Abstract
Objectives.
We tested the hypothesis that aging is associated with an increase in the effort and costs associated with cognitive activity using systolic blood pressure (SBP) as a measure of effort.
Method.
Younger and older adults engaged in an initial task (Phase 1) for 5 min that was relatively low (adding single digits) or high (subtracting by 3 s) in cognitive demands. They then solved a series of multiplication problems for 3 min (Phase 2). Cardiovascular measures were collected throughout, and reactivity was examined as a function of age, initial task difficulty, and test phase.
Results.
Older adults exhibited higher levels of reactivity than younger adults to cognitive engagement, with reactivity increasing with task difficulty. Difficulty of the initial task was also associated with greater effort and lower performance on the subsequent multiplication task, suggestive of fatigue or depletion. These fatigue effects were stronger for older adults.
Discussion.
The results were consistent with expectations and provided support for the utility of SBP reactivity as a measure of cognitive effort in studies of aging.
Keywords: Aging, Cardiovascular reactivity, Cognition, Effort, Motivation
Many aspects of basic cognitive functioning become less efficient with increasing age in adulthood. Relative to younger adults, older adults have more difficulty initiating cognitive operations, take longer to perform such operations, and are more negatively affected by task complexity. These age-related variations in cognitive efficiency have often been attributed to declines in basic resources or processes thought to underlie cognition, such as speed of processing, working memory, and executive functions (e.g., McCabe, Roediger, McDaniel, Balota, & Hambrick, 2010; Park & Payer, 2006; Salthouse, 1996).
An alternative way of conceptualizing the mechanisms underlying these age differences in efficiency is in terms of the effort undergirding performance. Specifically, we hypothesize that aging is associated with an increase in the effort associated with performing a given cognitive activity. This leads to the straightforward expectation that older adults must expend more effort than younger adults to both initiate cognitive activity and achieve similar objective levels of performance. To the extent that effort requirements also place upper limits on task performance, aging-related decrements in performance would be expected when task demands (e.g., complexity) are high. Evidence consistent with this perspective can be found in research demonstrating that, relative to younger adults, older adults require greater environmental support to initiate strategic memory behaviors (Craik & Anderson, 1999), are more negatively affected by cognitive loads (Verhaeghen, Steitz, Sliwinski, & Cerella, 2003), and need to recruit additional areas of the brain to achieve successful performance (Cabeza, 2002).
This focus on age differences in effort leads to the additional expectation that engaging in demanding cognitive activity will have greater costs in later adulthood because more effort is required to achieve a specified level of performance. This assertion is supported by research showing that, relative to younger adults, older adults (a) display stronger cortisol responses during cognitive testing (Neupert, Miller, & Lachman, 2006), (b) recover more slowly from such stress-related responses (e.g., Seeman & Robbins, 1994), and (c) are slower at replenishing blood glucose levels in the brain following cognitive activity (Gold, 2005).
Although the aforementioned research appears consistent with increased effort and costs associated with cognitive activity in later life, support would be enhanced through more objective observations of effort. Behavioral indices of effort associated with self-report and performance have been used elsewhere but may be problematic in terms of reliability (self-report) and validity (performance). For example, time spent on a task might reflect engagement but may be relatively independent from intensity of effort. Given the energetic aspect of effort, it might be especially useful to examine valid indices linked to relevant physiological processes. In this regard, investigations with young adults have shown that certain aspects of the cardiovascular (CV) response reflect cognitive effort (for review, see Gendolla & Wright, 2005).
Obrist (1981) has argued that active coping (i.e., task engagement, as reflected by expenditure of effort) is mediated by sympathetic nervous system (SNS) activation of the CV system. Mobilization of energy to meet task demands is thought to increase myocardial β-adrenergic activity, resulting in increased stroke volume and heart rate (HR). Extending this idea, Brehm and Self (1989) have argued that the energy expended in a given task is positively associated with task difficulty, as long as the individual perceives that success is possible and that the task is worthwhile. Research based on an integration of Obrist’s active coping hypothesis and motivational intensity theory (Brehm & Self, 1989) has used standard measures of CV activity (e.g., HR, systolic blood pressure [SBP] and diastolic blood pressure [DBP]) to assess task engagement across a variety of contexts and has found that SBP is the more reliable standard CV index (for review, see Gendolla & Wright, 2005). More so than HR and DBP, SBP responds in a similar way to task difficulty as more direct indices of myocardial β-adrenergic activity (e.g., pre-ejection period; Richter, Friedrich, & Gendolla, 2008).
Consistent with it being a reliable index of mental effort, SBP has been found to increase systematically with task difficulty but only to the point where successful performance is perceived to be still possible (Wright & Dill, 1993). This qualification results in somewhat different expectations for performance functions when perception of ability is low versus high. Low ability perception resulted in greater SBP at lower levels of task difficulty and a tailing off of SBP—suggestive of withdrawal of effort—at an earlier point as task difficulty increased (e.g., Wright & Dill, 1993; Wright, Murray, Storey, & Williams, 1997). Thus, effort expenditure as reflected in SBP change is a function of task difficulty and ability perception.
SBP also appears be a useful index of fatigue or depletion, in that expenditure of effort in an initial task is related to expenditure in a cognitively demanding subsequent task. Specifically, Wright and colleagues (Wright, Martin, & Bland, 2003; Wright et al., 2007, Experiment 1) found that the greater the demands associated with an initial task, the more effort participants had to exert to achieve a specified level of performance in a second task. Demanding initial tasks deplete energy needed to sustain effort, necessitating increased exertion in subsequent tasks. Importantly, continued exertion across tasks is predicated on the individual perceiving successful performance as possible and worthwhile. Otherwise, cessation of exertion or disengagement is likely (Wright et al., 2007, Experiment 2). This can be viewed as analogous to physical fatigue, which results in an increase in both the perception of difficulty and the effort required to perform a specific function relative to when one is in a more rested state. To achieve the same effectiveness as a rested person, a physically fatigued person must work harder.
