Abstract
Patients who recover from leishmaniasis usually show development of strong immunity and induction of interferon-γ (IFN-γ) and delayed type hypersensitivity. In a randomized trial, we analyzed the IFN-γ response by using a Quantiferon–Leishmania assay against three Leishmania peptide antigens and compared it with results of the leishmanin skin test (LST) in persons residing in areas in Iran to which zoonotic cutaneous leishmaniasis (ZCL, 181 persons), anthroponotic cutaneous leishmaniasis (ACL, 104 persons), and zoonotic visceral leishmaniasis (ZVL, 67 persons) are endemic. The percentage of persons with an IFN-γ-positive response (> 0.2 IU/mL) to three antigens and the mean concentration of IFN-γ induced by the antigens were higher for persons from areas endemic for ZVL than for persons from areas endemic for ZCL and ACL. The percentage of persons with LST-positive results (≥ 5 mm indurations) was 99%, 94%, and 70% for areas with ZCL, ACL, and ZVL, respectively. Our data indicate that the LST is significantly more sensitive than IFN-γ levels in persons who have been cured of cutaneous leishmaniasis than in persons who have been cured of ZVL.
Introduction
Leishmaniasis, caused by different species of protozoan parasites of genus Leishmania, is prevalent in different parts of Asia, Africa, southern Europe, and Latin America. These parasites are intra-macrophage organisms in mammalian hosts and can cause a wide range of diseases including cutaneous, mucosal, and visceral forms of leishmaniasis. Visceral leishmaniasis is fatal if not treated.1,2
Three types of leishmaniasis are endemic in different regions of Iran: zoonotic cutaneous leishmaniasis (ZCL) caused by L. major, anthroponotic cutaneous leishmaniasis (ACL) caused by L. tropica, and zoonotic visceral leishmaniasis (ZVL) caused by L. infantum.3 We recruited volunteers from all these three regions in this study.
In experimental murine models, the outcome of the disease depends upon development of cell-mediated immune response and is greatly influenced by expansion of specific T cell subsets. In disease-resistant mice, infection with L. major parasites results in a self-healing lesion associated with proliferation of CD4+ T cell populations and production of interferon-γ (IFN-γ), the Th1 response. In contrast, injection of the same parasite into disease-susceptible mice will cause development of a non-healing lesion with high level of interleukin-4 (IL-4), IL-10, and other cytokines related to the Th2 response.4–6
In humans, clinical features of leishmaniasis are probably multi-factorial but also depend upon the response of specific T cell sub-populations. In vitro stimulation of peripheral blood monocytes from patients who recovered from CL with Leishmania antigens leads to expansion of specific T cell populations, which indicates up-regulation of the Th1-like subset that produces IFN-γ and low levels of IL-4.7,8
A delayed-type hypersensitivity (DTH) reaction is an indicator for cell-mediated immunity and is used in epidemiologic investigations to determine exposure to Leishmania.9 A positive DTH reaction is a common feature after recovery from leishmaniasis, which can be evaluated by using the leishmanin skin test (LST).10 The LST response remains positive for decades after recovery,11,12 making it possible to identify almost all members of a population who have been infected.13 However, the LST is not a precise quantitative test, and exposes persons to leishmanial antigens.
The QuantiFERON (QFN) (Cellestis, Chadstone, Victoria, Australia) test is an in vitro procedure developed for measurement of IFN-γ under field conditions. In this study, we evaluated induction of IFN-γ by three specific antigens by using the QFN test and compared its results with those of the LST in volunteers in Iran who recovered from ZCL, ACL, and VL. The aim of this study was to evaluate and compare the performance of the QFN–Leishmania assay against that of the LST in leishmaniasis-endemic foci in Iran.
Patients And Methods
Study population and study design.
Four groups of persons were included in the study. The first group was composed of 121 persons who had recovered from ZCL (group ZCL-1, 34 males and 87 females, age range = 15–67 years, mean ± SD age = 33.53 ± 14.84 years) and 60 persons who had recovered from ZCL (group ZCL-2, 17 males and 43 females, age range = 12–70 years, mean ± SD = 39.78 ± 21.44 years) who resided in rural disease-endemic areas of Damghan, 324 km east of Tehran. For ZCL-1, Leishmania (Leish B) antigen was used. However, for ZCL-2, Leish C, which contained additional peptides to enhance reactivity, was used in the same area but at a different time, as recommended by Cellestis.
The second group was composed of 104 persons who showed healing of ACL (35 males and 69 females, age range = 9–70 years, mean ± SD = 30.89 ± 13.64 years) who resided in urban disease-endemic areas of Mashhad, 924 km east of Tehran.
