Abstract
Objectives.
Previous studies showed that negative self-stereotypes detrimentally affect the cognitive performance of marginalized group members; however, these findings were confined to short-term experiments. In the present study, we considered whether stereotypes predicted memory over time, which had not been previously examined. We also considered whether self-relevance increased the influence of stereotypes on memory over time.
Method.
Multiple waves of memory performance were analyzed using individual growth models. The sample consisted of 395 participants in the Baltimore Longitudinal Study of Aging.
Results.
Those with more negative age stereotypes demonstrated significantly worse memory performance over 38 years than those with less negative age stereotypes, after adjusting for relevant covariates. The decline in memory performance for those aged 60 and above was 30.2% greater for the more negative age stereotype group than for the less negative age stereotype group. Also, the impact of age stereotypes on memory was significantly greater among those for whom the age stereotypes were self-relevant.
Discussion.
This study shows that the adverse influence of negative self-stereotypes on cognitive performance is not limited to a short-term laboratory effect. Rather, the findings demonstrate, for the first time, that stereotypes also predict memory performance over an extended period in the community.
Keywords: Ageism, Aging, Memory, Self-perception, Stereotypes
Research has shown that negative stereotypes can adversely affect the cognitive performance of marginalized group members, including African Americans, women, and older individuals (e.g., Levy, 2003; Shih, Pittinsky, & Ambady, 1999; Wheeler & Petty, 2001). However, these various studies have been limited by laboratory methodologies. Thus, it has been impossible to know if the adverse cognitive outcomes of negative stereotypes result solely from short-term effects in controlled settings or if everyday life exposure to negative stereotypes is longitudinally associated with cognitive trajectories. The question of whether stereotypes predict memory over time has not been previously answered.
The current study focuses on negative age stereotypes. Ample evidence exists regarding the impact of these stereotypes in the laboratory (e.g., Horton, Baker, Pearce, & Deakin, 2008; Levy, 1996; Levy & Leifheit-Limson, 2009; Meisner, 2011). For example, a meta-analysis of nine experiments showed that older individuals who were explicitly primed with negative age stereotypes tended to exhibit worse memory performance than those who were explicitly primed with positive age stereotypes (Horton et al., 2008). This influence has also been found in implicit experiments, such as one in which primed age stereotypes affected the memory performance of older participants but not younger participants; it was assumed that the primes activated internalized stereotypes that were relevant to the older individuals’ self-images (Levy, 1996).
Several studies have demonstrated that the process of age stereotype internalization begins in childhood (e.g., Kwong See & Nicoladis, 2009). Additionally, research has shown that these stereotypes tend to become self-views in old age (Rothermund, 2005). These sets of findings correspond to stereotype embodiment theory, which posits age stereotypes are internalized across the lifespan and can influence functioning in old age when they become self-relevant (Levy, 2009). In the present study, therefore, we expected that age stereotypes measured earlier in life would be transportable across time and that the experimental memory findings would be transportable across space—from the laboratory to the community.
We selected memory as the outcome for this study because it is influenced by age stereotypes in the laboratory (e.g., Levy, 1996), thereby allowing us to consider whether an equivalent effect is found over time. The measure we used, the Benton Visual Retention Test (BVRT; Benton, 1974), is well-suited to our research goal, for it is sensitive to visual-memory decline (Lamar, Zonderman, & Resnick, 2002), valid with younger and older adults (Giambra, Arenberg, Kawas, Zonderman, & Costa, 1995), and ecologically valid (Dawson, Anderson, Uc, Dastrup, & Rizzo, 2009). Further, BVRT scores exhibit considerable variability in later life (Brant, Sheng, Morrell, & Zonderman, 2005). We considered whether this variability was due, in part, to age stereotypes.
Research on patterns of memory decrements in later life has tended to focus on biological processes (e.g., Bishop, Lu, & Yankner, 2010) rather than psychosocial processes that may be more amenable to interventions. We predicted that (a) individuals with more negative age stereotypes would have worse memory over time than those with less negative age stereotypes and (b) the impact of negative age stereotypes on memory would be greater when they were self-relevant than when they were not self-relevant.
METHOD
Participants
The Baltimore Longitudinal Study of Aging (BLSA) is the longest running investigation of memory and aging (Shock et al., 1984). Criteria for the original sample included community dwelling and willingness to visit the National Institute of Aging for regular assessments. For our cohort, participants needed to have age stereotype, memory, and covariate assessments, and they had to be at least aged 22 years at baseline. This age criterion ensured that the participants had reached 60 years of age or had the potential to turn 60 during the 38-year study.
