Background: Little tertiary structure information is available for the toxic intermediates in the amyloid-β (Aβ) fibrillation process.
Results: Aβ protofibrils show tertiary contacts between Glu-22 and Ile-31, which are not present in mature fibrils.
Conclusion: Aβ protofibrils share tertiary structure features with oligomers but not with mature fibrils.
Significance: Aβ protofibrils must undergo a major structural reorientation in the development of mature Aβ fibrils.
Keywords: Alzheimer Disease, Amyloid, Peptides, Solid-state NMR, Structural Biology, Fibrillation Process, Protofibril, Tertiary Structure
Abstract
We have studied tertiary contacts in protofibrils and mature fibrils of amyloid-β (Aβ) peptides using solid-state NMR spectroscopy. Although intraresidue contacts between Glu-22 and Ile-31 were found in Aβ protofibrils, these contacts were completely absent in mature Aβ fibrils. This is consistent with the current models of mature Aβ fibrils. As these intramolecular contacts have also been reported in Aβ oligomers, our measurements suggest that Aβ protofibrils are structurally more closely related to oligomers than to mature fibrils. This suggests that some structural alterations have to take place on the pathway from Aβ oligomers/protofibrils to mature fibrils, in agreement with a model that suggests a conversion of intramolecular hydrogen-bonded structures of Aβ oligomers to the intermolecular stabilized mature fibrils (Hoyer, W., Grönwall, C., Jonsson, A., Ståhl, S., and Härd, T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5099–5104).
Introduction
Alzheimer disease is characterized by extracellular deposition of plaques of amyloid-β (Aβ)2 peptides in the brain (1). These protein aggregates are composed of mature Aβ fibrils, which represent the end product of a long, complex, and not well understood fibrillation process (2, 3). The fibrillation pathway initiates with soluble unstructured monomeric Aβ peptides, which are converted into oligomers, protofibrils, and finally into mature fibrils (4–6). Recently, interest in the transient Aβ intermediate structures has been growing rapidly because these species are considered to represent the cytotoxic intermediates in Alzheimer disease (7). In addition to the well studied structure (8–12) and dynamics (13) of mature Aβ fibrils, a growing amount of data for oligomers (14–19) and protofibrils (20, 21) has become available. With regard to the secondary structure elements, these studies revealed that oligomers and protofibrils already exhibit the characteristic two β-strand sections connected by a short loop also present in mature Aβ fibrils. However, there are several significant differences between theses species. For instance, the first β-strand of Aβ oligomers and protofibrils is significantly shorter than that in mature Aβ fibrils and has to elongate during the conversion from protofibrils to mature fibrils (20). In addition, many questions about the tertiary structure, the fibrillation process, and the conversion from one intermediate to another are still unanswered.
Härd and co-workers have resolved the structure of Aβ(1–40) oligomers stabilized by an Affibody and also proposed a model for the arrangement of the two β-strands (14, 15, 22). In this model, these β-strands form intramolecular hydrogen bonds in the oligomeric state, in contrast to the known intermolecular hydrogen-bonded structure of mature Aβ fibrils (8, 11, 23). Such an arrangement is necessary to form the characteristic cross-β-structure, which is present in all amyloid protein fibrils. Therefore, the structural transition from Aβ oligomers into mature Aβ fibrils necessitates a 90° rotation of the β-strands upon maturation (see Fig. 1). The current model suggests that this switch from intra- to intermolecular hydrogen bonds occurs during the conversion from protofibrils to mature fibrils (14, 15). However, neither the tertiary structure nor the nature of the intra- versus intermolecular contacts in protofibrils has been investigated until now. So far, support for this model comes from the observation that double cysteine mutants of Aβ(1–40) and also Aβ(1–42), which are forced to retain the molecular structure of an oligomer by the cysteine bond, can form only protofibrils but not mature fibrils (14, 22). In addition, it was shown by IR spectroscopy that, in oligomers and protofibrils, the β-sheets are antiparallel (18, 24), whereas mature Aβ fibrils exhibit parallel β-sheets as shown by solid-state NMR (25).