Adult age differences in CV reactivity have been assessed in a number of different settings (for review, see Uchino, Birmingham, & Berg, 2010). This research has suggested that HR is a poor index of engagement in studies of aging due to the fact that normative changes in the heart restrict reactivity with increasing age. Although SBP also changes, exhibiting a normative increase in later life, reactivity in older adults does not appear to be suppressed and has been shown to be sensitive to context. Thus, the potential exists for using SBP to assess responses to task-related factors (e.g., difficulty) as a marker of cognitive effort in studies of aging. Its utility is further enhanced by the strong theoretical and empirical foundation in research with younger adults. Unfortunately, there is little systematic work examining aging and CV reactivity as an index of effort during cognitive activity (see Uchino et al., 2010).
The present study examined age differences in cognitive effort using SBP. We used a procedure similar to that of Wright and colleagues (2007, Experiment 1) to examine the impact of task demands and fatigue effects associated with cognitive engagement. Specifically, young and older adults first engaged in an extended period of cognitive activity involving relatively low or high cognitive demands. Following this initial fatigue induction period, all participants were then given the same challenging task to perform in order to determine the effects of fatigue. Our primary interest was in examining age differences in effort as a function of phase of experiment (fatigue induction vs. fatigue influence) and level of difficulty of the fatigue induction task. Wright and colleagues found that younger adults exerted more effort during the initial phase when task demands were high. When participants viewed success as possible in the subsequent task (i.e., fatigue influence), they also found that individuals in the high-demand condition exhibited higher overall levels of reactivity than those in the low-demand condition. This suggests that extended engagement in a cognitively demanding activity depletes energy, requiring greater subsequent effort to achieve a specified level of performance.
We tested the hypothesis that aging is associated with an increase in the cognitive effort necessary to support a fixed level of performance. In other words, older adults must exert more effort than younger adults to attain similar levels of performance. We also hypothesized that effort would increase as task demands increased and that older adults would exhibit greater effort (i.e., change in reactivity from baseline) than younger adults at each level of objective task difficulty. Another way of expressing this same idea is that identical levels of performance between age groups would be associated with greater effort in the older adults. Finally, we hypothesized that sustained effortful cognitive activity would have greater costs (i.e., fatigue effects) to the old than to the young.
Method
Participants
We originally tested 127 community-dwelling adults from the North Carolina State University Adult Development Lab participant database, each of who received $30 for participation. Participants were excluded (N = 17) if they had blood pressure readings during screening or baseline that were suggestive of hypertension (i.e., SBP ≥ 140 mmHg or DBP ≥ 90 mmHg; Chobanian et al., 2003) or if they scored above 8 (N = 2) on the Short Blessed test (Katzman, Brown, Peck, Schechter, & Schimmel, 1983). Five participants whose CV measures exceeded 3 SD from the sample mean were also excluded. The final sample consisted of 52 young (26 female; M age = 31.6 years, range = 19–45) and 51 older (24 female; M age = 70.9 years, range = 62–84) adults. Participant characteristics are displayed in Table 1. One younger and 28 older adults reported taking hypertensive medication in the last six months. Analyses revealed, however, that medication use was unrelated to any of our CV measures, and inclusion of antihypertensives as a covariate did not alter any results. Thus, this variable was not considered further.
Table 1.
Participant Characteristics
| Young adults | Older adults | |||
| Measure | M | SD | M | SD |
| Education (years) | 16.1 | 2.1 | 16.3 | 2.5 |
| SF-36: physical healtha | 51.1 | 4.3 | 44.9 | 8.6 |
| SF-36: mental healtha | 50.4 | 8.9 | 57.7 | 5.7 |
| Baseline HRa | 76.4 | 12.4 | 69.6 | 8.2 |
| Baseline SBPa | 112.0 | 10.6 | 117.3 | 12.1 |
| Baseline DBPa | 65.8 | 7.2 | 61.2 | 5.7 |
| Vocabulary | 52.1 | 7.7 | 54.7 | 6.6 |
| Digit-symbol substitutiona | 84.0 | 13.5 | 64.5 | 14.5 |
| Letter-number sequencinga | 11.9 | 2.7 | 10.5 | 2.6 |
Note. DBP = diastolic blood pressure; HR = heart rate; SBP = systolic blood pressure.
Age group difference significant at p ≤ .02.
Measures and Equipment
CV responses.—
The Finometer MIDI (Finapres Medical Systems [FMS], Amsterdam, the Netherlands) was used to collect continuous SBP, DBP, and HR responses using a finger cuff. Brachial artery systolic and diastolic pressures were extrapolated from finger arterial pressure through the use of a height correction unit and waveform filtering and level correction methods supplied by the BeatScope software package (FMS). The technology used in the Finometer MIDI has demonstrated reliability and validity (e.g., Gerin, Pieper, & Pickering, 1993; Podlesny & Kircher, 1999).
Current state questionnaire.—
To assess current emotional status, ratings on 10 different states were made using an 11-point scale (0 = not at all; 10 = extremely). Of primary interest were ratings of threat, arousal (tense, calm), and energy states (mentally tired, mentally sharp, physically tired, energetic).
Posttest questionnaire.—
A second questionnaire served as a manipulation check, using items similar to those in Wright et al. (2003) to assess perceptions of the test context. Using an 11-point scale, participants rated the difficulty of the tasks in each phase, their desire to meet performance standards in Phase 2 (see below), evaluation concerns during testing, and whether the presence of the tester made them feel nervous.
Cognitive ability.—
The vocabulary subtest from the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997) was used to assess verbal ability. The WAIS-III digit-symbol substitution and letter-number sequencing (LNS) subtests were used to assess perceptual speed and working memory, respectively.
Procedure
Prior to their test session, participants completed a background questionnaire and the SF-36 Health Survey (Ware, 1993). Upon arriving in the laboratory, participants had their blood pressure screened using a HEM-780 automatic blood pressure monitor (Omron Healthcare, Inc., Kyoto, Japan). Next, a finger blood pressure cuff was attached to the index, middle, or ring finger of the participant’s nondominant hand. The choice of finger was primarily based on comfort and fit of the finger cuff and did not impact the measurement accuracy. CV measures were collected continuously throughout baseline and Phases 1 and 2 of the experiment.
