Abstract
Objectives.
Typical measures of the useful field of view (UFOV) involve many components of attention. The objective of the current research was to examine the attentional operations that might underlie declines in the UFOV.
Method and Results.
We used 2 basic attention tasks to characterize the profile of visual attention in UFOV-impaired and -unimpaired observers. Our results suggested that declines in the UFOV result from a deficit in attentional disengagement, not a decrease in attentional breadth or scope.
Discussion.
The results suggested that UFOV decline in normal aging can be associated with a specific attentional operation, namely attentional disengagement. These results suggest that the underlying cause of UFOV decline may not be a restriction in the breadth or scope of attention. Because the UFOV is a reliable predictor of driving safety, our results point to attentional components that are critical for the visual behavior of older adults.
Keywords: Aging, Attention, Attentional disengagement, Cognitive decline, Useful field of view
Normal aging produces declines in many cognitive processes, including attention, and attentional difficulties may compromise everyday behavior for older adults. For example, older adults are at greater risk for automobile accidents than younger drivers. This increased risk is due, in part, to visual declines during aging, such as the ability to extract visual information from the environment. Although some age-related visual decline is associated with changes in low-level vision (e.g., visual acuity), substantial age-related visual decline results from decrements in higher level visual processes, such as visual attention (e.g., Kramer & Madden, 2008).
Several studies hypothesize that a primary cause of attentional limitations in older adults is a constriction in attentional breadth or scope, which reduces the area over which observers can process visual information in a single glance (e.g., Ball, Owsley, Sloane, Roenker, & Bruni, 1993; Kosslyn, Brown, & Dror, 1999). A constriction in this “functional” or “useful” field of view (UFOV; Ball, Owsley, & Beard, 1990; Sanders, 1970) is supported by studies showing that older individuals exhibit decrements in peripheral target localization and discrimination, particularly in cluttered or noisy visual environments (Ball et al., 1990; Scialfa, Kline, & Lyman, 1987; R. Sekuler & Ball, 1986). However, both localizing and discriminating visual targets involve not only the scope of visual attention but several other attentional processes. In the current research, we challenge the “attentional constriction” hypothesis of visual decline in normal aging, suggesting that adults with accelerated attentional decline might show a disengage deficit, that is, an impairment in disengaging attention. This disengage deficit may, in turn, produce patterns of behavior similar to an attentional constriction on some tasks.
In normal and clinical populations, attentional scope is often measured with a computerized screening task that measures the UFOV (Ball & Owsley, 1993; Edwards et al., 2005, 2006). The UFOV screening task predicts driving safety and performance on other everyday tasks. However, a careful consideration of this screening task suggests that the underlying difficulty might lie in disengaging attention from its current locus, rather than a constriction of attentional scope. There are several versions of the UFOV task (see Edwards et al., 2005), and each is composed of several subtests of varying difficulty. Each subtest assesses the exposure duration required for an observer to perform at an accuracy level of 75%. The subtests that are predictive of driving and other complex behaviors involve both selective and divided attention. In the four-subtest version of the UFOV screening, Subtest 1 asks observers to identify a single object (car or truck) presented at fixation; Subtest 2 requires observers to identify the object at fixation and to simultaneously localize a peripheral target that appears in an otherwise clear field; Subtest 3 is identical to Subtest 2, except that the peripheral target appears among distractors; finally, Subtest 4 involves reporting if two objects at fixation are the same or different while performing peripheral localization among distractors.
An attentional constriction account readily explains UFOV decline on Subtests 3 and 4. If attention becomes narrowed during normal aging, through mild cognitive impairment or following brain injury, then attention will be directed disproportionately to the task at fixation. Processing of the peripheral target will suffer, requiring increased exposure durations to reach performance criteria, a result found in both aging and clinical populations (e.g., Rizzo et al., 2005; Uc et al., 2005).
However, disruptions in other attentional processes could also predict results characteristic of UFOV decline. Because the UFOV subtests are complex, they tap multiple attentional components. For example, rather than dividing attention between central discrimination and peripheral localization, observers may instead disengage attention from the central object and rapidly search for the peripheral stimulus when it appears among distractors. Furthermore, in some subtests, observers must actively search for the peripheral target because it appears among visual noise. Thus, rather than strictly measuring the breadth of attention, the UFOV assay may place demands on basic attentional control processes that are important for efficiently extracting visual information from the environment. Thus, UFOV impairment may result from dysfunction in a number of basic control functions, which may include reduced attentional breadth but could also include an inability to disengage attention from its current focus. Given that basic aspects of attentional function, such as the ability to disengage and voluntarily shift (i.e., move) attention, have been associated with age-related declines in cognitive function (e.g., Castel, Chasteen, Scialfa, & Pratt, 2003; Rösler, Mapstone, Hays-Wicklund, Gitelman, & Weintraub, 2005; Trick & Enns, 1998), it is likely that age-related declines in the UFOV may be directly related to basic attentional operations other than attentional constriction.
