Abstract
Antimalarials are widely used in African and Southeast Asian countries, where they are combined with other drugs for the treatment of concurrent ailments. The potential for P-glycoprotein (P-gp)-mediated drug-drug interactions (DDIs) between antimalarials and P-gp substrates was examined using a Caco-2 cell-based model. Selected antimalarials were initially screened for their interaction with P-gp based on the inhibition of rhodamine-123 (Rho-123) transport in Caco-2 cells. Verapamil (100 μM) and quinidine (1 μM) were used as positive inhibition controls. Lumefantrine, amodiaquin, and artesunate all showed blockade of Rho-123 transport. Subsequently, the inhibitory effect of these antimalarials on the bi-directional passage of digoxin (DIG) was examined. All of the drugs decreased basal-to-apical (B-A) P-gp-mediated DIG transport at concentrations of 100 μM and 1 mM. These concentrations may reflect therapeutic doses for amodiaquin and artesunate. Therefore, clinically relevant DDIs may occur between certain antimalarials and P-gp substrates in general.
Introduction
Malaria is an enormous public health concern, with approximately one-half of the world's population currently at risk.1 Globally, about 250 million people are infected with malaria, and the disease is responsible for an estimated one million deaths annually.2 Sub-Saharan Africa accounts for the majority of cases, whereas Asia, Latin America, and some parts of the Middle East and Europe are also affected.3 Polypharmacy is a common feature of antimalarial therapy caused by the pandemicity of the disease and the need to treat concurrent ailments. Hence, malaria patients are likely to be at high risk for drug-drug interactions (DDIs).4,5
Membrane transporters play a crucial role in the modulation of absorption, distribution, metabolism, and excretion of antimalarial agents and other drugs.6,7 ATP-binding cassette transporters are known to function as barrier proteins to extrude toxins and xenobiotics from cells. P-glycoprotein (P-gp) is critical among these transporters. P-gp is an efflux transporter that is expressed on barrier epithelia, including those that line the intestine, kidney, and liver. P-gp is also found at the blood-brain barrier. P-gp mediates passage across cell membranes8 and is a determinant in the pharmacokinetics, efficacy, and toxicity of xenobiotics. The intestine plays a vital role in the absorption of xenobiotics, including drugs and toxins. In the intestine, uptake transporters such as organic anion transporting polypeptides and peptide transporter 1 are involved in drug absorption, whereas P-gp, breast cancer resistance protein, and multidrug resistance-associated proteins function as efflux transporters. Co-administered pharmaceuticals may therefore inhibit the absorption and efflux of drug substrates of these transporters. After oral administration, the concentration of the inhibitory agents can be much higher in the intestine than in the systemic circulation. This may cause DDIs in the intestine rather than in the liver upon oral administration of the inhibitory agent, even at therapeutic doses.9 Several methods for predicting the likelihood of intestinal enzyme and transporter-mediated DDIs have been suggested.10–12 This study used the drug-interaction number (DIN, the ratio of inhibitor dose/inhibition constant) as an index for predicting the occurrence of clinically relevant intestinal DDIs.
The issue of transporter-mediated DDIs is a subject of increasing recognition, and a number of clinically significant DDIs involving P-gp substrates have recently been reported. In particular, DDIs with digoxin (DIG), a P-gp drug substrate used to treat congestive heart failure, have been observed following concomitant administration of quinidine (QD), erythromycin, verapamil (VER), itraconazole, ketoconazole, and cyclosporine, among others.13–15
This study used a Caco-2 cell-based model and DIG as a representative P-gp substrate to investigate the potential of certain antimalarial drugs to undergo intestinal P-gp-related DDIs when co-administered with agents that are transported by the glycoprotein. Although a variety of approaches can be taken to study drug uptake in the intestine and other functions of differentiated intestinal cells, Caco-2 cell monolayers are a widely accepted model for such studies, including investigations of P-gp actions in the gut.16,17 The current results suggest that intestinal DDIs between some antimalarial agents and P-gp substrates in general may be an important, albeit underappreciated, therapeutic concern.
Materials And Methods
Materials.
