study is the ability to compare outcomes in the same site with the same population and the same study team over an 8-year period. We also had a 100% follow-up rate and closely followed WHO protocol recommendations for therapeutic efficacy studies. Our study was unique in that it contributed for the first time a parasite clearance curative benchmark for a high-intensity transmission setting after artesunate treatment.
It is important to note that we do not suggest artesunate be used in this or any other population as a monotherapy for the treatment of uncomplicated malaria despite its demonstrated efficacy. Artesunate monotherapy can only be justified for small research studies such as the present one, when it is imperative to get a clear measurement of efficacy without confounding by ACT partner drugs. Instead, our study supports the continued use of ACTs as first-line treatment of uncomplicated malaria in Mali. The WHO currently recommends that malaria-endemic countries conduct clinical efficacy studies every 24 months at sentinel sites to detect changes in the therapeutic efficacy of ACTs.27 We would further suggest that, given the devastation that artemisinin resistance would cause on the African continent should it follow in the footsteps of chloroquine and the antifolate antimalarial drugs,28 it is feasible, safe, and prudent to also perform periodic surveillance studies in high-intensity transmission areas to monitor the parasite clearance dynamics after artesunate treatment. Only with such proactive vigilance can we hope to have any possibility of identifying and reversing alarming trends before they have a disastrous public health impact.
ACKNOWLEDGMENTS
We thank the Village council, the entire population of Bougoula-Hameau, and Health and Administrative authorities of Sikasso for their help and support during the study.
Footnotes
Financial support: This study was primarily funded by the European and Developing Countries Clinical Trials Partnership (EDCTP IP_07_31060_002) and by the West African Network for Clinical Trials of Antimalarial Drugs (WANECAM). This work was also supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, and NIH Office of Women's Health and Research through the International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
Authors' addresses: Amelia W. Maiga, Duke University School of Medicine, Durham, NC, E-mail: aw70@duke.edu. Bakary Fofana, Issaka Sagara, Demba Dembele, Antoine Dara, Oumar Bila Traore, Sekou Toure, Kassim Sanogo, Souleymane Dama, Bakary Sidibe, Aminatou Kone, Mahamadou A. Thera, Ogobara K. Doumbo, and Abdoulaye A. Djimde, Malaria Research and Training Center, Department of Epidemiology of Parasitic Diseases, Faculty of Medicine, Pharmacy, and Odonto-stomatology, University of Bamako, Bamako, Mali, E-mails: bfofana@icermali.org, isagara@icermali.org, ddembele@icermali.org, tonydara@icermali.org, bila@icermali.org, sekout@icermali.org, sanogok@icermali.org, dama@icermali.org, bakary@icermali.org, amina@icermali.org, mthera@icermali.org, okd@icermali.org, and adjimde@icermali.org. Christopher V. Plowe, Center for Vaccine Development Howard Hughes Medical Institute, University of Maryland School of Medicine, Baltimore, MD, E-mail: cplowe@medicine.umaryland.edu.
References
- 1.WHO . Guidelines for the Treatment of Malaria. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 2.Maude RJ, Pontavornpinyo W, Saralamba S, Aguas R, Yeung S, Dondorp AM, Day NP, White NJ, White LJ. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malar J. 2009;8:31. doi: 10.1186/1475-2875-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alker AP, Lim P, Sem R, Shah NK, Yi P, Bouth DM, Tsuyuoka R, Maguire JD, Fandeur T, Ariey F, Wongsrichanalai C, Meshnick SR. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am J Trop Med Hyg. 2007;76:641–647. [PubMed] [Google Scholar]
- 4.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 5.Imwong M, Dondorp AM, Nosten F, Yi P, Mungthin M, Hanchana S, Das D, Phyo AP, Lwin KM, Pukrittayakamee S, Lee SJ, Saisung S, Koecharoen K, Nguon C, Day NP, Socheat D, White NJ. Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:2886–2892. doi: 10.1128/AAC.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000–2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 8.Djimde AA, Fofana B, Sagara I, Sidibe B, Toure S, Dembele D, Dama S, Ouologuem D, Dicko A, Doumbo OK. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am J Trop Med Hyg. 2008;78:455–461. [PubMed] [Google Scholar]