The final cohort (N = 395) consisted of 113 women and 282 men who were aged 22–77 (M = 45 years) at baseline, had a high level of self-rated health (M = 4.51, with 5 = excellent), and were highly educated (77% at least completed college). The attrition rate was 6% for reasons other than death (Shock et al., 1984).
To address the second hypothesis, we added two criteria: responded to the self-relevance item and aged 40 or older. We selected this older subset because 40 years was the youngest age mentioned when participants were asked the age at which they thought old age begins. The final cohort (N = 87) consisted of 27 women and 60 men, aged 40–74 years (M = 53 years). They had a high level of self-rated health (M = 4.52) and were highly educated (82% at least completed college). This subset of participants did not significantly differ from the larger cohort in percentage of women, χ2 = 0.65, p = .72, self-rated health, t = 0.05, p = .96, nor education, t = −0.60, p = .55.
Measures
Age stereotypes.—
BLSA participants responded to the 16-item age-stereotype subscale derived from negative items on the Attitudes toward Old People scale (ATOP; Tuckman & Lorge, 1953) between 1968 and 1980. The first assessment served as the predictor. The scale and subscale are reliable and valid (Levy, Zonderman, Slade, & Ferrucci, 2009; Tuckman & Lorge, 1953). The subscale includes such items as “Old people are absent-minded” and “Old people cannot concentrate well.” The range of scores was 0–16; a higher score signified more negative age stereotypes.
Memory.—
We assessed memory with the BVRT (Benton, 1974), a reliable and valid measure (Giambra et al., 1995). The interviewer presented each of 10 geometric-figure cards for 10 s, removed each card, and then asked participants to reproduce the figure from memory. Three equivalent and reliable sets of figures enabled testing at multiple waves while minimizing practice effects (Shock et al., 1984). Two researchers independently scored each drawing; participants received error scores ranging from 0 to 36. To allow a higher score to represent better memory performance, we subtracted error scores from 36. The BVRT was administered every 6 years from 1968 to 1991 and then every 2 years, with a range of 1–16 assessments and a median of seven. In our sample, 88% of the participants had three or more BVRT assessments, giving a total of 4,252 assessments.
Self-relevance.—
At baseline, immediately after participants responded to the ATOP measure, they were asked, “At what age does someone become old?” This measure has discriminant validity: In a sample of older individuals, responses to “At what age does the average man or woman become old?” did not significantly correlate with participants’ actual ages (Kaufman & Elder, 2002). We created a self-relevance variable by scoring a response equal to or below the participant’s actual age as “1” or “0” if an older age was mentioned. For example, if a 70-year-old participant stated old age begins at the age of 65, her age stereotypes would be categorized as self-relevant; whereas, if she stated old age begins at the age of 75, her age stereotypes would be categorized as not self-relevant.
Covariates.—
We included the following covariates that are associated with memory decline: age, depression, education, marital status, number of chronic conditions based on hospital records, race, self-rated health, and sex (e.g., Giambra et al., 1995; Håkansson et al., 2009; Marquis et al., 2002). Depression was measured by the Center for Epidemiologic Studies Depression (CES-D) scale (Radloff, 1977); scores ranged from 0 to 60, with higher scores indicating more depressive symptoms. Self-rated health was assessed with a five-item scale ranging from 1 (very poor) to 5 (excellent). To account for the range of years in which participants took the first age stereotype measure, and thus joined our study, baseline calendar year was also included as a covariate.
Covariates were included at the single timepoint of baseline, except for the time-dependent covariates of depression and age. Age was included at baseline and matched to age at each BLSA assessment. The CES-D, first included in 1980 and then in regular BVRT assessments, had a range of 1–14 assessments, with a median of four. We used the closest available measure of CES-D for each BVRT assessment; in most cases, this occurred in the same session as the BVRT assessment.
