Abstract
Background
While it is recommended that all patients with heart failure (HF) have advance directives (AD) in place before the end of life is imminent, the use of AD in HF has not been well studied.
Methods and Results
We enrolled consecutive Olmsted County residents presenting with HF from October 2007 through October 2011 into a longitudinal study. Information from AD completed prior to enrollment and hospitalizations in the month prior to death were abstracted. Among 608 patients (mean age 74.0 years, 54.9% male, 65.3% NYHA functional class 3 or 4) 164 (27.0%) patients died after a mean follow-up of 1.8 years. At enrollment, only 249 (41.0%) patients had an AD. While most AD appointed a proxy decision-maker (90.4%), less than half addressed wishes regarding use of cardiopulmonary resuscitation (41.4%), mechanical ventilation (38.6%), or hemodialysis (10.0%) at the end of life. The independent predictors of AD completion were older age (adjusted OR per 10-year increase 1.82, 95% CI 1.51–2.20), malignancy (OR 1.58, 95% CI 1.05–2.37) and renal dysfunction (OR for eGFR<60 mL/min 1.55, 95% CI 1.05–2.29). At the end of life, patients with AD specifying limits in the aggressiveness of care less frequently received mechanical ventilation (OR 0.26 95% CI 0.07–0.88) with a trend toward decreased intensive care unit admission (OR 0.45, 95% CI 0.16–1.29).
Conclusions
Despite a high mortality rate, over half of patients with HF do not have an AD, and existing AD fail to address important end of life medical decisions.
Keywords: heart failure, epidemiology, prognosis
Advance directives (AD) enable patients to document their end-of-life preferences and/or to appoint a surrogate decision-maker in the event they are no longer capable of making their own medical decisions. It has been estimated that 18–30% of U.S. adults have completed an AD, with slightly higher completion in patients with life-threatening chronic diseases or those receiving long-term care1–4. While conflicting evidence exists, AD may reduce morbidity, including reducing hospitalizations in nursing home residents5 and decreasing in-hospital death in Medicare beneficiaries6. Despite the fact that heart failure (HF) is a common chronic and fatal disease with a median mortality of only five years in the community7, little is known about completion of AD in patients with HF.
End of life care in HF is particularly important as HF imposes a staggering economic burden on the healthcare system8, and the end of life is associated with remarkably high healthcare utilization9, 10. This increase in healthcare use is driven by increased hospitalizations9, despite the fact that >80% of patients with chronic diseases report that they would want to avoid hospitalization as they near death11. Guidelines advocate that providers discuss advance care planning including advance directives (AD) with their patients with HF12, 13, yet few report having these discussions14 and it is unclear if advance care planning impacts end of life decisions and resource use.
We are well positioned in Olmsted County to examine AD completion in HF as we have a well-defined community cohort of patients with HF followed prospectively under the auspices of the Rochester Epidemiology Project, which enables capture of health care events in the county. Herein, we first examined the prevalence and predictors of AD completion in community patients with HF. Second, we tested the hypothesis that AD specifying limits in the aggressiveness of care patients wished to receive at the end of life were associated with decreased end of life hospitalizations, intensive care unit (ICU) admissions, and mechanical ventilation.
Methods
Study design
This is a population-based study conducted in Olmsted County in southeastern Minnesota (2010 U.S. Census population 144248, 90% Caucasian, 50% female). This type of research is possible in Olmsted County as all providers, including Mayo Clinic, have maintained extensively indexed medical records. Through the Rochester Epidemiology Project15, a centralized record linkage system, all medical records are retrievable such that medical information is complete and easily searchable for persons living in the county.
Patient Population
To identify potential HF cases, natural language processing of the electronic medical record text was utilized7. After a clinical visit, documentation is transcribed and appears in the record within 24 hours, making prompt ascertainment of newly diagnosed HF cases possible. The search was restricted to patients at least 20 years old residing in Olmsted County. This approach yields 100% sensitivity compared with billing data16. Records of potential cases are reviewed by trained abstractors to collect data and verify patients had evidence of active HF meeting Framingham criteria. Patients are contacted to obtain consent for study participation, which involves Doppler echocardiography, a venous blood sample, and questionnaires to assess health status. Hospitalized patients are contacted in the hospital, and patients recruited from a clinical setting are contacted at their next clinic visit for consent, study enrollment and data collection. All patients provided written authorization to participate in the study, which was approved by the Mayo Clinic Institutional Review Board.