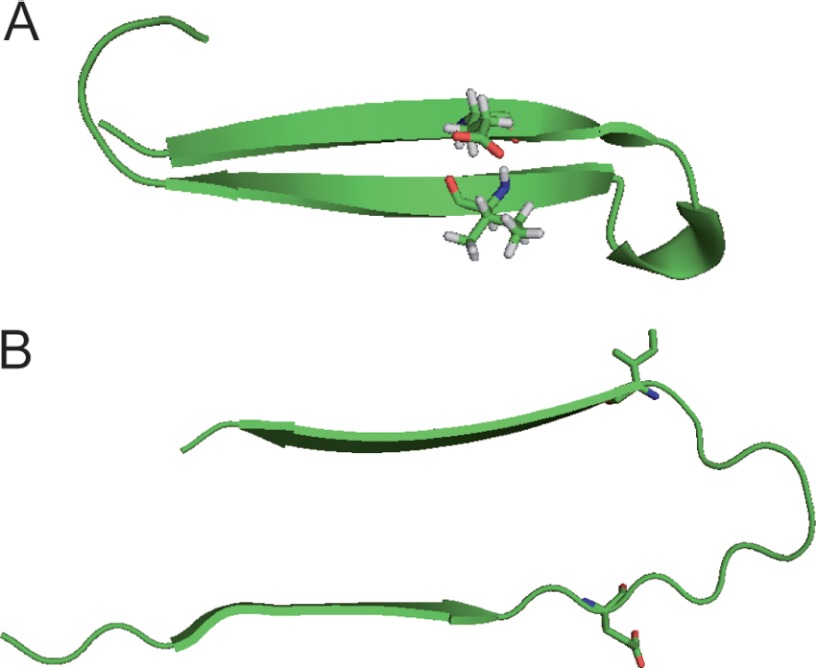
FIGURE 1.
Molecular structures of Aβ(1–40) oligomers (A; Protein Data Bank code 2OTK (15)) and mature Aβ fibrils (B; Ref. 11 and I. Bertini, personal communication). Glu-22 and Ile-31 are shown in their molecular structure. For Aβ protofibrils, no molecular structure is available so far.
To obtain more structural data, we used 13C solid-state NMR to investigate the contact between Glu-22 and Ile-31 of Aβ(1–40) protofibrils, which are by stabilized by the B10AP antibody as reported previously (24). Glu-22 and Ile-31 and, in particular, the side chains of these residues should become very close in space in the oligomeric structure as illustrated in Fig. 1. From the Protein Data Bank coordinates (15), one can calculate a distance of 5.3 Å between the α-carbons of Glu-22 and Ile-31; the side chain carbons show similar close proximities. In contrast, in mature Aβ fibrils (11), these residues point into different directions out of the cross-β-core of the fibrils, yielding a distance of 12.4 Å between the α-carbons of Glu-22 and Ile-31 and up to ∼18 Å between the side chain carbons. This is illustrated in Fig. 1 using the Protein Data Bank coordinates of these two structures. For protofibrils, no molecular structure with atomic resolution is available so far, but solid-state NMR work revealed that the secondary structure elements of protofibrils are more closely related to oligomers than to mature Aβ fibrils (20). Therefore, solid-state NMR measurements should reveal the arrangement of the two β-sheets in protofibrils and give insights in their tertiary fold.
EXPERIMENTAL PROCEDURES
The Aβ(1–40) peptides were produced by standard solid-phase synthesis according to the Fmoc (N-(9-fluorenyl)methoxycarbonyl) protocol using fully 13C/15N-labeled Ser-8, Glu-22, and Ile-31. B10AP-stabilized protofibrils were prepared in 1-ml samples (50 mm Hepes (pH 7.4) and 50 mm NaCl) containing 4 mg/ml labeled Aβ(1–40) and B10AP at a 10:1 molar ratio. The samples were incubated for 3 days (37 °C).
For mature Aβ(1–40) fibrils, the labeled peptide was solubilized in 50 mm sodium borate buffer (pH 9) at a concentration of 6 mg/ml. The sample was seeded and incubated at 37 °C for 1 week. Seeds consisted of Aβ(1–40) mature fibrils previously grown and seeded under the same conditions (second generation) and were sonicated for 10 min before addition to the sample. It has been shown that, also under these conditions, the structural properties of the mature fibrils agreed well with fibrils grown at pH 7.4 in phosphate buffer (13).
