Abstract
Leishmaniasis is an emerging disease in Thailand. Herein, we report on two human immunodeficiency virus (HIV)-infected patients with leishmaniasis who presented with overlapping manifestations between cutaneous and visceral leishmaniasis. Sequencing analysis of the internal transcribed spacer 1 (ITS1) of the ribosomal RNA gene showed that the species was identical to a new species recently described in Thailand. The detection of DNA of this Leishmania species in saliva may have important implications for transmission and epidemiological studies.
Introduction
Leishmaniasis is caused by a flagellate intracellular protozoan belonging to the genus Leishmania, which is transmitted to vertebrate hosts by a bite from infected female phlebotomine sand flies.1,2 The diverse clinical syndromes caused by infection with Leishmania parasites are characterized into the traditionally described clinical syndromes of visceral leishmaniasis (VL), mucosal leishmaniasis (ML) and cutaneous leishmaniasis (CL).3 Leishmaniasis is also recognized as an opportunistic infection in human immunodeficiency virus (HIV)-infected patients after reports from endemic areas, including various countries in South America, the Middle East, and India.4 Reports of imported or indigenous leishmaniasis in Thailand are scarce and even rarer in HIV-infected patients, with only one case report.5–9 Leishmaniasis is recently of increasing concern, because it is one of the potential opportunistic infections of the estimated 370,000 people living with HIV/acquired immunodeficiency syndrome (AIDS) in Thailand.10 Distinguishing leishmaniasis in tissue sections from the disseminated infections of Histoplasma capsulutum and Penicillium manefeii, the more common opportunistic infections in this patient group, can be difficult because of the disappearance of leishmanias-containing macrophages in the late stage of CL, unusual sites of parasitic presentation or multiple opportunistic infections besides VL among HIV patients, and escape of amastigotes from necrotic tissue.11–14
Because of its high sensitivity and specificity, polymerase chain reaction (PCR) has been used to detect Leishmania ribosomal RNA (rRNA) or DNA, including the new species of the leishmaniasis protozoa from Thailand, from clinical specimens, including tissue, blood, urine, feces, and nasal secretions, in several reports.7–9,15,16 Although previous reports have shown viable Leishmania sp. in saliva, reports of detection of Leishmania rRNA or DNA by PCR remain scanty.17–19 In this report, we add cases of two autochthonous HIV-infected patients with the new to Thailand Leishmania species; it was confirmed in both cases with conventional culture and rRNA detection from clinical specimens, including saliva.
Case Reports
Case 1.
A 46-year-old male Thai rubber planter from Songkhla, a province on the Pacific coast in southern Thailand, presented with progressive anemia for a few months. He had never traveled outside Thailand, and he had no history of blood transfusion, intravenous drug use, or needle sharing. He was diagnosed with HIV infection in 2003, and at the time that we first saw him, he was receiving boosted Lopinavir (LPV/rtv) and Lamivudine (3TC). An immunological profile showed CD4+ T-cell count of 175 cells/mm3, and a virological profile showed undetectable virus (< 40 copies/mL). The physical examination revealed marked pallor and petechiae without hepatosplenomegaly. A single painless, well-defined, and punched-out ulcer surrounded by erythematous plaque with serous oozing and granulation tissue on top (3 × 3 cm in size) was found at his left knee with left groin lymphadenopathy. During his admission, he developed an intracranial hemorrhage at the right parietal lobe and also, the right vitreous hemorrhage. His complete blood count revealed anemia (hematocrit decreased from 34% to 21%) and thrombocytopenia (platelet count of 20,000/μL). A bone marrow study revealed increasing megakaryocytic and erythroid stem cells. A specific organism was not identified, however, and fungal and mycobacterial cultures were negative. The man was diagnosed with Evans syndrome, an autoimmune disease in which an individual's antibodies attack their own red blood cells and platelets, and he was treated with intravenous immunoglobulin (IVIG) with 40 mg dexamethazone coadministered. A craniotomy was performed, and the blood clot was successfully removed. His clinical condition improved thereafter, and he was discharged with antiretroviral agents, cotrimoxazole for Pneumocystis jerovecii prophylaxis, and prednisolone at 60 mg/day.