Statistical Analysis
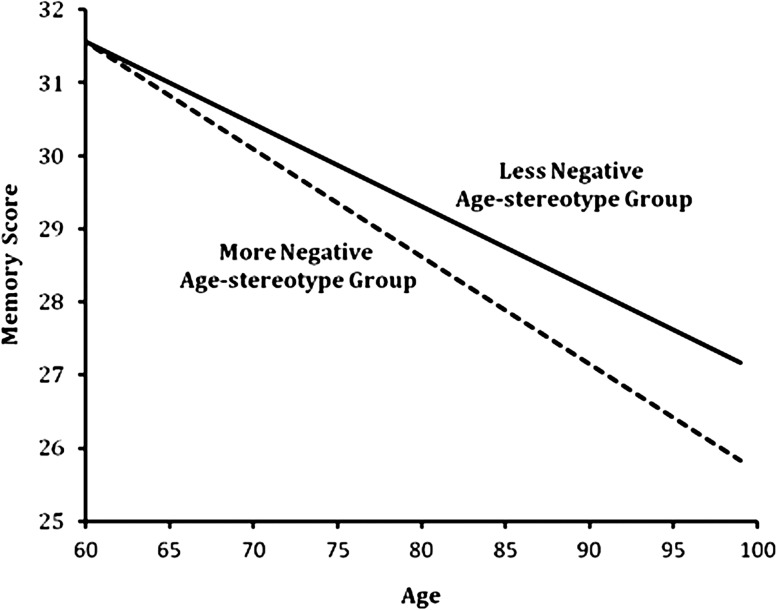
To examine both hypotheses, individual growth models were conducted (Singer & Willett, 2003), with the first age stereotype assessment treated as baseline and all covariates included. Marital status, race, and sex were included as categorical variables; the remaining covariates were treated as continuous variables. An unstructured covariance matrix was specified, allowing the data to define the covariance between each participant’s repeated memory assessments. For the first hypothesis, we examined whether the interaction of age stereotypes and age significantly predicted memory over time. For the second hypothesis, following the Baron and Kenny (1986) definition of a moderator, we examined whether the interaction of self-relevance and age stereotypes significantly predicted memory after adjusting for the main effects of self-relevance and age stereotypes. Standardized parameter estimates are reported. For Figure 1, we set all covariates at their median value and based age-stereotype categories on one standard deviation above and below the mean age-stereotype value of seven.
Figure 1.
Effect of age-stereotype groups on older individuals’ memory over time. Figure is based on model that includes all covariates. A higher memory score indicates better performance on the Benton Visual Retention Test.
RESULTS
Hypothesis 1
As predicted by our first hypothesis, individuals with more negative age stereotypes had worse memory over time than those with less negative age stereotypes (see Figure 1). The interaction between age stereotypes and age over time reached significance, β = −.24, SE = 0.12, t = −2.01, p = .04, d = 2.00, after adjusting for all covariates in an individual growth model. The covariates that significantly contributed to memory over time, consistent with previous studies (e.g., Giambra et al., 1995), were age β = −.68, SE = 0.16, t = −4.14, p < .0001, d = 5.00, education β = .57, SE = 0.12, t = 4.61, p < .0001, d = 4.42, self-rated health β = .25, SE = 0.12, t = 2.07, p = .04, d = 2.05, and sex β = −.88, SE = 0.38, t = −2.31, p = .02, d = 2.32, such that men had higher BVRT scores.
According to the model, there were increasingly greater disparities between the two groups as they aged, so that at the age of 70, the memory performance of those with more negative age stereotypes was equivalent to 73.14-year-old participants with less negative age stereotypes; at the age of 80, the interval was 6.16 years; and at the age of 90, it was 9.18 years. Overall, there was a 30.2% greater memory decline for those aged 60 and above in the more negative age-stereotype group compared to the less negative age-stereotype group.
Although we adjusted for both subjective and objective health in the models to increase our confidence that the pattern of more negative age stereotypes predicting worse memory over time was not due to its being related to worse health, we conducted an additional analysis with a healthy subset which reported excellent self-rated health and had no more than one chronic condition (N = 213). In this subset, more negative age-stereotypes still predicted worse memory over time, β = –.11, SE = 0.05, t = 2.16, p = .03, d = 2.14, adjusting for the covariates.
Hypothesis 2
As predicted by our second hypothesis, memory decline was greater among individuals for whom the negative age stereotypes were self-relevant than among those for whom these stereotypes were not self-relevant. Consistent with a moderator effect (Baron & Kenny, 1986), a significant interaction emerged between age stereotypes and self-relevance on memory performance in an individual growth model that included all covariates, β = −31.10, SE = 8.41, t = −3.70, p = .0002, d = 3.70.