In both cases, the peptide aggregates were recovered by ultracentrifugation at 100,000 rpm for 2 h at 4 °C (TLA-120.2 rotor, Beckman Optima TLX centrifuge). The pellet was lyophilized, rehydrated with 50 weight % H2O, and homogenized by freezing the sample in liquid nitrogen and thawing it at 37 °C. The morphology of the samples was checked by transmission electron microscopy (supplemental Fig. S1).
The 13C cross-polarization magic angle spinning NMR spectra were obtained using a Bruker AVANCE 750 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) operating at resonance frequencies of 749.7 MHz for 1H and 188.5 MHz for 13C. A 4-mm double-resonance magic angle spinning probe was used. The length of the cross-polarization contact time was 700 μs, and the 90° pulse was 5 μs for 13C and 4 μs for 1H. For heteronuclear two-pulse phase modulation decoupling, a 1H radiofrequency field of 65 kHz was applied. 13C chemical shifts were referenced externally relative to TMS. The peak assignment was taken from the literature (13, 20). The two-dimensional 13C-13C proton-driven spin exchange spectra were acquired with a mixing time of 600 ms and covariance-processed (26). A total of 64 complex data points were acquired in the indirect dimension at a spectral width of 190 ppm. For the protofibrils, 640 transients per increment were acquired, whereas 2096 scans were acquired for two-dimensional spectra of the mature Aβ fibrils at 58 t1 increments.
To measure 13C-1H dipolar couplings, constant time DIPSHIFT experiments with FSLG (frequency-switched Lee-Goldburg) (27) or MREV-8 (28) for homonuclear decoupling (80-kHz decoupling field) were performed. The order parameter was derived by dividing the determined coupling by the known rigid limits (29, 30). All NMR experiments were carried out at a temperature of 30 °C and a magic angle spinning frequency of 7 or 5 kHz (DIPSHIFT or MREV-8).
RESULTS