Amodiaquin (ADQ), DIG, VER, sulphadoxin (SDX), chloroquine (CQ), artemisinin (ASN), and artesunate (ART) were purchased from Sigma-Aldrich (St. Louis, MO). Artemether (ATM) was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan); lumefantrine (LUM) was obtained from May & Baker (Lagos, Nigeria); QD was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan); [3H]-DIG (40 Ci/mmol) was purchased from PerkinElmer (Boston, MA); and rhodamine-123 (Rho-123) was purchased from Acros Organics (Somerville, NJ). All other chemicals and reagents were of the highest analytical grade available.
Cell culture.
Immortalized human colon carcinoma Caco-2 cells (passage nos. 17–43) were routinely cultured in Dulbecco's modified Eagle's medium (Sigma) containing 10% fetal bovine serum and 1% antibiotic-antimycotic (Invitrogen, Grand Island, NY). Cells were maintained in an incubator at 37°C with 95% relative humidity and a 5% CO2 atmosphere. The culture medium was changed two to three times weekly. Upon reaching near confluence (80% to 90%), the cells were detached from the culture flask by the addition of 0.05% trypsin in 0.02% EDTA. Cells were then seeded at a density of 1 × 105 cells/cm2 on Transwell collagen-coated membrane inserts (6.5 mm membrane diameter, 0.4 μm pore size, 0.33 cm2 surface area) that were pre-loaded into Transwell 24-well cluster plates (Corning, NY). They were used 18 to 25 days post-seeding to obtain differentiated monolayers and an anticipated high expression level of transport proteins (i.e., P-gp). Monolayer integrity on the permeable membrane was assessed by transepithelial electrical resistance, which was tested using a Millicell ERS-2 Volt-Ohm meter (Millipore, Billerica, MA). Only monolayers with transepithelial electrical resistance values above 200 Ωcm2 were used in the transport experiments.
Transport experiments.
The transport experiments were conducted in transport medium consisting of Hank's balanced salt solution buffered with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4) at 37°C. Before the transport experiments, the monolayers were washed twice with transport medium and pre-incubated for 30 min. The test agents were dissolved in < 1% of the respective dissolving solvent. Each test agent was added to either the apical (A) or basolateral (B) side of the monolayer, with the apical side having a final volume of 100 μL, and the basolateral side having a final volume of 600 μL. The side of the monolayer that received the test agent was termed the donor compartment, whereas the other side was termed the recipient compartment. Samples (50 μL) were taken from either the donor or the recipient compartment at time intervals of 30, 60, 90, and 120 min, and the removed volume was replaced with a corresponding volume of pre-warmed, drug-free Hank's balanced salt solution at 37°C. To examine the effects of ADQ, ART, LUM, ATM, CQ, ASN, and SDX on P-gp-mediated transport, the impact of drug addition to both the A and the B side of the monolayer was independently assessed. VER (100 μM) and QD (1 μM) were used as positive inhibitor controls.
Quantification of Rho-123 and DIG transport.
The transport of Rho-123 was quantified using a MTP-600 fluorescence microplate reader (Corona Electric, Ibaraki, Japan) equipped with a 490 nm excitation, 590 nm emission filter set. For [3H]-DIG determination, a 50 μL sample of transport medium from either the donor or the recipient compartment was mixed with 2 mL of Clear-sol I scintillation cocktail (Nacalai Tesque, Kyoto, Japan) and the radioactivity was measured with a LSC-6100 liquid scintillation counter (Aloka, Tokyo, Japan).
Data analysis.
The apparent permeability coefficient (Papp) was calculated as
where dQ/dt is the rate of appearance of drug in the recipient compartment; A represents the membrane surface area of the Caco-2 monolayer (0.33 cm2); and C0 is the initial drug concentration in the donor compartment.
The efflux ratio (ER) was obtained as
where Papp B-A and Papp A-B are the mean Papps obtained for transport in the basolateral-to-apical (B-A) direction and the apical-to-basolateral (A-B) direction, respectively.
The DIN was calculated as
where Dosei is the maximum therapeutic dose of the inhibitor, and Ki is the inhibition constant. The Ki values were generated from ER12 and calculations made using Microsoft Excel software (Redmond, WA).