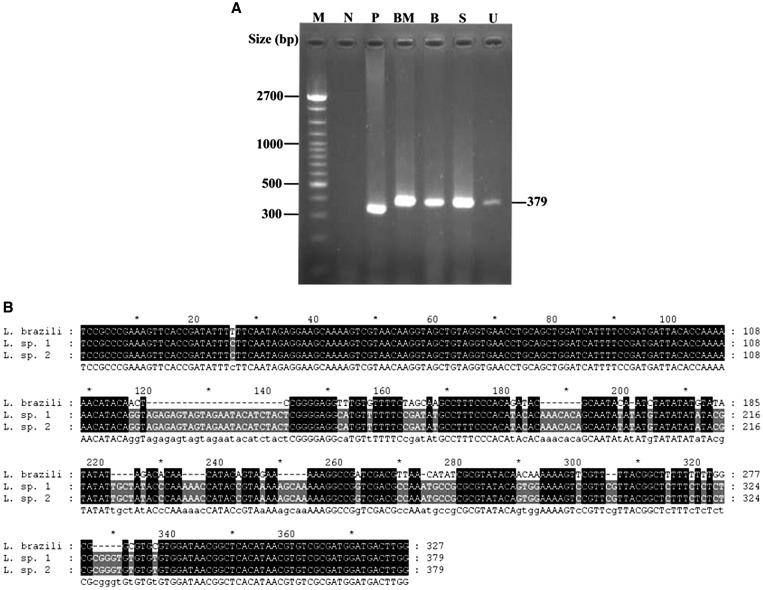
The patient returned in 4 weeks for a follow-up appointment, at which time the physical examination found that he was still markedly pale but without abnormal bleeding. An abdominal examination showed 16-cm-span liver enlargement and significant splenomegaly. The ulcer at his left knee and multiple left groin lymph nodes had not improved. He had no mucosal involvement, no cervical lymphadenopathy, and no parotid gland enlargement. His hematocrit was 23%, and the platelet count was 80,000/μL. A repeat bone marrow study showed amastigotes of Leishmania within macrophages and bar-shaped kinetoplasts (Figure 1A). The histology of the knee ulcer that had been performed at his prior admission was reviewed, and on close examination, amastigotes of Leishmania with kinetoplasts within the macrophage were identified. Bone marrow, discharge from the ulcer, and urine and oral fluid samples were sent to the Department of Parasitology, Chulalongkorn University. The specimens were cultured for Leishmania parasites in Schneider's medium, whereas the promastigotes were observed in only bone marrow culture (Figure 1B ). Leishmania species was identified using 18S rRNA gene primers, and the species of Leishmania was identified by nucleotide sequences of the amplified PCR products of the internal transcribed spacer 1 (ITS1) region of the rRNA gene. PCR was performed using a primer set described in the work by Spanakos and others.20 Leishmania DNA was detected in all samples (Figure 2A ). L. braziliensis DNA was used as a positive control; the PCR amplicon of L. braziliensis was 327 bp, whereas the amplicon of the novel species was 379 bp (Figure 2B). The sequencing was identical to the new species of Leishmania in specimens that were previously isolated in Thai patients and assessed in GenBank (accession nos. EF200011 and GQ226034).7,8 Nucleotide sequences of the 18S rRNA gene and the ITS1 region of the rRNA gene of this report were submitted to GenBank under accession no. JQ001751.
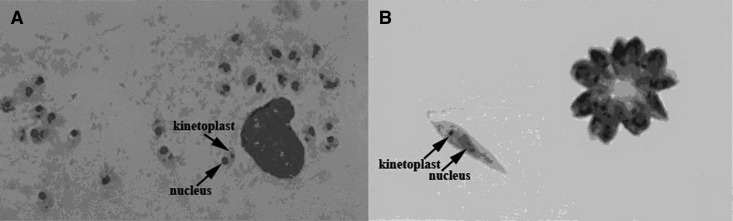
Figure 1.
(A) Bone marrow Giemsa staining. Amastigotes can be seen within a macrophage. The nucleus and kinetoplast can be observed within the amastigotes. (B) Promastigotes cultured in Schneider's medium Giemsa staining.
Figure 2.
(A) The PCR amplicons of patient 1 were analyzed on a 1.5% agarose gel electrophoresis and stained with ethidium bromide. Lane B = blood; lane BM = bone marrow; lane M = marker 100 bp; lane N = negative; lane P = positive from L. braziliensis; lane S = saliva; lane U = urine. (B) Comparison of the ITS1 gene sequences (L. brazili = L. braziliensis; L.sp 1 and L.sp 2 = Leishmania spp. sequences from cases 1 and 2, respectively).