Baseline.—
Participants were told to relax for 10 min and were provided with general interest magazines (e.g., The Smithsonian) to help pass the time. Baseline CV measures were assessed during the last 5 min of this period. Immediately following baseline, participants completed the current state questionnaire.
Phase 1: fatigue induction.—
Participants were randomly assigned to a high- or low-difficulty condition. Following Wright and colleagues (2007), low-difficulty participants counted forward by ones in 3-s intervals from the number 375, whereas those in the high-difficulty condition counted backwards by threes from the same point. Counting was paced using an auditory cue, and participants wrote their responses on a piece of paper. This phase lasted for 5 min.
Phase 2: fatigue influence.—
Immediately following Phase 1, participants were provided with four pages containing 175 single-digit multiplication problems. Participants were told that they would have 3 min to complete the problems as quickly and accurately as possible. To ensure high levels of motivation, they were informed that they would receive an additional $5 if they performed better than 80% of same-aged participants tested in a previous study. (All participants were, in fact, given the extra $5, which is included in the earlier mentioned compensation.)
Immediately following the influence period, participants completed the current state questionnaire for the second time as well as the posttest questionnaire. Finally, participants were administered the Short Blessed and the three ability tests.
CV data preparation.—
Continuous CV data collected during baseline and Phases 1 and 2 were used to calculate mean responses. To allow for the examination of potential change over time, mean SBP, DBP, and HR scores were obtained for each minute during baseline and Phases 1 and 2. Reliability was high across these time points for all measures in all three of these phases (α = .98–.99). Age Group × Task Difficulty × Time Period (minutes) analyses of variance (ANOVA) performed on each measure also revealed that changes in reactivity during each phase were small and nonsystematic. Thus, single mean reactivity scores for each phase were used as our primary dependent variables.
RESULTS
Preliminary Analyses
We first conducted 2 × 2 (Age Group × Task Difficulty) ANOVAs on the participant characteristics (Table 1) to identify inadvertent biases in our condition assignments. A significant interaction was observed for LNS scores, F(1,99) = 8.37, p = .01, = .08. Therefore, we entered LNS score as a covariate in all analyses. A significant main effect of task was also obtained for baseline DBP, F(1,99) = 3.97, p = .05, = .04, and a significant interaction was obtained for baseline HR, F(1,99) = 5.18, p = .03, = .05. These effects were already considered, however, due to CV baseline responses being entered as covariates to control for possible variation in CV reactivity as a function of initial response levels.
Subjective Ratings
Interpretation of CV reactivity effects is dependent upon satisfying assumptions regarding participants’ subjective responses to and perceptions of the tasks. On the current state questionnaire (Table 2, top), we were primarily interested in affective and energetic states that might influence participants’ responses to the task. Ratings of “tense” and “calm” suggested that participants in both age groups experienced low levels of anxiety and moderate levels of arousal, and threat perception was uniformly low. Participants also reported low levels of physical and mental fatigue and moderately high levels of energy in both domains. The only significant effects on any of these ratings were due to (a) decreases in ratings of calm (p < .001) and mental sharpness (p = .03) over time and (b) greater variation for younger adults than for older adults across time in reports of physical fatigue and across task difficulty for ratings of energetic states, although the observed variation was not dramatic. Thus, age differences in energetic and affective states were minimal.
Table 2.
Mean Ratings on Current State and Posttest Questionnaires
| Young adults | Old adults | |||||||
| Low difficulty | High difficulty | Low difficulty | High difficulty | |||||
| M | SD | M | SD | M | SD | M | SD | |
| A. Current state questionnaire | ||||||||
| Threat | ||||||||
| Pre | 0.3 | 0.6 | 0.4 | 1.0 | 0.5 | 0.7 | 0.3 | 0.5 |
| Post | 0.2 | 0.6 | 0.8 | 1.7 | 1.4 | 2.4 | 0.6 | 0.8 |
| Tense | ||||||||
| Pre | 1.5 | 1.3 | 1.7 | 1.8 | 1.7 | 2.3 | 1.4 | 1.9 |
| Post | 3.1 | 2.3 | 4.1 | 2.4 | 3.3 | 2.4 | 3.4 | 2.4 |
| Calm | ||||||||
| Pre | 8.0 | 1.6 | 8.1 | 1.4 | 8.4 | 1.7 | 8.7 | 1.7 |
| Post | 5.9 | 2.0 | 4.6 | 2.4 | 5.1 | 2.5 | 6.0 | 2.4 |
| Physically tired | ||||||||
| Pre | 2.7 | 2.7 | 2.9 | 2.5 | 2.0 | 2.3 | 2.3 | 2.2 |
| Post | 1.6 | 2.1 | 2.0 | 2.0 | 2.3 | 2.2 | 2.2 | 2.0 |
| Mentally tired | ||||||||
| Pre | 2.4 | 2.4 | 2.5 | 2.2 | 1.8 | 2.1 | 2.0 | 2.2 |
| Post | 2.5 | 2.1 | 3.5 | 2.3 | 2.3 | 1.9 | 2.5 | 1.9 |
| Energetic | ||||||||
| Pre | 6.0 | 1.8 | 5.3 | 1.6 | 5.5 | 2.2 | 6.1 | 1.8 |
| Post | 7.1 | 1.5 | 6.0 | 1.9 | 6.0 | 2.3 | 6.3 | 2.1 |
| Mentally sharp | ||||||||
| Pre | 7.4 | 1.5 | 7.3 | 1.6 | 7.1 | 1.9 | 7.5 | 1.7 |
| Post | 6.8 | 1.9 | 6.1 | 2.1 | 6.7 | 2.1 | 7.2 | 1.9 |
| B. Posttest questionnaire | ||||||||
| Phase 1 difficulty | 0.6 | 1.5 | 3.7 | 3.0 | 1.6 | 2.5 | 2.9 | 2.9 |
| Phase 2 difficulty | 4.3 | 3.0 | 4.9 | 2.7 | 3.6 | 2.5 | 3.9 | 3.3 |
| Phase 1 confidence | 9.6 | 0.8 | 6.3 | 2.8 | 8.4 | 2.0 | 7.7 | 2.5 |
| Phase 2 confidence | 7.6 | 1.9 | 7.1 | 2.1 | 7.7 | 2.0 | 8.2 | 2.3 |
| Motivation to do well | 8.3 | 2.0 | 8.7 | 1.4 | 7.8 | 2.5 | 8.5 | 1.3 |
| Performance concerns | 3.8 | 3.4 | 5.7 | 3.6 | 3.8 | 2.9 | 2.2 | 2.4 |
| Nervous about tester | 1.6 | 2.2 | 1.6 | 2.0 | 0.4 | 0.7 | 0.4 | 0.8 |
Responses on the posttest questionnaire (Table 2, bottom) were used as a manipulation check. Consistent with expectations, a significant Difficulty × Phase interaction was observed for perceptions of task difficulty, F(1,98) = 9.14, p = .01, = .09; ratings in Phase 1 were greater in the high-difficulty than in the low-difficulty condition but no differences were observed in Phase 2, where all participants performed the same task. Participants ratings of confidence in their ability to perform the task were relatively high, but a significant Difficulty × Phase interaction, F(1,98) = 23.98, p < .001, = .20, mirrored the task difficulty ratings, with confidence during Phase 1 being higher in the low-difficulty than in the high-difficulty condition but no differences emerging in Phase 2. Motivation to achieve a score higher than the specified standard was also high and stable across age and task difficulty groups. No significant age effects were observed in any of these cases. Finally, older adults were actually less nervous about the presence of the tester than were younger adults, F(1,98) = 12.09, p = .001, = .11. Younger adults also exhibited significantly higher levels of concern that the results would reflect poorly on their ability than did older adults, F(1,98) = 10.01, p = .002, = .09, and a significant Age × Difficulty interaction, F(1,98) = 5.02, p = .03, = .05, was due to this effect being stronger in the high-difficulty condition than in the low-difficulty condition.
