Abstract
There are contradictory data regarding older individuals’ sensitivity to pain stimulation and opioid administration. Adult (12–16 months; n = 10) and aged (27–31 months; n = 7) male F344xBN rats were tested in a thermal sensitivity procedure where the animal chooses to remain in one of two compartments with floors maintained at various temperatures ranging from hot (45°C) through neutral (30°C) to cold (15°C). Effects of morphine were determined for three temperature comparisons (ie, hot/neutral, cold/neutral, and hot/cold). Aged rats were more sensitive to cold stimulation during baseline. Morphine produced antinociception during hot thermal stimulation, but had no effect on cold stimulation. The antinociceptive (and locomotor-altering) effects of morphine were attenuated in aged rats. These data demonstrate age-related differences in baseline thermal sensitivity and responsiveness to opioids. Based on behavioral and physiological requirements of this procedure, it is suggested that thermal sensitivity may provide a relevant animal model for the assessment of pain and antinociception.
Keywords: Opioids, Chronic pain, Operant testing, Thermal preference, Animal models
Opioids are among the most commonly used and effective pain relievers available, and although the therapeutic value of morphine has been recognized for centuries, it is still considered the “gold standard” among pain medications (1–3). At the preclinical level, the effects of morphine have been assessed in essentially every nociceptive (pain assessment) procedure developed, and many factors have been shown to influence the potency and effectiveness of morphine. A large majority of preclinical testing of nociceptive sensitivity use reflexive pain assessment testing (eg, tail withdrawal from radiant heat or hot water, nocifensive behaviors on a hot plate), and the influential factors include organismic (eg, genetic background, sex) (4–8), procedural (eg, stimulus intensity, antinociceptive task) (5,8–11), pharmacokinetic (eg, dose, route of administration, pretreatment time) (12), and historical (eg, behavioral and drug history, development of tolerance) (10,13–15)variables.
Given the aging of the population (16,17) and the anticipated corresponding increase in age-related chronic pain (18–20), one factor that is of great translational interest is the role of age in determining the effects of morphine (21–25). In humans, opioids including morphine are typically described as having larger pain-relieving effects in older populations and these findings are attributed to changes in pharmacokinetics (eg, decreased rate of metabolism or excretion, changes in volume of distribution) (26, 27). These findings result in physicians being recommended to initially use lower doses in the elderly patients (eg, [28]), however, there are no compelling data in humans demonstrating that once pharmacokinetic variables are controlled for, that age does not influence the effectiveness of morphine. In the preclinical literature (for review see [22]), the antinociceptive effects of opioids in aged animals have been demonstrated to be greater (29,30) lesser (31–39), and no different (30,40,41) (although this group found a difference in tolerance development) relative to younger groups of animals. All the previously mentioned factors probably contribute to the disparate findings across studies.
We have recently reported differential sensitivity to thermal stimulation across ages using an operant thermal sensitivity procedure (24,42). In this procedure, animals choose to remain in one of two compartments, with the only difference being the temperature of the floor (see also [43]). In general, it was demonstrated that older female rats were more sensitive to cold stimulation, which manifested in part by a preference for hot stimulation over cold stimulation (42). Importantly, the thermal stimulation used in these procedures (eg, 45°C) does not elicit reflexive nocifensive behaviors such as paw licking, guarding, or withdrawal (42–44). Furthermore, this moderate thermal stimulation is believed to preferentially activate C-fiber nociceptors (generally associated with second, slow pain) rather than Aδ-fiber nociceptors (fast, first, or acute pain). Given that chronic pain conditions in humans are likely associated with C-fiber rather than Aδ-fiber activation (45–47), these experimental procedures may tap into physiological systems that subserve chronic pain conditions in humans and may be particularly suitable for studying changes or differences in sensitivity across ages (for discussion, see [24, 42, 48, 49]). Finally, there is a trend in the literature to use pain assessment procedures that involve cortical processing (eg, the type of thermal sensitivity assessed here), as opposed to spinal cord–mediated reflexive responding (eg, [50]).
The overall aims of the current experiment were to extend our previous findings to male rats and to characterize the effects of morphine across age-groups in this procedure. The data suggest that older animals are more sensitive to cold stimulation, sensitivity to the hot but not cold stimulation is attenuated by morphine, and that morphine was somewhat more effective in the adult rats relative to the aged rats.
METHODS
Subjects
Male Fischer 344 × Brown Norway F1 (F344xBN) rats were obtained from the National Institute of Aging colony maintained by Harlan Industries (Indianapolis, IN), and the experiments began at approximately 12 months (adult, n = 10) or 27 months of age (aged, final n = 7). Animals were individually housed in a climate-controlled colony room with a 12-hour light/dark cycle with food and water available ad libitum. Animals were treated in accordance with the regulations of the Institutional Animal Care and Use Committee and with the “Guide for the Care and Use of Laboratory Animals” (51). Animals were handled and weighed daily, and assessed on a weekly basis for signs of overt health problems with measures including, but not limited to, sudden declines in body weight, redness around the eyes and nostrils, ruffled coat, open sores on tail, and hunched posture. One aged animal died during the course of the experiment (apparently from causes unrelated to the experiment) and the data from this animal were excluded from the analyses.