The patient improved after 2 weeks of intravenous amphotericin B deoxycholate (1 mg/kg per day) followed with 400 mg itraconazole. The ulcerative lesion on his left knee improved, with only a hyperpigmented scar remaining. He suffered recurrence after 2 months of itraconazole and was retreated with 3 weeks of intravenous liposomal amphotericin B (3 mg/kg per day) followed with 400 mg itraconazole. There was no recurrence after 9 months follow-up.
Case 2.
A 30-year-old pet store owner from Trang, a province on the Indian Ocean coast of southern Thailand, had been suffering from generalized papules at the trunk and lower extremities for 4 years. He had never traveled outside Thailand. He denied any intravenous drug abuse. He had been diagnosed with HIV in 1999. His antiretroviral agents when we first saw him were Tenofovir (TDF), 3TC, and Nevirapine (NVP). The CD4+ T-cell count was 111 cells/mm3, and no virus was detectable (< 40 copies/mL). Medical records from his primary care hospital revealed multiple papules at his lower extremities and a single ulcerative lesion at the right leg. Pallor, hepatosplenomegaly, and lymphadenopathy were not detected. He was diagnosed with chronic dermatitis and received 0.1% triamcinolone acetonide cream and triamcinolone acetonide lotion for 1 year. One month before he was transferred to our institution, which is the primary referral hospital for southern Thailand, the patient developed anemia and abdominal distention. On admission, our physical examination found the patient markedly pale, with 14-cm-span liver enlargement and mild splenomegaly and generalized, painless, discrete, well-defined dusky red infiltrative papules and plaques with ulcers, some with serous oozing and some developing collarettes of scales on his torso, face and extremities, spare palms, and soles (Figure 3). A punched-out ulcer surrounded by erythematous plaque with purulent discharge and granulation tissue on top (2 × 2 cm in size) was identified on his right leg as well as bilateral small groin lymph nodes. His hematocrit was 21%, and the platelet count was 70,000/μL. Bone marrow, papule, and ulcer biopsies showed amastigotes of Leishmania within the macrophages and bar-shaped kinetoplasts. Bone marrow and oral fluid samples were sent to the Department of Parasitology, Chulalongkorn University. 18S rRNA of Leishmania was identified, and the sequencing of the ITS1 region of the rRNA gene was compatible with the new species of Leishmania. Comparison of the ITS1 sequences is shown in Figure 2B. Nucleotide sequences of the 18S rRNA gene and the ITS1 region of the rRNA gene of this report were submitted to GenBank under accession no. JQ001752.
Figure 3.
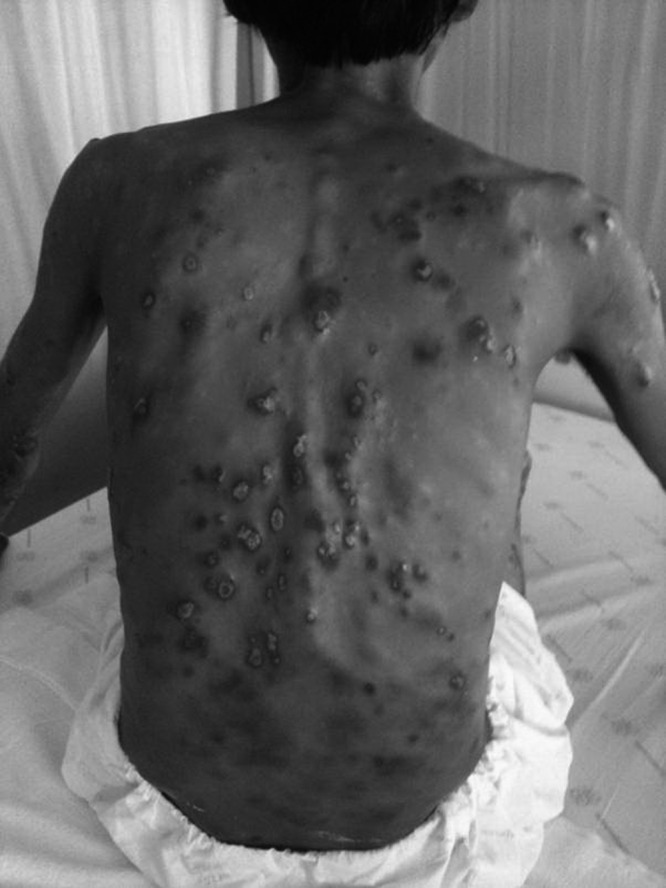
Multiple papular and ulcerative lesions on the back of patient 2.