It does not appear that the moderator effect of self-relevance was due to health differences between participants for whom age stereotypes were either self-relevant or not self-relevant. After adjusting for age, these groups did not differ on depression (χ2 = 0.36, p = .16), number of chronic conditions (χ2 = 0.15, p = .70), nor self-rated health (χ2 = 0.11, p = .74).
DISCUSSION
As expected, memory decline was significantly greater over time for participants who held more negative age stereotypes compared to those with less negative age stereotypes. Also as expected, the stereotype–memory effect was significantly greater when the stereotypes were self-relevant.
The findings demonstrate, for the first time, that these psychosocial influences can predict memory decline over an extended period. More specifically, memory trajectories were predicted by the baseline-stereotype measure that was administered as much as 38 years before memory was assessed and often well before old age was reached.
The 30.2% greater memory decline for those aged 60 and above in the more negative age-stereotype group was the result of increasingly wider gaps between the two groups as they aged. The robustness of this finding, which meets the definition of a large effect size (Cohen, 1988), is suggested by the stereotypes significantly predicting memory in the model after adjusting for relevant covariates.
Results of the current analyses converge with short-term experimental studies (e.g., Horton et al., 2008; Levy, 1996 ) by showing the detrimental influence of negative age stereotypes on cognitive performance over time. In this way, the results convey the utility of a long-term approach because there is otherwise a risk that the conclusions drawn from laboratory studies will reflect the limitations of the setting. To illustrate, according to stereotype threat theory, the prospect of confirming a negative stereotype about the cognitive ability of one’s group will induce stress that adversely affects performance in a specific short-term context (Schmader, Johns, & Forbes, 2008; Steele & Aronson, 1995). Consistent with this limited time and space, the theory presupposes that the stereotypes are not internalized (Steele & Aronson, 1995). In contrast, the outcome of the current study is bound by neither time nor space, which suggests stereotypes operate through internalization.
It is likely that the findings of laboratory studies involving older individuals and this longitudinal study share a mechanism. That is, the adverse effect on memory found in the experiments was due to exposing participants to negative age stereotype primes, and older individuals in the community continually encounter interpersonal and institutional incidents of ageism that serve as primes by activating internalized negative age stereotypes (e.g., Levy, Chung, & Canavan, 2011). Consequently, to the extent these everyday-life serial primes activate and reinforce these stereotypes, they would exert an ongoing effect (e.g., Levy et al., 2009).
Negative age stereotypes present memory decline in later life as the monolithic consequence of physiologic inevitability. Yet, this study demonstrates that the stereotypes themselves can contribute to memory over time.
FUNDING
This research was supported by the National Institute on Aging Intramural Research Program and grants from the Patrick and Catherine Weldon Donaghue Medical Research Foundation, National Heart, Lung, and Blood Institute (R01HL089314), and National Institute on Aging (R01AG032284) to the first author.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. doi:10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Benton AL. The revised visual retention test: Clinical and experimental applications. New York: Psychological Corporation; 1974. [Google Scholar]
- Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. doi:10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant LJ, Sheng SL, Morrell CH, Zonderman AB. Data from a longitudinal study provided measurements of cognition to screen for Alzheimer’s disease. Journal of Clinical Epidemiology. 2005;58:701–707. doi: 10.1016/j.jclinepi.2005.01.003. doi:10.1016/j.jclinepi.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Dawson JD, Anderson SW, Uc E, Dastrup E, Rizzo M. Predictors of driving safety in early Alzheimer disease. Neurology. 2009;10:521–527. doi: 10.1212/01.wnl.0000341931.35870.49. doi:10.1212/01.wnl.0000341931.35870.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambra LM, Arenberg D, Kawas C, Zonderman AB, Costa PT., Jr. Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging. 1995;10:123–139. doi: 10.1037//0882-7974.10.1.123. doi:10.1037/0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- Håkansson K, Rovio S, Helkala EL, Vilska AR, Winblad B, Soininen H, Kivipelto M. Association between mid-life marital status and cognitive function in later life: Population based cohort study. BMJ. 2009;339:99. doi: 10.1136/bmj.b2462. doi:10.1136/bmj.b2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton S, Baker J, Pearce GW, Deakin JM. On the malleability of performance: Implications for seniors. Journal of Applied Gerontology. 2008;27:446–465. doi:10.1177/0733464808315291. [Google Scholar]