The third group was composed of 67 persons (46 males and 21 females, age range = 2–21 years, mean ± SD age = 5.93 ± 3.16) from three areas endemic for ZVL caused by L. infantum. These areas included Kalibar, Ahar, and Meshkin Shahr, which are located approximately 700 km northwest of Tehran.
The fourth group was composed of 102 persons (71 males and 31 females, age range = 17–72 years, mean ± SD age = 38 ± 12 years) from non-endemic areas of Tehran (relatives of children who attended the Vaccination Division of the Pasteur Institute of Iran, Tehran, and personnel from the Institute). These persons were used as negative controls, had not spent extensive periods of time in leishmaniasis-endemic areas, especially during the sand fly season, and their LST results were zero. Persons with a history of infection were selected from those foci, where on the basis of previous studies and epidemiologic characteristics, the causative agents were most likely L. major, L. tropica, or L. infantum.14–16
All persons in the four groups were clinically examined. Those who met inclusion criteria, but not exclusion criteria, were enrolled in the study. Exclusion criteria were chronic illness, immunosuppression, such as infection with human immunodeficiency virus, silicosis, cancer (leukemia or lymphoma), gastrointestinal bypass surgery, diabetes mellitus, renal failure, recent major surgery, pregnancy, and lactation. After persons completed a questionnaire and informed consent was obtained, peripheral blood samples were collected and the LST was performed. The study was approved by the ethics committee of Pasteur Institute of Iran and registered in the Iranian Registry of Clinical Trials (IRCT138705081033N1).
Sampling.
A unique identifier (five-digit number) was assigned to each person after he or she completion of an informed consent form. Persons were identified only by their numeric identifier on all data collection forms. Four milliliters of peripheral blood was collected from each person, and 1 mL was added to each of four QFN–Leishmania test blood collection tubes (Cellestis), which included a negative control tube, a Leish A tube, a Leish B tube, and a mitogen-positive control tube in the first study of ZCL. Because responses to Leish B in the ZCL-endemic area were low, Leish C was used for 60 persons in ZCL area (group ZCL-2) and then in ACL and ZVL areas. The trial of ZCL-2 was conducted in the same disease-endemic area as for ZCL-1, but at different times and with different persons. The mitogen tube may also serve as a control for correct blood handling and incubation. Within 16 hours of collection, the tubes were incubated at 37°C for 16–24 hours, centrifuged, and plasma was removed and stored at 2–8°C until used for assessment of IFN-γ.
Leishmania antigens.
Three QFN-Leish test tubes were used in this study for induction of IFN-γ: The first tube was Leish A, which contained peptides from histone 2B (L. major and L. infantum). The second tube was Leish B, which contained the same antigen as Leish A plus additional peptides corresponding to regions of promastigote surface antigen 2 (L. major and L. Infantum). The third tube was Leish C, which contained Leish A peptides plus five peptides from nuclear phosphoprotein 12.
IFN-γ assessment.
Measurement of IFN-γ was carried out by using an enzyme immunoassay (QFN–enzyme-linked immunosobent assay [ELISA] Kit; Cellestis) according to the procedure described by manufacturer. Each ELISA plate used four dilutions of a standard preparation of IFN-γ to construct standard curves from which IFN-γ levels in plasma can be calculated. In brief, 50 μL of freshly prepared working-strength conjugate was added to the required ELISA plate wells. Fifty microliters of prepared standards or test plasma samples was then added to appropriate wells. The plates were covered and incubated at room temperature (22°C ± 5°C) for 120 ± 5 minutes in the dark. One hundred microliters of enzyme substrate solution was added to each well and mixed thoroughly by using a micro-plate shaker. Plates were covered with a lid and incubated at room temperature (22°C ± 5°C) for 30 minutes in the dark. Fifty microliters of enzyme stopping solution was then added. The optical density (OD) of each well was measured within 5 minutes of stopping the reaction by using a micro plate reader (Anthos 2020; Anthos Labtec, Salzburg, Austria) fitted with a 450 nm filter and with a 620–650 nm reference filter. The OD values were used to calculate results. Reported IFN-γ concentrations are those from Leish-stimulated samples minus the unstimulated negative control samples. Interferon-γ values ≥ 0.2 IU/mL were considered a positive response as reported to give the highest specificity and sensitivity.17 Mitogen control tubes from all persons showed positive results, which indicated correct blood handling and incubation of samples.
Calculation and interpretation.
Data were entered into a Microsoft (Redmond, WA) Excel spreadsheet. Regression analysis was performed and the coefficient of variation for the standards and the correlation coefficient (r) of the standard curve were calculated. Each ELISA was considered valid when the mean OD value for 1 IU/mL IFN-γ was ≥ 0.600 and when the correlation coefficient calculated for the standard curve was ≥ 0.98.
Leishmanin skin test.
