Abstract
The glycosylphosphatidylinositol (GPI)-anchored mucins of Trypanosoma cruzi trypomastigotes play an important immunomodulatory role during the course of Chagas disease. Here, some biological activities of tGPI-mucins from four T. cruzi isolates, including benznidazole-susceptible (BZS-Y), benznidazole-resistant (BZR-Y), CL, and Colombiana, were evaluated. GPI-mucins were able to differentially trigger the production of interleukin-12 and nitric oxide in BALB/c macrophages and modulate LLC-MK2 cell invasion. The significance of these variations was assessed after analysis of the terminal α-galactosyl residues. Enzymatic treatment with α-galactosidase indicated a differential expression of O-linked α-galactosyl residues among the strains, with higher expression of this sugar in BZS-Y and BZR-Y T. cruzi populations followed by Colombiana and CL. Unweighted pair group method analysis of the carbohydrate anchor profile and biological parameters allowed the clustering of two groups. One group includes Y and CL strains (T. cruzi II and VI), and the other group is represented by Colombiana strain (T. cruzi I).
Introduction
Chagas disease, a neglected illness caused by Trypanosoma cruzi, affects ∼15 million people in 18 countries in Latin America. It is transmitted by hematophagous triatomine bugs that are widely distributed from southern regions of United States to northern parts of Argentina. Also, congenital, transfusional, and oral transmissions occur.1 Its clinical manifestations initiate with a short acute phase characterized by high parasitemia and various symptoms such as myocarditis. After the development of an acquired immune response during the chronic phase, the parasite numbers decrease in the bloodstream and tissues, remaining very low for most Chagasic patients. During this phase, some symptomatic forms appear in the heart and digestive organs.2 Chagas disease chemotherapy is limited to two drugs, nitrofuran nifurtimox (NFX) and 2-nitroimidazole benznidazole (BZ). Both drugs present low cure rates in chronic patients and considerable side effects.3,4 Differences in the susceptibility to BZ and NFX among T. cruzi strains and the genetic background of the host may explain variations in therapeutic success.5–7
During early stages of infection, the pattern of susceptibility/resistance may be determined before acquired immunity, where innate immune mechanisms are crucial for parasite control.8 T. cruzi employs a highly elaborated array of molecules and strategies to invade a wide range of host cells and escape from host's immune defense mechanisms.9 In the site of infection, T. cruzi triggers the production of chemokines, proinflammatory cytokines (interleukin-12 [IL-12] and tumor necrosis factor-α [TNF-α]), and reactive oxygen (ROI) and nitrogen (RNI) intermediates by cells from the macrophage lineage.10 Glycosylphosphatidylinositol (GPI) anchors expressed in the surface of T. cruzi such as the GPI-mucins and the glycoinositolphospholipids (GIPLs) are determinant in this process.8 The GPI-mucins of T. cruzi are composed of two subfamilies (TcMUC and TcSMUG), with a total of 863 gene members clustered with other multigene surface protein families.11 The major subfamily of TcMUC (i.e., TcMUC II [844 gene members]) is mainly expressed in the mammalian trypomastigote stage.12,13 It consists of a highly antigenic coat, with variations that account for interstrain features, such as virulence and immunomodulatory properties.14 Early studies showed that T. cruzi glycoconjugates are involved in attachment/invasion of hosts cells,9 escape from host immunity, and induction of protective lytic antibodies.14,15 Most of the studies focused in the host innate immune response used the GPI-mucins from the Y strain of T. cruzi.10 In contrast to epimastigote- or metacyclic-derived GPI-mucins (eGPI- and mGPI-mucins, respectively), GPI-mucins and their GPI-moieties alone have a potent proinflammatory activity in murine and human macrophages. This activity is probably caused by the presence of an unsaturated fatty acid (C18:1 or C18:2) in the phosphatidylinositol (PI) moiety16 (Figure 1). Finally, a heterodimer composed by Toll-like receptor 2 (TLR2) and TLR6 is involved in the recognition of T. cruzi tGPI-mucins.17,18
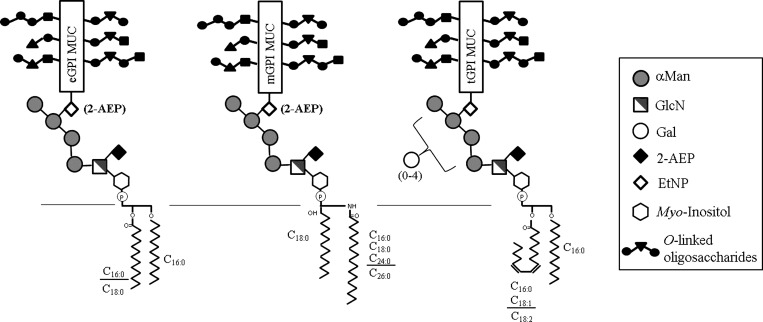
Figure 1.
Schematic representation of T. cruzi GPI-mucins from different lifecycle stages.14 Only the major GPI-mucins species are shown. All mucin GPI-anchors are composed of the same linear glycan core Manα1-2Manα1-2Manα1-6Manα1-4GlcN; however, only the core from cell-derived trypomastigotes can be modified by a branch of galactose residues, and the location is not known. The GPI-mucin lipid anchor varies according to stage. In epimastigotes, they are composed of unsaturated fatty acids (C18:1 and C18:2); in metacyclic trypomastigotes, they are predominantly inositol-phosphoceramides, and in the cell-derived trypomastigotes, they are composed exclusively of alkylacyl-PI structures. The O-linked oligosaccharides size and composition may vary depending on the strain (details in Discussion). α-Man = α-mannose; eGPI MUC = GPI-mucins from epimastigotes; EtNP/2-AEP = ethanolamine phosphate and aminoethylphosphonic acid; Gal = galactose; GlcNAc = N-acetylglucosamine; mGPI MUC, GPI-mucins from metacyclic trypomastigotes; tGPI MUC, GPI-mucins from cell-derived trypomastigotes. The text has other structural details.
Biochemically, the GPI moiety of T. cruzi mucins has the conserved core of Manα1-2Manα1-2Manα1-6Manα1-4GlcNAcα1-6myo-inositol-1-HPO4, which can be extended by phosphorylated substituents, including ethanolaminephosphate (EtNP), 2-aminoethylphosphonate (2-AEP), and extra carbohydrate residues. The EtNP or 2-AEP group serves as the point of attachment for the surface mucin glycoprotein moiety (Figure 1). The hydrophobic tail linked to the myo-inositol phosphate consists of an alkylacylglycerol or ceramide lipid.10 The GPI-mucins of T. cruzi are heavily O-glycosylated with glycan chains containing N-acetylglucosamine (GlcNAc) and β-galactose (β-Gal) residues, which are acceptors for sialic acid (Neu5Ac) units transferred by a trans-sialidase (TS) reaction.19,20 One of the features of tGPI-mucins that distinguishes them from the other parasite stages is the presence of terminal α-galactopyranosyl (α-Gal) residues in O-glycans attached to polypeptide core. These epitopes are highly immunogenic and elicit anti–α-galactosyl antibodies (anti–α-Gal). These antibodies have protective lytic activity against trypomastigotes and are determinant in both acute and chronic phases of Chagas disease.15,21 The O-linked sugar structures in the GPI-mucins have already been fully characterized in epimastigotes and metacyclic stages of Colombiana, Y, and CL22–25 but only partially defined in trypomastigotes of the Y strain.15 Currently, after a consensus, the molecular studies enabled the classification of T. cruzi strains into six (TcI–TcVI) discrete typing units (DTUs).26 The biological activities of GPI-mucins from different DTUs are still mostly unknown. In our study, the strains analyzed (Colombiana, Y, and CL) belong to T. cruzi DTUs I, II, and VI, respectively. Here, those features were explored in four T. cruzi strains/isolates (BZ-resistant population [BZR-Y], BZ-susceptible Y strain [BZS-Y], CL, and Colombiana) during interaction with macrophages and LLC-MK2 cells. Moreover, intraspecies variation in the terminal α-galactosyl residues in the O-linked glycans was assessed.