The patient received intravenous amphotericin B deoxycholate (1 mg/kg per day) for 2 weeks followed with 400 mg itraconazole. There was no recurrence after 3 months follow-up.
Conclusions
To the best of our knowledge, there have been only two species of Leishmania identified in five autochthonous patients in Thailand.6–9 L. infantum was identified in the bone marrow of a non-HIV–infected patient with VL from Bangkok, the capital of Thailand, by PCR amplification of ITS1 of the small subunit (SSU)-rRNA gene and miniexon gene with sequencing analysis.9 The new species of Leishmania was also identified in the bone marrow of two patients with VL in two reports by Sukmee and others7 and Suankratay and others.8 The nucleotide sequences of the ITS1 region of the rRNA gene of our patients were identical to the new species that were previously submitted to GenBank under accession nos. EF200011 and GQ226034.7,8 This novel species closely resembles L. colombiensis, which is a sister taxon of the clade of L. brasiliensis and L. guyanensis based on the phylogenetic analysis of miniexon genes.7
It is interesting that our two patients had overlapping manifestations of both visceral and cutaneous leishmaniasis. Infections caused by a particular species of Leishmania contribute to different forms of clinical manifestation and also result in various styles of visceral or dermal manifestations in different patients.3 Few species are found in patients with both VL and CL, with sometimes overlapping manifestations.3,21 Among reported old world CL cases, L. tropica was reported to be the causative organism of visceral infections in Desert Storm veterans from the Middle East.21 L. infantum, the Mediterranean and Caspian sea region Leishmania species, and the variety previously reported in indigenous Thai patients have also been reported in patients with both cutaneous and visceral infections.22 L. colombiensis, the species with which our new species is closely clustered, contributes to both American tegumentary leishmaniasis (ATL) and American VL (AVL).23 The single ulcerative skin lesion in our patient 1 is common in new world CL, whereas the multiple papules and nodules with a few shallow ulcers in our patient 2 were similar to the manifestations found in both old world and new world CL.24 However, in Thailand, the differential diagnosis of such ulcerative lesions includes histoplasmosis and penicillosis, which are the more common opportunistic infections in HIV-infected patients.10
We also believe that the altered host immunity caused by the HIV contributed to the diverse disease spectrum of our patients. Patient 1 presented with a large ulcer at the lower extremity and unilateral groin lymphadenopathy and developed hepatosplenomegaly after only 4 weeks of administration of high-dose steroids. It is believed that even a short course of high-dose steroids can delay the type hypersensitivity, antigen-specific T-cell reactivity, and activating cytokine secretions that normally maintain asymptomatic or local cutaneous infection in such cases.25 Patient 2 presented with diffused CL for 4 years and developed visceral involvement after 1 year of topical steroid use. Specific data on the effects of topical steroids on the clinical course of leishmaniasis are sparse, but the inhibition of epidermal regeneration and parasitic clearance has been postulated.26
To our knowledge, this report is the first report identifying the DNA of this species of Leishmania in human oral secretions. Although it is common knowledge that the Leishman–Donovan bodies in an individual suffering from VL exist in many organs of the body and several reports from India and Africa have shown viable Leishmania in nasal, oral, and nasopharyngeal secretions and even feces, the data are limited concerning L. donovani.27–29 Among new world leishmaniasis, one report showed L. braziliensis DNA in the saliva of a patient with ATL without clinical visceral or mucosal infection.30 Tonsils and adenoids have been identified as sites suspected of containing macrophage-engulfed Leishmania.17,27
Although this study identified DNA of Leishmania in oral secretions, the transmission of the disease from person to person by way of the upper respiratory and alimentary tracts has to be further studied. We consider PCR as the useful adjunct investigation tool for microscopic and/or serologic examinations for identification of Leishmania. The major problem with smear is relatively lower sensitivity compared with in vitro culture or PCR, and it is difficult to distinguish amastigotes from yeast, bacterial, and cellular debris. Also, PCR is suitable for new species identification proceeding to sequencing analysis. Although serological examinations, even immunofluorescence assays or enzyme-linked immunosorbent assays, are limited because of potential cross-reactivity with another infection and not routinely available in Thailand because of the low incidence of leishmaniasis, the application of this laboratory test should be further evaluated in endemic areas.