Advance Directives
AD are scanned into the medical record when provided by the patient or completed in the outpatient setting. Information from AD on patients enrolled in the study was manually abstracted from the medical record. Information recorded included the presence of AD and timing of their completion, whether a surrogate decision-maker was appointed and the relationship of that person to the patient, whether patients stated their wishes regarding use of cardiopulmonary resuscitation, mechanical ventilation, artificial nutrition, and hemodialysis if they experienced critical illness and the end of life were imminent, and their preferences regarding organ donation, autopsy, and burial after their death. Whether the patient reported desiring limits in the aggressiveness of care at the end of life was also assessed. This was defined by the presence of any statement in the document(s) conveying that the patient would want certain therapies or procedures withheld if the end of life were imminent. The first author (SMD) abstracted AD data on all study participants and information from a sample of patients (N=25) were abstracted by an experienced research nurse and the level of agreement was 100%.
Data Collection
Patient Baseline Characteristics
Baseline patient characteristics were abstracted from the medical record by trained research nurses. Prior myocardial infarction (MI) was defined by standardized criteria, which have been previously described and validated17. Physician’s diagnosis was used to document history of cerebrovascular disease, peripheral vascular disease, and chronic obstructive pulmonary disease (COPD). Hypertension was defined as systolic blood pressure >140mmHg, diastolic blood pressure >90mmHg, or use of anti-hypertensive medications. Diabetes mellitus was defined using American Diabetes Association criteria18 or use of diabetes medications. Patient height and weight at HF diagnosis were used to calculate body mass index (BMI). Malignancy was defined as a history of cancer other than basal cell skin cancer. Creatinine at HF diagnosis was collected and creatinine clearance was calculated using the Modification of Diet in Renal Disease Equation19. New York Heart Association (NYHA) functional class was assessed using standard definitions. The Charlson comorbidity index20 was used to assess the burden of comorbidity. A patient’s ability to perform activities of daily living is assessed annually for all patients at the Mayo Clinic by a self-administered survey. Difficulty with activities of daily living was defined by reported difficulty with one of more of the following activities within 90 days pre- and post- study enrollment: dressing, climbing stairs, bathing, difficulty walking, and getting in and out of bed.
Psychosocial Questionnaires
Following study enrollment, psychosocial questionnaires were administered to patients by a study nurse at a scheduled study visit. Questionnaires included the Patient Health Questionnaire (PHQ-9) to assess depression21, ENRICHD Social Support Instrument (ESSI) to assess social support22, 23, and Short Form 12 (SF-12) to assess health status and physical function24. For the PHQ-9, patients were categorized by score into no depression (score 0–4), mild (5–9), and moderate or severe (10 and above). Low social support was defined as an ESSI ≤ 22. The first question of the SF-12 which asks patients to rate their general health as excellent, very good, good, fair, or poor was used to assess general health. Poor perceived health status has been associated with increased healthcare resource use including hospitalizations in community HF patients25. Poor physical function was defined by an SF-12 physical function score less than the median in the population.
Echocardiography
All echocardiograms were obtained and analyzed at Mayo Clinic Echocardiography laboratory according to the American Society of Echocardiography guidelines. Left ventricular ejection fraction (EF) was measured using M-mode, quantitative, and semi-quantitative methods as previously described and validated with excellent correlation between methods26, 27. EF was dichotomized (reduced <50%, preserved ≥50%)28, 29.
Outcomes
Hospitalizations
Information from hospitalizations occurring in the last month of life among patients that died during follow-up was abstracted from the medical record.
Mortality
Follow-up took place through passive surveillance of the medical record. The ascertainment of death included death certificates filed in Olmsted County, obituary notices and electronic files of death certificates obtained from the State of Minnesota Department of Vital and Health Statistics. Whether the patient died during follow-up and the date of death when applicable were collected.
Statistical Analysis
Differences in patient baseline characteristics by AD status at study enrollment were compared using 2-sample t tests for continuous variables or χ2 for binary variables. Logistic regression was used to examine the predictors of AD completion at study enrollment. All characteristics with a potential association (p<0.25) with AD completion on univariate analyses were included in the multivariable model. Only 6% of patients were missing one or more variables from the multivariable model. There were no patients missing any outcomes including the presence or absence of AD and death. Kaplan Meier curves and Cox proportional hazard regression models were used to examine the association between AD and mortality. Logistic regression was used to examine the association between AD specifying limits in the aggressiveness of care and hospitalization, ICU care, and mechanical ventilation in the last month of life. For those patients who died within one month of study enrollment, any hospitalizations occurring after enrollment were included. A p value of <0.05 was used as the level of significance. Analyses were performed using SAS Version 9.2.1 (Cary, NC).