Together, these ratings suggest that both young and older participants (a) perceived task difficulty as intended, (b) viewed the tasks as within their capabilities, and (c) were highly motivated. This satisfies basic assumptions regarding the validity of the current approach to assessing effort (see Brehm & Self, 1989), allowing us to infer that participants in all groups generally perceived success on all tasks as possible and worthwhile. In addition, there was no evidence that older adults experienced higher levels of anxiety or threat in response to the test context, minimizing a potential alternative explanation for any observed effects (e.g., Hess, Auman, Colcombe, & Rahhal, 2003).
CV Responses
CV reactivity (Table 3) during Phases 1 (fatigue induction) and 2 (fatigue influence) was determined by subtracting mean baseline values from the mean values obtained during each period (Llabre, Spitzer, Saab, Ironson, & Schneiderman, 1991). Although SBP was of primary interest, we conducted 2 × 2 × 2 (Age Group × Task Difficulty × Test Phase) analyses of covariance (ANCOVAs) for each of our three CV measures using LNS scores and baseline response as covariates. (Realizing that the use of baseline CV response as a covariate could introduce a potential bias with ANCOVA (Jamieson, 2004), we calculated residualized change scores for each CV response using the appropriate baseline CV responses as predictors. The pattern of results obtained with the residualized changes scores was no different than that observed using baseline CV as a covariate.) Test phase was a within-participants factor.
Table 3.
Mean Adjusted Cardiovascular Reactivity Scores as a Function of Age Group, Test Phase, and Phase 1 Task Difficulty
| Age group and task difficulty | ||||||||
| Young adults | Old adults | |||||||
| Low | High | Low | High | |||||
| M | SE | M | SE | M | SE | M | SE | |
| Systolic blood pressure | ||||||||
| Phase 1 | 8.4 | 1.8 | 9.2 | 1.5 | 12.1 | 1.7 | 21.6 | 1.5 |
| Phase 2 | 21.0 | 2.5 | 17.6 | 2.2 | 25.7 | 2.5 | 31.2 | 2.2 |
| Diastolic blood pressure | ||||||||
| Phase 1 | 5.3 | 0.8 | 6.4 | 0.7 | 5.3 | 0.7 | 8.4 | 0.7 |
| Phase 2 | 11.7 | 1.2 | 11.2 | 1.0 | 11.4 | 1.1 | 12.0 | 1.1 |
| Heart rate | ||||||||
| Phase 1 | 2.1 | 0.8 | 6.6 | 0.7 | 2.7 | 0.8 | 3.1 | 0.8 |
| Phase 2 | 10.8 | 1.5 | 13.2 | 1.4 | 7.8 | 1.5 | 6.7 | 1.4 |
Systolic blood pressure.—
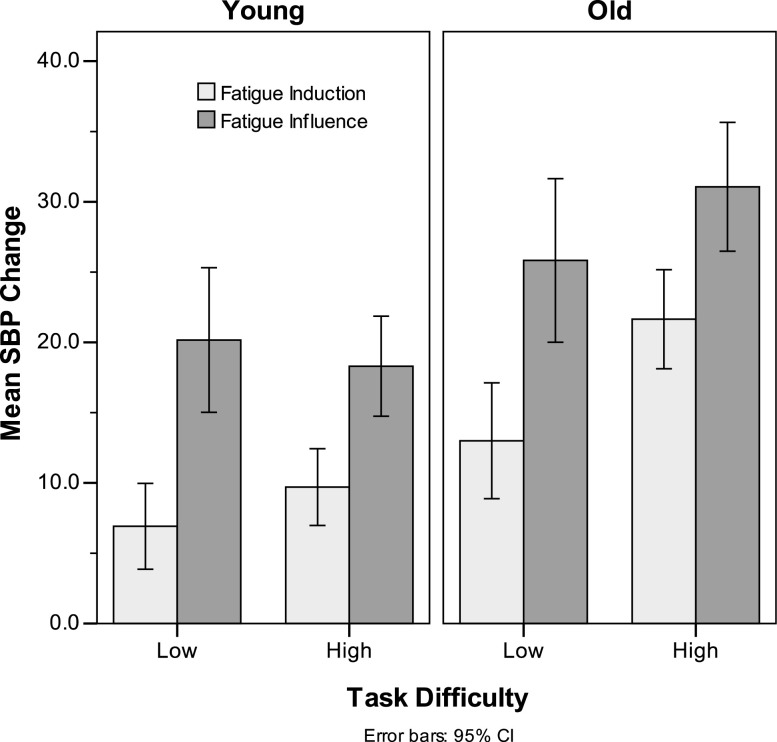
As predicted, older adults exhibited higher levels of reactivity than younger adults, F(1,97) = 19.69, p < .001, = .19. A significant Task Difficulty × Phase interaction was also obtained, F(1,97) = 6.06, p = .02, = .06, due to the change in reactivity being greater in the low-difficulty condition (10.3 vs. 23.3) than in the high-difficulty condition (15.4 vs. 24.4). Of greater interest is a significant interaction between age and task difficulty, F(1,97) = 5.48, p = .02, = .05. Difficulty had a stronger impact on older adults’ responses than on those of younger adults (Figure 1). The three-way interaction was not significant. Thus, consistent with expectations, older adults exhibited higher levels of reactivity in general and in response to increments in difficulty.