Materials And Methods
T. cruzi strains and mammalian cells.
The four populations/strains of T. cruzi used in this study are listed in Table 1 and were not cloned. BZR-Y was derived from the BZS-Y after in vivo selection after 25 successive passages in mice treated with a single high dose of BZ (500 mg/kg).6 CL and Colombiana strains were susceptible and naturally resistant to BZ, respectively.5 All T. cruzi strains had been classified previously as belonging to T. cruzi DTUs I, II, and VI.26 Epimastigote forms were maintained at 28°C in liver infusion tryptose (LIT) medium supplemented with 10% fetal bovine serum (FBS; Cultilab, Campinas, SP, Brazil).27 Mammalian tissue culture-derived trypomastigotes (TCTs) from the four T. cruzi samples were obtained after infection of green monkey (Rhesus) kidney LLC-MK2 epithelial cells obtained from America Type Culture Collection (Manassas, VA).28 Parasite cultures and cells were tested for Mycoplasma as previously described.29
Table 1.
T. cruzi strains/populations analyzed in this study
Extraction and purification of GPI-mucins.
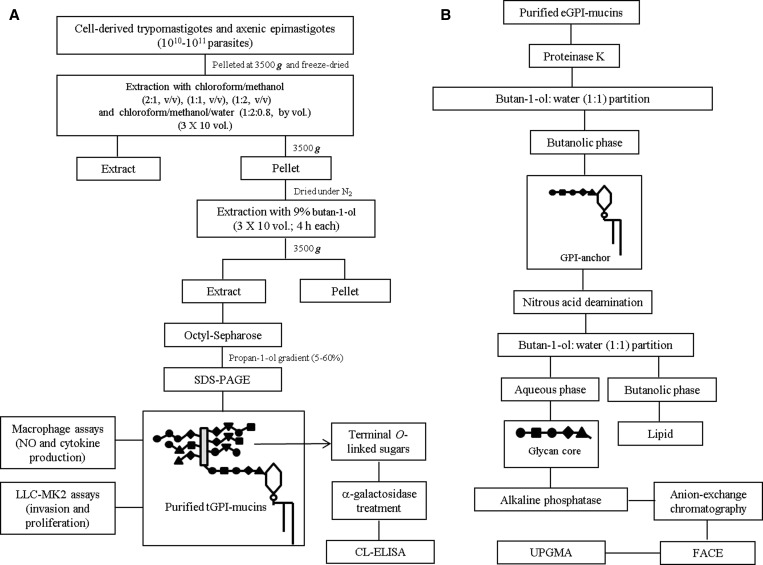
T. cruzi GPI-mucins from trypomastigote and epimastigote forms were purified as described (Figure 2A).15 Purified GPI-mucins were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining.15 Protein concentration was determined at 214 nm using bovine serum albumin (BSA; Sigma, St. Louis, MO) as standard.
Figure 2.
Procedures for the extraction, purification, and characterization of T. cruzi GPI-mucins. (A) Parasite cell pellets were lyophilized and subject to extraction with organic solvents. The resulting pellet was subjected to 9% butan-1-ol extraction. The extract was dried and resuspended in 0.1 M ammoniun acetate in 5% propan-1-ol. The material was applied onto an octyl-Sepharose column followed by a 0.1 M ammonium acetate in propan-1-ol gradient (5–60%). Fractions were resolved in SDS-PAGE, and GPI-mucins containing fractions were pooled. (B) Chemical and enzymatic treatments. eGPI-mucins were digested with Proteinase K (10 mg/mL, 16 hours), and the O-linked-sugars and GPI-anchors were separated after butal-1-ol:water (1:1) partition. After butan-1-ol:water partition, GPI-anchors were treated with alkaline phosphatase (15 mM Tris buffer, pH 9.0, 1 unit, 16 hours, 37°C). The sample was desalted, and GPI-anchor oligosaccharides were fluorescently labeled (0.05 N 8-aminonaphthalene-1,3,6-trisulfate and 1 M cyanoborohydride, 37°C, 16 hours). The oligosaccharides were subjected to FACE and visualized by a UV imager.
Purification of murine peritoneal macrophages and cytokine and nitrite measurements.
The animals were kept in the Animal Facility of the Centro de Pesquisas René Rachou/FIOCRUZ in strict accordance to the Guide for the Care and Use of Experimental Animals.30 All animals and experiments were approved by the Ethical Committee of Animal Handling (CEUA) from Oswaldo Cruz Foundation (FIOCRUZ protocol P-0289/06). Thioglycollate-elicited peritoneal macrophages were removed from 8- to 11-week-old female BALB/c mouse by peritoneal washing and enriched by plastic adherence. Cells (5 × 106 cells/mL) were kept in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (37°C, 5% CO2; Cultilab, Campinas, Brazil).31 Peritoneal macrophages were primed with 50 U/mL recombinant interferon-γ (IFN-γ; 18 hours, 37°C; B&D Biosciences, San Jose, CA) and subsequently incubated with 1 and 10 μg/mL tGPI-mucins from BZS-Y, BZR-Y, CL, and Colombiana strains. Supernatants were collected after 48 hours, and IL-12 (subunit p40) cytokine measurement was performed by sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions using the Opteia kit (B&D Biosciences). Nitric oxide (NO) production was measured indirectly by Griess reaction.32 All experiments were performed in triplicate. Negative controls were incubated with medium with IFN-γ (50 U/mL) (BD Biosciences, San Jose, CA) only.
Infection of LLC-MK2 epithelial Cells.
LLC-MK2 cells were grown in a 24-well dish and washed two times with Dulbecco's Modified Eagles Medium (DMEM; Invitrogen, Carlsbad, CA). Cells were incubated with 1 μg/mL tGPI-mucins from BZS-Y, BZR-Y, CL, and Colombiana strains in DMEM supplemented with 10% FBS (Cultilab, Campinas, CA) for 24 hours. Negative controls were incubated with DMEM medium only. After incubation, these cells were infected with TCT from the BZS-Y population with a multiplicity of infection (MOI) of 10 for 1 hour at 37°C, and then, they were washed two times with DMEM without serum. Non-adherent parasites were removed by the addition of Lymphoprep (Axis-Shield, Norton, MA) to the cell layers followed by two washes with DMEM without serum. Cells were incubated with DMEM with 10% FBS (24 hours, 37°C), fixed with 4% methanol and acetone and stained with Hoescht fluorescent dye (Molecular Probes; Invitrogen, Carlsbad, CA). All experiments were performed in triplicate, and 10 photos of each replicate were made using a digital video-imaging, fluorescent-inverted microscope (Nikon Inc., Melville, NY), enabling the counting of infected and non-infected cells.
Chemiluminescence immunoassay and specificity.
To ascertain the level of α-galactosylation in the O-linked sugars in the protein motif (Figure 2A), tGPI-mucins were subjected to chemiluminescent CL-ELISA against sera containing α-galactosyl antibodies purified from Chagasic patients.33 Briefly, 96-well ELISA microplates (NUNC; Thermo Scientific, Rochester, NY) were sensitized overnight at 4°C with tGPI-mucins (5 μg/mL) from all strains in 100 mM carbonate-bicarbonate buffer (pH 9.6). To avoid nonspecific binding in the assay, free-wells sites were blocked for 2 hours at 37°C with 1% BSA in phosphate buffered saline (PBS/BSA). Two-fold serial dilutions of primary purified anti–α-Gal antibody, obtained as described,20 were made in wash buffer (1×PBS 0.05% Tween 20, pH 7.4), added to antigen-coated wells, and incubated for 1 hour at 37°C. Wells were washed three times, and plates were incubated (1 hour, 37°C) with secondary donkey anti-goat immunoglobulin G (IgG)-biotin conjugate (1:2,000; eBioscience, San Diego, CA) in wash buffer. Wells were washed five times, and streptavidin-peroxidase (1:2,000; Zymed, San Francisco, CA) was added for 1 hour at 37°C. In parallel, for the anti–α-Gal antibody specificity, tGPI-mucins were treated overnight at 28°C with 0.1 U/well coffee beans α-galactosidase (Sigma, St. Louis, MO) before antibody incubations. The reaction was developed using luminol (ECL, Thermo Scientific, Pierce Protein Research Products, Rockford, IL) diluted in 100 mM carbonate-bicarbonate buffer (pH 9.6). The intensity of light emission was expressed in relative luminescent units (RLUs) using a Luminoskan luminometer (eBiosystems). Negative controls were incubated with secondary antibodies only.