The TDR/World Health Organization reference leishmanin,18 which was produced under conditions of good manufacturing practices, was used for skin testing according to a reported procedure.19 In brief, 0.1 mL of leishmanin was injected intradermal into the inner surface of the left forearm. Diameters of indurations at the injection site was measured 48 hours later by using a millimeter-graduated ruler and the ball point pen procedure.20 Indurations ≥ 5 mm were considered as positive LST results.
Statistical analysis.
Data were analyzed by using the z-test for comparison of differences between percentage of LST-positive persons and IFN-γ positive persons. Amounts of IFN-γ were also compared in different groups by using the t-test and SigmaStat 2.0 software (Jandel Scientific, Chicago, IL). P values < 0.05 were considered significant. Correlations between LST indurations and IFN-γ production against all antigens in different groups were analyzed by using linear regression with log b2 values and SPSS statistical software (SPSS Inc., Chicago, IL).
Results
Positive responses for IFN-γ and LST in persons with ZCL.
Two studies were carried out in ZCL-endemic areas. In the first study, sensitivity of the Leish A and B QFN assays and sensitivity of the LST were tested for 121 persons residing in the disease-endemic area. As shown in Table 1 , percentages of IFN-γ responders to Leish A and Leish B in persons who had recovered from CL were 45.5% and 43.8%, respectively. The LST showed 100% sensitivity in this area. In the second study, which was conducted with 60 persons, the percentage of IFN-γ responders to Leish A and Leish C was 46.7% and 50.0%, respectively. Sensitivity of the LST was 98.3% in this disease-endemic area. The LST-positive response was significantly higher than IFN-γ response against Leish A and Leish B (z-test value = 9.38, P < 0.001 and z-test value = 9.58, P < 0.001, respectively) in the first study and also against Leish A and Leish C in the second study (z-test value = 6.13, P < 0.001 and z-test value = 5.84, P < 0.001, respectively). The percentage of IFN-γ responders to Leish A in persons from the ZCL-1 and ZCL-2 areas was 45.86% (83 of 181).
Table 1.
Frequency of IFN-γ-positive persons in response to Leishmania (Leish) antigens A, B, or C compared with LST-positive persons residing in areas of Iran endemic for ZCL, ACL, and ZVL and in a non-endemic area*
| Area | IFN-γ responder to Leish A (no. positive/no. tested) | IFN-γ responder to Leish B (no. positive/no. tested) | IFN-γ responder to Leish C (no. positive/no. tested) | Frequency of LST-positive response (no. positive/no. tested) | Magnitude of LST reactions (mean ± SD diameter, mm) |
|---|---|---|---|---|---|
| ZCL-1 | 45.5% (55/121)† | 43.8% (53/121)† | – | 100% (121/121)† | 15.55 ± 4.96 |
| ZCL-2 | 46.7% (28/60)† | – | 50% (30/60)† | 98.3% (59/60)† | 14.16 ± 4.60 |
| ACL | 33.7% (35/104)† | – | 36.5% (38/104)† | 94.4% (85/90)† | 15.10 ± 7.18 |
| ZVL | 53.7% (36/67) | – | 58.2% (39/67) | 70.2% (47/67) | 8.84 ± 2.44 |
| Non-endemic | 9.8% (10/102) | – | 13.7% (14/102) | 0 | 0 |
IFN-γ = interferon-γ ZCL = zoonotic cutaneous leishmaniasis; ACL = anthroponotic cutaneous leishmaniasis; ZVL = zoonotic visceral leishmaniasis.
A significant difference was observed between LST-positive persons and IFN-γ responders to antigens A, B, or C.
Positive responses for IFN-γ and LST in persons with ACL.
The percentages of IFN-γ responders with a history of ACL against Leish A and Leish C were 33.7% and 36.5%, respectively. The sensitivity of the LST in persons with ACL was 94.4%. The LST responses were significantly higher than the IFN-γ responses against Leish A (z-test value = 8.43, P < 0.001) and Leish C (z-test value = 8.09, P < 0.001), as shown in Table 1.
Positive responses for IFN-γ and LST persons cured of ZVL.
The percentages of IFN-γ responders with a history of ZVL against Leish A and Leish C were 53.7% and 58.2% respectively, which were not significantly different from persons with positive LST results (70.2%) in this area. Responses of persons from the area endemic for ZVL were significantly higher than those from the area endemic for ACL against the same antigens (z-test value = 2.43, P = 0.015 and z-test value = 2.63 P = 0.009, respectively).
Responses for IFN-γ in healthy persons.
Specificity of IFN-γ production was evaluated in healthy persons without a history of leishmaniasis whose LST results were zero. Results showed positive IFN-γ responses in 10 of 102 healthy persons for Leish A (specificity = 90.2%), and 14 of 102 persons for Leish C (specificity = 86.3%). The difference between two groups was not significant (z-test value = 0.854, P = 0.393).