Figure 1.
Systolic blood pressure (SBP) reactivity as a function of age and Phase 1 task difficulty.
Diastolic blood pressure.—
A Difficulty × Phase interaction was the only significant effect observed for DBP, F(1,97) = 7.55, p = .01, = .07. This effect was similar to that observed for SBP, with the change in reactivity from Phase 1 to Phase 2 being greater in the low-difficulty (5.3 vs. 11.6) than in the high-difficulty condition (7.4 vs. 11.6).
Heart rate.—
HR reactivity was significantly greater in the influence than in the induction phase (Ms = 9.6 vs. 3.6), F(1,97) = 5.82, p = .02, = .06. Consistent with observations by Uchino and colleagues (2010), older adults exhibited less HR reactivity than did younger adults (Ms = 5.1 vs. 8.2), F(1,97) = 8.41, p = .01, = .08. This age-related reduction in HR reactivity was also evident in the significant Age × Difficulty interaction, F(1,97) = 6.33, p = .02, = .06, with the difference in reactivity to the low- and high-demand tasks being greater in the young group (Ms = 6.4 vs. 9.9) than in the old group (Ms = 5.2 vs. 4.9). Thus, consistent with expectations, both DBP and HR were less sensitive to context than SBP. The apparent reversal of age differences in HR reactivity relative to SBP most likely reflects the dampening of HR reactivity in later life, which further decreases sensitivity to context.
Predictors of Reactivity and Performance
We next conducted a series of regression analyses to examine specific predictions derived from the conceptual framework used to design our study. First, we examined the hypothesis that effort expended during the initial task would influence the amount of effort expended in the second task. We also examined whether this effect would be moderated by participant age. To do this, we performed a regression analysis in which baseline SBP, education, and WAIS-III subtest scores were entered as covariates in the first step. This was followed by entry of age group (dummy coded, with the young group as the referent), Phase 1 SBP reactivity, and finally the interaction between these two factors. Task difficulty was not included in this and subsequent regression analyses because degree of effort expended in Phase 1 is essentially a proxy for difficulty. All predictor variables here and in subsequent analyses were standardized for purposes of centering to control for multicollinearity effects. As seen in Table 4, age was positively associated with reactivity in Step 2 but was reduced to nonsignificance when Phase 1 SBP reactivity was included. Addition of reactivity in Step 3 also resulted in an R 2 increment of .38. Thus, greater reactivity (i.e., effort) in Phase 1 was associated with greater reactivity in Phase 2, and this relationship subsumed the age effect, which presumably reflected normative changes in reactivity.
Table 4.
Prediction of Phase 2 SBP Reactivity
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Predictor | β | p | β | p | β | p | β | p |
| Letter-number sequencing | −.12 | .28 | −.05 | .66 | .06 | .40 | .07 | .36 |
| Digit-symbol substitution | −.21 | .05 | −.01 | .96 | .02 | .78 | .02 | .83 |
| Vocabulary | −.07 | .51 | −.15 | .16 | −.04 | .63 | −.04 | .56 |
| Education | .21 | .05 | .20 | .05 | .22 | .002 | .23 | .001 |
| Age group | — | — | .39 | .001 | .04 | .61 | .04 | .69 |
| Phase 1 ΔSBP | — | — | — | — | .77 | <.001 | .85 | <.001 |
| Age × Phase 1 ΔSBP | — | — | — | — | — | — | −.09 | .46 |
| Model R 2 | .10 | .19 | .61 | .62 | ||||
| df | 4,98 | 5,97 | 6,96 | 7,95 | ||||
| p | .04 | .001 | <.001 | <.001 | ||||
| R 2 change | — | .09 | .42 | .01 | ||||
| df | — | 1,97 | 1,96 | 1,95 | ||||
| p | — | .001 | <.001 | .46 | ||||
Note. SBP = systolic blood pressure.
We next examined the relationship between reactivity and performance on the multiplication task. There were no significant differences (ps > .12) between younger and older adults in accuracy (96.5% vs. 96.6%) or in the percent of problems attempted (52.7% vs. 57.5%). The absence of age differences in performance along with the previously noted significant increase in SBP reactivity with age is notable in suggesting that older adults had to exert more effort than younger adults to achieve similar levels of task performance. Going beyond these observations based on aggregate data, however, we examined this relationship more directly using a regression-based procedure similar to the aforementioned to predict performance. Number of problems attempted was used as the performance measure because it appears most related to effort on task.
We entered baseline SBP, education, and measures of ability as covariates in Step 1, age group in Step 2, both Phase 1 and Phase 2 reactivity in Step 3, and the interaction between these last two factors and age group in the final step (Table 5). Reactivity in both phases was associated with performance but in different ways. Specifically, Phase 2 reactivity was positively associated with performance, but Phase 1 reactivity was negatively associated with performance. These effects illustrate the positive linkage between effort expenditure and performance within a specific task as well as the negative consequences associated with previous effortful cognitive activity.
Table 5.