Fig. 2 shows characteristic 13C-13C proton-driven spin diffusion correlation spectra of Aβ(1–40) protofibrils (panel A) and mature Aβ fibrils (panel B) both with uniformly 13C/15N-labeled Ser-8, Glu-22, and Ile-31. The spectra show all of the trivial intraresidue correlations within the labeled amino acids. As a long mixing time of 600 ms was used, also interresidue correlations can be observed. All magnetization exchange, which gives rise to a cross-peak in the proton-driven spin diffusion spectra, is caused by dipolar interactions with a distance dependence of r−6 (31).
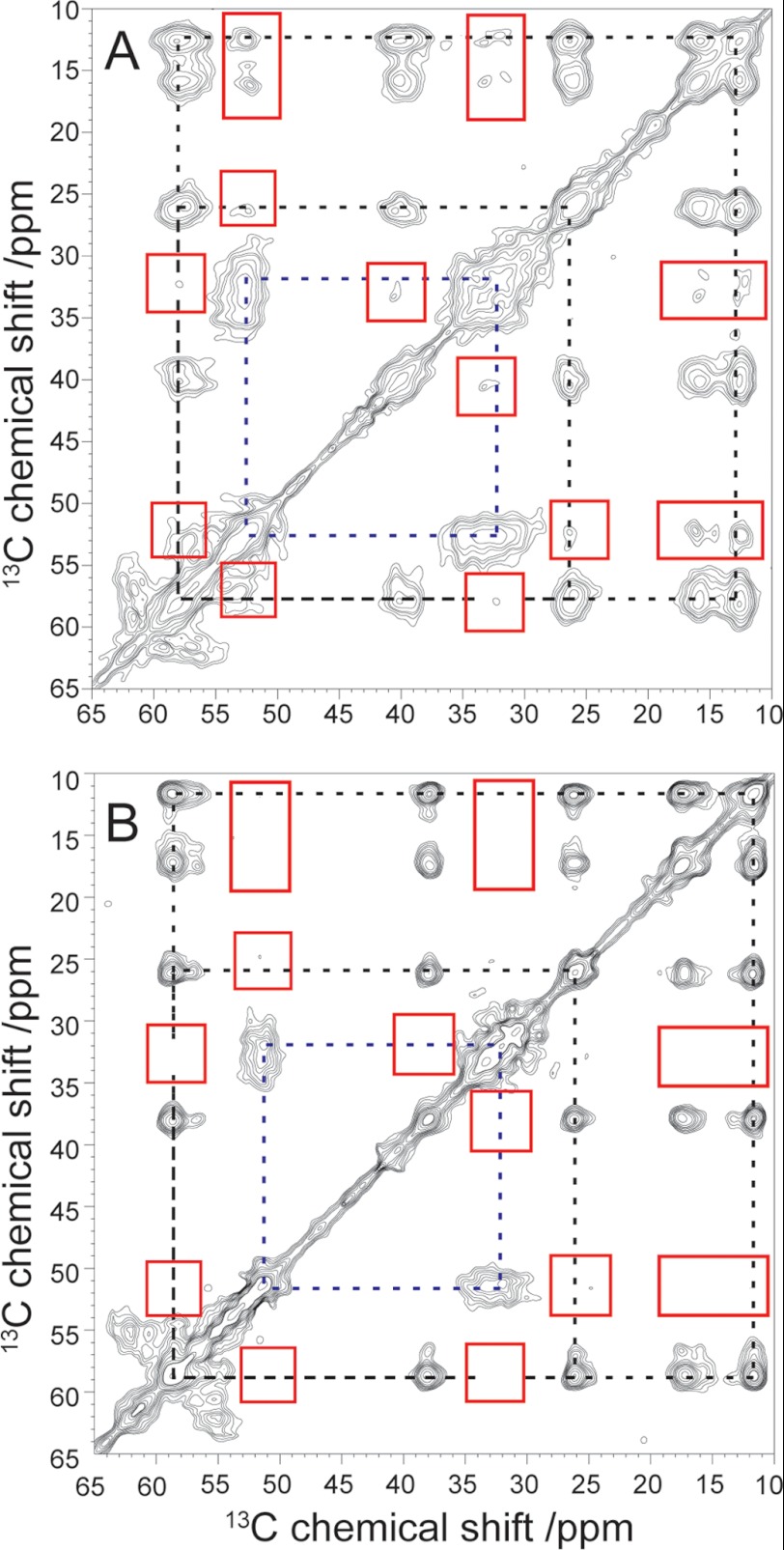
FIGURE 2.
Aliphatic region of the covariance-processed 13C-13C correlation spectra (by proton-driven spin diffusion with a mixing time of 600 ms) for B10AP-stabilized Aβ protofibrils (A) and mature Aβ fibrils (B). The major correlations inside one and the same amino acid for Glu-22 (dashed blue lines) and Ile-31 (dashed black lines) are highlighted. Interresidue cross-peaks between Glu-22 and Ile-31 in A and the lack of these cross-peaks in B are marked with red boxes.
In addition, the 13C-13C correlation spectra of Aβ protofibrils clearly show cross-peaks between Glu-22 and Ile-31, especially between the Cα and Cβ signals of Glu-22 at 52.6 and 32.6 ppm and between the Cδ and Cϵ signals of Ile-31 at 16.1 and 12.5 ppm. This means that, in Aβ protofibrils, Ile-31 and Glu-22 are in close proximity (<6–7 Å), which is observable by 13C-13C correlation spectroscopy (32). The cross-peaks between the other carbons of these two amino acids (including the cross-peak Glu-22 Cα–Ile-31 Cα) are weaker and only sparsely above the noise level, even when using covariance NMR processing, which is known to enhance small cross-peaks (33). For comparison, the standard Fourier transform-processed two-dimensional NMR spectrum is shown in supplemental Fig. S2.
For comparison, we conducted the same experiment using mature Aβ fibrils grown from Aβ(1–40) peptides using the same amino acid labeling procedure. In the NMR spectrum of this sample (Fig. 2B and supplemental Fig. S2B), no cross-peaks between Glu-22 and Ile-31 are visible, as expected from the molecular structure of mature fibrils (Fig. 1B). Please note that there are some differences in the chemical shift values between Aβ protofibrils and mature Aβ fibrils for these amino acids as reported previously (13, 20).