- Kaufman G, Elder GH. Revisiting age identity: A research note. Journal of Aging Studies. 2002;16:169–176. doi:10.1016/S0890-4065(02)00042-7. [Google Scholar]
- Kwong See S, Nicoladis E. Impact of contact on the development of children's positive stereotyping about aging language competence. Educational Gerontology. 2009;36:52–66. doi:10.1080/03601270903018352. [Google Scholar]
- Lamar M, Zonderman AB, Resnick S. Contribution of specific cognitive processes to executive functioning in an aging population. Neuropsychology. 2002;16:156–162. doi: 10.1037//0894-4105.16.2.156. doi:10.1037//0894-4105.16.2.156. [DOI] [PubMed] [Google Scholar]
- Levy B. Improving memory in old age through implicit self-stereotyping. Journal of Personality and Social Psychology. 1996;71:1092–1107. doi: 10.1037//0022-3514.71.6.1092. doi:10.1037//0022-3514.71.6.1092. [DOI] [PubMed] [Google Scholar]
- Levy BR. Mind matters: Cognitive and physical effects of aging self-stereotypes. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2003;58:203–211. doi: 10.1093/geronb/58.4.p203. doi:10.1093/geronb/58.4.P203. [DOI] [PubMed] [Google Scholar]
- Levy BR. Stereotype embodiment: A psychosocial approach to aging. Current Directions in Psychological Science. 2009;18:332–336. doi: 10.1111/j.1467-8721.2009.01662.x. doi:10.1111/j.1467-8721.2009.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Chung P, Canavan M. Impact of explanatory style and age stereotypes on health across the lifespan. In: Fingerman KL, Berg CA, Smith J, Antonucci TC, editors. Handbook of life-span development. New York: Springer; 2011. pp. 437–456. [Google Scholar]
- Levy BR, Leifheit-Limson E. The stereotype-matching effect: Greater influence on functioning when age stereotypes correspond to outcomes. Psychology and Aging. 2009;24:230–233. doi: 10.1037/a0014563. doi:10.1037/a0014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Zonderman AB, Slade MD, Ferrucci L. Age stereotypes held earlier in life predict cardiovascular events in later life. Psychological Science. 2009;20:296–298. doi: 10.1111/j.1467-9280.2009.02298.x. doi:10.1111/j.1467-9280.2009.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis S, Moore M, Howieson DB, Sexton G, Payami H, Kaye JA, Camicioli R. Independent predictors of cognitive decline in healthy elderly persons. Archives of Neurology. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. doi:10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- Meisner BA. A meta-analysis of positive and negative age stereotype priming effects on behavior among older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2011;66 doi: 10.1093/geronb/gbr062. Advance online publication. doi:10.1093/geronb/gbr062. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Rothermund K. Effects of age stereotypes on self-views and adaptation. In: Greve W, Rothermund K, Wentura D, editors. The adaptive self: Personal continuity and intentional self-development. Cambridge, MA: Hogrefe; 2005. pp. 223–242. [Google Scholar]
- Schmader T, Johns M, Forbes C. An integrated process model of stereotype threat effects on performance. Psychological Review. 2008;115:336–356. doi: 10.1037/0033-295X.115.2.336. doi:10.1037/0033-295X.115.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M, Pittinsky TL, Ambady N. Stereotype susceptibility: Identity salience and shifts in quantitative performance. Psychological Science. 1999;10:80–83. doi: 10.1111/1467-9280.00371. doi:10.1111/1467-9280.00111. [DOI] [PubMed] [Google Scholar]
- Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. Normal human aging: The Baltimore Longitudinal Study of Aging. Washington, DC: U. S. Government Printing Office; 1984. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. doi:10.1093/acprof:oso/9780195152968.001.0001. [Google Scholar]
- Steele CM, Aronson J. Stereotype threat and the intellectual test performance of African Americans. Journal of Personality and Social Psychology. 1995;69:797–811. doi: 10.1037//0022-3514.69.5.797. doi:10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- Tuckman J, Lorge I. Attitudes toward old people. Journal of Social Psychology. 1953;37:249–260. [Google Scholar]
- Wheeler SC, Petty RE. The effects of stereotype activation on behavior: A review of possible mechanisms. Psychological Bulletin. 2001;127:797–826. doi: 10.1037/0033-2909.127.6.797. doi:10.1037//0033-2909.127.6.797. [DOI] [PubMed] [Google Scholar]