Results
Patient Population
A total of 608 patients were enrolled from October 2007 through October 2011, reflecting a 73.5% consent rate (608 of 827 eligible patients). The baseline characteristics of the population are shown in Table 1. Patients were elderly with a mean age of 74.0 years, 54.9% were men, 49.9% had preserved EF, and 65.3% reported NYHA functional class 3 or 4 symptoms. A total of 365 (60.0%) had a prior diagnosis of HF, and the remainder had incident HF. Patients who did not consent to participate in the study were more likely to be (older [78.6 vs. 74.0 years] and female [53.4 vs. 45.1%]).
Table 1.
Baseline Characteristics of 608 Patients with Heart Failure
| Characteristic | Missing (N) | Overall (n=608) | Advance Directive (n=249) | No Advance Directive (n=359) | * P value |
|---|---|---|---|---|---|
| Age (years) | 0 | 74.0 (13.2) | 79.8 (10.3) | 70.0 (13.5) | <0.001 |
| Male | 0 | 334 (54.9) | 121 (48.6) | 213 (59.3) | 0.009 |
| Preserved EF (≥50%) | 39 | 284 (49.9) | 134 (58.0) | 150 (44.4) | 0.001 |
| Comorbidities | |||||
| Hypertension | 1 | 551 (90.8) | 229 (92.0) | 322 (89.9) | 0.40 |
| Diabetes Mellitus | 1 | 231 (38.1) | 89 (35.9) | 142 (39.6) | 0.36 |
| COPD | 0 | 169 (27.8) | 78 (31.3) | 91 (25.3) | 0.11 |
| Peripheral Vascular Disease | 0 | 143 (23.5) | 76 (30.5) | 67 (18.7) | <0.001 |
| Cerebrovascular Disease | 0 | 164 (27.0) | 88 (35.3) | 76 (21.2) | <0.001 |
| Prior MI | 1 | 160 (26.4) | 75 (30.1) | 85 (23.7) | 0.08 |
| Malignancy | 0 | 174 (28.6) | 92 (37.0) | 82 (22.8) | <0.001 |
| NYHA Class 3 or 4 | 2 | 396 (65.3) | 156 (62.7) | 240 (67.2) | 0.24 |
| eGFR<60 mL/minute | 0 | 350 (57.6) | 169 (67.9) | 181 (50.4) | <0.001 |
| BMI (kg/m2) | 0 | 31.4 (8.0) | 30.0 (7.0) | 32.4 (8.4) | <0.001 |
| Psychosocial Characteristics | |||||
| Low social support | 121 | 52 (10.7) | 17 (8.8) | 35 (11.9) | 0.28 |
| Moderate/severe depression | 121 | 70 (14.4) | 24 (12.4) | 46 (15.7) | 0.32 |
| Poor perceived health | 121 | 65 (13.4) | 27 (14.0) | 38 (12.9) | 0.74 |
| Poor physical function | 121 | 243 (49.9) | 102 (52.9) | 141 (48.0) | 0.29 |
| Difficulty with ADLs | 193 | 222 (53.5) | 95 (57.2) | 127 (51.0) | 0.21 |
EF= ejection fraction, COPD= chronic obstructive pulmonary disease, MI= myocardial infarction, NYHA= New York Heart Association, eGFR= estimated glomerular filtration rate, BMI= body mass index, ADLs= activities of daily living
The test statistics reported are based on those individuals with observed values
Advance Directive Completion
At study enrollment, only 249 (41.0%) patients had an AD, and they were completed an average of 3.3 years prior. Most of the population (60.0%) had pre-existing HF (the remainder were newly diagnosed), and AD completion was similar (age-adjusted p value 0.53) regardless of the timing of diagnosis. The characteristics of completed AD are shown in Table 2. Most AD appointed a surrogate decision-maker (90.4%), who was most frequently a spouse (41.8% of cases) or son/daughter (27.7%). However, a minority of AD addressed the patient’s preferences regarding use of cardiopulmonary resuscitation (41.4% of AD), mechanical ventilation (38.6%), artificial nutrition and hydration (38.6%), or hemodialysis (10.0%).
Table 2.