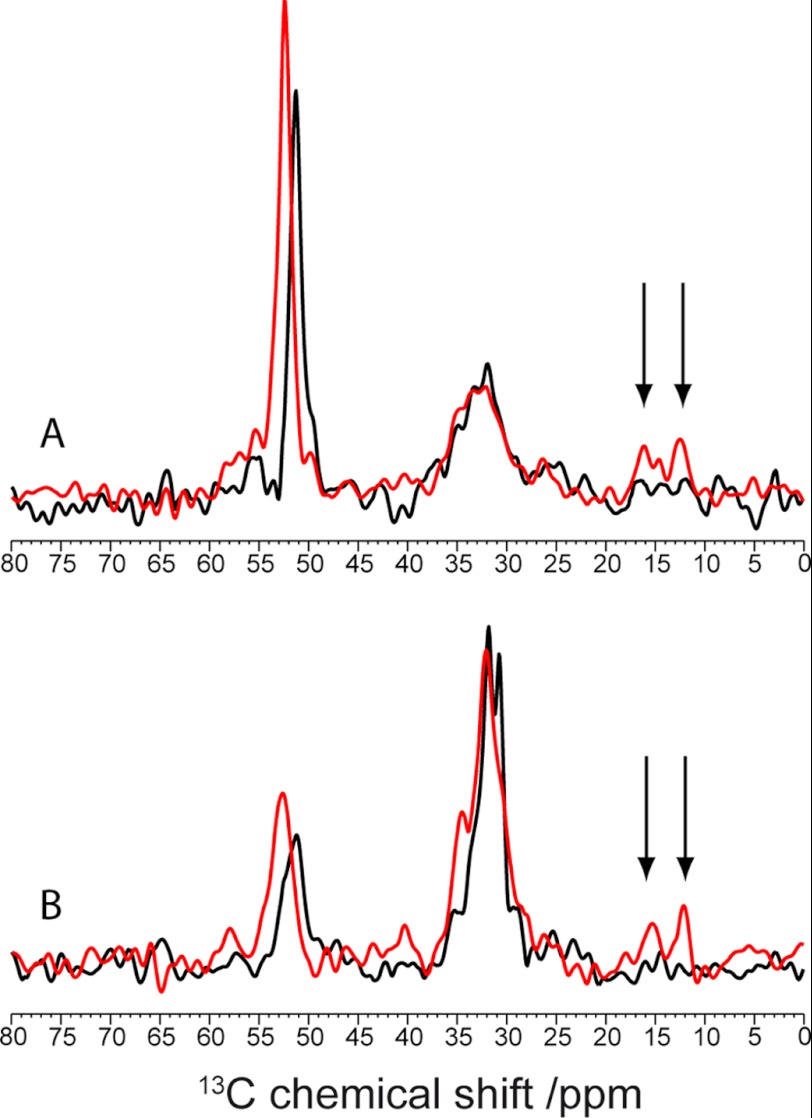
To confirm that the differences in the NMR spectra are not just the result of the choice of contour levels in the two-dimensional plots, we also extracted slices from the two-dimensional spectra, which are shown in Fig. 3 (see also supplemental Fig. S3 for the standard Fourier transform-processed spectra). Again, the cross-peaks from Glu-22 to the side chain of Ile-31 are only observable for the Aβ protofibrils but not for the mature Aβ fibrils.
FIGURE 3.
Slices through the Cα (A) and Cβ (B) peaks of Glu-22 for B10AP-stabilized Aβ protofibrils (red) and mature Aβ fibrils (black) of the covariance-processed proton-driven spin diffusion spectra. Interresidue cross-peaks between Glu-22 and Ile-31 are highlighted by the arrows.