Responses for IFN-γ in persons who recovered from leishmaniasis.
As shown in Table 2 , persons who recovered from ZVL produced significantly higher levels of IFN-γ against Leish A (mean ± SE = 2.66 ± 0.61 IU/mL) than persons in the ZCL-2 area (P = 0.029). No other significant difference was observed for other groups or other antigens. Values for positive controls (cells from all persons who responded to mitogen) were 7–18 times higher than those to all three antigens, which indicated that the conditions of culture were satisfactory.
Table 2.
Production of IFN-γ against Leishmania (Leish) antigens A, B, or C by volunteers in areas of Iran endemic for ZCL, ZVL, and ACL*
| Area | IFN-γ to mitogen | IFN-γ to Leish C | IFN-γ to Leish B | IFN-γ to Leish A |
|---|---|---|---|---|
| ZCL-1 | 14.901 ± 0.40 | – | 1.813 ± 0.369 | 1.573 ± 0.316 |
| ZCL-2 | 18.444 ± 1.419 | 1.104 ± 0.230 | – | 1.000 ± 0.169† |
| ACL | 17.069 ± 0.643 | 1.464 ± 0.482 | – | 1.454 ± 0.559 |
| ZVL | 19.551 ± 0.856 | 2.238 ± 0.551 | – | 2.664 ± 0.606† |
Values are mean ± SE (IU/mL). IFN-γ = interferon-γ; ZCL = zoonotic cutaneous leishmaniasis; ACL = anthroponotic cutaneous leishmaniasis; ZVL = zoonotic visceral leishmaniasis.
A statistically significant difference was shown between IFN-γ production against Leish Aantigen between persons who were cured of ZVL and healed cases of ZCL-2 (P = 0.029).
Response related to time of assay after clinical cure.
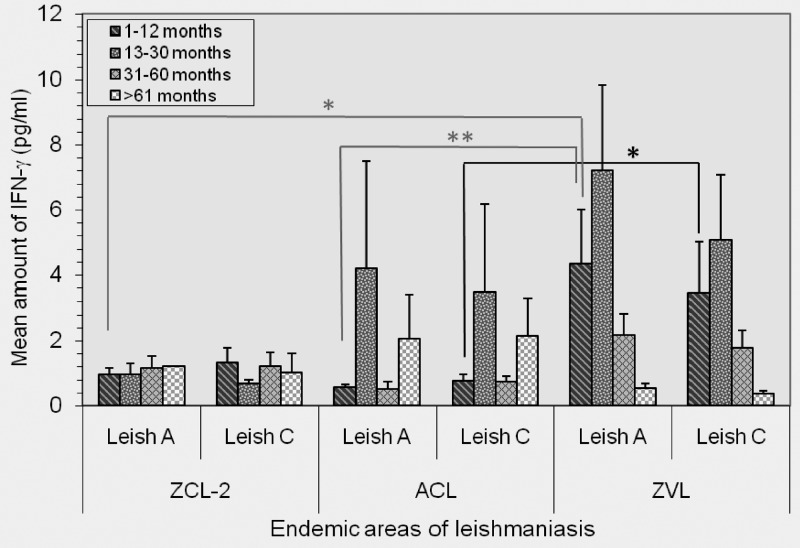
The IFN-γ response against Leish A and Leish C was analyzed in cured persons from ZCL-2-, ACL-, and ZVL-endemic areas for different periods (1–12 months, 13–30 months, 31–60 months, and > 61 months post-recovery). The pattern of responses for different periods was different for the three groups. After visceral disease, as in persons with ZVL, IFN-γ- production against Leish A and Leish C starts during the early periods, peaks by 13–30 months, and decrease thereafter. In persons from in the ZCL-2 group, no difference in IFN-γ production could be discerned throughout the study periods (Figure 1). The magnitude of IFN-γ response for a visceral disease (ZVL) during the early period (1–12 month post-recovery) was significantly higher than that of persons with ACL against Leish A (P = 0.003) and Leish C (P = 0.030). However, the difference for persons in the ZCL-2 group was significant only for Leish A (P = 0.017) during the same period. No statistically significant differences were observed in other periods between persons from different disease-endemic areas.
Figure 1.
Mean concentration of interferon-γ (IFN-γ against Leishmania antigen A (Leish) A and Leish C antigens in persons cured of zoonotic cutaneous leishmaniasis (ZCL), anthroponotic cutaneous leishmaniasis (ACL), and zoonotic visceral leishmaniasis (ZVL) in leishmaniasis-endemic areas in Iran, according to the time of assay in relation to recovery from the disease. The IFN-γ response to either Leish A or Leish C did not vary in persons from the ZCL area throughout the study. The magnitude of the IFN-γ response in persons from the ZVL area was significantly higher than that of persons from the ACL area against Leish A (P = 0.003, by t-test) and Leish C (P = 0.030, by t-test) during the early period (1–12 months post-recovery). However for ZCL, the difference was only significant for Leish A (P = 0.017) during the same period. No significant difference was seen in other periods between persons from different regions. *P = 0.017 and 0.030; **P = 0.003.