In the current study, we studied two aspects of attention in older observers who either did or did not show UFOV impairments (also see Weaver, Bédard, Jim McAuliffe, & Parkkari, 2009), namely attentional breadth and attentional disengagement. Based on performance on a standardized UFOV task (Edwards et al., 2006; Rizzo et al., 2004), observers were classified as either “UFOV impaired” or “UFOV unimpaired.” Observers in both groups performed two basic attention tasks that provide measures of different core attentional functions. Observers performed a flanker task (B. A. Eriksen & Eriksen, 1974) in which they discriminated targets that appeared at fixation. Two irrelevant flanking distractors appeared in the periphery at varying eccentricities, and these distractors were either congruent or incongruent with the target. Congruent flankers produce shorter discrimination response times than incongruent flankers. The basic flanker effect has been used extensively as a general assay of selective attention and as an assay of the spatial profile of selective attention in particular (B. A. Eriksen & Eriksen, 1974; C. W. Eriksen & St. James, 1986; Pan & Eriksen, 1993). Furthermore, attention appears to spill over obligatorily to task irrelevant flankers in low perceptual load displays that contain few distractors (e.g., Lavie, 1995), particularly when flankers are salient (Cosman & Vecera, 2009, 2010). By varying the eccentricity of the flankers, we can indirectly measure the breadth of attention to test the constriction hypothesis of UFOV decline.
Observers also performed a spatial cuing task (Posner, 1980) in which a target is preceded by a spatial precue that either predicts the target’s location (valid cues) or does not predict the target’s location (invalid cues). The spatial cuing task can distinguish many different attentional operations, including the movement, engagement, and disengagement of attention (see Posner, 1988).
Impairments in different attentional operations lead to different predictions on each of our attention tasks. If attention is constricted in UFOV decline, then we should observe a reduced flanker interference effect in UFOV-impaired observers than in unimpaired observers. Additionally, reaction times (RTs) in the spatial cuing task would be longer overall in UFOV-impaired individuals because both the precue and target would fall in the periphery outside the primary focus of attention.
In contrast, if UFOV decline was produced by a disengage deficit, then only spatial cuing would be affected in UFOV-impaired individuals because attention remains engaged on the target location in the flanker task; neither disengagement nor visual search is required in this task because the target’s position is fixed. In spatial cuing, UFOV-impaired individuals would show disproportionately slow response times to invalid trials compared with unimpaired observers because invalid trials require an attentional disengagement from the cued location before directing attention to the target at another location. On valid trials, the spatial cue pulls attention to the target’s location; only on invalid trials must attention disengage from the cued location. Similar response times on valid trials for the UFOV-impaired and -unimpaired groups would indicate that attentional engagement by the cue, as well as the movement of attention to the cued location, are similar between the two participant groups.
METHOD
Participants
Eight males and 12 females between the ages of 66 and 87 participated; observers were recruited from the Iowa City, IA, area through newspaper ads, flyers, and screening sessions. We obtained 10 observers with UFOV impairments (mean age = 79.1, SD = 5.4), age matched with 10 observers who did not (mean age = 78.3, SD = 6.9). All observers had normal or corrected vision of at least 20/40, and no observers met screening criteria for dementia, assessed with the Mini-Mental State Examination (UFOV unimpaired, M = 29.1; UFOV impaired, M = 28.9). The impaired and unimpaired groups did not differ on contrast sensitivity, measured by the Pelli–Robson chart (UFOV unimpaired, M = log contrast 1.575; UFOV impaired, M = log contrast 1.485), or on complex figure copying (Rey–Osterrieth figure; mean copying time for UFOV impaired and unimpaired = 1.8 min). Observers performed two tasks, described below, and we counterbalanced the order of these tasks.
The UFOV Classification
The standard UFOV test (see Edwards et al., 2005) has several subtests, as described above. Our observers performed all subtests. We measured the presentation duration required to maintain 75% accuracy in each subtest. To identify participants with UFOV decline, we examined performance on the subtests that required (a) central discrimination and peripheral localization among distractors and (b) central discrimination and peripheral discrimination among distractors (i.e., Subtests 3 and 4, respectively). Previous work has defined UFOV impairment as a score of 800 ms or higher on the sum of Subtests 3 and 4 (Vance et al., 2007). Because of difficulties recruiting participants that met this criterion, we defined UFOV impairment less stringently: We defined UFOV-impaired individuals as those having a score of 500 ms on Subtest 4 and having a Subtests 3 and 4 total of 690 ms or greater. We chose these criteria in part because our sample did not contain any profound difficulties on Subtest 3 that would be required to meet Vance and colleagues’ (2007) inclusion criterion of an 800 ms total. The UFOV results for our two groups appear in Table 1.
Table 1.
UFOV Task Scores for the Impaired and Unimpaired Groups
| UFOV-impaired group (ms) | UFOV-unimpaired group (ms) | |
| Subtest 1 | 17.1 | 17.2 |
| Subtest 2 | 54.9 | 95.7a |
| Subtest 3 | 294.5 | 176.2 |
| Subtest 4 | 500 | 402.3 |
Notes. Scores are the mean exposure durations required to achieve 75% correct on a subtest.