Apparatus
Experimental sessions were conducted in a custom-built experimental apparatus. Two aluminum temperature-controlled floor plates (18 × 29 cm; Smalls Design and Manufacturing, Portland, TN) were connected to two water chiller/heaters (Model RTE-7; Thermo Scientific) and surrounded by a white Plexiglas chamber composed of two compartments separated by a divider with a cutout allowing free access to each side (Magnum Wood LLC, Gainesville, FL). Each compartment measured 18 × 29 cm. For data analysis purposes, a 5-cm ring around the outside of the compartment was designated as the “wall,” a 5 × 5–cm square at the cutout between compartments was designated the “entrance,” and the remaining 19 × 8–cm portion of the floor was designated the “center.” An overhead camera recorded video, and sessions were analyzed using EthoVision software (Noldus Information Technology, Wageningen, The Netherlands).
Experimental Timeline
Animals were obtained and allowed to acclimate to the colony room, the laboratory, and handling procedures for approximately 4 weeks. Habituation and exposure to the testing apparatus and a variety of temperature comparisons to train the animals to “sample” each side occurred over the next 4 weeks. The initial assessment of thermal sensitivity (described in the following) was conducted over the next 4 weeks. Subsequently, the morphine testing phase was conducted over 9 weeks. In total, these experiments were conducted over a 4- to 5-month period. Experiments were conducted in the middle of the light phase at approximately the same time every day, 5 days per week.
Initial A ssessment of Thermal Sensitivity
For the initial assessment, one compartment’s floor was set to 30°C (hereafter referred to as “neutral”), and the other compartment was set to a temperature that ranged between 15°C and 45°C (in 3°C increments). Each temperature comparison was assessed two times over a 4-week period.
Morphine Testing
Once the initial assessment of thermal sensitivity was completed, baseline and drug testing commenced. Initially, there was a 2-week period of baseline testing where behavior during three temperature comparisons (hot vs cold, 45°C vs 15°C; hot vs neutral, 45°C vs 30°C; and neutral vs cold, 30°C vs 15°C) was repeatedly assessed. Subsequently, baseline testing occurred every Monday and Thursday during the morphine testing phase. The effects of morphine were assessed on Tuesdays and Fridays. Saline tests were typically conducted on Wednesdays. Temperature comparisons and morphine doses were tested in a pseudorandom order across days. The effects of saline were determined twice for each animal at each temperature comparison, and each compartment (ie, warmer or cooler) was used as the “start side” for one of the sessions. Each dose of morphine was tested at a particular temperature comparison once in each animal, and the “start side” was counterbalanced across rats.
Drug
Morphine sulfate was purchased from Sigma–Aldrich, dissolved in sterile saline, and passed through a 22-μm filter. Morphine and saline were administered intraperitoneally in a volume of 1.0 mL/kg.
Statistics
Data are displayed as mean ± standard error of the mean. In general, a two-way repeated measures analysis of variance, followed by Student Newman–Keuls post hoc comparisons, was conducted with the alpha level set at .05. Age (adult vs aged) was the between-subject variable, with session, temperature comparison, or morphine dose as the within-subject variables. Dependent variables included the absolute duration of the session in a particular component, the relative preference for one side over the other, locomotor activity (ie, distance traveled in centimeter), the number of times an animal switched from one compartment to another (“crosses”), the latency to make the first cross from the start compartment to the other, and time spent in a particular spatial location (ie, center, wall, or entrance).
RESULTS
Initial Sensitivity to Thermal Stimulation
Animals were exposed to the testing apparatus during daily 10-minute sessions. During these sessions, one compartment contained a floor that was maintained at 30°C and the temperature of the “comparison” floor was altered across sessions. When the “comparison” temperature was similar to the neutral temperature (eg, 27°C–33°C), there was no temperature preference (Figure 1; 300 seconds indicate equal time on each side) in either age-group. When the temperature of the comparison floor was decreased to 24°C and below, or increased to 39°C and above, there were temperature-dependent decreases in the amount of time spent in that compartment (main effect of temperature: F 9,144 = 6.7, p < .001). At the highest and lowest temperatures tested, rats from both age-groups spent approximately 80%–85% of the session in the “neutral” compartment. It is important to note that even at these temperatures, there was no indication of reflexive, nocifensive behaviors such as paw licking or guarding during these sessions. Overall, the aged rats spent more time on the “neutral” floor indicated by a temperature–effect curve that was “lower” compared with the adult rats (main effect of age: F 1,144 = 5.0, p = .04).
Figure 1.
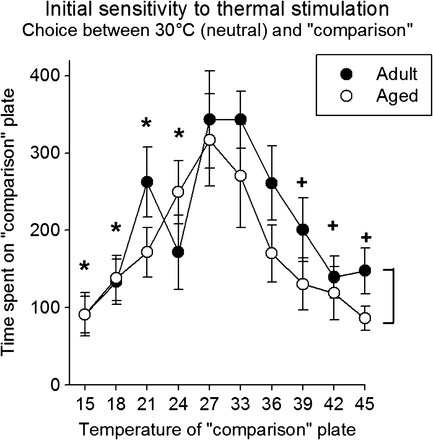
Initial sensitivity to thermal stimulation. Thermal sensitivity was assessed in adult (12–16 months old) and aged (27–31 months old) rats. During 600-second sessions, the duration of time spent on either the 30°C or “comparison” floor plate was determined. The figure shows the time spent on the “comparison” plate as a function of that temperature. In general, the intermediate temperatures resulted in near equal times (∼300 seconds) spent in each compartment (ie, there was no preference). As the temperature of the floor plate increased or decreased, less time was spent in that compartment (ie, the temperature was aversive). Brackets indicate an overall significant main effect of age; * indicates a significant difference from 27°C; and + indicates a significant difference from 33°C.