Characteristics of 249 Advance Directives at Study Enrollment
| N (%) | |
|---|---|
| Appointed proxy decision-maker | 225 (90.4) |
| Spouse | 104 (41.8) |
| Son/daughter | 69 (27.7) |
| Other/unclear | 52 (20.9) |
| Expressed wishes regarding: | |
| Cardiopulmonary resuscitation | 103 (41.4) |
| Mechanical ventilation | 96 (38.6) |
| Artificial nutrition and hydration | 96 (38.6) |
| Hemodialysis | 25 (10.0) |
| Organ donation | 122 (49.0) |
| Burial | 114 (45.8) |
| Autopsy | 51 (20.5) |
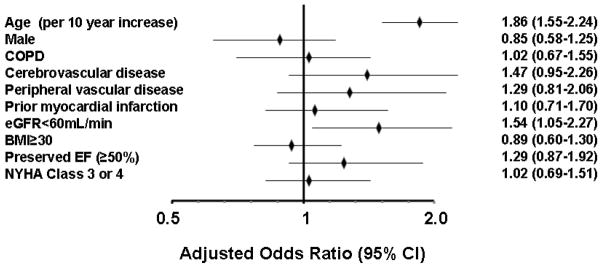
As shown in Table 1, in unadjusted analyses, AD were more likely to be completed prior to study enrollment among patients who were older, female, and who had cerebrovascular disease, peripheral vascular disease, lower BMI, preserved EF, history of malignancy, and renal dysfunction (eGFR<60 mL/min). However, there were no differences based on severity of HF as assessed by NYHA functional class, in patients who had poor perceived health, or in those with decreased physical function or difficulty completing activities of daily living. The adjusted association between patient baseline characteristics and AD completion (at study enrollment) are shown in Figure 1. Older age, history of malignancy, and renal dysfunction (eGFR<60 mL/min) were independent predictors of AD completion. Age provided the majority of prognostic power of the model (model C-statistic 0.75, age alone C-statistic 0.72). In total, 60.7%, 31.3%, and 13.6% of those age ≥80 years, 60–79 years, and <60 years, respectively had an AD. In patients with a history of malignancy, 52.9% had an AD compared with 36.2% of those without malignancy. In patients with renal dysfunction, 48.3% had AD compared with 31.0% of those with normal eGFR. Some patients enrolled in the study (n=121, 19.9%) did not return for their visit to complete the psychosocial questionnaires. However, as the psychosocial data, physical function score, and difficulty with activities of daily living demonstrated no association with AD use, these variables were not included in the final model. Sensitivity analyses were conducted adding each variable shown in Table 1 to the final model sequentially and none were statistically significant predictors of AD completion.
Figure 1.
The adjusted odds ratios (OR) and 95% confidence intervals (CI) predicting advance directive use at study enrollment are shown. All factors shown were included in the multivariable model. COPD= chronic obstructive pulmonary disease, eGFR= estimated glomerular filtration rate, NYHA= New York Heart Association, EF= ejection fraction, BMI= body mass index
Impact of AD on End of Life Care
After a mean follow-up of 1.8 years (through December 1, 2011), 164 (27.0%) patients had died. The Kaplan-Meier predicted 2-year mortality rate was 26% (22–30%). There was no difference in mortality in patients with AD completed prior to study enrollment compared to those without (unadjusted hazard ratio for death 1.30, 95% CI 0.96–1.77, p=0.092, age-adjusted hazard ratio for death 0.94, 95% CI 0.68–1.30, p=0.70). Patients had the opportunity to complete AD after enrollment but prior to death. Among the 164 patients who died, 75 (45.7%) had an AD at study enrollment, and an additional 31 completed an AD during follow-up, such that 106 (64.6%) had an AD in place at the time of death. In 25/106 (23.6%) cases, the AD specified that the patient did not wish to have cardiopulmonary resuscitation or mechanical ventilation (Do Not Resuscitate/Do Not Intubate [DNR/DNI]). An additional 39 (36.8%) AD stated limitations on the aggressiveness of care the patient would like to receive if death was felt to be imminent. The remaining 42 (39.6%) AD either did not address resuscitation preference or comment on limits in care at the end of life or stated they wanted no limits on the aggressiveness of care.