The high Subtest 2 score for the UFOV-unimpaired group was caused by one participant in this group, who had an abnormally long exposure duration. This participant appeared typical for the group on the other subtests, and when this participant is removed, the average Subtest 2 score for the unimpaired group is 45.1 ms. This participant was included in the reported results, but excluding this participant did not alter the pattern of results we report.
Flanker Interference Task: Stimuli and Procedure
Observers sat approximately 55 cm from the monitor in a dimly lit room, and viewing distance was monitored by the experimenter throughout testing. Observers were instructed to focus attention at fixation and respond to the identity of a target letter (E or H) while trying to ignore the flanker letters on either side. At the beginning of each trial, a black fixation point appeared on a white background for 500 ms, followed by the stimulus array, which remained on the screen until observers responded. The stimulus array consisted of a single 1.0° by 1.3° black target letter centered at fixation and two identical black flanker letters (one to the left and one to the right of the target letter) that were either congruent (e.g., E target/E flankers) or incongruent with the target (e.g., E target, H flankers). The flanker letters could appear at one of four eccentricities (1°, 2°, 4°, and 8°), and each flanker letter was cortically scaled to match the size of the target letter. This resulted in flanker sizes of 1.0° by 1.3°, 1.3° by 1.7°, 1.7° by 2.1°, and 2.2° by 2.9° for flanker eccentricities of 1°, 2°, 4°, and 8°, respectively. All trial types were intermixed, such that each trial was equally likely to contain congruent or incongruent flankers, at any of the four eccentricities. Following a 32-trial practice block, observers performed 6 blocks of 32 trials.
Spatial Cuing Task: Stimuli and Procedure
Observers sat 55 cm from the monitor in a dimly lit room; viewing distance was again monitored by the experimenter throughout testing. Observers identified a single target that appeared at one of two locations, either 5.0° to the left or right of fixation. Prior to the target, a nonpredictive peripheral cue, a 0.4° diameter red dot appearing 5.0° from fixation (the center of a possible target location), was presented for 100 ms either at the upcoming target location (valid cue) or the opposite location (invalid cue) with equal frequency. Following a fixed stimulus-onset asynchrony (SOA) of 100 ms, a target appeared until observers responded; we used a short SOA to minimize the possibility of eye movements to the cue. The target was a black Landolt square measuring 1.5° by 1.5°, with a 0.6° gap either in the top or bottom. Observers reported the location of the gap (top or bottom) as quickly and accurately as possible. Valid and invalid trials were intermixed. Following a 24-trial practice block, observers performed 10 blocks of 24 trials.
RESULTS
Flanker Interference Task
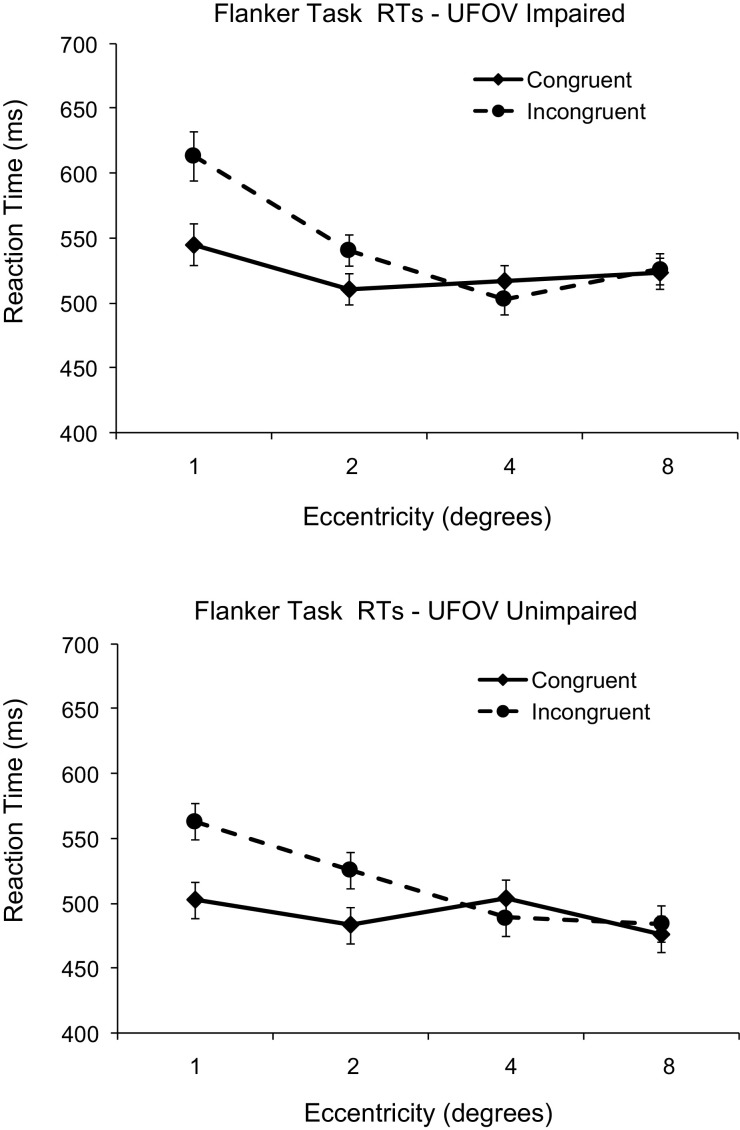
Flanker interference effects were defined as the difference in RT between congruent trials and incongruent trials. The RTs from the flanker task for both UFOV unimpaired and impaired observers are depicted in Figure 1; accuracy data appear in Table 2. Data were analyzed using a mixed-model three-factor ANOVA, with UFOV status (impaired vs. unimpaired) as a between-subjects factor and flanker eccentricity (1°, 2°, 4°, and 8°) and flanker compatibility (congruent vs. incongruent) as within-subjects factors.