- 9.WHO . Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. Geneva: World Health Organization; 2003. [Google Scholar]
- 10.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster Trans R Soc Trop Med Hyg. 2000;94((Suppl 1)):S1–S90. [PubMed] [Google Scholar]
- 11.von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am J Trop Med Hyg. 2001;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- 12.Beshir KB, Hallett RL, Eziefula AC, Bailey R, Watson J, Wright SG, Chiodini PL, Polley SD, Sutherland CJ. Measuring the efficacy of anti-malarial drugs in vivo: quantitative PCR measurement of parasite clearance. Malar J. 2010;9:312. doi: 10.1186/1475-2875-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flegg J, Guerin P, White N, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . Methods for Surveillance of Antimalarial Drug Efficacy. Geneva: World Health Organization; 2009. [Google Scholar]
- 15.Stepniewska K, Ashley E, Lee SJ, Anstey N, Barnes KI, Binh TQ, D'Alessandro U, Day NP, de Vries PJ, Dorsey G, Guthmann JP, Mayxay M, Newton PN, Olliaro P, Osorio L, Price RN, Rowland M, Smithuis F, Taylor WR, Nosten F, White NJ. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2010;201:570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrara VI, Zwang J, Ashley EA, Price RN, Stepniewska K, Barends M, Brockman A, Anderson T, McGready R, Phaiphun L, Proux S, van Vugt M, Hutagalung R, Lwin KM, Phyo AP, Preechapornkul P, Imwong M, Pukrittayakamee S, Singhasivanon P, White NJ, Nosten F. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS ONE. 2009;4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ittarat W, Pickard AL, Rattanasinganchan P, Wilairatana P, Looareesuwan S, Emery K, Low J, Udomsangpetch R, Meshnick SR. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am J Trop Med Hyg. 2003;68:147–152. [PubMed] [Google Scholar]
- 18.Sowunmi A, Adewoye EO, Gbotsho GO, Happi CT, Sijuade A, Folarin OA, Okuboyejo TM, Michael OS. Factors contributing to delay in parasite clearance in uncomplicated falciparum malaria in children. Malar J. 2010;9:53. doi: 10.1186/1475-2875-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704–709. doi: 10.1016/S0140-6736(97)02255-1. [DOI] [PubMed] [Google Scholar]
- 20.Michael OS, Gbotosho GO, Folarin OA, Okuboyejo T, Sowunmi A, Oduola AM, Happi CT. Early variations in Plasmodium falciparum dynamics in Nigerian children after treatment with two artemisinin-based combinations: implications on delayed parasite clearance. Malar J. 2010;9:335. doi: 10.1186/1475-2875-9-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bethell D, Se Y, Lon C, Tyner S, Saunders D, Sriwichai S, Darapiseth S, Teja-Isavadharm P, Khemawoot P, Schaecher K, Ruttvisutinunt W, Lin J, Kuntawungin W, Gosi P, Timmermans A, Smith B, Socheat D, Fukuda MM. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS ONE. 2011;6:e19283. doi: 10.1371/journal.pone.0019283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung S, Van Damme W, Socheat D, White N, Mills A. Access to artemisinin combination therapy for malaria in remote areas of Cambodia. Malar J. 2008;7:96. doi: 10.1186/1475-2875-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyunt MM, Plowe CV. Pharmacologic advances in the global control and treatment of malaria: combination therapy and resistance. Clin Pharmacol Ther. 2007;82:601–605. doi: 10.1038/sj.clpt.6100361. [DOI] [PubMed] [Google Scholar]
- 24.Thanh NV, Toan TQ, Cowman AF, Casey GJ, Phuc BQ, Tien NT, Hung NM, Biggs BA. Monitoring for Plasmodium falciparum drug resistance to artemisinin and artesunate in Binh Phuoc Province, Vietnam: 1998–2009. Malar J. 2010;9:181. doi: 10.1186/1475-2875-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giao PT, Binh TQ, Kager PA, Long HP, Van Thang N, Van Nam N, de Vries PJ. Artemisinin for treatment of uncomplicated falciparum malaria: is there a place for monotherapy? Am J Trop Med Hyg. 2001;65:690–695. doi: 10.4269/ajtmh.2001.65.690. [DOI] [PubMed] [Google Scholar]
- 27.WHO . Global Plan for Artemisinin Resistance Containment. Geneva: World Health Organization; 2011. [Google Scholar]
- 28.Plowe CV. The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg. 2009;103((Suppl 1)):S11–S14. doi: 10.1016/j.trstmh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]