All results are expressed as the mean ± SEM (N ≥ 3 for each experiment). Values of P < 0.05 were deemed statistically significant using Dunnett's test.
Results
Transport and inhibition of Rho-123 across Caco-2 cell monolayers.
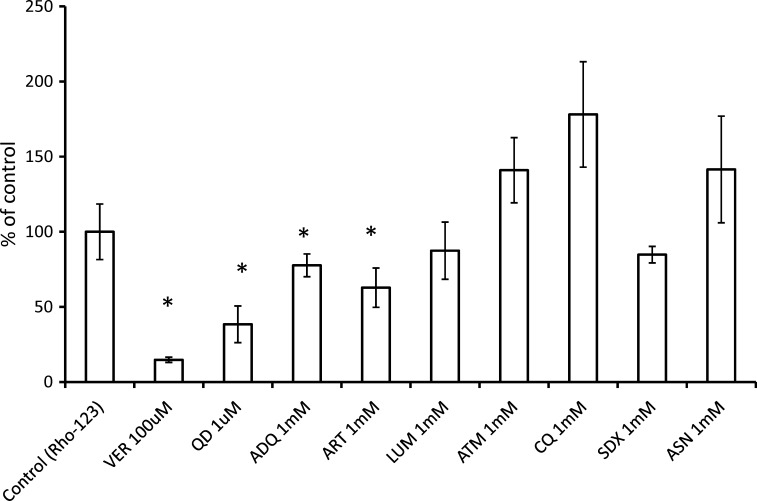
The B-A transport of Rho-123 (5 μM), a typical P-gp substrate, was first investigated at 120 min in the presence or absence of drugs (Figure 1). VER (100 μM) and QD (1 μM) were used as positive controls for P-gp inhibition and significantly decreased Rho-123 transport across the Caco-2 cell monolayer. The effect of seven antimalarials (ART, ASN, ATM, ADQ, CQ, LUM, and SDX, each used at a concentration of 1 mM) on Rho-123 transport was next examined. Among these antimalarials, ART and ADQ showed the most significant inhibitory effects. LUM also exhibited some inhibition of Rho-123 transport, although its actions were less pronounced than those of ART and ADQ. ART, ADQ, and LUM were therefore used in the subsequent experiments.
Figure 1.
Screening for the inhibition of P-glycoprotein (P-gp)-mediated rhodamine-123 (Rho-123) transport by test antimalarials. Rho-123 (5 μM) was added to the basolateral side of a Caco-2 monolayer, and the basolateral-to-apical (B-A) transport of the tracer dye was measured at 120 min in the presence of test drugs (amodiaquin (ADQ), artemisinin (ASN), artesunate (ART), lumefantrine (LUM), artemether (ATM), chloroquine (CQ), and sulphadoxin (SDX)). Each antimalarial was used at a concentration of 1 mM. Verapamil (VER, 100 μM) and quinidine (QD, 1 μM) were used as the positive inhibition controls. Each column represents the mean ± SEM (N ≥ 3). *Significant difference, P < 0.05 versus control (transport in the absence of drugs).
Transepithelial transport of DIG across Caco-2 cell monolayers.