Statistical analysis.
For the biological studies, the following statistical analysis was performed. The Kolmogorov–Smirnov test was made to test the null hypothesis that the data were sampled from a Gaussian distribution (P < 0.01). In this case, parametric Student t test and analysis of variance (ANOVA) were performed to test equality of population medians among groups and independent samples. When samples deviated from Gaussian distribution, a non-parametric Kruskal–Wallis test was performed to compare two independent samples. Data were analyzed by GraphPad Prism 4.0 software, and P < 0.05 was considered significant.
Purification of GPI-anchors from epimastigotes.
The biological experiments described above used purified GPI-mucins from trypomastigotes (tGPIs). The unweighted pair group method analysis (UPGMA) analysis of the carbohydrate profile was based only in the epimastigote anchors (eGPIs) for comparison with reported DTUs.26 eGPI-mucins were subjected to Proteinase K treatment (5 mg/mL; Qiagen Inc., Valencia, CA) in 10 mM Tris·HCl, pH 7.8 (18 hours, 37°C) (Figure 2B). The material was heated (100°C, 5 minutes) to deactivate enzyme and subjected to butanol:water partition (2:1). The remaining GPI-anchors in the butanolic phase were subjected to nitrous acid deamination (300 μL 0.25 M sodium acetate and 300 μL 0.5 M NaNO2) for 16 hours at 37°C to release the lipid. The suspension was then dried and subjected again to butanol:water partition, and polysaccharides were recovered in the aqueous phase. The oligosaccharides were desalted by passage through a two-layered column of AG50W-X12 over AG1-X8 (acetate; Bio-Rad, Hercules, CA). Anchor oligosaccharides were fluorescently labeled with 0.05 N 8-aminonaphthalene-1,3,6-trisulfate (ANTS; Sigma) and 1 M cyanoborohydride (16 hours, 37°C; Sigma) and subjected to fluorophore-assisted carbohydrate electrophoresis (FACE). Samples were run in polyacrylamide gels and visualized by an ultraviolet (UV) imager.34
Unweighted pair group method analysis.
All strains/populations used in this work were previously characterized as T. cruzi I, II, or VI (Table 1).26 To evaluate if such discrimination would be observed in this work, a taxon/character matrix was constructed based on the presence/absence of bands from FACE analysis and biological techniques performed.35 The relationship among the strains was determined after a phenogram construction using the Dice similarity coefficient36 from the NTSYSpc software package (version 2.02).
Results
Purification of GPI-mucins.
T. cruzi GPI-mucins from either epimastigotes or trypomastigotes were extracted and purified from different strains/populations (Table 1). Fractions eluted from Octyl-Sepharose were resolved by SDS-PAGE and silver-stained. All GPI-mucins were eluted between 30% and 50% propan-1-ol gradient in 0.1 M ammonium acetate as previously described15 (data not shown). The GPI-mucins containing fractions were pooled, dried, and resuspended in sterile and lipopolysaccharide-free water before biological and immunological assays. Due to amount limitation, only GPI-mucins from epimastigotes (eGPIs) were used for UPGMA analysis (described later).
Cytokine and nitrite levels on supernatants of stimulated macrophages.
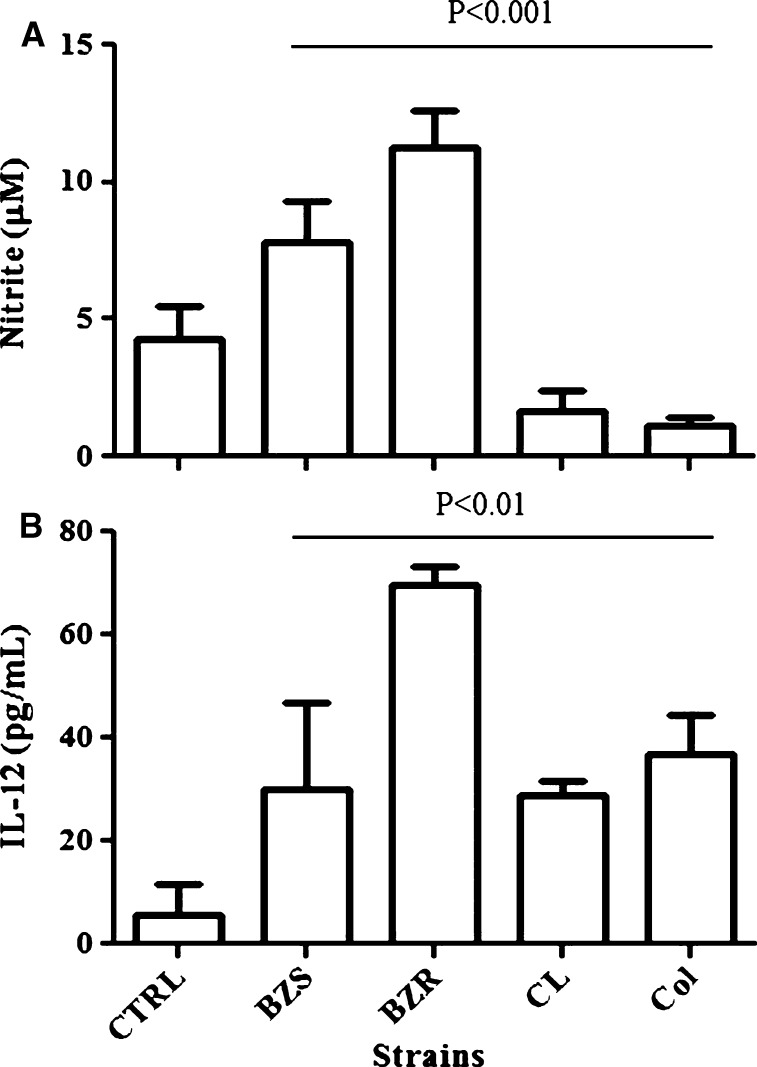
Previous studies16,31,37 have shown the production of NO and proinflammatory cytokines by macrophages after exposure with either parasites or tGPI-mucins of T. cruzi (Y strain). In our work, we extended the number of the strains, including an in vivo-induced BZR-Y, its susceptible counterpart BZS-Y, and Colombiana and CL strains. There were considerable differences in the NO and IL-12 production after exposure to tGPI-mucins from all isolates in the concentration of 1 μg/mL (P < 0.001 and P < 0.01, respectively). A higher production of NO and IL-12 was observed for the BZR-Y population compared with the other T. cruzi samples (Figure 3A and B). In the concentration of 10 μg/mL, those differences were abolished (P > 0.05; data not shown).
Figure 3.
NO and IL-12 production by Balb/c macrophages stimulated with GPI-mucins (1 μg/mL) obtained from trypomastigotes from different strains in the presence of IFN-γ. Negative controls (CTRLs) were incubated with IFN-γ only. The levels of (A) NO and (B) IL-12 were measured 48 hours post-stimulation by Griess reaction and ELISA, respectively. Values are means for two experiments in triplicate. Bars represent standard errors. BZR = benznidazole-resistant population from Y strain; BZS = benznidazole-susceptible population from Y strain; CL = CL strain; Col = Colombiana strain.
LLC-MK2 invasion after tGPI-mucins exposure.