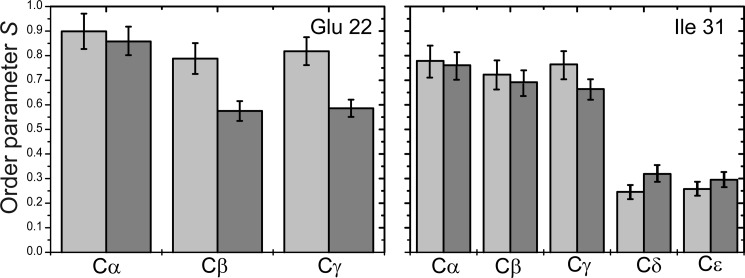
The structural differences in Aβ protofibrils and mature fibrils are also reflected in the molecular dynamics of the residues investigated. As shown in Fig. 4, the order parameters of the amino acid side chains determined from motionally averaged dipolar couplings show a clear tendency to be lower in mature Aβ fibrils than in Aβ protofibrils. If one assumes a similar structural arrangement of the β-sheets in Aβ oligomers (Fig. 1) and in protofibrils, the interaction of the side chains of Glu-22 and Ile-31 leads to a motional restriction and therefore the higher order parameters in protofibrils. The decreased order parameters of these side chains in mature Aβ fibrils are caused by the additional degrees of motional freedom the side chains can undergo when they are pointing out of the cross-β-core of the fibrils (Fig. 1). It should be noted that binding of the B10 antibody has no significant influence on the order parameters of Glu-22 and Ile-31 (13, 20).
FIGURE 4.
C-H order parameters for Glu-22 and Ile-31 in B10AP-stabilized Aβ protofibrils (light gray bars) and mature Aβ fibrils (dark gray bars).
DISCUSSION
We conclude that Glu-22 and Ile-31 are in close proximity in Aβ protofibrils but are significantly more distant in mature Aβ fibrils (as suggested by the structural model of both species sketched in Fig. 1B). This means that the Aβ protofibrils share some similarity in tertiary structure with Aβ oligomers, a finding that was already suggested from the analysis of secondary chemical shifts (20). Further support for this conclusion comes from a previous experimental finding that Phe-19 and Leu-34 are in close proximity in Aβ protofibrils (20) as well as in oligomers (16), where the distance between the Phe ring and Leu δ-carbons is 3.9 Å. However, contacts between the side chains of these residues have also been observed in mature Aβ fibrils (distance of ∼7 Å) (10, 34), rendering this pair of residues less indicative of a tertiary structural conversion from protofibrils to mature Aβ fibrils.
Our findings suggest that there has to be a rearrangement of the two β-strands of Aβ protofibrils during the conversion to mature Aβ fibrils. Of course, we can only speculate about the nature of the hydrogen bonds in protofibrils, but because the close proximity between Glu-22 and Ile-31 is already known from Aβ oligomers (15), one can assume that the β-strands in Aβ protofibrils may also form intramolecular hydrogen bonds. This can be comprehended by a closer structural relationship between Aβ oligomers and protofibrils compared with protofibrils and mature fibrils as was suggested on the basis of 13C chemical shift data (20), the capability of the cysteine mutant to form protofibrils (14, 22), and the known IR data, which indicate antiparallel β-sheets in protofibrils (18, 24). Therefore, it seems possible that the rearrangement of the intramolecular hydrogen bonds to the intermolecular hydrogen bonds takes place in the final structural transition from Aβ protofibrils to mature fibrils. Consequently, our solid-state NMR data would support the model for the aggregation mechanism of Aβ fibrils suggested by Härd and co-workers (15).
Supplementary Material
Acknowledgments
We thank the Deutsche Forschungsgemeinschaft and the Institute of Experimental Physics of the University of Leipzig for measuring time on the AVANCE 750 spectrometer.
This work was supported by the Deutsche Forschungsgemeinschaft (Grants Transregio-SFB 102 and A6 and a personal grant to I. M.).

This article contains supplemental Figs. S1–S4.
- Aβ
- amyloid-β.