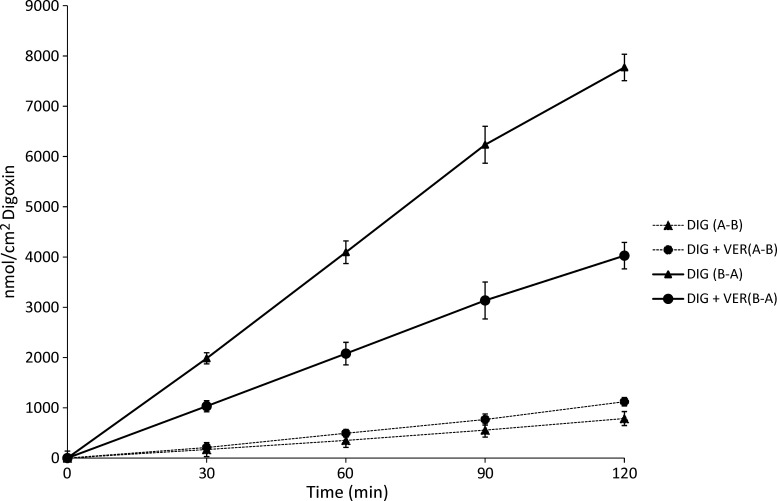
The bi-directional transport of [3H]-DIG was then examined. In the body, DIG is primarily transported in the B-A direction across the intestinal barrier epithelium by P-gp. Figure 2 illustrates the basic transport characteristics of DIG across the model Caco-2 cell monolayer in the presence or absence of VER (100 μM), the positive inhibition control. As shown in Figure 2, the transport rate of DIG in the B-A direction was greater than that in the A-B direction by over 10-fold. These data are consistent with a high expression of P-gp by the Caco-2 cell monolayer and with P-gp-mediated transport as the predominant means of DIG conveyance. The introduction of VER significantly decreased transport of DIG in the B-A direction (P < 0.05), and only slightly increased transport in the A-B direction.
Figure 2.
Digoxin (DIG) transport kinetics. The bi-directional transport of DIG with or without verapamil (VER, 100 μM) across Caco2 cell monolayers was determined as a function of time. Each data point represents the mean ± SEM of four to seven replicate assays. Solid lines represent basolateral-to-apical (B-A) transport, and dashed lines represent apical-to-basolateral (A-B) transport.
Effect of antimalarials on transepithelial transport of DIG.
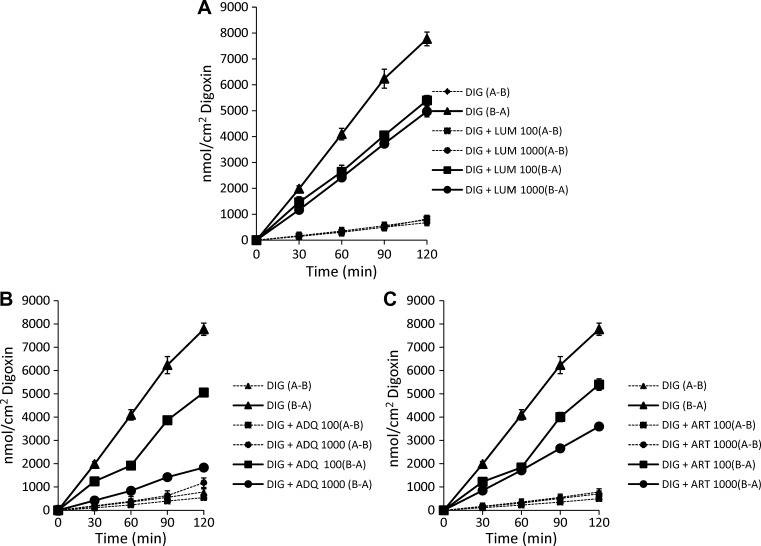
The effect of LUM, ADQ, and ART (100 μM and 1 mM) on P-gp-mediated DIG transport was next investigated (Figure 3 and Table 1). Figure 3A shows the bi-directional transport of DIG across the Caco-2 cell monolayer in the presence or absence of LUM (100 μM and 1 mM). In the B-A direction, significant 33% and 38% decreases in the transport rate of DIG were observed for LUM at concentrations of 100 μM and 1 mM, respectively, which is suggestive of P-gp inhibition (P < 0.05). However, transport was only minimally affected by LUM in the A-B direction. Figure 3B shows that the transport of DIG in the B-A direction was also significantly impaired by ADQ. The B-A transport of DIG was reduced by 38% and 77% in the presence of ADQ at concentrations of 100 μM and 1 mM, respectively (P < 0.05). As for LUM, this result is indicative of P-gp inhibition. Here, ADQ (1 mM) showed more potent inhibition than the positive inhibition control, VER (100 μM). In the A-B direction, ADQ (1 mM) significantly stimulated DIG transport by 25%. An increase in the A-B transport of DIG was also seen with the co-administration of VER (100 μM). The ART was the last antimalarial to be investigated. Figure 3C shows significant decreases in DIG transport in the B-A direction by 36% and 56% in the presence of ART at concentrations of 100 μM and 1 mM, respectively (P < 0.05). No significant impact was observed on A-B transport. Therefore, LUM, ADQ, and ART all inhibited B-A transport of DIG, with 1 mM ADQ exhibiting the most potent inhibition. These results confirm the P-gp inhibitory effect of the antimalarials that was initially observed in the Rho-123 transport assay. Table 1 shows the Papps of DIG in the presence or absence of LUM, ADQ, ART, and VER in the A-B and B-A directions, calculated based on the data shown in Figures 1–3. The DIG alone showed an ER of 10.8, further confirming it as a P-gp substrate. Significant effects were observed for ART, ADQ, LUM (1 mM) and VER in the B-A transport of DIG, whereas ADQ (1 mM) and VER significantly increased its A-B transport.