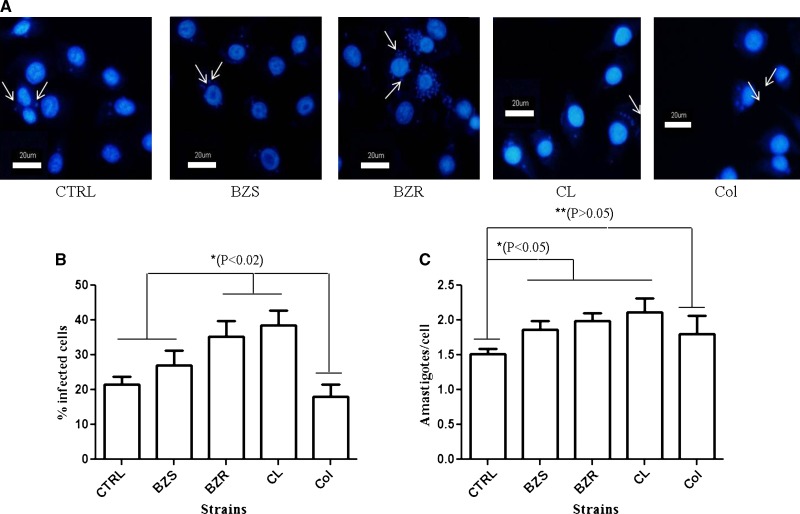
T. cruzi trypomastigotes are able to release extracellular vesicles containing Tc85, trans-sialidase (TS), and tGPI-mucins that increase parasite invasion and cardiac tissue lesions in mice.38 To evaluate the ability of purified tGPI-mucins in modulating invasion of LLC-MK2 cells by trypomastigotes, the cells were pre-incubated with the glycoconjugates (1 μg/mL) from each strain/populations for 24 hours. Trypomastigotes were able to invade and multiply inside LLC-MK2 cells (Figure 4A). After acquisition of pictures and evaluation of 10 fields per experiment, it was observed that the cells pre-incubated with tGPI-mucins of BZR-Y and CL had a higher infection rate than those cells pre-treated with tGPI-mucins from BZS-Y, Colombiana, and control (P < 0.02, t test) (Figure 4B). Moreover, a slight increase in the number of intracellular amastigotes (∼0.5-fold) was noted for the strains BZS-Y, BZR-Y, and CL compared with control (P < 0.05, t test) (Figure 4C).
Figure 4.
Cell invasion by T. cruzi trypomastigotes (Y strain) previously incubated with GPI-mucins from trypomastigotes (tGPI-mucins). Negative controls (CTRLs) were incubated with DMEM medium only. (A) Intracellular amastigotes are indicated by arrows. (B) Percentage of LLC-MK2–infected cells and (C) number of intracellular amastigotes of T. cruzi previously incubated with tGPI-mucins. Values are means for two experiments in triplicate. Bars represent standard errors. Definitions of abbreviations are in Figure 3.
Reactivity with anti–α-Gal antibodies from chronic Chagasic patients.
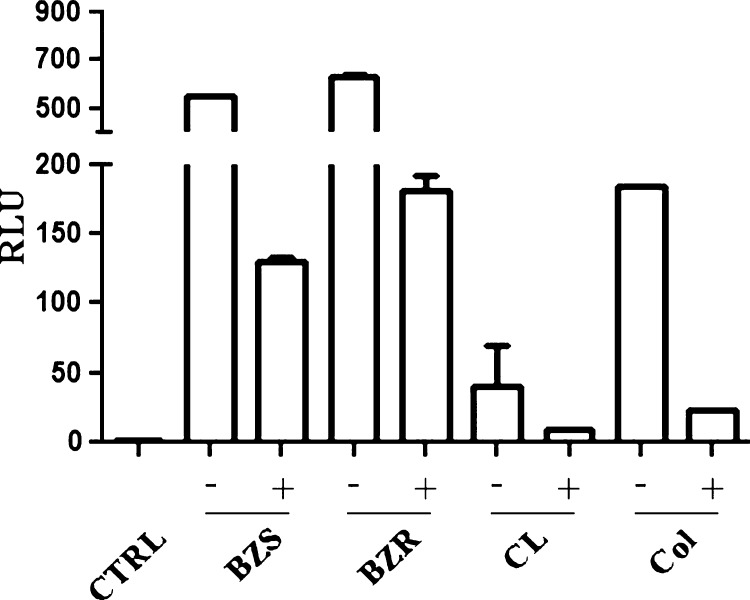
As observed above, tGPI-mucins from BZR-Y, BZS-Y, Colombiana, and CL strains had differential biological and immunological activities. Next, we evaluated whether these tGPI-mucins could also be differentially recognized by Chagasic anti–α-Gal antibodies. For this evaluation, tGPI-mucins were immobilized in CL-ELISA microplate and subjected or not to α-galactosidase treatment to remove terminal α-Gal residues and then reacted with Chagasic anti–α-Gal antibodies (Figure 5). Higher reactivity was observed for BZS-Y and BZR-Y followed by Colombiana and CL (P < 0.0001, ANOVA). A significant decrease (above 70%) in anti–α-Gal reactivity was noticed after enzymatic treatment in all strains. These data are consistent with the presence of not only variable amounts of terminal α-Gal residues but also other changes in the structure of this epitope that may affect antibody binding.
Figure 5.
Reactivity of tGPI-mucins from different T. cruzi strains treated/untreated with α-galactosidase and incubated with purified α-galactosyl antibodies from Chagasic patients. Negative controls (CTRLs) used secondary antibodies only. BZR = benznidazole-resistant population from Y strain; BZS = benznidazole-susceptible population from Y strain; CL = CL strain; Col = Colombiana strain; RLU = relative luminescent unit; − = untreated tGPI-mucins; + = tGPI-mucins treated with α-galactosidase.
Analysis of the glycan core moiety.
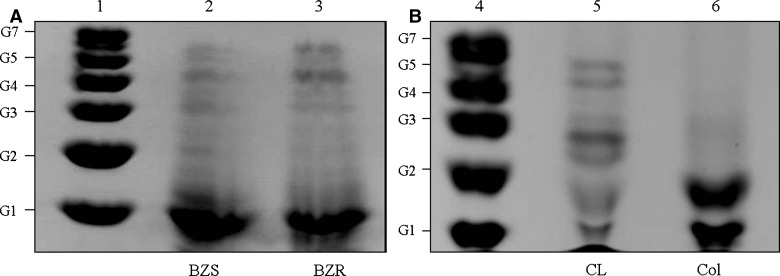
Epimastigotes and trypomastigotes have a similar a linear glycan core of Manα(1-2)Manα(1-2)Manα(1-6)Manα(1-4)GlcNα(1-6)Ins-1-phosphate. Although they share the same glycan core, the latter may exhibit zero to four galactoses (Figure 1).16 For this reason, the preliminary analysis of carbohydrate anchors was based only in GPI-mucins derived from epimastigotes. To evaluate if carbohydrate variations could reflect previous described DTUs,26 GPI-mucins from epimastigotes were subjected to proteinase K treatment, and anchors were purified (Figure 2B). Those anchors were labeled and subjected to FACE (Figure 6A and B). It was observed that there were longer carbohydrate oligosaccharides for BZS and BZR (lanes 2 and 3) that reached up to six sugars (Figure 6A). CL strain (lane 5) had approximately four to five sugars, and strain Colombiana (lane 6) very low glucosylated, with up to three sugars in its glycan core (Figure 6B).
Figure 6.
FACE of GPI-anchor oligosaccharides from different T. cruzi strains. (A) Lane 1 = oligoglucose ladder represented by G1–G7; lane 2 = BZS strain; lane 3 = BZR strain. (B) Lane 4 = oligoglucose ladder represented by G1–G7; lane 5 = CL strain; lane 6 = Colombiana strain.
Unweighted pair group method analysis.