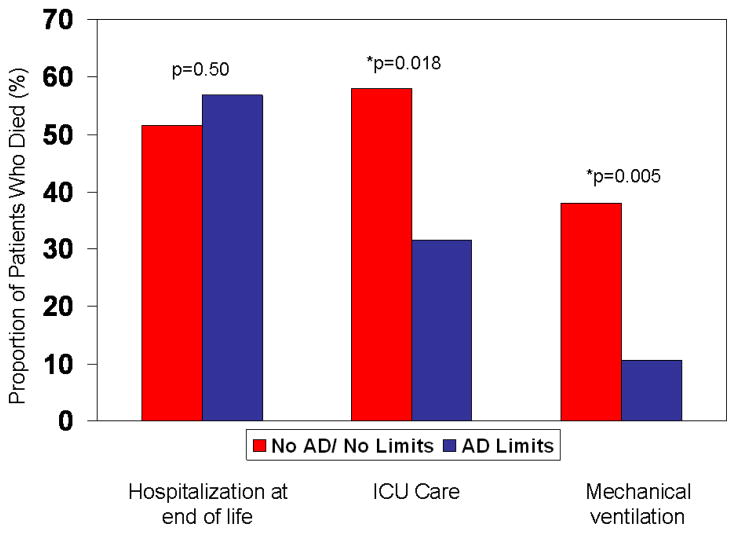
Among patients that died, 88 (53.7%) were hospitalized in their final month of life, of which 50 (30.5%) died in the hospital. During hospitalization, 41 of 88 patients (46.6%) were cared for in an ICU and 23 (26.1%) received mechanical ventilation. There were no differences in the proportion of patients hospitalized in the last month of life in those with an AD specifying limits (DNR/DNI or other limits in the aggressiveness of care) compared with the remaining patients who died (Figure 2). However, among those hospitalized within the last month of life, patients with an AD specifying limits were less frequently cared for in the ICU and less frequently received mechanical ventilation. After adjustment for age, sex and comorbidity (Charlson comorbidity index), patients with AD specifying limits were less likely to receive mechanical ventilation compared with others who died without an AD or with an AD without limits (adjusted OR 0.26, 95% CI 0.06–0.88, p=0.03), though the decreased risk of ICU care was no longer statistically significant (OR 0.45, 95% CI 0.16–1.29, p=0.14). There was no difference in the risk of hospitalization in the last month of life in those with an AD with limits compared with those without (adjusted OR 1.26, 95% CI 0.64–2.48, p=0.51).
Figure 2.
The proportion of patients with each outcome (hospitalization, ICU care, mechanical ventilation) at the end of life among the 164 patients who died are shown according to whether they had an advance directive specifying limits in care at the time of death. AD= advance directive, ICU= intensive care unit
Discussion
HF is a disabling disease with a high associated morbidity and mortality. While advance care planning is acknowledged to be an important component of patient care in HF, very little is known about the use and impact of AD in patients with HF. Herein, we found that less than half of community patients with HF had an AD, and most AD failed to address important medical decisions common at the end of life, including use of cardiopulmonary resuscitation, mechanical ventilation, and hemodialysis. However, those with AD in place at the time of death that specified limits in the aggressiveness of care were less likely to receive mechanical ventilation, and had a trend toward decreased ICU care.
Completion of AD
It has been more than 20 years since Congress passed the Patient Self-Determination Act in 1990 mandating that all Medicare-certified institutions provide written information to patients about their rights to execute AD30. The intent of AD is to allow patients to document their end of life preferences and to appoint a proxy decision-maker if they become incapable of making medical decisions. Despite their endorsement, most studies have reported that <50% of severely or terminally ill patients have an AD in place3, 31–34. Very little is known about AD completion in patients with cardiovascular diseases such as HF. Among 112 patients admitted to a coronary care unit, 23% had AD31. Swetz et al reported that only 37% of 68 patients with advanced HF had AD in place prior to left ventricular assist device implantation, and of those that had AD, none mentioned management of the ventricular assist device as one approached end of life3. To the best of our knowledge, there are no reports of the prevalence of AD in the general HF population. We found that only 41% of Olmsted County residents with HF had an AD at study enrollment. While some patients may have been recently diagnosed with HF and not yet had the opportunity to participate in advance care planning, even at the time of death, 35% of patients with HF still did not have an AD.
A lack of awareness of AD in patients with HF may be one barrier to their use. In a small Canadian study, 76% of patients with HF did not know what AD were, though 80% wanted more information about them35. Minnesota lacks a formal hierarchy for surrogate-decision making if one has not been formally named via a healthcare power of attorney. As this hierarchy is variable from state-to-state, the naming of a surrogate decision-maker is encouraged, particularly in this environment. While we found that the vast majority of AD appointed a proxy decision-maker, their assignment alone may not be sufficient, as the appointed surrogates are frequently absent at the end of life36 and the language of AD may be too general in nature to guide all treatment decisions33. We found that AD in patients with HF infrequently discussed specific preferences for medical interventions such as cardiopulmonary resuscitation and hemodialysis. Similar to previous reports in the general medical population, directives more commonly stated a general desire to avoid life-prolonging therapies if the end of life was imminent37. AD may provide more meaningful guidance to surrogates making critical decisions in an emotionally-charged environment if they contained more clinically relevant data.
AD completion appeared to have little correlation with the patient’s diagnosis of HF, as there was no difference in the proportion of patients with AD completed by duration of HF or NYHA functional class. Further, we found no difference in AD completion according to key psychosocial and mobility characteristics including poor perceived health, depression, low social support, poor physical function and difficulty completing activities of daily living, many of which have been associated with increased morbidity and mortality in cardiovascular disease25, 38, 39. Advanced age was by far the strongest predictor of AD completion, with malignancy and renal dysfunction representing the only other factors associated with AD use. Therefore, key characteristics traditionally associated with adverse prognosis are failing to trigger completion of AD in HF patients. This may be particularly important for young patients with HF, who are very unlikely to have their end of life preferences documented in the form of AD.