Baseline Testing Throughout Study
Baseline sessions were conducted throughout the study. There were three types of baseline sessions: hot versus neutral (45°C vs 30°C), cold versus neutral (15°C vs 30°C), and hot versus cold (45°C vs 15°C). Data from the eight baseline sessions for each condition are shown in Figure 2. In tests of sensitivity to hot (left panel) and cold (center panel) stimulation, animals of both age-groups demonstrated an aversion indicated by an average time of approximately 100 and 50 seconds (out of 600 seconds total) spent in the hot and cold compartments, respectively, and this aversion was maintained throughout the study. In the hot versus cold preference sessions, there was a statistically significant interaction (F 7,112 = 3.1, p = .005) and a main effect of both age (F 1,112 = 5.2, p = .037) and session (F 7,112 = 4.3, p < .001). In general, the aged rats showed a preference for the hot side (statistically significant, pairwise comparisons at sessions 3, 4, and 5). During all experimental sessions, distance traveled and the number of “crosses” between compartments were measured (Figure 3). There were significant main effects of age (F 1,32 = 17.5, p <.001, and F 1,32 = 5.2, p = .037, for distance and crosses, respectively) and temperature comparison (F 2,32 = 62.4, p < .001, and F 2,32 = 27.9, p < .001, for distance and crosses, respectively). Overall, adult rats were more active than the aged animals. Furthermore, both measures of activity were highest during the hot versus cold comparison, and lowest during the cold versus neutral comparison.
Figure 2.
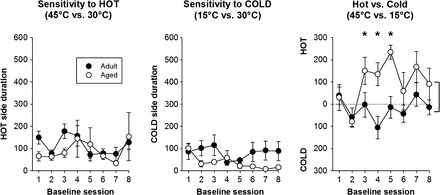
Baseline sensitivity across the study. Baseline thermal sensitivity was assessed in adult (12–16 months old) and aged (27–31 months old) rats throughout the experiment. Following testing of initial sensitivity, baseline sessions were composed of three temperature comparisons: hot vs neutral, cold vs neutral, or hot vs cold. The figure shows data from the eight sessions at each comparison. Adult and aged rats showed an aversion to both the hot and cold thermal stimulation (left and center panels) that remained stable and did not differ across ages. During the hot vs cold assessment, there were differences between the age-groups during sessions 3, 4, and 5, with the aged animals showing a marked preference for the hot side relative to the adult rats. Brackets indicate an overall significant main effect of age; * indicates a significant difference from adult rats.
Figure 3.
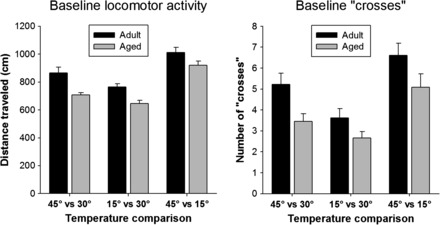
Baseline activity. Locomotor activity in the testing chambers and the number of crosses from one chamber compartment to the other were determined during baseline testing. For both measures, there was a significant main effect of age (adult > aged rats) and temperature comparison (45°C vs 15°C > 45°C vs 30°C > 15°C vs 30°C).
Effects of Morphine Treatment
Saline and a range of morphine doses (0.56–5.6 mg/kg, intraperitoneally) were administered 15 minutes before experimental sessions, and the effects on thermal sensitivity were assessed. In both experimental situations using the “hot” floor (ie, hot vs neutral and hot vs cold), morphine decreased aversion to this 45°C stimulus, indicated by a loss of preference during the hot versus neutral sessions (Figure 4, left panel) or the development of a “hot” compartment preference during hot versus cold sessions (Figure 4, right panel). This effect was observed in a dose-dependent manner such that the effects following 1.7, 3.0, and 5.6 mg/kg morphine administration were significantly different from those of saline. Similar effects were observed in both age-groups (ie, there was no significant interaction), although the level of antinociception across all doses was greater in the adult rats in the hot versus neutral session (main effect of age). Morphine failed to alter thermal sensitivity in the cold versus neutral test sessions (Figure 4, center panel). When a hot or cold stimulus was compared with the 30°C (neutral) floor, aged animals showed a greater aversion to the extreme temperature, indicated by a significant main effect of age (Figure 4, left and center panels).
Figure 4.
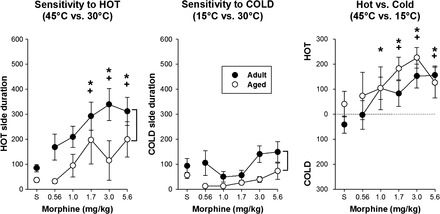
Effects of morphine on thermal sensitivity. Morphine resulted in a decreased aversion toward the hot side (left and right panels) in a dose-dependent manner, indicated by an increase in time spent on the hot side during these testing sessions relative to saline administration. There was no effect of morphine on thermal sensitivity when assessed in the cold vs neutral condition (center panel). There was an overall age effect in the “hot vs neutral” and “cold vs neutral” testing conditions (left two panels), with aged rats spending more time on the “neutral” side compared with adult rats. Brackets in the left and center panels indicate a significant age difference; * indicates a statistically significant difference from saline; and + indicates a statistically significant difference from 0.56 mg/kg morphine.
Across all temperature testing conditions and regardless of morphine pretreatment, activity was higher in the adult relative to aged rats (Figure 5; main effect of age in all conditions). There was a trend toward a statistically significant interaction in the cold versus neutral condition, with morphine resulting in larger increases in activity in the adult relative to aged rats (F 5,80 = 2.02, p = .085). Morphine administration failed to alter locomotor activity in the hot versus cold testing condition in either age-group.