Prediction of Phase 2 Performance
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Predictor | β | p | β | p | β | p | β | p |
| Letter-number sequencing | −.02 | .81 | .04 | .67 | −.01 | .982 | −.02 | .832 |
| Digit-symbol substitution | .23 | .02 | .42 | <.001 | .41 | <.001 | .40 | <.001 |
| Vocabulary | .02 | .88 | −.06 | .56 | −.05 | .60 | −.05 | .59 |
| Education | .35 | .001 | .34 | <.001 | .22 | .02 | .23 | .02 |
| Age group | — | — | .37 | .001 | .38 | .001 | .37 | .001 |
| Phase 1 ΔSBP | — | — | — | — | −.50 | <.001 | −.68 | .002 |
| Phase 2 ΔSBP | — | — | — | — | .55 | <.001 | .89 | <.001 |
| Age × Phase 1 ΔSBP | — | — | — | — | — | — | .26 | .20 |
| Age × Phase 2 ΔSBP | — | — | — | — | — | — | −.45 | .01 |
| Model R2 | .19 | .27 | .39 | .43 | ||||
| df | 4,98 | 5,97 | 7,95 | 9,93 | ||||
| p | <.001 | <.001 | <.001 | <.001 | ||||
| R2 change | — | .08 | .12 | .04 | ||||
| df | — | 1,97 | 2,95 | 2,93 | ||||
| p | — | .001 | <.001 | .04 | ||||
Note. SBP = systolic blood pressure.
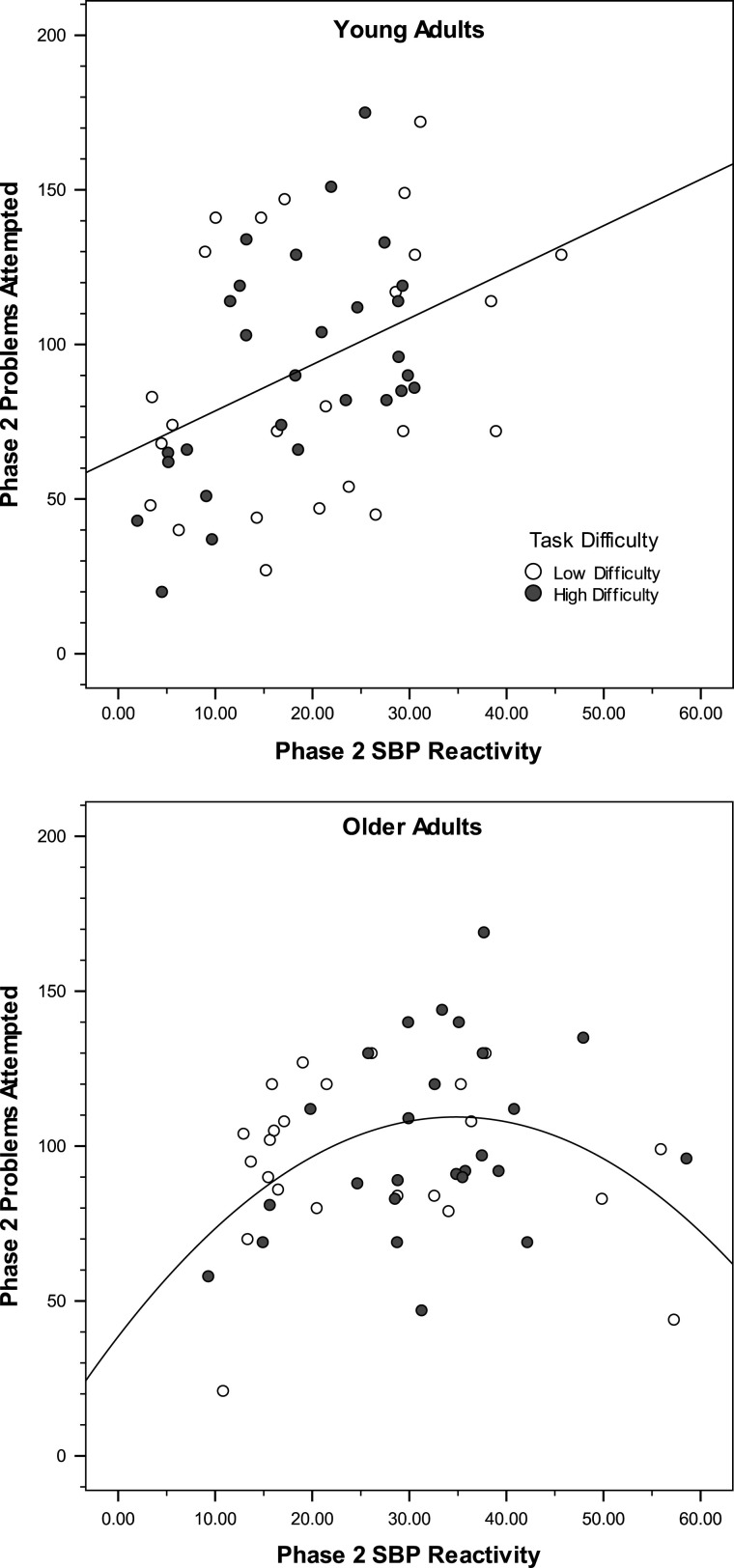
Age also moderated the effect of Phase 2 reactivity. Regressions within each age group suggest that the impact of effort on performance was more systematic in the young than in the old: young—β = .68, t = 4.23, p < .001; old—β = .38, t = 2.02, p = .05. Visual inspection of the data, however, suggested a curvilinear relationship in the data of the older group. When the quadratic term associated with Phase 2 reactivity was added to each of these within-age analyses in a final step, a significant increment in model strength was only observed in the older group, with the quadratic component being significantly associated with performance (β = −1.55, t = −2.38, p = 02). As seen in Figure 2, performance increased initially with effort in the old group but then declined. This latter trend appears to reflect increased levels of effort associated with fatigue (68% of the older adults in the high task–difficulty condition are represented in the upper end of the effort distribution for that age group), which may result in relatively inefficient performance. Note that there is minimal overlap with the younger adults’ effort distribution at the upper end of the older adults’ distribution, suggesting that reactivity is quite high and perhaps disruptive of performance.
Figure 2.
Phase 2 performance as a function of Phase 2 systolic blood pressure (SBP) reactivity and age.