Both the American College of Cardiology/American Heart Association and Heart Failure Society of American Guidelines recommend that physicians discuss AD with their patients who have HF12, 13, though studies have shown that only 12% of patients with AD received input from their physician in its development32. As physician perceptions of the end of life preferences of their patients with HF has been shown to be frequently inaccurate40, AD may represent one way to facilitate patient-provider communication about end of life. There are several potential reasons why physicians may not routinely discuss end of life planning with their HF patients. First, estimating prognosis is difficult41. While models exist to predict prognosis in HF, most have only modest accuracy. The potential uncertainty regarding prognosis in HF makes routine advance care planning even more appropriate in this population to avoid forcing patients and family members to make abrupt decisions when patients are facing critical illness unexpectedly. While timing of these discussions may be difficult in a busy clinical practice, as a recent Scientific Statement on decision-making in advanced HF notes “on the day of hospital admission, it is far better to review rather than introduce advanced care decisions.”41 Second, though data has shown that patient-physician advance care planning discussions improve patient satisfaction42, many physicians are hesitant to partake in such conversations at the risk of taking away hope and hastening death. However, recent evidence suggests that having discussions about AD does not decrease survival43. While our study was not designed to test the impact that AD completion has on survival, there was no difference in survival in patients with an AD compared to those without.
There has been substantial debate as to whether AD impact medical care at the end of life, with studies demonstrating that AD both decrease6, 44, 45 or have no effect1, 32, 46 on healthcare resource at the time of death. In the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT), patients with AD receive care inconsistent with their written preferences up to half of the time33. However, recent studies have demonstrated decreased inhospital death, less aggressive care, and lower end of life healthcare expenditures in elderly patients with AD specifying preferences6, 44. We found that among community patients with HF who died, those with AD specifying limits in the aggressiveness of care they wished to receive at the end of life were far less likely to receive mechanical ventilation (OR 0.23), and found a trend toward a decrease in ICU care (OR 0.45) in the last month of life. While we found no association between AD specifying limits and hospitalization in the last month of life, the number of patients who died in this study was small, and these analyses should be replicated in larger populations of patients with HF. Though our findings would suggest that use of AD in patients with HF may have no impact on the number of hospitalizations at the end of life, ICU care and mechanical ventilation are expensive47, and reductions in these endpoints in patients who do not wish to receive this type of care may result in cost savings. Therefore, AD may facilitate less aggressive care in alignment with patient preferences at the end of life. The mechanism for this facilitation is unclear. This may be related to AD being directly used by family members and providers in making medical decisions. Alternatively, the presence of AD may serve as a marker of patient engagement in an end of life planning process, and act as a conduit for communication of wishes in advance of end of life. Although this requires further study, either of these mechanisms may be beneficial in promoting patient autonomy and planning care that is consistent with a patient’s goals.
Limitations
Our study had several limitations. First, AD that were not a part of the patient’s medical record were not captured. Further, patients were not asked at study enrollment whether they had AD that they did not provide to clinic personnel. However, as these patients are residents of Olmsted County and receiving medical care at Mayo Clinic, AD would likely be provided to augment patient care. Second, we do not have information on why patients do not have AD including whether they declined to complete them, but this would be of interest in future studies. Third, the consent rate for the study was 73.5%, which is similar to other community epidemiologic studies48. As non-participants were an average of 4.6 years older than participants, their AD use may have been slightly higher than the results we reported. Finally, while Olmsted County is becoming increasingly diverse, the population remains 85% Caucasian, and these results may not apply to communities of varying racial and ethnic diversity. However, there are important advantages of these data. First, they reflect the comprehensive experience of a community cohort of patients with HF who are followed longitudinally for outcomes. Second, they provide detailed information on the content and predictors of AD, and their association with hospitalizations at the end of life.
Conclusions
Healthcare resource use is high in HF patients, particularly those with advanced disease. In an era of increasing focus on patient-centered medicine and respect for patient autonomy, the matching of patient preferences to healthcare delivery is of mounting importance. We found that AD were underutilized and inadequate, as they frequently failed to address patient preferences regarding end of life care. However, when they were formulated, AD specifying limits were associated with lesser use of invasive care in alignment with patient preferences. AD completed in detail may represent a simple, useful tool to facilitate appropriate healthcare resource use at the end of life, particularly in patients with life-limiting illnesses such as HF.
What Is Known
Cardiology guidelines advocate that providers discuss advance care planning including advance directives with their patients with heart failure, but little is known about their use in heart failure.
There has been substantial debate as to whether completion of advance directives impacts medical care at the end of life.