Figure 5.
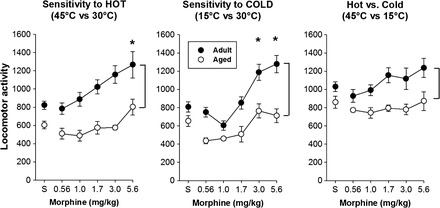
Effects of morphine on locomotor activity. Morphine resulted in dose-dependent increases in locomotor activity in both age-groups when assessed in the “hot vs neutral” (left) and “cold vs neutral” (center), but not during the “hot vs cold” (right) condition. In all cases, locomotor activity was higher in the adult, relative to aged, rats. * indicates a significant difference from saline; the brackets in all panels indicate a significant age difference.
One variable of interest was the latency to escape from the initial compartment, as this could represent a measure analogous to “pain threshold“ (ie, the first subjective feeling of a painful sensation). Table 1 shows the latency (in seconds) to escape as a function of age, starting compartment temperature, and test condition (eg, morphine dose). There were no significant differences or interactions between ages or test condition within a particular temperature condition. Analysis of latencies across temperatures during the baseline sessions revealed that latencies to cross when the start side was “cold” were significantly lower relative to either “neutral” or “hot” start sides (F 2,32 = 5.11, p = .012; no significant interaction or main effect of age). These data suggest that the cold temperature was more aversive than the neutral and hot temperatures.
Table 1.
Latency (seconds) to Leave the “Start Chamber” at the Beginning of the Session
| From 45°C | From 30°C | From 15°C | ||||
| Adult | Old | Old | Old | Adult | Old | |
| Baseline | 48.8 ± 12.4 | 29.2 ± 11.3 | 37.6 ± 12.2 | 35.1 ± 11.3 | 12.9 ± 3.3 | 14.2 ± 3.9 |
| Saline | 40.1 ± 14.8 | 30.9 ± 7.7 | 50.7 ± 24.8 | 14.6 ± 3.5 | 1.5 ± 1.7 | 8.6 ± 0.7 |
| 0.56 | 54.5 ± 14.7 | 8.9 ± 2.8 | 23.5 ± 14.6 | 6.5 ± 2.8 | 6.7 ± 0.9 | 9.9 ± 1.5 |
| 1.0 | 75.8 ± 40.4 | 18.7 ± 14.1 | 73.1 ± 64.9 | 32.0 ± 12.4 | 12.9 ± 3.4 | 7.5 ± 0.2 |
| 1.7 | 112.5 ± 55.8 | 100.6 ± 76.6 | 25.0 ± 15.2 | 28.5 ± 17.0 | 9.5 ± 1.8 | 7.0 ± 1.8 |
| 3.0 | 87.9 ± 53.2 | 17.0 ± 10.0 | 15.0 ± 6.0 | 105.0 ± 72.9 | 10.2 ± 3.1 | 8.4 ± 1.5 |
| 5.6 | 81.1 ± 41.4 | 64.5 ± 57.0 | 29.4 ± 11.4 | 72.1 ± 45.8 | 13.8 ± 7.1 | 7.5 ± 1.9 |
Note: Temperature of the start side is indicated in the first row, and data are shown for adult and aged rats for baseline, saline, and morphine test sessions.
To further explore this potential aversion to the cold temperature, the spatial location of the rat on each side was assessed. Figure 6 shows the spatial location (ie, center, wall, or entrance) during saline sessions for each temperature comparison, and it is clear that the animals spend most of the time in the center regardless of temperature comparison. For the cold or hot versus neutral sessions, animals spent more time on the neutral side (center and right panels). For the hot versus neutral condition, there was a significant interaction between age and location on the hot side (F 2,32 = 5.25, p = .011), although the absolute amount of time spent on this side was very low. In the hot versus cold comparison (left panel), when spatial location on the hot side was analyzed, there was a trend (p = .09) toward an effect of age (aged > adult) but no interaction between factors. When data from the cold side were analyzed, there was a significant interaction between age and location (F 2,32 = 3.35, p = .047) such that the aged animals spent less time in the center relative to adult animals (p < .05). These data suggest a greater aversion to the cold side for the aged rats.
Figure 6.
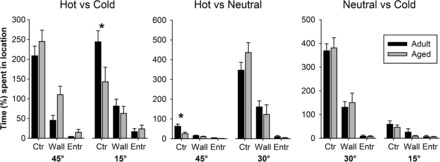
Spatial location during each temperature comparisons following saline administration. Each compartment was divided into “wall,” “center,” and “entrance” areas, and the absolute duration spent in each area for each compartment is shown for the three temperature comparisons. Aged rats spent less time in the center of the cold floor during the hot vs cold comparison and on the hot floor during the hot vs neutral comparison. * indicates a significant interaction and age difference.
DISCUSSION
The purpose of the present study was to extend previous findings from females to males regarding differences in thermal sensitivity across ages (42) and to examine the responsiveness of different aged rats to the prototypical opioid pain reliever morphine. The general findings were that (a) older animals were more sensitive to the extreme temperatures; (b) older animals preferred hot to cold thermal stimulation and/or were more averse to the cold stimulation; (c) morphine effectively reduced the aversion produced by hot, but not cold, thermal stimulation; and (d) morphine was slightly less effective in producing antinociception (and altering locomotor activity) in the older rats.