REFERENCES
- 1. Holtzman D. M., Morris J. C., Goate A. M. (2011) Alzheimer disease: the challenge of the second century. Sci. Transl. Med. 3, 77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finder V. H., Glockshuber R. (2007) Amyloid-βaggregation. Neurodegener. Dis. 4, 13–27 [DOI] [PubMed] [Google Scholar]
- 3. Morgado I., Fändrich M. (2011) Assembly of Alzheimer Aβ peptide into nanostructured amyloid fibrils. Curr. Opin. Colloid Interface Sci. 16, 508–514 [Google Scholar]
- 4. Goldsbury C. S., Wirtz S., Müller S. A., Sunderji S., Wicki P., Aebi U., Frey P. (2000) Studies on the in vitro assembly of Aβ(1–40): implications for the search for Aβ fibril formation inhibitors. J. Struct. Biol. 130, 217–231 [DOI] [PubMed] [Google Scholar]
- 5. Harper J. D., Wong S. S., Lieber C. M., Lansbury P. T. (1997) Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem. Biol. 4, 119–125 [DOI] [PubMed] [Google Scholar]
- 6. Dasilva K. A., Shaw J. E., McLaurin J. (2010) Amyloid-β fibrillogenesis: structural insight and therapeutic intervention. Exp. Neurol. 223, 311–321 [DOI] [PubMed] [Google Scholar]
- 7. Lashuel H. A., Lansbury P. T., Jr. (2006) Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q. Rev. Biophys. 39, 167–201 [DOI] [PubMed] [Google Scholar]
- 8. Petkova A. T., Ishii Y., Balbach J. J., Antzutkin O. N., Leapman R. D., Delaglio F., Tycko R. (2002) A structural model for Alzheimer β-amyloid fibrils based on experimental constraints from solid-state NMR. Proc. Natl. Acad. Sci. U.S.A. 99, 16742–16747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paravastu A. K., Petkova A. T., Tycko R. (2006) Polymorphic fibril formation by residues 10–40 of the Alzheimer β-amyloid peptide. Biophys. J. 90, 4618–4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paravastu A. K., Leapman R. D., Yau W. M., Tycko R. (2008) Molecular structural basis for polymorphism in Alzheimer β-amyloid fibrils. Proc. Natl. Acad. Sci. U.S.A. 105, 18349–18354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bertini I., Gonnelli L., Luchinat C., Mao J., Nesi A. (2011) A new structural model of Aβ40 fibrils. J. Am. Chem. Soc. 133, 16013–16022 [DOI] [PubMed] [Google Scholar]
- 12. Lührs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Döbeli H., Schubert D., Riek R. (2005) 3D structure of Alzheimer amyloid-β(1–42) fibrils. Proc. Natl. Acad. Sci. U.S.A. 102, 17342–17347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scheidt H. A., Morgado I., Rothemund S., Huster D. (2012) Dynamics of amyloid-β fibrils revealed by solid-state NMR. J. Biol. Chem. 287, 2017–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandberg A., Luheshi L. M., Söllvander S., Pereira de Barros T., Macao B., Knowles T. P., Biverstål H., Lendel C., Ekholm-Petterson F., Dubnovitsky A., Lannfelt L., Dobson C. M., Härd T. (2010) Stabilization of neurotoxic Alzheimer amyloid-β oligomers by protein engineering. Proc. Natl. Acad. Sci. U.S.A. 107, 15595–15600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoyer W., Grönwall C., Jonsson A., Ståhl S., Härd T. (2008) Stabilization of a β-hairpin in monomeric Alzheimer amyloid-β peptide inhibits amyloid formation. Proc. Natl. Acad. Sci. U.S.A. 105, 5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed M., Davis J., Aucoin D., Sato T., Ahuja S., Aimoto S., Elliott J. I., Van Nostrand W. E., Smith S. O. (2010) Structural conversion of neurotoxic amyloid-β(1–42) oligomers to fibrils. Nat. Struct. Mol. Biol. 17, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chimon S., Shaibat M. A., Jones C. R., Calero D. C., Aizezi B., Ishii Y. (2007) Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer β-amyloid. Nat. Struct. Mol. Biol. 14, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 18. Cerf E., Sarroukh R., Tamamizu-Kato S., Breydo L., Derclaye S., Dufrêne Y. F., Narayanaswami V., Goormaghtigh E., Ruysschaert J. M., Raussens V. (2009) Antiparallel β-sheet: a signature structure of the oligomeric amyloid-β peptide. Biochem. J. 421, 415–423 [DOI] [PubMed] [Google Scholar]