Potency of LST.
Along with measurement of LST sensitivity, the magnitude of LST reactivity was determined for persons with histories of ZCL, ACL, and ZVL. Mean ± SD indurations were 15.55 ± 4.96, 14.15 ± 4.60, 15.10 ± 7.18, and 8.60 ± 2.58 mm for persons in groups ZCL-1, ZCL-2, ACL, and ZVL, respectively Table 1.
Correlation between LST indurations and IFN-γ production.
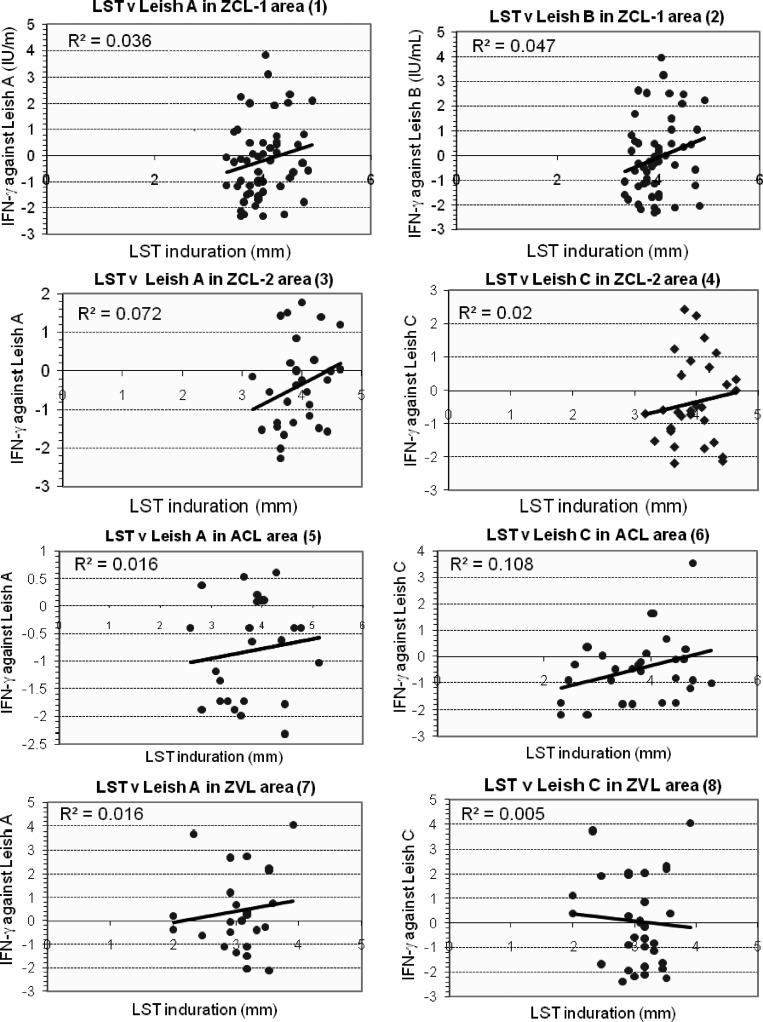
As shown in Figure 2, when we used log 2 values, there was no significant correlation between LST results and IFN-γ responses to any antigens in persons from any regions tested.
Figure 2.
Linear regression of log 2 values of the interferon-γ (IFN-γ response compared with leishmanin skin test indurations for Leishmania antigen A (Leish) A and Leish B in area of Iran endemic for zoonotic cutaneous leishmaniasis (ZCL-1) (panels 1 and 2), for Leish A and Leish C in areas endemic for ZCL-2 (panel 3 and 4), in areas endemic for anthroponotic cutaneous leishmaniasis (ACL) (panels 5 and 6), and in areas endemic for zoonotic visceral leishmaniasis (ZVL) (panel 7 and 8). No statistically significant correlation was found between leishmanin skin test indurations and IFN-γ responses of persons from different areas.
Discussion
Development of protective immunity against different clinical forms of human leishmaniasis is usually associated with production of IFN-γ, and a life-long positive LST result, both of which are induced by Leishmania-specific T cells.21–23 These cells respond to recall antigens and can be detected in two ways: 1) by production of IFN-γ,21 which can be assessed in vitro, and 2) by the LST, which shows a in vivo DTH reaction induced by intradermal injection of leishmanin. Although these reactions can be observed after curing of leishmaniasis when the host is generally immune, these responses do not always indicate protection because abundance of the Th2-type response in the presence of the Th1-response leads to persistent disease, as in the chronic non-healing form of CL or mucosal leishmaniasis, where IFN-γ and LST reactivities are high.24 It is not known whether in the absence of a chronic lesion (or absence of the Th2 response), strong LST and IFN-γ reactivity could be a marker for protection. Nevertheless, there is interest in measuring IFN-γ levels in vitro without exposing persons to leishmanial antigens by a simple field-adapted test, such as the QFN test, particularly in vaccine studies as a marker of immunogenicity and possibly protective immunity.