Although pain is a common problem in the elderly population, it remains unclear how mechanisms of nociception and pain perception change with age (22,52–54). It is becoming more widely accepted that the assessment of pain sensitivity and tolerance levels in humans is complicated by a number of factors including social influences, patient–physician interactions, expectations and beliefs, and cognitive status, in addition to the relative subjectivity of some measures of pain sensitivity (54–56). These factors highlight the potential importance of conducting animal studies of nociception and aging where many of these influences can be eliminated, controlled for, or explicitly studied. A large majority of preclinical testing of nociceptive sensitivity use reflexive pain assessment testing (eg, tail withdrawal from radiant heat or hot water, nocifensive behaviors on a hot plate); this is also true in the study of aging and pain, and has resulted in seemingly contradictory findings that are often attributed to differences in experimental designs and methodologies. We recently reported differences in thermal sensitivity across ages when assessed using various thermal sensitivity procedures in female rats (42), and the present study extended these findings to male rats. In general, the same pattern of differential sensitivity was observed, although the absolute magnitude of the effect was smaller in the present study. That is, older animals appear to be more sensitive to the aversive effects of more extreme temperatures (both hot and cold), indicated by a greater preference for the neutral plate when compared with a variety of other temperatures (Figure 1). Similarly, when repeated hot-versus-cold comparisons were made under baseline conditions, older animals spent less time on the relatively more aversive cold plate (Figures 2 and 6). These results are similar to previous studies (24,42), although it should be noted that the parameters used across these experiments were not identical; this suggests a generality to the overall finding of greater sensitivity to the aversive effects of thermal stimulation (especially cold) in older animals. Given the long duration of testing trials used in this procedure (compared with reflex-based procedures), future studies will be conducted to determine whether aspects of behavior in this task are related to thermal thresholds or thermal pain tolerance levels (two measures commonly assessed in humans), and/or the phenomenon of temporal summation (eg, [57,58]), and the reported differences across ages demonstrated in humans with all of these measures (eg, [49,59,60]). In the present study, the initial latency to leave the “start side” was dependent on the floor temperature (ie, lower latencies during the “cold” conditions; Table 1), suggesting that this may be a measure analogous to thermal or pain threshold. However, it should be noted that the animals have extensive experience in the procedure and tended to “sample” both compartments relatively early in the session.
Administration of morphine increased the amount of time spent on the “hot” plate in a dose-dependent manner for both adult and aged rats. This increase in duration occurred regardless of the comparison side temperature (neutral or cold), suggesting that morphine decreased sensitivity to hot thermal stimulation. These data are consistent with an extensive literature demonstrating that hot thermal stimulation is sensitive to opioid modulation. This finding provides the basis for many nociceptive procedures that are used as screens for potential pharmacotherapies for pain relief; these methods include radiant heat, warm water, or hot plate stimulation of a rodent’s tail, paw, or snout. There is a contradictory literature regarding the effects of morphine (or any opioid) in older versus younger subjects (humans and nonhumans). Many of the differences are likely due to various organismic, environmental, procedural, and historical variables. In the present study, the antinociceptive effects of morphine appeared to be a greater magnitude in the adult rats—this was indicated by a slightly larger absolute level of antinociception (Figure 4, left panel) and a shift from a small preference for the cold side to a dramatic preference for the hot side (Figure 4, right panel). Taken together, these data along with the extant literature suggest that there are age-related differences in sensitivity to opioids; however, the magnitude and direction of the age effect is controlled by numerous variables. An important translational extension of this research will be to identify the conditions under which these factors play a role in human clinical situations.
Morphine failed to alter sensitivity to cold thermal stimulation (Figure 4, center panel). There is a large body of research in humans demonstrating opioid-induced antinociception using the “cold-pressor” test (typically, immersion of forearm into cold water) (61–65), and a more limited literature showing mu-opioid–mediated antinociception using cold-water, tail-withdrawal tests in rats (eg, [66]). The use of “cold plate” procedures with rodents typically involves the use of more extreme temperatures than those used here, utilize reflex-based dependent measures (eg, paw shakes), and are primarily used for assessing hyperalgesia following chronic constriction injuries as a model of neuropathic pain (67–70). Unfortunately, there are not enough data available to explain the lack of morphine’s effect on cold thermal stimulation in the present study using moderate cold temperatures in the context of a choice situation.
It should be noted that the dependent measures assessed in this procedure require coordinated movements of the entire organism with an intact brain or central nervous system. This is unlike many reflex-based nociceptive testing situations in which spinal rats are capable of making the pain-related response (eg, [71]). In fact, there is a trend in the field to move away from (or at least supplement) assessment of standard, reflex-based, pain-elicited behaviors toward other dependent variables, which are either pain suppressed or operant in nature. For example, several research groups have examined pain-suppressed locomotion, eating, or schedule-controlled operant behavior (maintained by food or intracranial self-stimulation; eg, [72–76]). Similarly, there are several laboratories developing behavioral procedures in which animals escape from an aversive situation (for conceptually similar procedures to the methods employed here, see [77–80]). In experimental situations where operant behavior (including locomotor activity) is required, an important consideration is that performance (eg, in older animals) could be altered simply because of compromised behavioral function (eg, the animal cannot move, is slower, is unable to escape from a situation). In the present study, distance traveled was measured during testing sessions and it was demonstrated that there was reliably higher levels of locomotion in the younger animals across all testing conditions. However, activity is high enough in the oldest animals to suggest that an inability to locomote was not responsible for the differences on changes in thermal sensitivity at baseline or following administration of morphine. Given that both aversion or preference and activity were measured at the same time, it was of interest to determine if these were dissociable measures; that is, does an increase in activity necessarily result in a change in preference, and conversely, could a change in preference be observed without changes in overall levels of activity? Comparison of Figures 4 and 5 suggests that each of these situations can be observed. For example, the center panels show increases in activity without changes in preference, the right panels show dramatic changes in preference with no overall change in activity, and the panels on the left show changes in both measures. Together, these data suggest that thermal sensitivity and locomotor activity are distinct measures that the effects of morphine on thermal sensitivity are not simply a consequence of the locomotor-altering effects.