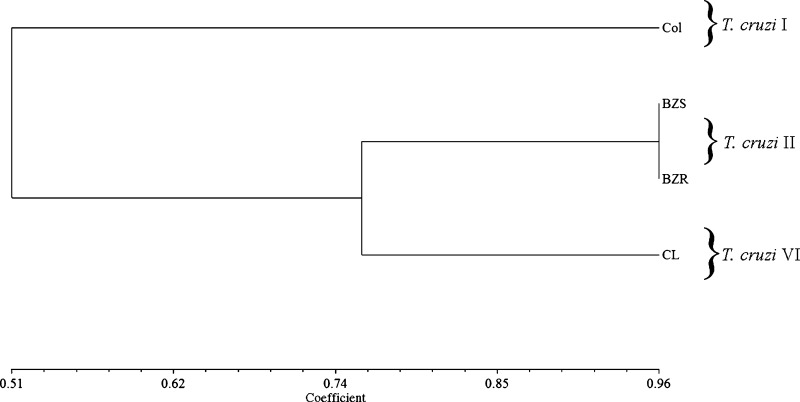
The previous results showed variations among the strains with no expected pattern. In some experiments, CL strain, for example, was similar to Colombiana (Figures 3, 4C, and 5) or Y (Figures 3B, 4B, and 6). To better describe the relationships among the strains and evaluate their position compared with molecular designations,26 a UPGMA analysis was performed (Figure 7). Based on the taxon/character similarity matrix constructed on the presence/absence of a given character, the strains fell into two main clusters. BZS and BZR populations (T. cruzi II) were grouped together with CL (T. cruzi VI) with a similarity coefficient index of ∼76%. Colombiana strain (T. cruzi I) was completely discriminated from the others with a similarity coefficient index of ∼51%.
Figure 7.
UPGMA of T. cruzi strains based on presence/absence of characters from all biological and biochemical parameters studied. The similarity coefficient (horizontal scale) was derived from the Dice index.36 On the right, the respective DTUs (T. cruzi I–VI) are indicated as previously reported.26 Definitions of abbreviations are in Figure 3.
Discussion
T. cruzi belongs to a heterogeneous species consisting of a pool of strains, stocks, or isolates circulating between the wild and domestic environments through different vertebrate and invertebrate hosts.2 Recently, it has been proposed that T. cruzi has six DTUs (T. cruzi I–VI).26 T. cruzi I strains are known to induce low parasitism in human Chagasic patients, whereas T. cruzi II strains seem to be responsible for tissue lesions and high parasitemia.39 T. cruzi VI comprises a group of strains that share common features with hybrid strains. For example, the CL Brener clone, with a genome that was sequenced, is considered a hybrid.11 In this work, we reported some biological roles in GPI-mucins exhibiting intraspecies polymorphism from the strains Colombiana, Y (BZS-Y and BZR-Y), and CL belonging to T. cruzi groups I, II, and VI, respectively.
Glycoconjugates are not translated directly from the genome, being secondary genomic products. They are synthesized in the lumen of the Golgi and endoplasmic reticulum because of the action of membrane transporters and glycosyltransferases.40 In trypanosomatids, some of those components have been reported for Leishmania,41,42 T. rangeli,43 and T. cruzi.44,45 The intra- and interspecies variations in glycoconjugate assembly may originate from innumerous combinatory sequences dependent on the action and specificity of glycosidases and glycosyltransferases. Consequently, this variation allows carbohydrates to be important sources of biological diversity. Such variability is likely to have implications in antigenicity, pathogenesis, and virulence among different strains.46 Despite this finding, a number of parasite and host cell molecules are known to be involved in this process. Those molecules include proteins, glycoproteins, and glycoconjugates that are crucial for parasite recognition and invasion,9,47 which may include the Gp85/trans-sialidase (Gp85/TS) and the mucin-like superfamilies located on the parasite surface.14,20,48–50 The GPI-mucins of T. cruzi, also known as sialoglycoproteins, are mucin-like molecules that are highly glycosylated and have a conserved GPI-anchor linked to the parasite surface.14 The O-linked oligosaccharides account for the polymorphisms among T. cruzi strains and are targets for lytic anti–α-Gal antibodies from chronic Chagasic patients.15,51,52 A number of publications described intraspecies polymorphisms in the O-glycosilations of GPI-mucins from different T. cruzi strains (G, Colombiana, CL-Brener, and Y).15,22–25,53 Extensive studies have reported on the biological activities of GPI-mucins from Y strain trypomastigotes, especially in the induction of NO and proinflammatory cytokines (IL-12 and TNF-α) by macrophages.16,31,37 Furthermore, the molecular mechanisms of this induction were reported to involve members of the nuclear factor κB (NF-κB) pathway, including TLR2,17,18 MyD88,54 extracellular signal-related (ERK) -1/ERK2 kinases, mitogen-activated protein kinase (MAPK), stress-activated protein kinase (SAPK-2/p38), and inhibitor of κB (IκB).55,56 However, an unknown aspect of T. cruzi glycobiology is how tGPI-mucins from different strains/DTUs would trigger different responses in murine macrophages. Here, a higher production of NO and IL-12 was observed for BZR-Y followed by BZS-Y, CL, and Colombiana. This result is consistent with the previous studies on the ability of tGPI-mucins from Y strain to trigger the production of proinflammatory cytokines and NO.16,31,37 Interestingly, this ability was lower for strains CL and Colombiana, showing that differential stimulation by tGPI-mucins may occur in different degrees in the innate immune system.
Because of the fact that mostly T. cruzi glycoconjugates are on the external surface of cells or secreted, they are able to modulate and mediate important cell–cell, cell–matrix, and cell–molecule interactions.14,51,52,57 Infections and immune responses suffer major interferences and depend on N- and O-linked carbohydrates during innate and adaptive responses.58 During its life cycle, T. cruzi trypomastigotes are able to release extracellular vesicles containing glycoproteins from the parasite surface. Those purified vesicles exhibited a proinflammatory pattern during host–parasite interaction determinant in the immunopathology and tissue lesions. The pre-treatment of BALB/c mice with vesicles, followed by parasite challenge, could significantly exacerbate parasite load and inflammation of the heart, and it could hasten animal mortality.38 Here, similarly to the vesicles, purified tGPI-mucins were also able to modulate trypomastigote invasion during interaction with LLC-MK2 cells. Higher invasion ability was observed for Y strain after the cells were incubated with tGPI-mucins from BZR-Y population and CL strain. However, pre-incubation with Tc-mucins slightly increased (∼0.5-fold) amastigote proliferation for all strains, except for Colombiana. However, this pattern did not vary among the strains, suggesting a role for tGPI-mucins in invasion with no apparent interference in intracellular parasite development. Because the lipid moiety of GPI-anchors tends to be conserved among strains,17 one hypothesis that could explain the differential invasion pattern could be related to the polymorphisms in its glycan part. Because of its hydrophobic nature, the GPI-anchors from GPI-mucins could be inserted in the plasma membrane, enabling its O-linked sugars to act as a site for parasite recognition.