Figure 3.
Digoxin (DIG) and antimalarial drug kinetics. DIG transport across a Caco-2 cell monolayer was examined as a function of time. Apical-to-basolateral (A-B) transport and basolateral-to-apical (B-A) transport of DIG was investigated in the presence or absence of A, lumefantrine (LUM, 100 μM and 1 mM); B, amodiaquin (ADQ, 100 μM and 1 mM); and C, artesunate (ART, 100 μM and 1 mM). Each data point represents the mean ± SEM of at least four determinations. Solid lines represent basolateral-to-apical (B-A) transport, and dashed lines represent apical-to-basolateral (A-B) transport.
Table 1.
Apparent permeability coefficient (Papp) and efflux ratio (ER) of digoxin (DIG) in combination with artesunate (ART), amodiaquin (ADQ), and lumefantrine (LUM) at the indicated concentrations in the apical-to-basolateral (A-B) and basolateral-to-apical (B-A) direction across a Caco-2 cell monolayer
| Papp*10–6 cm/s | Papp*10–6 cm/s | ER | |
|---|---|---|---|
| A-B | B-A | ||
| DIG alone | 3.14 ± 0.04 | 33.8 ± 1.63 | 10.8 |
| DIG + 100 μM ART | 2.00 ± 0.22 | 20.98 ± 0.24* | 10.5 |
| DIG + 1 mM ART | 2.84 ± 0.22 | 14.85 ± 0.41* | 5.2 |
| DIG + 100 μM ADQ | 2.15 ± 0.07 | 20.35 ± 0.81* | 9.5 |
| DIG + 1 mM ADQ | 4.03 ± 0.06* | 7.60 ± 0.55* | 1.9 |
| DIG + 100 μM LUM | 3.01 ± 0.08 | 22.81 ± 1.39 | 7.6 |
| DIG + 1 mM LUM | 2.74 ± 0.06 | 20.67 ± 0.76* | 7.5 |
| DIG + 100 μM VER | 4.36 ± 0.13* | 17.30 ± 0.50* | 4.0 |
Significant difference, P < 0.05 vs. control.
Finally, the DINs for ADQ, ART, and LUM were calculated. The DINs for ADQ, ART, and LUM were 46.7, 12.6, and 0.7, respectively, indicating the greatest risk of DDIs for ADQ.
Discussion
The inhibition and induction of transporters that mediate the uptake and efflux of xenobiotics are important mechanisms underlying DDIs. The P-gp transporter is known to modulate intestinal absorption of its substrates. The expression of P-gp on the apical membrane of mucosal cells may play an important role in extruding orally administered drugs into the intestinal lumen, resulting in lower bioavailability of pharmaceutical agents. Therefore, the potential of intestinal P-gp-mediated DDIs of antimalarials with P-gp substrates was examined in this study. The test concentrations of the antimalarials (100 μM and 1 mM) were chosen to simulate theoretical drug concentrations in the gut, which were determined in this study to be 5.16 mM for ADQ, 2.08 mM for ART, and 3.63 mM for LUM. These theoretical concentrations were calculated by dividing the maximum therapeutic dose by 250 mL (assuming complete dissolution in a glass of water).18 VER, a widely used P-gp inhibitor, was used as a positive inhibitor control at a concentration of 100 μM.