DISCUSSION
This study represents one of the first systematic attempts to use CV reactivity as a measure of effort to examine the impact of aging on cognitive performance. In doing so, we tested three specific hypotheses. First, our results supported the hypothesis that aging would be associated with an increase in the cognitive effort necessary to support a fixed level of performance. The easiest way to see this is by examining reactivity in Phase 1, where older adults exhibited a greater increase in SBP over baseline than did younger adults, suggestive of greater effort expenditure.
We also hypothesized that effort would increase as task demands increased in Phase 1 (fatigue induction) and that older adults would exhibit greater effort than younger adults at each level of objective task difficulty. This hypothesis was generally supported in that older adults exhibited higher levels of reactivity in all conditions and their reactivity increased with task difficulty. The fact that younger adults did not exhibit the anticipated increase in reactivity with difficulty was somewhat unexpected, although they did exhibit higher SBP reactivity to the multiplication task in Phase 2, which all participants rated as the most difficult task.
Finally, we hypothesized that the effort associated with cognitive activity would have greater costs to the old than to the young. The effects of exertion can be seen in two primary ways. First, effort expenditure in Phase 1 was positively associated with effort expenditure in Phase 2, suggesting that high levels of effort devoted to earlier tasks resulted in participants having to exert even greater effort to support subsequent task performance. Using our previous analogy, a physically fatigued person would have to work harder to achieve the same effectiveness as a rested person when performing a physical activity. Second, the amount of effort exerted during Phase 1 was negatively correlated with performance in Phase 2. This effect, taken along with the positive association between Phase 2 effort and performance, helps illustrate the fact that participants in the high-difficulty condition had to exert more effort than those in the low-difficulty condition to achieve essentially identical levels of performance. The greater effort in the former group essentially reflects the “penalty” associated with previous effortful cognitive activity—as reflected in the aforementioned negative correlation.
As expected, fatigue effects were stronger for the old than the young. Specifically, older adults exhibited higher levels of effort than younger adults in Phase 2, and this appeared to mainly reflect their overall higher levels of effort during Phase 1. In other words, the increased effort in Phase 2 reflected costs associated with earlier effort expenditure. In addition, older adults displaying the highest levels of reactivity in Phase 2 exhibited disruption in task performance. This suggests that extremely high levels of effort following initial depletion may result in relatively inefficient performance, perhaps reflecting disruption at high levels of arousal. Such disruption might be particularly strong for those with reduced cognitive resources (e.g., older adults; Labouvie-Vief, 2009; Labouvie-Vief, Grühn, & Studer, 2010).
An alternative way of viewing this latter trend is that the low levels of performance associated with high effort in the older adults may simply reflect lower levels of ability. That is, those who are low in ability will have to exert the highest levels of effort, with lower performance levels simply being reflective of their capabilities. The fact that this trend was observed while controlling for ability, however, attenuates the viability of such an explanation.
Consistent with expectations, we also found that both the age and task-related effects were strongest with SBP. The observed age effects may, in part, be related to the less consistent DBP reactivity and reduced HR reactivity in later life (e.g., Uchino et al., 2010). To the extent that SBP reactivity is a cleaner reflection of SNS response and myocardial β-adrenergic activity, however, these results are also in line with the idea that task demands and age influence the effort associated with cognitive activity. One potential concern about interpreting the age effects associated with SBP is that age-related increases in reactivity could be due to an aging CV system. In spite of such age-related changes, however, Uchino and colleagues (2010) have argued that SBP reactivity, unlike HR reactivity, may still be a valid indicator of age differences in psychological processes due to the apparent sensitivity of this measure in assessing age effects across contexts. It is perhaps also notable that the controlling for health status did not affect the obtained results, suggesting that the age-related reactivity effects were not simply reflections of poorer health status in older adults but rather something more fundamental to the aging process.
Assuming that the observed CV reactivity effects reflect age differences in the effort and costs associated with cognitive activity, the present results have broad-ranging implications for understanding aging. The increased reactivity on the part of older adults suggests greater expenditure of effort to support task performance. This plus the related observations that older adults experience greater fatigue and disruption of performance at higher levels of effort suggest that a focus on effort may be useful in understanding commonly observed age effects on cognition. The results also have significance for motivational perspectives relevant to understanding age differences in cognitive performance. Research has shown that age-related variation in physical resources—which presumably underlie the age differences in CV reactivity demonstrated here—are linked to intrinsic motivational states associated with engagement in complex cognitive processing (Hess, 2001; Hess, Emery, & Neupert, 2011). Hess (2006); Hess & Emery, (2011) has further hypothesized that the increased selectivity in engagement of cognitive resources in later life (e.g., Germain & Hess, 2007; Hess, Germain, Swaim, & Osowski, 2009) is, in part, an adaptive response to age-related increases in the effort and costs associated with cognitive activity. Our study supports the assumptions underlying this hypothesis, although examination of specific linkages between aging, effort, and motivation awaits further study.
FUNDING
Support for this study was provided by National Institute on Aging grants AG05552 and AG034580.
Acknowledgments
The authors would like to thank Carla Strickland for her assistance in participant recruitment and data entry.