Figure 1.
Magnitude of the flanker effect at each eccentricity in both useful field of view (UFOV)-impaired and -unimpaired observers. Error bars represent 95% confidence intervals for reaction times (RTs) to the central target for each compatibility condition (Cousineau, 2005; Loftus & Masson, 1994).
Table 2.
Accuracy Data From the Flanker Task
| UFOV-impaired group | UFOV-unimpaired group | |
| 1° Separation | ||
| Congruent | 99.2% (0.83) | 98.8% (0.83) |
| Incongruent | 90.2% (4.7) | 92.5% (2.8) |
| 2° Separation | ||
| Congruent | 98.8% (0.83) | 96.3% (1.3) |
| Incongruent | 98.3% (0.87) | 97.5% (1.4) |
| 4° Separation | ||
| Congruent | 98.5% (0.76) | 98.1% (1.4) |
| Incongruent | 98.8% (0.64) | 97.8% (0.95) |
| 8° Separation | ||
| Congruent | 96.9% (1.3) | 99.4% (0.63) |
| Incongruent | 98.1% (1.3) | 98.1% (0.95) |
Note. Standard errors appear in parentheses. UFOV = useful field of view.
We observed significant main effects of flanker eccentricity, F(3, 54) = 4.0, p < .02, = .18, and flanker compatibility, F(1, 18) = 4.81, p < .05, = .21, in the accuracy data, as well as a significant flanker eccentricity by compatibility interaction, F(3, 54) < 4.67, p < .01, = .21. No other main effects or interactions in the accuracy data were significant, Fs < 0.63, ps >.60, all < .04. For RTs, there was a nonsignificant difference in overall RTs between UFOV-impaired (M = 529 ms) and -unimpaired (M = 503 ms) observers, F(1, 18) < 1, p > .41, = .04. There were main effects of both flanker compatibility, with shorter RTs to trials with congruent flankers (M = 505 ms) than to those with incongruent flankers (M = 528 ms), F(1, 18) = 57.88, p < .001, = .76. There was also a main effect of flanker eccentricity, with shorter RTs as the flankers fell further in the periphery, F(3, 54) = 38.99, p < .001, = .68.
As can be seen in Figure 1, there was a two-way interaction between compatibility and eccentricity, F(3, 54) = 29.37, p < .001, = .62. Clearly, there was a large flanker effect at the closest two flanker eccentricities (1° and 2°), but these effects were eliminated at the farthest eccentricities (4° and 8°). The difference between congruent and incongruent flankers was significant at both the 1° separation, t(19) = 11.01, p < .001, and the 2° separation, t(19) = 5.66, p < .001. But, most important, there was neither an interaction between UFOV status and compatibility, F(1, 18) < 1, p > .62, = .014, nor an interaction between UFOV status and eccentricity, F(3, 54) = 2.09, p > .11, = .10. Finally, there was no three-way interaction, F(3, 54) < 1, p > .68, = .027. The lack of any interaction between UFOV status and flanker compatibility indicates that the flanker interference effect was similar for each UFOV group.
Because of the numeric difference in baseline RTs between the impaired and unimpaired groups, we performed a further analysis on log RTs to further explore the effect of UFOV status on the overall pattern of results. In general, log RTs produced results similar to untransformed RTs. There was a main effect of eccentricity, F(3, 54) = 15.56, p < .001, = .46, and of compatibility, F(1, 18) = 6.74, p < .02, = .27. There was a difference between log RTs for impaired and unimpaired participants, F(1, 18) = 24.95, p < .01, = .58. The interaction between eccentricity and congruency remained, F(3, 54) = 10.43, = .37. As with the nontransformed RTs, there was neither an interaction between UFOV status and compatibility, F(1, 18) = 1.57, p > .22, = .08, nor an interaction between UFOV status and eccentricity, F(3, 54) = 2.35, p > .08, = .116. Finally, there was no three-way interaction, F(3, 54) < 1, p > .37, = .036.