Although morphine remains the mainstay of pain management and there is an extensive body of preclinical and clinical research, we do not know everything there is to know about the conditions under which it functions as an effective analgesic in the human situation. Here, we demonstrate subtle but reliable differences in nociception to hot and cold thermal stimulation and responsiveness to the mu-opioid morphine across rats of different ages.
Funding
This work was primarily supported by the National Institutes of Health (R21 DA023022 to D.M.). C.S.C. was supported by R01 AG024526, J.D.M. was supported by T32 AG00196, and additional support was provided by P30 AG028740.
References
- 1.Mather LE. 1994 John J. Bonica Lecture. The clinical effects of morphine pharmacology. Reg Anesth. 1995;20:263–282. [PubMed] [Google Scholar]
- 2.Zöllner C, Stein C. Opioids. Handbook of Experimental Pharmacology. 2007;177:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
- 3.Yaksh TL, Wallace. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York, NY:: McGraw-Hill; MS. Chapter 18. Opioids, analgesia, and pain management. In: Brunton LL, Chabner BA, Knollmann BC, eds. http://www.accessmedicine.com/content/aspx?aID=16683526. Accessed: November 21, 2011. [Google Scholar]
- 4.Elmer GI, Pieper JO, Negus SS, Woods JH. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain. 1998;75:129–140. doi: 10.1016/S0304-3959(97)00215-7. [DOI] [PubMed] [Google Scholar]
- 5.Morgan D, Cook CD, Picker MJ. Sensitivity to the discriminative stimulus and antinociceptive effects of mu opioids: role of strain of rat, stimulus intensity, and intrinsic efficacy at the mu opioid receptor. J Pharmacol Exp Ther. 1999;289:965–975. [PubMed] [Google Scholar]
- 6.Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther. 1999;289:1370–1375. [PubMed] [Google Scholar]
- 7.Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology. 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- 8.South SM, Edwards SR, Smith MT. Antinociception versus serum concentration relationships following acute administration of intravenous morphine in male and female Sprague-Dawley rats: differences between the tail flick and hot plate nociceptive tests. Clin Exp Pharmacol Physiol. 2009;36:20–28. doi: 10.1111/j.1440-1681.2008.05019.x. [DOI] [PubMed] [Google Scholar]
- 9.Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology. 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- 11.Lemberg K, Kontinen VK, Viljakka K, Kylanlahti I, Yli-Kauhaluoma J, Kalso E. Morphine, oxycodone, methadone and its enantiomers in different models of nociception in the rat. Anesth Analg. 2006;102:1768–1774. doi: 10.1213/01.ane.0000205751.88422.41. [DOI] [PubMed] [Google Scholar]
- 12.Shang GW, Liu DN, Yan LH, et al. Nociceptive stimulus modality-related difference in pharmacokinetic-pharmacodynamic modeling of morphine in the rat. Pharmacol Biochem Behav. 2006;85:464–473. doi: 10.1016/j.pbb.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Lane DA, Morgan MM. Antinociceptive tolerance to morphine from repeated nociceptive testing in the rat. Brain Res. 2005;1047:65–71. doi: 10.1016/j.brainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 15.Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology. 2003;168:426–434. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- 16.Vincent GK, Velkoff VA. Current Population Reports, Washington, DC: : U.S. Census Bureau; 2010. The Next Four Decades, The Older Population in the United States: 2010 to 2050; pp. P25–1138. [Google Scholar]
- 17.Le Couteur DG, McLachlan AJ, de Cabo R. Aging, drugs, and drug metabolism. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr084. In press. [DOI] [PubMed] [Google Scholar]
- 18.Gagliese L, Melzack R. Chronic pain in elderly people. Pain. 1997;70:3–14. doi: 10.1016/s0304-3959(96)03266-6. [DOI] [PubMed] [Google Scholar]
- 19.Bruckenthal P, Reid MC, Reisner L. Special issues in the management of chronic pain in older adults. Pain Med. 2009;10(suppl 2):S67–S78. doi: 10.1111/j.1526-4637.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 20.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–431. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 21.Bodnar RJ, Romero MT, Kramer E. Organismic variables and pain inhibition: roles of gender and aging. Brain Res Bull. 1988;21:947–953. doi: 10.1016/0361-9230(88)90032-9. [DOI] [PubMed] [Google Scholar]
- 22.Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev. 2000;24:843–854. doi: 10.1016/s0149-7634(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 23.Won A, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Long-term effects of analgesics in a population of elderly nursing home residents with persistent nonmalignant pain. J Gerontol A Biol Sci Med Sci. 2006;61:165–169. doi: 10.1093/gerona/61.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan D, Carter CS, DuPree JP, Yezierski RP, Vierck CJ., Jr Evaluation of prescription opioids using operant-based pain measures in rats. Exp Clin Psychopharmacol. 2008;16:367–375. doi: 10.1037/a0013520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011;12:1336–1357. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugo RA, Kern SE. Clinical pharmacokinetics of morphine. J Pain Palliat Care Pharmacother. 2002;16:5–18. doi: 10.1080/j354v16n04_02. [DOI] [PubMed] [Google Scholar]