References
- Brehm JW, Self EA. The intensity of motivation. Annual Review of Psychology. 1989;40:109–131. doi: 10.1146/annurev.ps.40.020189.000545. doi:10.1146/annurev.ps.40.020189.000545. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. doi:10.1037/0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, Roccella EJ. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. Journal of the American Medical Association. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. doi:10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Anderson ND. Applying cognitive research to problems of aging. In: Gopher D, Koriat A, editors. Attention and performance: Vol. XVII. Cognitive regulation of performance: Interaction of theory and application. Cambridge, MA: MIT Press; 1999. pp. 583–615. [Google Scholar]
- Gendolla GHE, Wright RA. Motivation in social settings: Studies of effort-related cardiovascular arousal. In: Forgas JP, Williams K, von Hippel B, editors. Social motivation: Conscious and nonconscious processes. Cambridge University Press; 2005. [Google Scholar]
- Gerin W, Pieper C, Pickering TG. Measurement reliability of cardiovascular reactivity change scores. A comparison of intermittent and continuous methods of assessment. Journal of Psychosomatic Research. 1993;37:493–501. doi: 10.1016/0022-3999(93)90005-z. doi:10.1016/0022-3999(93)90005-Z. [DOI] [PubMed] [Google Scholar]
- Germain CM, Hess TM. Motivational influences on controlled processing: Moderating distractibility in older adults. Aging, Neuropsychology, and Cognition. 2007;14:462–486. doi: 10.1080/13825580600611302. doi:10.1080/13825580600611302. [DOI] [PubMed] [Google Scholar]
- Gold PE. Glucose and age-related changes in memory. Neurobiology of Aging. 2005;26(Suppl. 1):S60–S64. doi: 10.1016/j.neurobiolaging.2005.09.002. doi:10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Hess TM. Aging-related influences on personal need for structure. International Journal of Behavioral Development. 2001;25:482–490. doi:10.1080/01650250042000429. [Google Scholar]
- Hess TM. Adaptive aspects of social cognitive functioning in adulthood: Age-related goal and knowledge influences. Social Cognition. 2006;24:279–309. doi:10.1521/soco.2006.24.3.279. [Google Scholar]
- Hess TM, Auman C, Colcombe SJ, Rahhal TA. The impact of stereotype threat on age differences in memory performance. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2003;58:3–11. doi: 10.1093/geronb/58.1.p3. doi:10.1093/geronb/58.1.P3. [DOI] [PubMed] [Google Scholar]
- Hess TM, Emery L. Memory in context: The impact of age-related goals on performance. In: Naveh-Benjamin M, Ohta N, editors. Perspectives on memory and aging. London: Psychology Press; in press. [Google Scholar]
- Hess TM, Emery L, Neupert S. Longitudinal relationships between resources, motivation, and cognitive functioning. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. doi: 10.1093/geronb/gbr100. 2011. doi:10.1093/geronb/gbr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM, Germain CM, Swaim EL, Osowski NL. Aging and selective engagement: The moderating impact of motivation on older adults’ resource utilization. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2009;64:447–456. doi: 10.1093/geronb/gbp020. doi:10.1093/geronb/gbp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. Analysis of covariance (ANCOVA) with difference scores. International Journal of Psychophysiology. 2004;52(3):277–283. doi: 10.1016/j.ijpsycho.2003.12.009. doi:10.1016/j.ijpsycho.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schecter R, Shimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. American Journal of Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G. Cognition and equilibrium regulation in development and aging. Restorative Neurology and Neuroscience. 2009;27:551–565. doi: 10.3233/RNN-2009-0512. doi:10.3233/RNN-2009-0512. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, Grühn D, Studer J. Dynamic integration of emotion and cognition: Equilibrium regulation in development and aging. In: Lerner RM, Lamb ME, Freund AM, editors. The handbook of life-span development, Volume 2, social and emotional development. Hoboken, NJ: Wiley; 2010. pp. 79–115. [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GH, Schneiderman N. The reliability and specificity of delta versus residualized change as measure of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28:701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. doi:181659810.1111/j.1469-8986.1991.tb01017.x1992-34270-001. 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology. 2010;24:222–243. doi: 10.1037/a0017619. doi:10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert SD, Miller LMS, Lachman ME. Physiological reactivity to cognitive stressors: Variations by age and socioeconomic status. International Journal of Aging and Human Development. 2006;62:221–235. doi: 10.2190/17DU-21AA-5HUK-7UFG. doi:10.2190/17DU-21AA-5HUK-7UFG. [DOI] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular psychophysiology: A perspective. New York, NY: Plenum Press; 1981. [Google Scholar]
- Park DC, Payer D. Working memory across the adult lifespan. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. New York, NY: Oxford University Press; 2006. pp. 128–142. [Google Scholar]
- Podlesny JA, Kircher JC. The Finapres (volume clamp) recording method in psychophysiological detection of deception examinations. Experimental comparison with the cardiograph method. Forensic Science Communication. 1999;7:1–17. [Google Scholar]
- Richter M, Friedrich A, Gendolla GHE. Task difficulty effects on cardiac activity. Psychophysiology. 2008;45:869–875. doi: 10.1111/j.1469-8986.2008.00688.x. doi:10.1111/j.1469-8986.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. doi:10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Robbins RJ. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocrinology Review. 1994;15:233–260. doi: 10.1210/edrv-15-2-233. doi:10.1210/edrv-15-2-233. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Birmingham W, Berg CA. Are older adults less or more physiologically reactive? A meta-analysis of age-related differences in cardiovascular reactivity to laboratory tasks. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2010;65:154–162. doi: 10.1093/geronb/gbp127. doi:10.1093/geronb/gbp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: A meta-analysis. Psychology and Aging. 2003;18:443–469. doi: 10.1037/0882-7974.18.3.443. doi:10.1037/0882-7974.18.3.443. [DOI] [PubMed] [Google Scholar]
- Ware JE., Jr. SF-36 health survey. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- Wright RA, Dill JD. Blood pressure responses and incentive appraisals as a function of perceived ability and objective task demand. Psychophysiology. 1993;30:152–160. doi: 10.1111/j.1469-8986.1993.tb01728.x. doi:10.1111/j.1469-8986.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Wright RA, Junious TR, Neal C, Avello A, Graham C, Herrmann L, Walton N. Mental fatigue influence on effort-related cardiovascular response: Difficulty effects and extension across cognitive performance domains. Motivation and Emotion. 2007;31:219–231. doi:10.1007/s11031-007-9066-9. [Google Scholar]
- Wright RA, Martin RE, Bland JL. Energy resource depletion, task difficulty, and cardiovascular response to a mental arithmetic challenge. Psychophysiology. 2003;40:98–105. doi: 10.1111/1469-8986.00010. doi:10.1111/1469-8986.00010. [DOI] [PubMed] [Google Scholar]
- Wright RA, Murray JB, Storey PL, Williams BJ. Ability analysis of gender relevance and sex differences in cardiovascular response to behavioral challenge. Journal of Personality and Social Psychology. 1997;73:405–417. doi: 10.1037//0022-3514.73.2.405. doi:10.1037/0022-3514.73.2.405. [DOI] [PubMed] [Google Scholar]