In protozoa, inter- and intraspecies polymorphisms were reported in the major Leishmania glycoconjugate, the lipophosphoglycan (LPG). In L. infantum and L. major, a higher NO production was observed in the more complex LPG structures containing glucose and galactose side chains, respectively.59,60 In T. cruzi, early studies reported intraspecies variations in the GIPLs from epimastigotes in five strains (G, G-645, Tulahuen, CL, and Y).61 The work by Carreira and others61 suggested that those polymorphisms could be related to differences in pathogenicity and immunomodulatory activities during T. cruzi infection. Besides interspecies polymorphisms, intraspecies variation may occur among different T. cruzi parasite stages with biological relevance. When epimastigotes differentiate into metacyclic trypomastigotes, the PI moiety of the GPI anchor of the mucin is modified, but the O-linked carbohydrate chains stay essentially the same.22 However, in trypomastigote GPI-mucins, both the GPI-anchor and O-linked glycans undergo structural modifications, resulting in much higher proinflammatory activity of the GPI10 and strong recognition by protective anti–α-Gal antibodies.15
Here, we also evaluated the ability of human-purified anti–α-Gal antibodies against trypomastigote-derived tGPI-mucins from the different strains/populations. Those antibodies represent 1% of the IgGs circulating in the serum of healthy humans because of constant antigenic stimulation by LPS from intestinal bacteria such as Escherichia coli and Salmonella, suggesting the absence of α-galactosyl epitopes (Galα1-3 Galβ1-4GlcNAc-R) in the tissues.62 In T. cruzi and Leishmania-infected patients, the sera exhibited anti–α-Gal titers higher than the levels in healthy or bacteria-infected individuals.63 In both acute and chronic Chagasic patients, anti–α-Gal antibodies have a higher lytic power against T. cruzi trypomastigotes, which is crucial for decreasing parasite levels during infection.15,21,64 Our results showed that the levels of O-linked α-galactosylation may vary among the strains, and therefore, the ability of the host to clear the parasite using these antibodies might depend on the expression of those sugars on tGPI-mucins. Moreover, it is interesting to point out that Colombiana and CL strains expressed the lowest levels of those sugars among the strains. Our data are also consistent with previous biochemical studies showing important variations in O-linked oligosaccharides from strains Y, CL-Brener, and Colombiana. In the Y strain, they were represented by Galpβ1-3GlcNAc-ol, Galpβ1-6(Galpβ1-3)GlcNAc-ol, and Galpβ1-2Galpβ1-6(Galpβ1-3)GlcNAc-ol. Also, other O-linked oligosaccharides had a 1–4 linkage to N-acetylglucosaminitol with the structures Galpβ1-4GlcNAc-ol, Galpβ1-6(Galpβ1-4)GlcNAc-ol and Galpβ1-2Galpβ1-6(Galpβ1-4)GlcNAc-ol.23 In the CL-Brener strain, the O-linked oligosaccharides are structurally similar to those oligosaccharides found in the Y strain. However, the former exhibited novel syalylated O-glycans represented by Neu5Acα2–3Galp1-4[Galpβ1–6]GlcNAc-ol and Galpβ1–4[Neu5Acα2–3Galp1-6]GlcNAc-ol.24 Differently from Y and CL-Brener strains, Colombiana O-linked assignments were very similar to those assignments previously reported for G strains22,53 represented by O-glycans as belonging to a GPI-mucin family that contain β-galactofuranose (β-Galf) residue attached to an α-GlcNAc.24 It is interesting to notice that the CL-Brener strain, considered a hybrid, exhibited a carbohydrate profile similar to the Y strain. However, in the present study, it was very similar to Colombiana in triggering NO and cytokine production. For this reason, we decided to compare all parameters in this study using UPGMA analysis (Figure 7). As expected, no substantial differences were observed for the Y populations BZS and BZR (T. cruzi II) grouped together with CL strain (T. cruzi VI). However, the Colombiana strain (T. cruzi I) was completely discriminated from the other, a result consistent with the previous molecular studies.26 The data also confirmed the dual nature of CL strain as a possible consequence of its hybrid nature.11
The BZR-Y population was selected in vivo from the Y strain, and it is resistant to treatment with BZ.6 Using live trypomastigotes, biological differences regarding phagocytosis and cytokine release have shown that the BZR-Y population is less susceptible to destruction than BZS-Y.65 However, the P-glycoprotein (PGP), a multidrug resistance-associated glycoconjugate, presented the same levels of protein expression in BZS-Y and BZR-Y populations.66 Many molecular studies have compared those two populations to identify resistance markers.67–69 Although no correlation was detected, other studies have found differences in the in vitro-induced BZ-resistant strains (17WTS X 17LER).67 More recently, a proteomic analysis of BZS-Y/BZR-Y and 17WTS/17LER also identified many overexpressed proteins in the BZR-Y population.70 In this work, tGPI-mucins from the BZR-Y population were able to induce higher levels of NO and IL-12 than BZS-Y. Similarly, although in lower levels, tGPI-mucins from the naturally BZ-resistant Colombiana strain were more recognized by anti–α-Gal antibodies than the CL-susceptible strain. Although they were able to trigger different levels of NO and IL-12, the BZR-Y and BZS-Y populations had similar expression of α-Gal epitopes.
In conclusion, we have shown intraspecies variations in the anchor and O-linked sugars in the GPI-mucins in four T. cruzi strains. Those variations were important for differential NO and IL-12 production as well as modulation of trypomastigote invasion. Enzymatic treatments confirmed intraspecies polymorphisms in the O-linked α-galactosyl residues. Recently, it was proposed to use structural data for taxonomic purposes for comparing the O-glycans of T. cruzi I and II lineages.25,71 Here, we could also discriminate all lineages in T. cruzi I, II, and VI using the newly reported consensus.26 We could not correlate those variations to the BZ resistance phenotype, and they are probably strain-specific. However, because tGPI-mucins are important for inducing anti–α-Gal antibodies, variations in their structures are also likely to have implications in diagnosis and immunopathology of Chagas disease depending on the T. cruzi strain/DTU.
ACKNOWLEDGMENTS
The authors thank Dr. Egler Chiari for critical reading and helpful suggestions.
Footnotes
Financial support: R.P.S., S.M.M., and A.J.R. are research fellows supported by National Council for the Development of Research of Brazil (CNPq) Process 305042/2010-6). R.R.A. is supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). M.N.R. is supported by CNPq Grant 552072/2009-5. This work was supported by CNPq Grants PAPES-IV-400138/2006-9 and PDJ-150880/2005-7 and FAPEMIG. This work was also funded by National Institutes of Health Grants 1R01AI070655-04, 3R01AI070655-04S1, 2G12RR008124-16A1, and 2G12RR008124-16A1S1. We thank the Biomolecule Analysis Core Facility at the Border Biomedical Research Center/Biology/UTEP University of Texas at El Paso (National Institutes of Health Grants 2G12RR008124-16A1, 2G12RR008124-16A1S1 and G12MD007592).
Authors' addresses: Rodrigo P. Soares, Rafael R. Assis, Marcele N. Rocha, Felipe A. Moura e Castro, Gustavo F. Freitas, Silvane M. Murta, Sara L. Santos, and Alvaro J. Romanha, Centro de Pesquisas René Rachou, FIOCRUZ, Belo Horizonte, MG, Brazil, E-mails: rsoares@cpqrr.fiocruz.br, rafael.assis@cpqrr.fiocruz.br, marcele@cpqrr.fiocruz.br, felipeiters@hotmail.com, freitas@cpqrr.fiocruz.br, silvane@cpqrr.fiocruz.br, sara.lopes.santos@gmail.com, and romanha@cpqrr.fiocruz.br. Ana C. Torrecilhas, Departamento de Ciências Biológicas, Universidade Federal de São Paulo (UNIFESP), Diadema, SP, Brazil, E-mail: ana.trocoli@gmail.com. Alexandre F. Marques and Igor C. Almeida, Border Biomedical Research Center, Department of Biological Sciences, University of Texas, El Paso, TX, E-mails: afmarques@utep.edu and icalmeida@utep.edu.