Critically, across all of our analyses, we found no difference between flanker interference in UFOV-impaired and -unimpaired observers, indicating that UFOV decline may not be the result of attentional constriction. UFOV-impaired individuals showed normal selective attention ability across eccentricities, as evidenced by the similar magnitude of flanker interference effects between the groups. An attentional constriction account would have predicted that UFOV-impaired individuals show less of a flanker interference effect than unimpaired observers. Of course, it remains possible that the constriction in UFOV impairment constricts attention to the central 2° and that normal attentional breadth extends to 3°; such a scenario would explain the flanker effect in both groups at the 2° separation and the lack of a flanker effect in both groups at the 4° separation. However, this interpretation seems tenuous because it requires that UFOV impairment is caused by a slight 1° constriction difference between impaired and unimpaired individuals. Such a small difference seems unlikely to explain the myriad consequences of having a UFOV impairment that have been reported in the literature.
Spatial Cuing Task
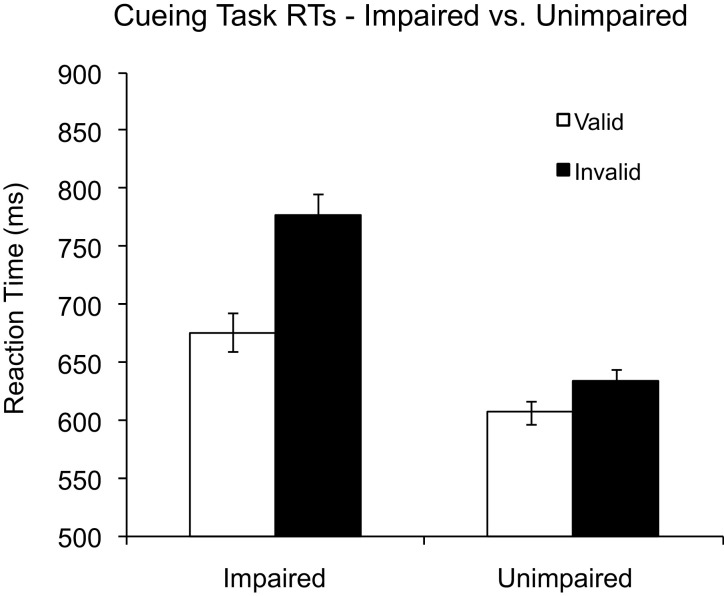
Observers’ mean RT data for each condition are shown in Figure 2, and these data were analyzed using a mixed-model ANOVA, with UFOV status (impaired vs. unimpaired) as a between-subjects factor and cue validity (valid vs. invalid) as a within-subjects factor. The accuracy data appear in Table 3.
Figure 2.
Mean reaction times for the spatial cuing task in both useful field of view (UFOV)-impaired and -unimpaired observers. Error bars represent 95% confidence intervals for reaction times (RTs) to the target for each cue validity condition (Cousineau, 2005; Loftus & Masson, 1994).
Table 3.
Accuracy Data From the Spatial Cuing Task
| UFOV-impaired group | UFOV-unimpaired group | |
| Valid trials | 97.8% (0.65) | 95.6% (2.1) |
| Invalid trials | 98.3% (0.79) | 96.0% (1.9) |
Notes. Standard errors appear in parentheses. UFOV = useful field of view.
There were no significant main effects or interactions in the accuracy data, Fs < 0.6, ps > .46. For RTs, we observed a main effect of validity, F(1, 18) = 27.1, p < .0001, = .60, with shorter RTs to valid trials (M = 617 ms) than to invalid trials (M = 670 ms). The main effect of UFOV status was significant, F(1, 18) = 4.2, p = .05, = .20. Most important, there was a significant interaction between UFOV status and cue validity, F(1, 18) = 9.0, p < .01, = .33. Both groups exhibited shorter RTs to valid trials than to invalid trials, for unimpaired, t(9) = 2.6, p < .04, and for impaired, t(9) = 4.5, p < .005. Planned comparisons revealed a significant between-group differences on invalid trials, with impaired observers responding significantly slower (M = 777 ms) than unimpaired observers (M = 635 ms), t(18) = 2.9, p < .01, but RTs to validly cued targets did not differ, t(18) = 1.6, p > .10.
As before, we analyzed log RTs to minimize the baseline RT differences between the UFOV-impaired and -unimpaired participants. Log RTs exhibited the same pattern as the untransformed RTs. There was a main effect of validity, F(1, 18) = 41.8, p < .0001, = .70, and a main effect of UFOV status, F(1, 18) = 4.7, p < .05, = .20. Importantly, the interaction remained significant, F(1, 18) = 12.9, p < .005, = .42. Again, both groups responded shorter to valid trials than to invalid trials, t(9) = 2.6, p < .02 for unimpaired and t(9) = 4.5, p < .001 for impaired. As with the untransformed RTs, the main effect of UFOV status was driven primarily by the invalid trials, which differed between the groups, t(18) = 2.6, p < .02; valid trials did not differ between the groups, t(18) = 1.5, p > .14.