- 27.Wilder-Smith OH. Opioid use in the elderly. Eur J Pain. 2005;9:137–140. doi: 10.1016/j.ejpain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often use World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 29.Islam AK, Cooper ML, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon morphine antinociception in rats. Physiol Behav. 1993;54:45–53. doi: 10.1016/0031-9384(93)90042-e. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Gray JD. Age-related differences in sensitivity to the antinociceptive effects of opioids in male rats: influence of nociceptive intensity and intrinsic efficacy at the mu receptor. Psychopharmacology. 2001;156:445–453. doi: 10.1007/s002130100750. [DOI] [PubMed] [Google Scholar]
- 31.Webster GW, Shuster L, Eleftheriou BE. Morphine analgesia in mice of different ages. Exp Aging Res. 1976;2:221–233. doi: 10.1080/03610737608257178. [DOI] [PubMed] [Google Scholar]
- 32.Chan SH, Lai YY. Effects of aging on pain responses and analgesic efficacy of morphine and clonidine in rats. Exp Neurol. 1982;75:112–119. doi: 10.1016/0014-4886(82)90011-5. [DOI] [PubMed] [Google Scholar]
- 33.Kavaliers M, Hirst M, Teskey GC. Aging, opioid analgesia and the pineal gland. Life Sci. 1983;32:2279–2287. doi: 10.1016/0024-3205(83)90427-7. [DOI] [PubMed] [Google Scholar]
- 34.Hoskins B, Burton CK, Ho IK. Differences in morphine-induced antinociception and locomotor activity in mature adult and aged mice. Pharmacol Biochem Behav. 1986;25:599–605. doi: 10.1016/0091-3057(86)90148-6. [DOI] [PubMed] [Google Scholar]
- 35.Kramer E, Bodnar RJ. Age-related decrements in morphine analgesia: a parametric analysis. Neurobiol Aging. 1986;7:185–191. doi: 10.1016/0197-4580(86)90041-2. [DOI] [PubMed] [Google Scholar]
- 36.Crisp T, Stafinsky JL, Hoskins DL, Perni VC, Uram M, Gordon TL. Age-related changes in the spinal antinociceptive effects of DAGO, DPDPE and beta-endorphin in the rat. Brain Res. 1994;643:282–286. doi: 10.1016/0006-8993(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 37.Crisp T, Giles JR, Cruce WLR, McBurney DL, Stuesse SL. The effects of aging on thermal hyperalgesia and tactile-evoked allodynia using two models of peripheral mononeuropathy in the rat. Neurosci Lett. 2003;339:103–106. doi: 10.1016/s0304-3940(03)00009-0. [DOI] [PubMed] [Google Scholar]
- 38.Jourdan D, Boghossian S, Alloui A, et al. Age-related changes in nociception and effect of morphine in the Lou rat. Eur J Pain. 2000;4:291–300. doi: 10.1053/eujp.2000.0188. [DOI] [PubMed] [Google Scholar]
- 39.Jourdan D, Pickering G, Marchand F, Gaulier JM, Alliot J, Eschalier A. Impact of ageing on the antinociceptive effect of reference analgesics in the Lou/c rat. Br J Pharmacol. 2002;137:813–820. doi: 10.1038/sj.bjp.0704944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Crugten JT, Somogyi AA, Nation RL, Reynolds G. The effect of old age on the disposition and antinociceptive response of morphine and morphine-6β-glucuronide in the rat. Pain. 1997;71:199–205. doi: 10.1016/s0304-3959(97)03363-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Mitchell J, Moriyama K, et al. Age-dependent morphine tolerance development in the rat. Anesth Analg. 2005;100:1733–1739. doi: 10.1213/01.ANE.0000152192.23851.40. [DOI] [PubMed] [Google Scholar]
- 42.Yezierski RP, King C, Morgan D, Carter C, Vierck CJ. Effects of age on thermal sensitivity in the rat. J Gerontol A Biol Sci Med Sci. 2010;65:353–362. doi: 10.1093/gerona/glq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vierck CJ, Acosta-Rua A, Nelligan R, Tester N, Mauderli A. Low dose systemic morphine attenuates operant escape but facilitates innate reflex responses to thermal stimulation. J Pain. 2002;3:309–319. doi: 10.1054/jpai.2002.125186. [DOI] [PubMed] [Google Scholar]
- 44.King CD, Devine DP, Vierck CJ, Rodgers J, Yezierski RP. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain Res. 2003;987:214–222. doi: 10.1016/s0006-8993(03)03339-0. [DOI] [PubMed] [Google Scholar]
- 45.Raja SN, Meyer RA, Campbell JN. Peripheral mechanisms of somatic pain. Anesthesiology. 1988;68:571–590. doi: 10.1097/00000542-198804000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–323. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmelz M. Translating nociceptive processing into human pain models. Exp Brain Res. 2009;196:173–178. doi: 10.1007/s00221-009-1809-2. [DOI] [PubMed] [Google Scholar]
- 48.Harkins SW, Davis MD, Bush FM, Kasberger J. Suppression of first pain and slow temporal summation of second pain in relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:M260–M265. doi: 10.1093/gerona/51a.5.m260. [DOI] [PubMed] [Google Scholar]
- 49.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 50.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 51.Institute of Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 1996. [Google Scholar]
- 52.Gibson SJ. IASP global year against pain in older persons: highlighting the current status and future perspectives in geriatric pain. Expert Rev Neurother. 2007;7:627–635. doi: 10.1586/14737175.7.6.627. [DOI] [PubMed] [Google Scholar]