References
- 1.Dias JC. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz. 2009;104((Suppl 1)):41–45. doi: 10.1590/s0074-02762009000900007. [DOI] [PubMed] [Google Scholar]
- 2.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Docampo R. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem Biol Interact. 1990;73:1–27. doi: 10.1016/0009-2797(90)90106-w. [DOI] [PubMed] [Google Scholar]
- 5.Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 6.Murta SM, Romanha AJ. In vivo selection of a population of Trypanosoma cruzi and clones resistant to benznidazole. Parasitology. 1998;116:165–171. doi: 10.1017/s0031182097002084. [DOI] [PubMed] [Google Scholar]
- 7.Toledo M, Tafuri W, Bahia MT, Tibayrenc M, Lana M. Genetic diversity and drug resistance in Trypanosoma cruzi, the agent of Chagas disease. Antimicrob Agents Chemother. 2004;4:11–22. [Google Scholar]
- 8.Gazzinelli RT, Ropert C, Campos MA. Role of the Toll/interleukin-1 receptor signaling pathway in host resistance and pathogenesis during infection with protozoan parasites. Immunol Rev. 2004;201:9–25. doi: 10.1111/j.0105-2896.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 9.Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 10.Almeida IC, Gazzinelli RT. Proinflammatory activity of glycosylphosphatidylinositol anchors derived from Trypanosoma cruzi: structural and functional analyses. J Leukoc Biol. 2001;70:467–477. [PubMed] [Google Scholar]
- 11.El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher AL, Blandin G, Westenberger SJ, Caler E, Cerqueira GC, Branche C, Haas B, Anupama A, Arner E, Aslund L, Attipoe P, Bontempi E, Bringaud F, Burton P, Cadag E, Campbell DA, Carrington M, Crabtree J, Darban H, da Silveira JF, de Jong P, Edwards K, Englund PT, Fazelina G, Feldblyum T, Ferella M, Frasch AC, Gull K, Horn D, Hou L, Huang Y, Kindlund E, Klingbeil M, Kluge S, Koo H, Lacerda D, Levin MJ, Lorenzi H, Louie T, Machado CR, McCulloch R, McKenna A, Mizuno Y, Mottram JC, Nelson S, Ochaya S, Osoegawa K, Pai G, Parsons M, Pentony M, Pettersson U, Pop M, Ramirez JL, Rinta J, Robertson L, Salzberg SL, Sanchez DO, Seyler A, Sharma R, Shetty J, Simpson AJ, Sisk E, Tammi MT, Tarleton R, Teixeira S, Van Aken S, Vogt C, Ward PN, Wickstead B, Wortman J, White O, Fraser CM, Stuart KD, Andersson B. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 12.Buscaglia CA, Campo VA, Di Noia JM, Torrecilhas AC, De Marchi CR, Ferguson MA, Frasch AC, Almeida IC. The surface coat of the mammal-dwelling infective trypomastigote stage of Trypanosoma cruzi is formed by highly diverse immunogenic mucins. J Biol Chem. 2004;279:15860–15869. doi: 10.1074/jbc.M314051200. [DOI] [PubMed] [Google Scholar]
- 13.Nakayasu ES, Sobreira TJ, Torres R, Jr, Ganiko L, Oliveira PS, Marques AF, Almeida IC. Improved proteomic approach for the discovery of potential vaccine targets in Trypanosoma cruzi. J Proteome Res. 2012;11:237–246. doi: 10.1021/pr200806s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Serrano A, Hutchinson C, Nakayasu ES, Almeida IC, Carrington M. Comparison and evolution of the surface architecture of trypanosomatid parasites. In: Barry JD, Mottram JC, McCulloch R, Acosta-Serrano A, editors. Trypanosomes: After the Genome. Norwich, United Kingdom: Horizon Scientific Press; 2007. pp. 319–337. [Google Scholar]
- 15.Almeida IC, Ferguson MA, Schenkman S, Travassos LR. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem J. 1994;304:793–802. doi: 10.1042/bj3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida IC, Camargo MM, Procopio DO, Silva LS, Mehlert A, Travassos LR, Gazzinelli RT, Ferguson MA. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 2000;19:1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos MA, Almeida IC, Takeuchi O, Akira S, Valente EP, Procopio DO, Travassos LR, Smith JA, Golenbock DT, Gazzinelli RT. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 18.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 19.Schenkman S, Eichinger D, Pereira ME, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 20.Frasch AC. Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol Today. 2000;16:282–286. doi: 10.1016/s0169-4758(00)01698-7. [DOI] [PubMed] [Google Scholar]
- 21.Almeida IC, Milani SR, Gorin PA, Travassos LR. Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-alpha-galactosyl antibodies. J Immunol. 1991;146:2394–2400. [PubMed] [Google Scholar]
- 22.Serrano AA, Schenkman S, Yoshida N, Mehlert A, Richardson JM, Ferguson MA. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J Biol Chem. 1995;270:27244–27253. doi: 10.1074/jbc.270.45.27244. [DOI] [PubMed] [Google Scholar]
- 23.Previato JO, Jones C, Xavier MT, Wait R, Travassos LR, Parodi AJ, Mendonca-Previato L. Structural characterization of the major glycosylphosphatidylinositol membrane-anchored glycoprotein from epimastigote forms of Trypanosoma cruzi Y-strain. J Biol Chem. 1995;270:7241–7250. doi: 10.1074/jbc.270.13.7241. [DOI] [PubMed] [Google Scholar]
- 24.Todeschini AR, da Silveira EX, Jones C, Wait R, Previato JO, Mendonca-Previato L. Structure of O-glycosidically linked oligosaccharides from glycoproteins of Trypanosoma cruzi CL-Brener strain: evidence for the presence of O-linked sialyl-oligosaccharides. Glycobiology. 2001;11:47–55. doi: 10.1093/glycob/11.1.47. [DOI] [PubMed] [Google Scholar]
- 25.Todeschini AR, de Almeida EG, Agrellos OA, Jones C, Previato JO, Mendonca-Previato L. Alpha-N-acetylglucosamine-linked O-glycans of sialoglycoproteins (Tc-mucins) from Trypanosoma cruzi Colombiana strain. Mem Inst Oswaldo Cruz. 2009;104((Suppl 1)):270–274. doi: 10.1590/s0074-02762009000900035. [DOI] [PubMed] [Google Scholar]
- 26.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG, Second Satellite M. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 27.Camargo EP. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev Inst Med Trop Sao Paulo. 1964;12:93–100. [PubMed] [Google Scholar]
- 28.Andrews NW, Colli W. Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J Protozool. 1982;29:264–269. doi: 10.1111/j.1550-7408.1982.tb04024.x. [DOI] [PubMed] [Google Scholar]
- 29.Uphoff CC, Drexler HG. Detection of mycoplasma contaminations. Methods Mol Biol. 2005;290:13–23. doi: 10.1385/1-59259-838-2:013. [DOI] [PubMed] [Google Scholar]
- 30.Ernest D, Olfert DVM, Brenda M, Cross DVM, McWilliam AA. Guide to the Care and Use of Experimental Animals. Ottawa, Ontario, Canada: Canadian Council on Animal Care; 1993. [Google Scholar]
- 31.Camargo MM, Almeida IC, Pereira ME, Ferguson MA, Travassos LR, Gazzinelli RT. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 32.Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida IC, Covas DT, Soussumi LM, Travassos LR. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion. 1997;37:850–857. doi: 10.1046/j.1537-2995.1997.37897424410.x. [DOI] [PubMed] [Google Scholar]
- 34.Soares RP, Barron T, McCoy-Simandle K, Svobodova M, Warburg A, Turco SJ. Leishmania tropica: intraspecific polymorphisms in lipophosphoglycan correlate with transmission by different Phlebotomus species. Exp Parasitol. 2004;107:105–114. doi: 10.1016/j.exppara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Rocha MN, Margonari C, Presot IM, Soares RP. Evaluation of 4 polymerase chain reaction protocols for cultured Leishmania spp. typing. Diagn Microbiol Infect Dis. 2010;68:401–409. doi: 10.1016/j.diagmicrobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Dice LR. Measures of the amount of ecological association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 37.Camargo MM, Andrade AC, Almeida IC, Travassos LR, Gazzinelli RT. Glycoconjugates isolated from Trypanosoma cruzi but not from Leishmania species membranes trigger nitric oxide synthesis as well as microbicidal activity in IFN-gamma-primed macrophages. J Immunol. 1997;159:6131–6139. [PubMed] [Google Scholar]