In this study, three Leishmania antigens prepared by QFN technology were used for assessment of IFN-γ. Crude whole-cell antigen (leishmanin) was used for eliciting a DTH response. Results indicate that all groups tested had significantly higher percentages of LST positive reactions than those with an IFN-γ–positive response, except in the ZVL area, where the difference was not significant. The reason for the lower positivity for IFN-γ than LST reactivity in persons from the ZVL area could be related to limited amounts of leishmanial antigens in the QFN test compared with leishmanin, which contains whole parasite antigens. Also, memory cells to these limited number of antigens are short lived compared with multiple antigens present in leishmanin.
The LST response in persons in the ZVL focus was significantly lower than that in persons from the ZCL and ACL foci, whereas the IFN-γ response was comparable in persons from all three foci. This finding may be caused by antigenic specificity of leishmanin produced from L. major, which may cross-react to a greater extent with L. major and L. tropica, than with L. infantum (L. chagasi). Conversely, comparable or higher levels of IFN-γ in the ZVL focus than in other foci is most likely caused by L. infantum antigens in Leish A and Leish C. Successful recovery of patients in ZCL and ACL foci is generally associated with increased production of IFN-γ, when crude leishmanial antigens from L. major are used.7,25–27 Thus, relatively lower LST results but higher IFN-γ responses in the ZVL focus determined by using the QFN test with a limited number of antigens is likely caused by antigenic specificity.
Our results are consistent with those of a study that used the QFN test and showed higher sensitivity in persons cured of ZVL (83%) than in persons cured of ACL (40%) to H2B antigens, which are nearly identical to Leish A.17 Moreover, the IFN-γ response induced by Leish A in persons cured of ZVL was significantly higher than in persons cured of disease from the ZCL-2 area, but not in other groups. Our results may be attributed to the nature of leishmanial antigens, particularly peptides from L. infantum, which were present in our test antigens. Although differences in IFN-γ levels were statistically significant, these differences may not be biologically important. However, we have no data regarding this issue.
Interesting patterns of IFN-γ responses emerged when data were analyzed on the basis of the time of assay in relation to recovery from disease. In patients who were cured of visceral disease, IFN-γ response was higher during the earlier periods tested (1–12 and 13–30 months post-cure) and then decreased. In persons from the ZCL-2 area, low levels of IFN-γ were detected throughout and no difference between different periods was observed (Figure 1). The magnitude of response from a person with a visceral disease (ZVL) during the early period (1–12 month post-cure) was significantly higher than those in the ZCL-area for Leish A and in persons with ACL for Leish A and Leish C. These findings might be caused by higher parasite load during visceral leishmaniasis than during CL.
Although the correlation of IFN-γ with protection is not universally accepted, there is evidence that IFN-γ is the main cytokine of the Th1 response and is involved in protective immunity against leishmaniasis. However, not all LST-converted persons in a disease-endemic focus are protected against subsequent Leishmania infection (Sharifi I and others, unpublished data). In first-generation vaccine trials, persons with negative LST results were recruited and those who became LST positive had a lower incidence of disease but not all were protected.28 Protection showed a maximum rate of 50%,29 and those protected may have produced IFN-γ. The QFN test would have been a useful tool to address this issue.
Specificity of Leish A and Leish C was also tested in healthy persons with negative LST results. Although specificity was higher for Leish A than for Leish C, the difference was not significant (z-test value = 0.854, P = 0.393). The reasons for production of IFN-γ by healthy unexposed persons are not clear. However, production of IFN-γ by unrelated T cells stimulated with leishmanial antigens has been reported.30 Pre-exposure to organisms with cross-reacting antigens (i.e., Mycobacteria spp.) might also be cause of the IFN-γ response.
In conclusion, the results of the present study indicate that the QFN test, as adapted for Leishmania, detected approximately half the persons who had positive LST test results. Most reactivity was caused by specific IFN-γ responses to histone 2B peptides. The proportion of QFN test responders was also highest in populations exposed to L. major and L. infantum than in populations exposed to L. tropica. Whether the QFN–Leishmania test provides a better correlation of protection than the LST requires further investigation.