A preliminary screening was first carried out using seven antimalarial drugs in a Rho-123 transport assay to investigate their potential as P-gp inhibitors or non-inhibitors. Of the seven antimalarials initially screened, ART, ADQ, and LUM showed the most efficacious blockade of Rho-123 transport in the B-A direction across a Caco-2 cell monolayer. This observation confirms previous reports that showed an inhibitory effect of ADQ against P-gp-mediated transport in Caco-2 cells, as well as an inhibitory effect of ART in K562/adr and GLC4/adr resistant cell lines.19,20
Next, the impact of ART, ADQ, and LUM on DIG transport was evaluated. In this study, all three antimalarials inhibited DIG transport at concentrations of both 100 μM and 1 mM. ADQ and ART (1 mM) exhibited potent inhibition that was comparable with that of VER (100 μM). Under the conditions of this study, LUM was shown to be a relatively weak P-gp inhibitor, even at a concentration of 1 mM. This was further exemplified by the similar ERs obtained with LUM at 100 μM and 1 mM (7.6 and 7.5, respectively).
Tachibana and others10 described the DIN as an index for predicting DDI potential (Equation 3). It was reported that P-gp inhibitors with a DIN below 10.8 have a low risk of interacting with P-gp substrates, whereas those with a DIN above 27.9 present a high risk. Therefore, the DINs for the potential interaction of the antimalarials with DIG were calculated. From these determinations, the DIN for ADQ (46.7) suggests a high potential for DDIs involving ADQ. The calculated DIN for ART was 12.6, suggestive of moderate DDI risk. The LUM had a DIN of 0.7, suggestive of low DDI risk. This implies that, although all three antimalarials inhibited P-gp-mediated DIG transport under the study conditions, there may be a higher risk of clinically relevant DDIs with ADQ and ART than with LUM. Because a higher DIN tends to correlate with a higher AUC ratio,10 it may cause toxicity when drugs with narrow therapeutic indices are involved. In addition, there is a likely increase in bioavailability when P-gp substrate drugs are co-administered with ADQ or ART. The DIN as an index for predicting DDI potential is similar to the U.S. Food and Drug Administration (FDA) draft guidance. For P-gp interactions, the guidance recommends an evaluation for potential clinical DDIs when [I2]/IC50 are greater than or equal to 10.21 Here, [I2] is the theoretical maximum gastrointestinal concentration. ADQ besides being used as an effective antimalarial especially in chloroquine-resistant malaria is also known to possess potent anti-inflammatory and antipyretic properties. Therefore, there is the potential of several DDIs occurring. In addition, ART (which has a moderate DDI risk from the computed DIN) belongs to the class of artemisinin-based antimalarials, which is the WHO recommended first-line therapy when used in combination with other antimalarials. It is therefore widely used and may pose a higher intestinal DDI potential when orally administered because of the higher exposure levels in the intestine.
In clinical settings, P-gp has been implicated in the disposition of some human immunodeficiency virus-1 (HIV-1) protease inhibitors (e.g., amprenavir, indinavir, nelfinavir, and saquinavir).22,23 Because malaria and HIV share a wide overlap in their socio-economic and geographical areas of occurrence, many locales with a high malaria burden also have a high HIV burden. Thus, co-administration of antimalarials with antiretroviral drugs is common. Therefore, there might be an increased likelihood of DDIs when HIV protease inhibitors are co-administered with LUM, ART, and ADQ, which may in turn require dose adjustments of the antimalarial or the antiretroviral agent, or both. In addition, colchicine, a drug used to treat gout and a well-known P-gp substrate, may be co-administered with antimalarials such as CQ, which can also be used to treat gout. Colchicine is notorious for its narrow therapeutic index, and hence DDIs with antimalarials may result in an increased risk of colchicine-induced toxicity. This potentially clinically relevant DDI may also require dose adjustments.24
Previous studies have shown that some antimalarial drugs exhibit low absorption after oral administration, leading to their classification as P-gp substrates or inhibitors.25–27 Current malaria treatment guidelines recommend combination therapy, preferably ART-based.28 Thus, ART (as well as ADQ and LUM) are clinically used in combination with additional antimalarial drugs and non-antimalarial drugs that, as described previously, may be P-gp substrates. This could result in unexpectedly high therapeutic outcomes for the co-administered drug, again necessitating dose adjustments to maximize therapeutic efficacy. Although several clinical reports detail treatment failure and antimalarial drug resistance, these reports have mainly emphasized mutations that mediate resistance in malarial parasites.29–31 However, the current study brings new light to the fact that altered therapeutic outcomes might be caused by DDIs between these antimalarials and other drugs that interact with P-gp. Poor oral bioavailability (as is the case with most P-gp substrates) with a resultant wide range in blood drug levels will favor the emergence of resistance because doses are mainly chosen based on therapeutic ratios. Co-administration of antimalarial P-gp substrate drugs with antimalarials that are P-gp inhibitors may improve oral bioavailability of the former and thereby reduce doses that are needed to clear infection.32 This is expected to reduce the cost of therapy, as well as the emergence and spread of resistance.