- 38.Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, E Silva NC, de Almeida Abrahamsohn I, Colli W, Manso Alves MJ. Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009;11:29–39. doi: 10.1016/j.micinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Freitas JM, Lages-Silva E, Crema E, Pena SD, Macedo AM. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int J Parasitol. 2005;35:411–417. doi: 10.1016/j.ijpara.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 40.McConville MJ, Mullin KA, Ilgoutz SC, Teasdale RD. Secretory pathway of trypanosomatid parasites. Microbiol Mol Biol Rev. 2002;66:122–154. doi: 10.1128/MMBR.66.1.122-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobson DE, Scholtes LD, Valdez KE, Sullivan DR, Mengeling BJ, Cilmi S, Turco SJ, Beverley SM. Functional identification of galactosyltransferases (SCGs) required for species-specific modifications of the lipophosphoglycan adhesin controlling Leishmania major-sand fly interactions. J Biol Chem. 2003;278:15523–15531. doi: 10.1074/jbc.M301568200. [DOI] [PubMed] [Google Scholar]
- 42.Segawa H, Soares RP, Kawakita M, Beverley SM, Turco SJ. Reconstitution of GDP-mannose transport activity with purified Leishmania LPG2 protein in liposomes. J Biol Chem. 2005;280:2028–2035. doi: 10.1074/jbc.M404915200. [DOI] [PubMed] [Google Scholar]
- 43.Miletti LC, Koerich LB, Pacheco LK, Steindel M, Stambuk BU. Characterization of D-glucose transport in Trypanosoma rangeli. Parasitology. 2006;133:721–727. doi: 10.1017/S0031182006000989. [DOI] [PubMed] [Google Scholar]
- 44.Tetaud E, Chabas S, Giroud C, Barrett MP, Baltz T. Hexose uptake in Trypanosoma cruzi: structure-activity relationship between substrate and transporter. Biochem J. 1996;317:353–359. doi: 10.1042/bj3170353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira MM, Einicker-Lamas M. Inositol metabolism in Trypanosoma cruzi: potential target for chemotherapy against Chagas' disease. An Acad Bras Cienc. 2000;72:413–419. doi: 10.1590/s0001-37652000000300015. [DOI] [PubMed] [Google Scholar]
- 46.Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 47.Burleigh BA, Woolsey AM. Cell signalling and Trypanosoma cruzi invasion. Cell Microbiol. 2002;4:701–711. doi: 10.1046/j.1462-5822.2002.00226.x. [DOI] [PubMed] [Google Scholar]
- 48.Colli W. Trans-sialidase: a unique enzyme activity discovered in the protozoan Trypanosoma cruzi. FASEB J. 1993;7:1257–1264. doi: 10.1096/fasebj.7.13.8405811. [DOI] [PubMed] [Google Scholar]
- 49.Eugenia Giorgi M, de Lederkremer RM. Trans-sialidase and mucins of Trypanosoma cruzi: an important interplay for the parasite. Carbohydr Res. 2011;346:1389–1393. doi: 10.1016/j.carres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Buscaglia CA, Campo VA, Frasch AC, Di Noia JM. Trypanosoma cruzi surface mucins: host-dependent coat diversity. Nat Rev Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- 51.Almeida IC, Krautz GM, Krettli AU, Travassos LR. Glycoconjugates of Trypanosoma cruzi: a 74 kD antigen of trypomastigotes specifically reacts with lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas disease. J Clin Lab Anal. 1993;7:307–316. doi: 10.1002/jcla.1860070603. [DOI] [PubMed] [Google Scholar]
- 52.Pereira-Chioccola VL, Acosta-Serrano A, Correia de Almeida I, Ferguson MA, Souto-Padron T, Rodrigues MM, Travassos LR, Schenkman S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J Cell Sci. 2000;113:1299–1307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 53.Previato JO, Jones C, Goncalves LP, Wait R, Travassos LR, Mendonca-Previato L. O-glycosidically linked N-acetylglucosamine-bound oligosaccharides from glycoproteins of Trypanosoma cruzi. Biochem J. 1994;301:151–159. doi: 10.1042/bj3010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campos MA, Closel M, Valente EP, Cardoso JE, Akira S, Alvarez-Leite JI, Ropert C, Gazzinelli RT. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol. 2004;172:1711–1718. doi: 10.4049/jimmunol.172.3.1711. [DOI] [PubMed] [Google Scholar]
- 55.Ropert C, Almeida IC, Closel M, Travassos LR, Ferguson MA, Cohen P, Gazzinelli RT. Requirement of mitogen-activated protein kinases and I kappa B phosphorylation for induction of proinflammatory cytokines synthesis by macrophages indicates functional similarity of receptors triggered by glycosylphosphatidylinositol anchors from parasitic protozoa and bacterial lipopolysaccharide. J Immunol. 2001;166:3423–3431. doi: 10.4049/jimmunol.166.5.3423. [DOI] [PubMed] [Google Scholar]
- 56.Ropert C, Closel M, Chaves AC, Gazzinelli RT. Inhibition of a p38/stress-activated protein kinase-2-dependent phosphatase restores function of IL-1 receptor-associate kinase-1 and reverses Toll-like receptor 2- and 4-dependent tolerance of macrophages. J Immunol. 2003;171:1456–1465. doi: 10.4049/jimmunol.171.3.1456. [DOI] [PubMed] [Google Scholar]
- 57.Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 58.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 59.Proudfoot L, Nikolaev AV, Feng GJ, Wei WQ, Ferguson MA, Brimacombe JS, Liew FY. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc Natl Acad Sci USA. 1996;93:10984–10989. doi: 10.1073/pnas.93.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coelho-Finamore JM, Freitas VC, Assis RR, Melo MN, Novozhilova N, Secundino NF, Pimenta PF, Turco SJ, Soares RP. Leishmania infantum: lipophosphoglycan intraspecific variation and interaction with vertebrate and invertebrate hosts. Int J Parasitol. 2011;41:333–342. doi: 10.1016/j.ijpara.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Carreira JC, Jones C, Wait R, Previato JO, Mendonca-Previato L. Structural variation in the glycoinositolphospholipids of different strains of Trypanosoma cruzi. Glycoconj J. 1996;13:955–966. doi: 10.1007/BF01053191. [DOI] [PubMed] [Google Scholar]
- 62.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avila JL, Rojas M, Galili U. Immunogenic Gal alpha 1-3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J Immunol. 1989;142:2828–2834. [PubMed] [Google Scholar]
- 64.Gazzinelli RT, Pereira ME, Romanha A, Gazzinelli G, Brener Z. Direct lysis of Trypanosoma cruzi: a novel effector mechanism of protection mediated by human anti-gal antibodies. Parasite Immunol. 1991;13:345–356. doi: 10.1111/j.1365-3024.1991.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 65.Murta SM, Ropert C, Alves RO, Gazzinelli RT, Romanha AJ. In-vivo treatment with benznidazole enhances phagocytosis, parasite destruction and cytokine release by macrophages during infection with a drug-susceptible but not with a derived drug-resistant Trypanosoma cruzi population. Parasite Immunol. 1999;21:535–544. doi: 10.1046/j.1365-3024.1999.00251.x. [DOI] [PubMed] [Google Scholar]
- 66.Murta SM, dos Santos WG, Anacleto C, Nirde P, Moreira ES, Romanha AJ. Drug resistance in Trypanosoma cruzi is not associated with amplification or overexpression of P-glycoprotein (PGP) genes. Mol Biochem Parasitol. 2001;117:223–228. doi: 10.1016/s0166-6851(01)00350-4. [DOI] [PubMed] [Google Scholar]
- 67.Murta SM, Krieger MA, Montenegro LR, Campos FF, Probst CM, Avila AR, Muto NH, de Oliveira RC, Nunes LR, Nirde P, Bruna-Romero O, Goldenberg S, Romanha AJ. Deletion of copies of the gene encoding old yellow enzyme (TcOYE), a NAD(P)H flavin oxidoreductase, associates with in vitro-induced benznidazole resistance in Trypanosoma cruzi. Mol Biochem Parasitol. 2006;146:151–162. doi: 10.1016/j.molbiopara.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Rego JV, Murta SM, Nirde P, Nogueira FB, de Andrade HM, Romanha AJ. Trypanosoma cruzi: characterisation of the gene encoding tyrosine aminotransferase in benznidazole-resistant and susceptible populations. Exp Parasitol. 2008;118:111–117. doi: 10.1016/j.exppara.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 69.Nogueira FB, Ruiz JC, Robello C, Romanha AJ, Murta SM. Molecular characterization of cytosolic and mitochondrial tryparedoxin peroxidase in Trypanosoma cruzi populations susceptible and resistant to benznidazole. Parasitol Res. 2009;104:835–844. doi: 10.1007/s00436-008-1264-1. [DOI] [PubMed] [Google Scholar]
- 70.Andrade HM, Murta SM, Chapeaurouge A, Perales J, Nirde P, Romanha AJ. Proteomic analysis of Trypanosoma cruzi resistance to Benznidazole. J Proteome Res. 2008;7:2357–2367. doi: 10.1021/pr700659m. [DOI] [PubMed] [Google Scholar]
- 71.Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]