ACKNOWLEDGMENTS
We thank our colleagues at the Pasteur Institute of Iran for support, Dr. A. Mostafavi for his assistance in statistical analyses. D. Iravani for technical assistance with skin testing and sampling, A. H. Javadi for administrative help, A. Bozarjomehr (Center for Control of Diseases, Mashhad University of Medical Sciences) and Z. Zareei (School of Public Health, Tehran University of Medical Sciences) for assistance during sampling, and Cellestis for providing Quantiferon Kits and antigens.
Footnotes
Financial support: This study was supported by Cellestis and grant #IPI-405 from the Pasteur Institute of Iran and LEISHDNAVAX, FP-7 EU.
Authors' addresses: Mohammad H. Alimohammadian, Haideh Darabi, Farhad Riazirad, and Soheila Ajdary, Immunology Department, Pasteur Institute of Iran, Pasteur Avenue, Tehran, Iran, E-mails: mhalimohammadian@yahoo.com, hdarabi@pasteur.ac.ir, riazirad@yahoo.com, and sohary@yahoo.com. Stephen L. Jones, Cellestis Limited, Level 1, Office Tower 2, PO Box 169, Chadstone, Victoria, Australia, E-mail: sjones@cellestis.com. Aliakbar Shabani, School of Public Health, Semnan University of Medical Sciences, Damghan, Iran, E-mail: aashaebani@yahoo.com. Mohammad A. Rezaee, Center for Control of Diseases, Mashhad University of Medical Sciences, Mashhad, Iran, E-mail: rezaeema1@mums.ac.ir. Mehdi Mohebali, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, E-mail: mohebali@tumes.ac.ir. Zohreh Hosseini, Vaccination Division, Pasteur Institute of Iran, Tehran, Iran. Farrokh Modabber, Center for Research and Training in Skin Diseases and Leprosy TUMS, and Drugs for Neglected Diseases Initiative, 15 Chemin Louis-Dunant, Geneva, Switzerland, E-mail: fmodabber@dndi.org.
References
- 1.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Nadim A, Seyedi-Rashti MA. A brief review of the epidemiology of various types of leishmaniasis in Iran. Acta Med Iran. 1971;XIV:99–106. [Google Scholar]
- 4.Reiner SL, Locksly RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 5.Muller I, Fruth U, Louis JA. Immunobiology of experimental leishmaniasis. Med Microbiol Immunol (Berl) 1992;181:1–12. doi: 10.1007/BF00193391. [DOI] [PubMed] [Google Scholar]
- 6.Launois P, Tacchini-Cottier F, Parra-Lopez C, Louis JA. Cytokines in parasitic diseases: the example of cutaneous leishmaniasis. Int Rev Immunol. 1998;17:157–180. doi: 10.3109/08830189809084491. [DOI] [PubMed] [Google Scholar]
- 7.Kemp M, Hey AS, Kurtzhals JA, Christensen CB, Gaffar A, Mustafa MD, Kordofani AA, Ismail A, Kharazmi A, Theander TG. Dichotomy of the human T cell response to Leishmania antigens. I. Th1-like response to Leishmania major promastigote antigen in individuals recovered from cutaneous leishmaniasis. Clin Exp Immunol. 1994;96:410–415. doi: 10.1111/j.1365-2249.1994.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajdary S, Alimohammadian MH, Eslami MB, Kemp K, Kharazmi A. Comparison of the immune profile of nonhealing cutaneous leishmaniasis patients with those with active lesions and those who have recovered from infection. Infect Immun. 2000;68:1760–1764. doi: 10.1128/iai.68.4.1760-1764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5:1–14. [PubMed] [Google Scholar]
- 10.TDR , World Health Organization . The Leishmaniases. Tropical Diseases Progress in International Research, 1987–1988. Ninth Programme Report. Geneva: UNDP/Word Bank/WHO, Special Programme for Research and Training in Tropical Disease; 1989. pp. 85–92. [Google Scholar]
- 11.Mauel J, Behin R. Leishmaniasis: immunity, immunopathology and immunodiagnosis. In: Cohen S, Warren KS, editors. Immunology of Parasitic Infection. Oxford United Kingdom: Blackwell Scientific Publication; 1982. pp. 299–314. [Google Scholar]
- 12.Manson-Bahr PEC. Diagnosis. In: Peters W, Killick-Kendrick R, editors. The Leishmaniases in Biology and Medicine. Clinical Aspects and Control. Volume 2. London: Academic Press; 1987. pp. 703–729. [Google Scholar]
- 13.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 14.Tashakori M, Kuhls K, Al-Jawabreh A, Mauricio I, Schonian G, Farajnia S, Alimohammadian MH. Leishmania major: genetic heterogenecity of Iranian isolates by single-strand conformation polymorphism and sequence analysis of ribosomal DNA internal transcribed spacer. Acta Trop. 2006;98:52–58. doi: 10.1016/j.actatropica.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Nadim A, Seyedi-Rashti MA, Fagihi A. Epidemiology of cutaneous leishmaniasis in Iran. B. Khorasan, part III, human infection. Bull Soc Pathol Exot. 1969;62:702–710. [PubMed] [Google Scholar]
