Abstract
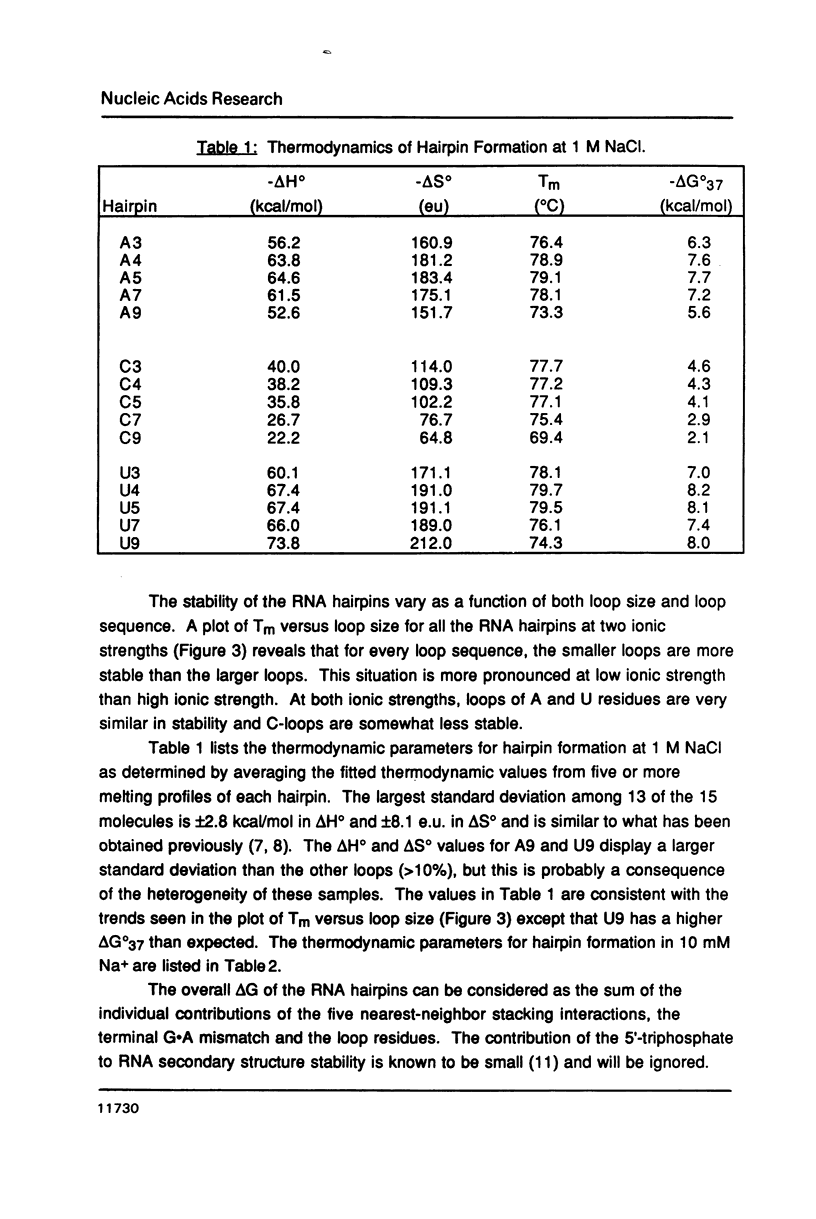
Fifteen RNA hairpins that share the same stem sequence and have homopolymer loops of A, C and U residues which vary in length from three to nine nucleotides were synthesized and their thermal stabilities determined. Tm varies as a function of loop size but is almost independent of loop composition. Loops of four or five nucleotides are found to be the most stable loop size. This is consistent with the observation that four-membered loops are the most prevalent loop size in 16S-like RNAs. The contribution of each loop to hairpin stability was calculated by subtracting the known contribution of the helical stem. These data should be useful for predicting the stability of other hairpins.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freier S. M., Petersheim M., Hickey D. R., Turner D. H. Thermodynamic studies of RNA stability. J Biomol Struct Dyn. 1984 Mar;1(5):1229–1242. doi: 10.1080/07391102.1984.10507514. [DOI] [PubMed] [Google Scholar]
- Fuller W., Hodgson A. Conformation of the anticodon loop intRNA. Nature. 1967 Aug 19;215(5103):817–821. doi: 10.1038/215817a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Maizels N., Maxam A. Sequences of controlling regions of the lactose operon. Cold Spring Harb Symp Quant Biol. 1974;38:845–855. doi: 10.1101/sqb.1974.038.01.087. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Hickey D. R., Turner D. H. Effects of terminal mismatches on RNA stability: thermodynamics of duplex formation for ACCGGGp, ACCGGAp, and ACCGGCp. Biochemistry. 1985 Jul 16;24(15):3987–3991. doi: 10.1021/bi00336a028. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Cantor C. R., Tinoco I., Jr Association of complementary oligoribonucleotides in aqueous solution. Biochemistry. 1968 Sep;7(9):3164–3178. doi: 10.1021/bi00849a020. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbons L. P., van der Marel G. A., van Boom J. H., Altona C. Hairpin and duplex formation of the DNA octamer d(m5C-G-m5C-G-T-G-m5C-G) in solution. An NMR study. Nucleic Acids Res. 1986 May 27;14(10):4187–4196. doi: 10.1093/nar/14.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Base-stacking and base-pairing contributions to helix stability: thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):256–263. doi: 10.1021/bi00271a004. [DOI] [PubMed] [Google Scholar]
- Sugimoto N., Kierzek R., Turner D. H. Sequence dependence for the energetics of dangling ends and terminal base pairs in ribonucleic acid. Biochemistry. 1987 Jul 14;26(14):4554–4558. doi: 10.1021/bi00388a058. [DOI] [PubMed] [Google Scholar]
- Tuerk C., Gauss P., Thermes C., Groebe D. R., Gayle M., Guild N., Stormo G., d'Aubenton-Carafa Y., Uhlenbeck O. C., Tinoco I., Jr CUUCGG hairpins: extraordinarily stable RNA secondary structures associated with various biochemical processes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1364–1368. doi: 10.1073/pnas.85.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Borer P. N., Dengler B., Tinoco I., Jr Stability of RNA hairpin loops: A 6 -C m -U 6 . J Mol Biol. 1973 Feb 5;73(4):483–496. doi: 10.1016/0022-2836(73)90095-8. [DOI] [PubMed] [Google Scholar]
- Van Charldorp R., Heus H. A., Van Knippenberg P. H., Joordens J., De Bruin S. H., Hilbers C. W. Destabilization of secondary structure in 16S ribosomal RNA by dimethylation of two adjacent adenosines. Nucleic Acids Res. 1981 Sep 11;9(17):4413–4422. doi: 10.1093/nar/9.17.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Charldorp R., Verhoeven J. J., Van Knippenberg P. H., Haasnoot C. A., Hilbers C. W. A carbon-13 nuclear magnetic resonance study of the 3'-terminus of 16S ribosomal RNA of Escherichia coli specifically labeled with carbon-13 in the methylgroups of the m6(2)Am6(2)A sequence. Nucleic Acids Res. 1982 Jul 24;10(14):4237–4245. doi: 10.1093/nar/10.14.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]