Taken together with the results from the flanker task, the current findings suggest that individuals with UFOV impairment have difficulties disengaging attention from a cued location, as evidenced by disproportionately longer RTs on invalid trials compared with unimpaired observers. Furthermore, the finding that our impaired observers were not significantly slower than the unimpaired observers to efficiently shift attention to the cued location on valid trials is important: This finding demonstrates that UFOV impairment is unlikely to be due to either the movement of or engagement of attention but rather to the disengagement of attention (see Losier & Klein, 2001; Posner, Walker, Friedrich, & Rafal, 1984; Vecera & Flevaris, 2005). UFOV-impaired individuals appear to have “sticky” attention, which slows the disengagement of attention from its current locus.
DISCUSSION
Our results suggest that impairments on the most difficult UFOV subtests—those involving attending to fixation and then to the periphery—are associated with deficits in basic attentional operations, namely the ability to disengage attention. Importantly, our impaired participants showed attentional disengagement difficulties even though our definition of UFOV impairment was not as extreme as other studies (e.g., Vance et al., 2007): The disengage deficit we reported appears to be very sensitive and can distinguish among individuals who would have been classified as unimpaired by other criteria.
UFOV impairment on the most difficult subtests does not appear to be due to a simple constriction in the breadth of attention. This interpretation is also consistent with the notion that efficient performance in the UFOV task relies on basic attentional control processes. We hypothesize that in the standardized UFOV assessment, subtests that require attention to be directed at fixation and then into the periphery show reliable aging differences because these subtests require attentional disengagement. To the extent that attentional disengagement is impaired, UFOV impairments will be observed. Note that impairments in simpler subtests (e.g., Subtest 1) may be caused by impairments in low-level visual processes, such as acuity or contrast sensitivity, although impairments in these subtests during normal aging are uncommon. Our claims regarding attentional disengagement hold for UFOV decline that does not include impairments in the lowest subtests or in low-level vision.
Our mechanistic description of UFOV impairment is in line with studies that have shown deficits in the efficiency of attentional shifting and the disengagement of attention in advancing age (Castel et al., 2003; Oken, Kishiyama, & Kaye, 1994; Rösler et al., 2005; Trick & Enns, 1998). Some recent work (Weaver et al., 2009) reported a modest correlation between a flanker task in the Attention Network Task (ANT; Fan, McCandliss, Sommer, Raz, & Posner, 2002) and UFOV scores. However, these correlations were modest and driven by a handful of outliers, and the flanker component of the ANT differs greatly from the version reported here. Further work using the ANT to understand UFOV performance should include an assessment of attentional disengagement as another predictor variable.
A more recent alternative view of the UFOV measure is that it taps the speed or efficiency of visual processing (Lunsman et al., 2008; A. B. Sekuler, Bennett, & Mamelak, 2000; Vance et al., 2007). Such accounts necessarily propose that speed of processing interacts with tasks because UFOV impairment is typically most pronounced for the more difficult subtests. Our results are not inconsistent with a speed of processing view, particularly if one views our findings as pointing to a specific attentional process (disengagement) that differs in speed or efficiency between UFOV-impaired and -unimpaired individuals.
One point for discussion is the baseline RT differences across UFOV-impaired and -unimpaired participants. Although we have attempted to minimize these baseline differences by analyzing log RTs, it remains possible that overall speed of processing differences might contribute to our findings. However, overall speed of processing does not predict an attentional disengagement in other work (e.g., Vecera & Flevaris, 2005) nor does speed of processing alone readily explain intact flanker interference yet impaired disengagement in UFOV-impaired participants.
We acknowledge that our results are correlational in demonstrating that individuals with UFOV impairment also show a disengage impairment. However, in light of our task analysis of the UFOV screening, it is more parsimonious to argue that a single deficit in attentional disengagement produces impairments in both UFOV screening and spatial cuing than to argue for separate causes to these impairments. Of course, further evidence for a disengage deficit will be important for making a stronger case that this attentional operation produces deleterious effects in older adults. Similarly, we acknowledge that the two tasks of interest, the UFOV assay and the spatial cuing task, are very different and likely require different attentional processes. These differences, coupled with intact attentional operations, might contribute to the correlated performance between the UFOV screening and spatial cuing. For example, participants with UFOV impairment might be unable to divide attention between central and peripheral stimuli, producing a UFOV impairment. These same participants might show a large spatial cuing effect, which appears as a disengage problem but is instead the result of participants’ attempt at ignoring the distracting, unpredictive cue in the spatial cuing task by constricting attention. Our results cannot address such possibilities, and future work should continue to explore the attentional processes that underlie UFOV performance. However, this potential difficulty is not specific to our tasks of interest; it can also occur for comparisons between pairs of very different cognitive tasks. Furthermore, we should acknowledge that manipulations within a single task could affect the relationship between a pair of tasks. For example, predictive spatial cues might require an attentional configuration very different from unpredictive spatial cues. Predictive cues might encourage a wider attentional focus than nonpredictive cues because predictive cues can be used to optimize performance; consequently, predictive cues might be more closely related to the task requirements imposed by UFOV screening in which two visual events must both be attended. One advantage of our approach is that we have relied on known attentional processes and a processing framework to link performance in the UFOV screening task to that in the spatial cuing task.