In conclusion, the current findings show that ART, ADQ, and LUM decreased P-gp-mediated transport across Caco-2 cell monolayers. Although DIG was used as a representative P-gp substrate in this study, there may be a potential for DDIs between certain antimalarials and P-gp substrates in general. These interactions may be of clinical relevance given the high incidence of co-administration of P-gp substrate drugs with antimalarials. Thus, this study is anticipated to provide useful information that will lead to more effective treatment policies for malaria. However, further in vivo investigations are recommended to confirm the in vitro findings.
Disclaimer: The authors declare no competing interests.
Footnotes
Authors' addresses: Enoche F. Oga, Shuichi Sekine, Yoshihisa Shitara, and Toshiharu Horie, Department of Biopharmaceutics, Graduate School of Pharmaceutical Sciences, Chiba University, Chuo-ku, Chiba, Japan, E-mails: enocheoga@chiba-u.jp, ssekine@p.chiba-u.ac.jp, shitara@faculty.chiba-u.jp, and horieto@p.chiba-u.ac.jp.
References
- 1.WHO . World Health Organization Expert Committee on Malaria. WHO, Geneva: 2000. http://www.rollbackmalaria.org/docs/ecr20.pdf 20th Report. Available at. Accessed April 28, 2011. [Google Scholar]
- 2.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 3.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brentlinger P, Behrens C, Mice M. Challenges in the concurrent management of malaria and HIV in pregnancy in sub-Saharan Africa. Lancet Infect Dis. 2006;6:100–111. doi: 10.1016/S1473-3099(06)70383-8. [DOI] [PubMed] [Google Scholar]
- 5.Fehintola FA, Akinyinka OO, Adewole IF, Maponga CC, Ma Q, Morse GD. Drug interactions in the treatment and chemoprophylaxis of malaria in HIV infected individuals in sub Saharan Africa. Curr Drug Metab. 2011;12:51–56. doi: 10.2174/138920011794520008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacomini K, Sugiyama Y. Membrane transporters and drug response. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's: The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2005. pp. 41–70. [Google Scholar]
- 8.Tanigawara Y. Role of P-glycoprotein in drug disposition. Ther Drug Monit. 2000;22:137–140. doi: 10.1097/00007691-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Shitara Y, Kitajima M, Tachibana T, Ishigai M, Horie T, Sugiyama Y. Extrapolation of in vitro metabolic and P-glycoprotein-mediated transport data to in vivo by modeling and simulation. In: Pang KS, Rodrigues AD, Peter R, editors. Enzyme-and Transporter-based Drug-Drug interactions, Progress and Future Challenges. New York: Springer; 2010. pp. 299–315. [Google Scholar]
- 10.Tachibana T, Kato M, Watanabe T, Mitsui T, Sugiyama Y. Method for predicting the risk of drug–drug interactions involving inhibition of intestinal CYP3A4 and P-glycoprotein. Xenobiotica. 2009;39:430–443. doi: 10.1080/00498250902846252. [DOI] [PubMed] [Google Scholar]
- 11.Rostami-Hodjegan A, Tucker G. In silico simulations to assess the in vivo consequences of in vitro metabolic drug–drug interactions. Drug Discov Today Technol. 2004;1:441–448. doi: 10.1016/j.ddtec.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto H, Matsumoto S, Tachibana M, Niwa S, Hirabayashi H, Amano N, Moriwaki T. Establishment of in vitro P-glycoprotein inhibition assay and its exclusion criteria to assess the risk of drug–drug interaction at the drug discovery stage. J Pharm Sci. 2011;100:4013–4023. doi: 10.1002/jps.22652. [DOI] [PubMed] [Google Scholar]