What This Article Adds
Only 41% of community heart failure patients have an advance directive in place, and 35% of patients did not complete an advance directive prior to death
Many completed advance directives did not address specific patient preferences regarding key end of life decisions including use of cardiopulmonary resuscitation, mechanical ventilation, and artificial nutrition.
At the end of life, patients with heart failure who had advance directives specifying limits in the aggressiveness of care they wished to receive were equally likely to be hospitalized, but less likely to receive mechanical ventilation.
Acknowledgments
We would like to thank Kay Traverse and Jill Killian for their assistance with data collection and statistical analysis.
Sources of Funding
This work was supported by grants from the NIH (HL72435) and the Rochester Epidemiology Project from the National Institute of Aging (R01 AG034676).
Footnotes
Disclosures
Dr. Mueller is a member of the Boston Scientific Patient Safety Advisory Board and lectures for the Boston Scientific Education Services. Dr. Swetz has received honoraria for speaking for Boston Scientific. Dr. Roger and Dr. Dunlay have no disclosures.
References
- 1.Halpern NA, Pastores SM, Chou JF, Chawla S, Thaler HT. Advance directives in an oncologic intensive care unit: a contemporary analysis of their frequency, type, and impact. J Palliative Med. 2011;14:483–489. doi: 10.1089/jpm.2010.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschman KB, Abbott KM, Hanlon AL, Prvu Bettger J, Naylor MD. What factors are associated with having an advance directive among older adults who are new to long term care services? J Am Med Directors Assoc. 2012;13:82 e87–11. doi: 10.1016/j.jamda.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swetz KM, Mueller PS, Ottenberg AL, Dib C, Freeman MR, Sulmasy DP. The use of advance directives among patients with left ventricular assist devices. Hosp Practice. 2010;39:78–84. doi: 10.3810/hp.2011.02.377. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson A, Wenger N, Shugarman LR. Literature review on advance directives. Washington, DC: Report prepared for the U.S. Department of Health and Human Services; Assistant Secretary for Planning and Evaluation; Office of Disability, Aging and Long-Term Care Policy; 2007. [Google Scholar]
- 5.O’Malley AJ, Caudry DJ, Grabowski DC. Predictors of nursing home residents’ time to hospitalization. Health Services Res. 2010;46:82–104. doi: 10.1111/j.1475-6773.2010.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR. Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA. 2011;306:1447–1453. doi: 10.1001/jama.2011.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplant. 1994;13:S107–112. [PubMed] [Google Scholar]
- 9.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, Peterson ED, Curtis LH. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 11.Fisher ES. The Dartmouth Atlas of Health Care. In: Bronner KK, editor. End of Life Care. 2007. [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 13.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann JC, Wenger NS, Davis RB, Teno J, Connors AF, Jr, Desbiens N, Lynn J, Phillips RS. Patient preferences for communication with physicians about end-of-life decisions. SUPPORT Investigators. Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment. Ann Intern Med. 1997;127:1–12. doi: 10.7326/0003-4819-127-1-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proceed. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. J Biomed Inform. 2005;38:145–153. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Roger VL, Killian J, Henkel M, Weston SA, Goraya TY, Yawn BP, Kottke TE, Frye RL, Jacobsen SJ. Coronary disease surveillance in Olmsted County objectives and methodology. J Clin Epidemiol. 2002;55:593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Garber A, Goldberg R, Havas S, Holman R, Lamendola C, Howard WJ, Savage P, Sowers J, Vega GL. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group IV: lifestyle and medical management of risk factors. Circulation. 2002;105:e153–158. doi: 10.1161/01.cir.0000014022.85836.96. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krumholz HM, Butler J, Miller J, Vaccarino V, Williams CS, Mendes de Leon CF, Seeman TE, Kasl SV, Berkman LF. Prognostic importance of emotional support for elderly patients hospitalized with heart failure. Circulation. 1998;97:958–964. doi: 10.1161/01.cir.97.10.958. [DOI] [PubMed] [Google Scholar]
- 23.Vaglio J, Jr, Conard M, Poston WS, O’Keefe J, Haddock CK, House J, Spertus JA. Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health Qual Life Outcomes. 2004;2:24. doi: 10.1186/1477-7525-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain AM, McNallan SM, Dunlay SM, Spertus JA, Moser DK, Weston SA, Redfield MM, Roger VL. Self-rated Health Predicts Healthcare Utilization in Heart Failure Patients. Circulation. 2011;124:A13869. doi: 10.1161/JAHA.114.000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amico AF, Lichtenberg GS, Reisner SA, Stone CK, Schwartz RG, Meltzer RS. Superiority of visual versus computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J. 1989;118:1259–1265. doi: 10.1016/0002-8703(89)90018-5. [DOI] [PubMed] [Google Scholar]
- 27.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 28.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 29.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 30.The Patient Self Determination Act of 1990. Vol 42 USC 1395 cc; 1990.