Although our results point to a disengage deficit in UFOV-impaired individuals, we do not necessarily take this to imply a neurally localizable disengage process (see Posner et al., 1984). Instead, attentional disengagement might arise from competitive interactions within the representation of objects or locations (e.g., Cohen, Romero, Servan-Schreiber, & Farah, 1994) or from an imbalance in bottom–up attentional inputs (Vecera & Flevaris, 2005). UFOV declines might, ultimately, be caused by a more general decline in attentional competition instead of a disengage deficit specifically. For example, attentional disengagement might rely on endogenous attentional processes under the control of “executive” processes; thus, the disengage deficit might be a sensitive measure of early executive decline, such as that hypothesized by some accounts of cognitive aging (e.g., West, 1996; West & Bowry, 2005).
A disengage deficit and the corresponding UFOV decline have a number of possible everyday consequences. For example, driving requires an operator to shift attention between in-vehicle task performance and the environment, and such attentional shifts will require disengagement from one item before attending to another. A disengage deficit could therefore lead to the increased accident rates that characterize UFOV-impaired individuals (Ball et al., 1993; Clay et al., 2005; Owsley, Ball, et al., 1998; Owsley McGwin et al., 1998). Furthermore, it may be possible that training UFOV-impaired observers in disengaging attention specifically could produce visual improvements, including improvements in everyday tasks such as driving (see Vance et al., 2007). Thus, a better understanding of the component attentional processes that lead to UFOV impairments may lead to more precisely tailored remediation of attention impairments seen in aging.
Funding
This research was supported in part by grants from the National Science Foundation (BCS 03-39171), the National Institutes of Health (R01AG026027), and by a research contract from the Nissan Motor Corporation.
References
- Ball K, Owsley C, Beard B. Clinical visual perimetry underestimates peripheral field problems in older adults. Clinical Vision Sciences. 1990;5:113–125. [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology and Visual Science. 1993;34:3110–3123. [PubMed] [Google Scholar]
- Castel AD, Chasteen AC, Scialfa CT, Pratt J. Adult age difference in the time course of inhibition of return. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2003;58:256–259. doi: 10.1093/geronb/58.5.p256. doi:10.1093/geronb/58.5.P256. [DOI] [PubMed] [Google Scholar]
- Clay OJ, Wadley VG, Edwards JD, Roth DL, Roenker DL, Ball KK. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: Current and future implications. Optometry and Vision Science. 2005;82:724–731. doi: 10.1097/01.opx.0000175009.08626.65. doi:10.1097/01.opx.0000175009.08626.65. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Romero RD, Servan-Schreiber D, Farah MJ. Mechanisms of spatial attention: The relation of macrostructure to microstructure in parietal neglect. Journal of Cognitive Neuroscience. 1994;6:377–387. doi: 10.1162/jocn.1994.6.4.377. doi:10.1162/jocn.1994.6.4.377. [DOI] [PubMed] [Google Scholar]
- Cosman JD, Vecera SP. Perceptual load modulates attentional capture by abrupt onsets. Psychonomic Bulletin & Review. 2009;16:404–410. doi: 10.3758/PBR.16.2.404. doi:10.3758/PBR.16.2.404. [DOI] [PubMed] [Google Scholar]
- Cosman JD, Vecera SP. Attentional capture by motion onsets is modulated by perceptual load. Attention, Perception, & Psychophysics. 2010;72:2096–2105. doi: 10.3758/bf03196686. doi:10.3758/APP. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology. 2005;1:42–45. [Google Scholar]
- Edwards JD, Ross LA, Wadley VG, Clay OJ, Crowe M, Roenker DL, Ball KK. The useful field of view test: normative data for older adults. Archives of Clinical Neuropsychology. 2006;21:275–286. doi: 10.1016/j.acn.2006.03.001. doi:10.1016/j.acn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. doi:10.3758/BF03203267. [Google Scholar]
- Eriksen CW, St. James JD. Visual attention within and around the field of focal attention: A zoom lens model. Perception & Psychophysics. 1986;40:225–240. doi: 10.3758/bf03211502. doi:10.3758/BF03211502. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz M, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. doi:10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Brown HD, Dror IE. Aging and the scope of visual attention. Gerontology. 1999;45:102–109. doi: 10.1159/000022071. doi:10.1159/000022071. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. New York, NY: Psychology Press; 2008. pp. 189–250. [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin and Review. 1994;1:476–490. doi: 10.3758/BF03210951. doi:10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Losier BJW, Klein RM. A review of the evidence for a disengage deficit following parietal lobe damage. Neuroscience and Biobehavioral Reviews. 2001;25:1–13. doi: 10.1016/s0149-7634(00)00046-4. doi:10.1016/S0149-7634(00)00046-4. [DOI] [PubMed] [Google Scholar]