- 53.McCleane G. Pharmacological pain management in the elderly patient. Clin Interv Aging. 2007;2:637–643. doi: 10.2147/cia.s1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takai Y, Yamamoto-Mitani N, Okamoto Y, Koyama K, Honda A. Literature review of pain prevalence among older residents of nursing homes. Pain Manag Nurs. 2010;11:209–223. doi: 10.1016/j.pmn.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Gibson SJ, Farrell MJ. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. doi: 10.1097/00002508-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med. 2001;17:433–456. doi: 10.1016/s0749-0690(05)70079-3. [DOI] [PubMed] [Google Scholar]
- 57.Cooper BY, Vierck CJ Jr, Yeomans DC. Selective reduction of second pain sensations by systemic morphine in humans. Pain. 1986;24:93–116. doi: 10.1016/0304-3959(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 58.Lomas LM, Picker MJ. Behavioral assessment of temporal summation in the rat: sensitivity to sex, opioids and modulation by NMDA receptor antagonists. Psychopharmacology. 2005;180:84–94. doi: 10.1007/s00213-005-2153-2. [DOI] [PubMed] [Google Scholar]
- 59.Helme RD, Meliala A, Gibson SJ. Methodologic factors which contribute to variations in experimental pain threshold reported for older people. Neurosci Lett. 2004;6:144–146. doi: 10.1016/j.neulet.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 61.Conley KM, Toledano AY, Apfelbaum JL, Zacny JP. Modulating effects of a cold water stimulus on opioid effects in volunteers. Psychopharmacology. 1997;131:313–320. doi: 10.1007/s002130050298. [DOI] [PubMed] [Google Scholar]
- 62.Luginbühl M, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Zbinden AM. Comparison of five experimental pain tests to measure analgesic effects of alfentanil. Anesthesiology. 2001;95:22–29. doi: 10.1097/00000542-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Koltzenburg M, Pokorny R, Gasser UE, Richarz U. Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain. 2006;126:165–174. doi: 10.1016/j.pain.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 64.Pud D, Yarnitsky D, Sprecher E, Rogowski Z, Adler R, Eisenberg E. Can personality traits and gender predict the response to morphine? An experimental cold pain study. Eur J Pain. 2006;10:103–112. doi: 10.1016/j.ejpain.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Comer SD, Cooper ZD, Kowalczyk WJ, et al. Evaluation of potential sex differences in the subjective and analgesic effects of morphine in normal, healthy volunteers. Psychopharmacology. 2010;208:45–55. doi: 10.1007/s00213-009-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen XH, Adams JU, Geller EB, DeRiel JK, Adler MW, Liu-Chen LY. An antisense oligodeoxynucleotide to mu-opioid receptors inhibits mu-opioid receptor agonist-induced analgesia in rats. Eur J Pharmacol. 1995;275:105–108. doi: 10.1016/0014-2999(95)00012-a. [DOI] [PubMed] [Google Scholar]
- 67.Vissers KC, Hoffmann VL, Adriaensen HF, Heylen RJ, Meert TF. Increased cold allodynia following intrathecal N-methyl-D-aspartate in rats with a mononeuropathy. Life Sci. 2005;77:414–422. doi: 10.1016/j.lfs.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 68.Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol. 2007;560:142–149. doi: 10.1016/j.ejphar.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Tanimoto-Mori S, Nakazato-Imasato E, Toide K, Kita Y. Pharmacologic investigation of the mechanism underlying cold allodynia using a new cold plate procedure in rats with chronic constriction injuries. Behav Pharmacol. 2008;19:85–90. doi: 10.1097/FBP.0b013e3282f3d0a3. [DOI] [PubMed] [Google Scholar]
- 70.Datta S, Chatterjee K, Kline RH, 4th, Wiley RG. Behavioral and anatomical characterization of the bilateral sciatic nerve chronic constriction (bCCI) injury: correlation of anatomic changes and responses to cold stimuli. Mol Pain. 2010;6:7. doi: 10.1186/1744-8069-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berge OG, Hole K. Tolerance to the antinociceptive effect of morphine in the spinal rat. Neuropharmacology. 1981;20:653–657. doi: 10.1016/0028-3908(81)90112-x. [DOI] [PubMed] [Google Scholar]
- 72.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 73.Martin TJ, Kahn WR, Eisenach JC. Abdominal surgery decreases food-reinforced operant responding in rats: relevance of incisional pain. Anesthesiology. 2005;103:629–637. doi: 10.1097/00000542-200509000-00028. [DOI] [PubMed] [Google Scholar]
- 74.Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- 76.Stevenson GW, Cormier J, Mercer H, et al. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LaBuda CJ, Fuchs PN. Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain. Neurosci Lett. 2000;290:137–140. doi: 10.1016/s0304-3940(00)01340-9. [DOI] [PubMed] [Google Scholar]
- 78.Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain. 2009;10:767–773. doi: 10.1016/j.jpain.2009.01.325. [DOI] [PubMed] [Google Scholar]
- 79.Mauderli AP, Acosta-Rua A, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. J Neurosci Methods. 2000;97:19–29. doi: 10.1016/s0165-0270(00)00160-6. [DOI] [PubMed] [Google Scholar]
- 80.Baliki M, Calvo O, Chialvo DR. Spared nerve injury rats exhibit thermal hyperalgesia on an automated operant dynamic thermal escape task. Mol Pain. 2005;1:18. doi: 10.1186/1744-8069-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