- 16.Soleimanzadeh G, Edrissian GH, Movahhed-Danesh AM, Nadim A. Epidemiological aspects of kala-azar in Meshkin-Shahr, Iran: human infection. WHO Bulletin OMS. 1993;71:759–762. [PMC free article] [PubMed] [Google Scholar]
- 17.Turgay N, Balcioglu C, Ozensoy S, Ozbel Y, Jones SL. Quantiferon-Leishmania as an epidemiological tool for evaluating the exposure to Leishmania infection. Am J Trop Med Hyg. 2010;83:822–824. doi: 10.4269/ajtmh.2010.09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.TDR, World Health Organization . Leishmaniasis. Tropical Disease Research. Progress 1975–1994, Highlights 1993–1994. Twelfth Programme Report. Geneva: UNDP/Word Bank/WHO, Special Programme for Research and Training in Tropical Disease; 1995. pp. 135–146. [Google Scholar]
- 19.Bahar K, Dowlati Y, Shidani B, Alimohammadian MH, Khamesipour A, Ehsasi S, Hashemi-Fesharaki R, Ale-Agha S, Modabber F. Comparative safety and immunogenicity trial of two killed Leishmania major vaccines with or without BCG in human volunteers. Clin Dermatol. 1996;14:489–495. doi: 10.1016/0738-081x(96)00071-5. [DOI] [PubMed] [Google Scholar]
- 20.Sokal JE. Measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 21.Wakil AE, Wang ZE, Ryan JC, Fowell DJ, Locksley RM. Interferon gamma derived from CD4 (+) T cells is sufficient to mediate T helper cell type 1 development. J Exp Med. 1998;188:1651–1656. doi: 10.1084/jem.188.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostami MN, Keshavarz H, Edalat R, Sarrafnejad A, Shahrestani T, Mahboudi F, Khamesipour A. CD8+ T cells as a source of IFN-γ production in human cutaneous leishmaniasis. PLoS Negl Trop Dis. 2010;4:e845. doi: 10.1371/journal.pntd.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller I. Role of T cell subsets during the recall of immunologic memory to Leishmania major. Eur J Immunol. 1992;22:3063–3069. doi: 10.1002/eji.1830221206. [DOI] [PubMed] [Google Scholar]
- 24.Matos DS, Azeredo-Coutinho RB, Schubach A, Conceicão-Silva F, Baptista C, Moreira JS, Mendonça SC. Differential interferon-γ production characterizes the cytokine responses to Leishmania and Mycobacterium leprae antigens in concomitant mucocutaneous leishmaniasis and lepromatous leprosy. Clin Infect Dis. 2005;40:e5–e12. doi: 10.1086/427069. [DOI] [PubMed] [Google Scholar]
- 25.Ajdary S, Riazi-rad F, Alimohammadian MH, Pakzad SR. Immune response to Leishmania antigen in anthroponotic cutaneous leishmaniasis. J Infect. 2009;59:139–143. doi: 10.1016/j.jinf.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Alimohammadian MH, Darabi H, Malekzadeh SH, Mahmoodzadeh-Niknam H, Ajdary S, Khamesipour A, Bahonar A, Mofarrah A. Exposure to Leishmania major modulates the proportion of CD4+ T cells without affecting cellular immune responses. Microbiol Immunol. 2007;51:1003–1011. doi: 10.1111/j.1348-0421.2007.tb03984.x. [DOI] [PubMed] [Google Scholar]
- 27.Castellano LR, Filho DC, Argiro L, Dessein H, Prata A, Dessein A, Rodrigues V. Th1/Th2 immune responses are associated with active cutaneous leishmaniasis and clinical cure is associated with strong interferon-γ production. Hum Immunol. 2009;70:383–390. doi: 10.1016/j.humimm.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Momeni AZ, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Labaf Ghassem R, Dowlati Y, Sharifi I, Aminjavaheri M, Shafiei A, Alimohammadian MH, Hashemi-Fesharaki R, Nasseri K, Godal T, Smith G, Modabber F. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1998;17:466–472. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- 29.Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Kieny MP, Tanner M. First generation leishmaniasis vaccines: a review of the field efficacy trials. Vaccine. 2008;26:6759–6767. doi: 10.1016/j.vaccine.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 30.Da-Cruz AM, Oliveira MP, Bertho AL, Mendes-Aguiar CO, Coutinho SG. T cells specific to Leishmania and other nonrelated microbial antigens can migrate to human leishmaniasis skin lesions. J Invest Dermatol. 2010;130:1329–1336. doi: 10.1038/jid.2009.428. [DOI] [PubMed] [Google Scholar]