In conclusion, this report has shown manifestations of the new species of Leishmania with visceral, cutaneous, and overlapping infections. We suspect that the variations were caused by host immune response modulations. Although this new species of Leishmania was detected in oral secretions, the potential for human-to-human transmission requires additional study.
ACKNOWLEDGMENTS
This work was partially supported by Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission Grant HR1160A-55. We thank Dr. Nattapa Preejawai and Mr. Dave Patterson for suggestions concerning revisions to this text.
Footnotes
Authors' addresses: Sarunyou Chusri, Thanaporn Hortiwakul, and Khachornsakdi Silpapojakul, Division of Infectious Disease, Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand, E-mails: sarunyouchusri@hotmail.com, hratri@medicine.psu.ac.th, and skhachor@medicine.psu.ac.th. Padet Siriyasatien, Department of Parasitology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, E-mail: padetcu@gmail.com.
References
- 1.Cupolillo E, Grimaldi G, Jr, Momen H. A general classification of New World Leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994;50:296–311. doi: 10.4269/ajtmh.1994.50.296. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Control of the Leishmaniasis. Technical Report Series 793. Geneva: WHO; 1990. pp. 21–25. [Google Scholar]
- 3.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 4.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viriyavejakul P, Viravan C, Riganti M, Punpoowong B. Imported cutaneous leishmaniasis in Thailand. Southeast Asian J Trop Med Public Health. 1997;28:558–562. [PubMed] [Google Scholar]
- 6.Thisyakorn U, Jongwutiwes S, Vanichsetakul P, Lertsapcharoen P. Visceral leishmaniasis: the first indigenous case report in Thailand. Trans R Soc Trop Med Hyg. 1999;93:23–24. doi: 10.1016/s0035-9203(99)90166-9. [DOI] [PubMed] [Google Scholar]
- 7.Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, Samung Y, Kongkaew W, Bumrungsana K, Chanachai K, Apiwathanasorn C, Rujirojindakul P, Wattanasri S, Ungchusak K, Leelayoova S. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38:617–622. doi: 10.1016/j.ijpara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Suankratay C, Suwanpimolkul G, Wilde H, Siriyasatien P. Case report: autochthonous visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient: the first in Thailand and review of the literature. Am J Trop Med Hyg. 2010;82:4–8. doi: 10.4269/ajtmh.2010.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maharom P, Siripattanapipong S, Mungthin M, Naaglor T, Sukkawee R, Pudkorn R, Wattana W, Wanachiwanawin D, Areechokchai D, Leelayoova S. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J Trop Med Public Health. 2008;39:988–990. [PubMed] [Google Scholar]
- 10.Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health, Thailand Annual reports of opportunistic infection in thai HIV infected-patient (Thai) 2010. http://www.boe.moph.go.th/files/report/20110401_19956923.pdf Available at: Accessed November 1, 2011.