- 19. Haupt C., Leppert J., Rönicke R., Meinhardt J., Yadav J. K., Ramachandran R., Ohlenschläger O., Reymann K. G., Görlach M., Fändrich M. (2012) Structural basis of β-amyloid-dependent synaptic dysfunctions. Angew. Chem. Int. Ed. Engl. 51, 1576–1579 [DOI] [PubMed] [Google Scholar]
- 20. Scheidt H. A., Morgado I., Rothemund S., Huster D., Fändrich M. (2011) Solid-state NMR spectroscopic investigation of Aβ protofibrils: implication of a β-sheet remodeling upon maturation into terminal amyloid fibrils. Angew. Chem. Int. Ed. Engl. 50, 2837–2840 [DOI] [PubMed] [Google Scholar]
- 21. Fawzi N. L., Ying J., Ghirlando R., Torchia D. A., Clore G. M. (2011) Atomic resolution dynamics on the surface of amyloid-β protofibrils probed by solution NMR. Nature 480, 268–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Härd T. (2011) Protein engineering to stabilize soluble amyloid-β protein aggregates for structural and functional studies. FEBS J. 278, 3884–3892 [DOI] [PubMed] [Google Scholar]
- 23. Paravastu A. K., Qahwash I., Leapman R. D., Meredith S. C., Tycko R. (2009) Seeded growth of β-amyloid fibrils from Alzheimer brain-derived fibrils produces a distinct fibril structure. Proc. Natl. Acad. Sci. U.S.A. 106, 7443–7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Habicht G., Haupt C., Friedrich R. P., Hortschansky P., Sachse C., Meinhardt J., Wieligmann K., Gellermann G. P., Brodhun M., Götz J., Halbhuber K. J., Röcken C., Horn U., Fändrich M. (2007) Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Aβ protofibrils. Proc. Natl. Acad. Sci. U.S.A. 104, 19232–19237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antzutkin O. N. (2004) Amyloidosis of Alzheimer Aβ peptides: solid-state nuclear magnetic resonance, electron paramagnetic resonance, transmission electron microscopy, scanning transmission electron microscopy, and atomic force microscopy studies. Magn. Reson. Chem. 42, 231–246 [DOI] [PubMed] [Google Scholar]
- 26. Zhang F., Brüschweiler R. (2004) Indirect covariance NMR spectroscopy. J. Am. Chem. Soc. 126, 13180–13181 [DOI] [PubMed] [Google Scholar]
- 27. Munowitz M. G., Griffin R. G., Bodenhausen G., Huang T. H. (1981) Two-dimensional rotational spin-echo nuclear magnetic resonance in solids: correlation of chemical shift and dipolar interactions. J. Am. Chem. Soc. 103, 2529–2533 [Google Scholar]
- 28. Rhim W. K., Elleman D. D., Vaughan R. W. (1973) Enhanced resolution for solid-state NMR. J. Chem. Phys. 58, 1772–1773 [Google Scholar]
- 29. Barre P., Zschornig O., Arnold K., Huster D. (2003) Structural and dynamical changes of the bindin B18 peptide upon binding to lipid membranes. A solid-state NMR study. Biochemistry 42, 8377–8386 [DOI] [PubMed] [Google Scholar]
- 30. Huster D., Xiao L., Hong M. (2001) Solid-state NMR investigation of the dynamics of the soluble and membrane-bound colicin Ia channel-forming domain. Biochemistry 40, 7662–7674 [DOI] [PubMed] [Google Scholar]
- 31. Schmidt-Rohr K., Spiess H. W. (1994) Multidimensional Solid-State NMR and Polymers, pp. 95–98, Academic Press, London [Google Scholar]
- 32. Huster D. (2005) Investigations of the structure and dynamics of membrane-associated peptides by magic angle spinning NMR. Prog. Nucl. Magn. Reson. Spectrosc. 46, 79–107 [Google Scholar]
- 33. Masuda Y., Fukuchi M., Yatagawa T., Tada M., Takeda K., Irie K., Akagi K., Monobe Y., Imazawa T., Takegoshi K. (2011) Solid-state NMR analysis of interaction sites of curcumin and 42-residue amyloid-β protein fibrils. Bioorg. Med. Chem. 19, 5967–5974 [DOI] [PubMed] [Google Scholar]
- 34. Tycko R. (2006) Molecular structure of amyloid fibrils: insights from solid-state NMR. Q. Rev. Biophys. 39, 1–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.