- 13.Cavet ME, West M, Simmons NL. Transport and epithelial secretion of the cardiac glycoside, digoxin, by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1996;118:1389–1396. doi: 10.1111/j.1476-5381.1996.tb15550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JH. Transporter-mediated drug interactions: clinical implications and in vitro assessment. Expert Opin Drug Metab Toxicol. 2007;3:81–92. doi: 10.1517/17425255.3.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Horn J, Hansten P. Drug interactions with digoxin: the role of P-glycoprotein. Pharm Times. 2004:45–46. October. [Google Scholar]
- 16.Takano M, Hasegawa R, Fukuda T, Yumoto R, Nagai J, Murakami T. Interaction with P-glycoprotein and transport of erythromycin, midazolam and ketoconazole in Caco-2 cells. Eur J Pharmacol. 1998;358:289–294. doi: 10.1016/s0014-2999(98)00607-4. [DOI] [PubMed] [Google Scholar]
- 17.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2001;46:27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 18.Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15:11–22. doi: 10.1023/a:1011984216775. [DOI] [PubMed] [Google Scholar]
- 19.Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Reungpatthanaphong P, Mankhetkorn S. Modulation of multidrug resistance by artemisinin, artesunate and dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines. Biol Pharm Bull. 2002;25:1555–1561. doi: 10.1248/bpb.25.1555. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhang YD, Zhao P, Huang SM. Predicting drug-drug interactions: an FDA perspective. AAPS J. 2009;11:300–306. doi: 10.1208/s12248-009-9106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol. 2007;73:1573–1581. doi: 10.1016/j.bcp.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, Pollak U, Koren G, Bentur Y. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. 2010;48:407–414. doi: 10.3109/15563650.2010.495348. [DOI] [PubMed] [Google Scholar]
- 25.Crowe A, Ilett KF, Karunajeewa HA, Batty KT, Davis TM. Role of P glycoprotein in absorption of novel antimalarial drugs. Antimicrob Agents Chemother. 2006;50:3504–3506. doi: 10.1128/AAC.00708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham YT, Regina A, Farinotti R, Couraud P, Wainer IW, Roux F, Gimenez F. Interactions of racemic mefloquine and its enantiomers with P-glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochim Biophys Acta. 2000;1524:212–219. doi: 10.1016/s0304-4165(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 27.Qi J, Wang S, Liu G, Peng H, Wang J, Zhu Z, Yang C. Pyronaridine, a novel modulator of P-glycoprotein-mediated multidrug resistance in tumor cells in vitro and in vivo. Biochem Biophys Res Commun. 2004;319:1124–1131. doi: 10.1016/j.bbrc.2004.05.099. [DOI] [PubMed] [Google Scholar]
- 28.WHO . Guidelines for the Treatment of Malaria. Second edition. Geneva: World Health Organization Press; 2010. p. 194. [Google Scholar]
- 29.Price RN, Dorsey G, Ashley EA, Barnes KI, Baird JK, d'Alessandro U, Guerin PJ, Laufer MK, Naidoo I, Nosten F, Olliaro P, Plowe CV, Ringwald P, Sibley CH, Stepniewska K, White NJ. World Antimalarial Resistance Network I: clinical efficacy of antimalarial drugs. Malar J. 2007;6:119. doi: 10.1186/1475-2875-6-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacon DJ, Jambou R, Fandeur T, Le Bras J, Wongsrichanalai C, Fukuda MM, Ringwald P, Sibley CH, Kyle DE. World Antimalarial Resistance Network (WARN) II: in vitro antimalarial drug susceptibility. Malar J. 2007;6:120. doi: 10.1186/1475-2875-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg. 2004;71:179–186. [PubMed] [Google Scholar]
- 32.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]