- 11.Ridley DS, Ridley MJ. The evolution of the lesion in cutaneous leishmaniasis. J Pathol. 1983;141:83–96. doi: 10.1002/path.1711410109. [DOI] [PubMed] [Google Scholar]
- 12.Weigle KA, Davalos MD, Heredia P. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg. 1987;36:489–496. doi: 10.4269/ajtmh.1987.36.489. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht H, Sobottka I, Emminger C. Visceral leishmaniasis emerging as an important opportunistic infection in HIV-infected persons living in areas nonendemic for Leishmania donovani. Arch Pathol Lab Med. 1996;120:189–198. [PubMed] [Google Scholar]
- 14.Hofman V, Marty P, Perrin C. The histological spectrum of visceral leishmaniasis caused by Leishmania infantum MON-1 in acquired immune deficiency syndrome. Hum Pathol. 2000;31:75–84. doi: 10.1016/s0046-8177(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 15.Mimori T, Sasaki J, Nakata M, Gomez EA, Uezato H, Nonaka S. Rapid identification of Leishmania species from formalin-fixed biopsy samples by polymorphism-specific polymerase chain reaction. Gene. 1998;210:179–186. doi: 10.1016/s0378-1119(97)00663-x. [DOI] [PubMed] [Google Scholar]
- 16.Pirmez C, da Silva Trajano V, Paes-Oliveira Neto MP, da-Cruz AM, Gonçalves-da-Costa SC, Catanho M. Use of PCR in diagnosis of human American tegumentary leishmaniasis in Rio de Janeiro, Brazil. J Clin Microbiol. 1999;37:1819–1823. doi: 10.1128/jcm.37.6.1819-1823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forkner CE, Zia LS. Viable Leishmania donovani in nasal and oral secretions of patients with kala-azar and the bearing of this finding on the transmission of the disease. J Exp Med. 1934;59:491–499. doi: 10.1084/jem.59.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero GA, Sampaio RN, Macedo V de O, Marsden PD. Sensitivity of a vacuum aspiratory culture technique for diagnosis of localized cutaneous leishmaniasis in an endemic area of Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz. 1999;94:505–508. doi: 10.1590/s0074-02761999000400014. [DOI] [PubMed] [Google Scholar]
- 19.Galaï Y, Chabchoub N, Ben-Abid M, Ben-Abda I, Ben-Alaya-Bouafif N, Amri F, Aoun K, Bouratbine A. Diagnosis of Mediterranean visceral leishmaniasis by detection of Leishmania antibodies and Leishmania DNA in oral fluid samples collected using an Oracol device. J Clin Microbiol. 2011;49:3150–3153. doi: 10.1128/JCM.00267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spanakos G, Piperaki ET, Menounos PG, Tegos N, Flemetakis A, Vakalis NC. Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans R Soc Trop Med Hyg. 2008;102:46–53. doi: 10.1016/j.trstmh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Magill AJ, Grogl M, Johnson SC, Gasser RA., Jr Visceral infection due to Leishmania tropica in a veteran of operation Desert Storm who presented 2 years after leaving Saudi Arabia. Clin Infect Dis. 1994;19:805–806. doi: 10.1093/clinids/19.4.805. [DOI] [PubMed] [Google Scholar]
- 22.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Bonfante C, Bonfante-Garrido R, Grimaldi G, Jr, Momen H, Cupolillo E. Genotypically distinct Leishmania colombiensis isolates from Venezuela cause both cutaneous and visceral leishmaniasis in humans. Infect Genet Evol. 2003;3:119–124. doi: 10.1016/s1567-1348(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 24.Dowlati Y. Cutaneous leishmaniasis: clinical aspects. Clin Dermatol. 1996;14:425–431. doi: 10.1016/0738-081x(96)00058-2. [DOI] [PubMed] [Google Scholar]
- 25.Weigle K, Saravia NG. Natural history, clinical evaluation, and the host-parasite interaction in New World cutaneous leishmaniasis. Clin Dermatol. 1996;14:433–450. doi: 10.1016/0738-081x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- 26.Marovich M, Lira R, Shepard M, Fuchs G, Kruetzer R, Nutmann T, Neva F. Leishmaniasis recidivans recurrence after 43 years: a clinical and immunologic report after successful treatment. Clin Infect Dis. 2001;33:1076–1079. doi: 10.1086/322643. [DOI] [PubMed] [Google Scholar]
- 27.Mebrahtu YB, Hendricks LD, Oster CN, Lawyer PG, Perkins PV, Pamba H. Leishmania donovani parasites in the nasal secretions, tonsillopharyngeal mucosa, and urine centrifugates of visceral leishmaniasis patients in Kenya. Am J Trop Med Hyg. 1993;48:530–535. doi: 10.4269/ajtmh.1993.48.530. [DOI] [PubMed] [Google Scholar]
- 28.Shorft HE, Smith ROA, D'Silva HAH, Swaminath CS. Leishmania donovani in human faeces in Indian kala-azar. Indian J Med Res. 1923;17:644–646. [Google Scholar]
- 29.Shortt HE, Swaminath CS. The viability of Leishmania donovani excreted in the nasal mucus in Indian kala-azar. Indian J Med Res. 1937;25:341–343. [Google Scholar]
- 30.Corvalan FH, Sampaio RNR, Brustoloni YM, Andreotti R, Lima MSC., Jr DNA identification of Leishmania (Viannia) braziliensis in human saliva from a patient with American cutaneous leishmaniasis. J Venom Anim Toxins Trop Dis. 2011;17:98–102. [Google Scholar]