- 31.Kirkpatrick JN, Guger CJ, Arnsdorf MF, Fedson SE. Advance directives in the cardiac care unit. Am Heart J. 2007;154:477–481. doi: 10.1016/j.ahj.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Teno J, Lynn J, Wenger N, Phillips RS, Murphy DP, Connors AF, Jr, Desbiens N, Fulkerson W, Bellamy P, Knaus WA. Advance directives for seriously ill hospitalized patients: effectiveness with the patient self-determination act and the SUPPORT intervention. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc. 1997;45:500–507. doi: 10.1111/j.1532-5415.1997.tb05178.x. [DOI] [PubMed] [Google Scholar]
- 33.Teno JM, Licks S, Lynn J, Wenger N, Connors AF, Jr, Phillips RS, O’Connor MA, Murphy DP, Fulkerson WJ, Desbiens N, Knaus WA. Do advance directives provide instructions that direct care? SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc. 1997;45:508–512. doi: 10.1111/j.1532-5415.1997.tb05179.x. [DOI] [PubMed] [Google Scholar]
- 34.van Oorschot B, Schuler M, Simon A, Flentje M. Advance directives: prevalence and attitudes of cancer patients receiving radiotherapy. Support Care Cancer. 2012 doi: 10.1007/s00520-012-1394-y. [DOI] [PubMed] [Google Scholar]
- 35.Habal MV, Micevski V, Greenwood S, Delgado DH, Ross HJ. How aware of advanced care directives are heart failure patients, and are they using them? Canadian J Cardiol. 2011;27:376–381. doi: 10.1016/j.cjca.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 36.Teno JM, Stevens M, Spernak S, Lynn J. Role of written advance directives in decision making: insights from qualitative and quantitative data. J Gen Intern Med. 1998;13:439–446. doi: 10.1046/j.1525-1497.1998.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura A, Mueller PS, Evenson LK, Downer LL, Bowron CT, Thieke MP, Wrobleski DM, Crowley ME. Patients who complete advance directives and what they prefer. Mayo Clin Proceed. 2007;82:1480–1486. doi: 10.1016/S0025-6196(11)61091-4. [DOI] [PubMed] [Google Scholar]
- 38.Burg MM, Barefoot J, Berkman L, Catellier DJ, Czajkowski S, Saab P, Huber M, DeLillo V, Mitchell P, Skala J, Taylor CB. Low perceived social support and post-myocardial infarction prognosis in the enhancing recovery in coronary heart disease clinical trial: the effects of treatment. Psychosom Med. 2005;67:879–888. doi: 10.1097/01.psy.0000188480.61949.8c. [DOI] [PubMed] [Google Scholar]
- 39.Faller H, Stork S, Schowalter M, Steinbuchel T, Wollner V, Ertl G, Angermann CE. Depression and survival in chronic heart failure: does gender play a role? Eur J Heart Fail. 2007;9:1018–1023. doi: 10.1016/j.ejheart.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Krumholz HM, Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, Califf RM, Vidaillet H, Davis RB, Muhlbaier LH, Connors AF, Jr, Lynn J, Goldman L. Resuscitation preferences among patients with severe congestive heart failure: results from the SUPPORT project. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Circulation. 1998;98:648–655. doi: 10.1161/01.cir.98.7.648. [DOI] [PubMed] [Google Scholar]
- 41.Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, Havranek EP, Krumholz HM, Mancini D, Riegel B, Spertus JA. Decision Making in Advanced Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2012 Mar 5; doi: 10.1161/CIR.0b013e31824f2173. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tierney WM, Dexter PR, Gramelspacher GP, Perkins AJ, Zhou XH, Wolinsky FD. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16:32–40. doi: 10.1111/j.1525-1497.2001.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer SM, Min SJ, Sauaia A, Kutner JS. “They’re going to unplug grandma”: Advance directive discussions and documentation do not decrease survival in patients at baseline lower risk of death. J Hosp Med. 2012;7:3–7. doi: 10.1002/jhm.930. [DOI] [PubMed] [Google Scholar]
- 44.Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. New Engl J Med. 2010;362:1211–1218. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, Block SD, Maciejewski PK, Prigerson HG. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelley AS, Ettner SL, Morrison RS, Du Q, Wenger NS, Sarkisian CA. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med. 2011;154:235–242. doi: 10.7326/0003-4819-154-4-201102150-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Critical care medicine. 2005;33:1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 48.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]