- Lunsman M, Edwards JD, Andel R, Small BJ, Ball KK, Roenker DL. What predicts changes in useful field of view test performance? Psychology and Aging. 2008;23:917–927. doi: 10.1037/a0013466. doi:10.1037/a0013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Kishiyama SS, Kaye JA. Age-related differences in visual-search task performance—Relative stability of parallel but not serial search. Journal of Geriatric Psychiatry and Neurology. 1994;3:163–168. doi: 10.1177/089198879400700307. doi:10.1177/089198879400700307. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Jr., Sloane ME, Roenker DL, White MF, Overley E. T. Visual processing impairment and risk of motor-vehicle crash among older adults. Journal of the American Medical Association. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. doi:10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Jr., Ball K. Vision impairment, eye disease, and injurious motor vehicle crashes in the elderly. Ophthalmic Epidemiology. 1998;5:101–113. doi: 10.1076/opep.5.2.101.1574. doi:10.1076/opep.5.2.101.1574. [DOI] [PubMed] [Google Scholar]
- Pan K, Eriksen CW. Attentional distribution in the visual field during same-different judgments as assessed by response competition. Perception & Psychophysics. 1993;53:134–144. doi: 10.3758/bf03211723. doi:10.3758/BF03211723. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:2–25. doi: 10.1080/00335558008248231. doi:10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Structures and functions of selective attention. In: Boll T, Bryant B, editors. Clinical neuropsychology and brain function: Research, measurement, and practice. Washington, DC: American Psychological Association; 1988. pp. 173–202. doi:10.1037/10063-005. [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal lobe injury on covert orienting of visual attention. Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M, Shi Q, Dawson J, Anderson S, Kellison I, Pietras T. Stops for cops: Impaired response implementation for older drivers with cognitive decline. Transportation Research Record. 2005;1922:1–8. doi:10.3141/1922-01. [Google Scholar]
- Rösler A, Mapstone M, Hays-Wicklund A, Gitelman DR, Weintraub S. The “zoom lens” of focal attention in visual search: Changes in aging and Alzheimer's disease. Cortex. 2005;41:512–519. doi: 10.1016/s0010-9452(08)70191-6. doi:10.1016/S0010-9452(08)70191-6. [DOI] [PubMed] [Google Scholar]
- Sanders AF. Some aspects of the selective process in the functional visual field. Ergonomics. 1970;13:101–117. doi: 10.1080/00140137008931124. doi:10.1080/00140137008931124. [DOI] [PubMed] [Google Scholar]
- Scialfa CT, Kline DW, Lyman BJ. Age differences in target identification as a function of retinal location and noise level: an examination of the useful field of view. Psychology and Aging. 1987;2:14–19. doi: 10.1037//0882-7974.2.1.14. doi:10.1037/0882-7974.2.1.14. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Ball K. Visual localization: Age and practice. Journal of the Optical Society of America. 1986;3:864–867. doi: 10.1364/josaa.3.000864. doi:10.1364/JOSAA.3.000864. [DOI] [PubMed] [Google Scholar]
- Sekuler AB, Bennett PJ, Mamelak M. Effects of aging on the useful field of view. Experimental Aging Research. 2000;26:103–120. doi: 10.1080/036107300243588. doi:10.1080/036107300243588. [DOI] [PubMed] [Google Scholar]
- Trick LM, Enns JT. Lifespan changes in attention: The visual search task. Cognitive Development. 1998;13:369–386. doi:10.1016/S0885-2014(98)90016-8. [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver landmark and traffic sign identification in early Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:764–768. doi: 10.1136/jnnp.2004.049338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D, Dawson J, Wadley V, Edwards J, Roenker D, Rizzo M, Ball K. The accelerate study: The longitudinal effect of speed of processing training on cognitive performance of older adults. Rehabilitation Psychology. 2007;52:89–96. doi:10.1037/0090-5550.52.1.89. [Google Scholar]
- Vecera SP, Flevaris AV. Attentional control parameters following parietal lobe damage: Evidence from normal subjects. Neuropsychologia. 2005;43:1189–1203. doi: 10.1016/j.neuropsychologia.2004.10.009. doi:10.1016/j.neuropsychologia.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Weaver B, Bédard M, Jim McAuliffe J, Parkkari M. Using the Attention Network Test to predict driving test scores. Accident Analysis and Prevention. 2009;41:76–83. doi: 10.1016/j.aap.2008.09.006. doi:10.1016/j.aap.2008.09.006. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. doi:10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West RL, Bowry R. The aging of cognitive control: Studies of conflict processing, goal neglect, and error monitoring. In: Engle RW, Sedek G, von Hecker U, McIntosh DN, editors. Cognitive Limitations in Aging and Psychopathology: Attention, Working Memory, and Executive Functions. Cambridge, UK: Cambridge University Press; 2005. pp. 97–121. doi:10.1017/CBO9780511720413.006. [Google Scholar]