Background: Vasculature is a key component of the brain neurogenic stem cell niche.
Results: Knockdown of matriptase/epithin in neural progenitor cells or blocking of Gs protein activity in brain endothelial cells impairs contact-induced endothelial signaling activation and gene stimulation.
Conclusion: Matriptase/epithin and Gs protein signaling guide contact communication in neurovascular niche.
Significance: The study advances our understanding of cell-cell communication in the neurogenic vascular niche.
Keywords: Cytokine Induction, Endothelial Cell, G Protein-coupled Receptors (GPCR), GTPase, Neural Stem Cell, Matriptase/Epithin, Neural Progenitor Cells, Neurovasculature, Niche, Type II Transmembrane Serine Protease
Abstract
Vasculature is an important component of the neural stem cell niche in brain. It regulates neural stem/progenitor (NS/P) cell self-renewal, differentiation, and migration. In the neurogenic niches of adult brain, NS/P cells lie close to blood vessels, and proliferating NS/P cells frequently contact the vasculature. In the present study we showed that NS/P cells in co-culture with brain endothelial (bEND) cells activated endothelial G proteins and p38 mitogen-activated protein kinase (p38 MAPK) and stimulated cytokine/chemokine expression. These NS/P cell-induced endothelial responses took place during NS/P cell and bEND cell direct contact and were critically dependent on the expression of the type II transmembrane serine protease matriptase (MTP) by NS/P cells, because knocking down of MTP in NS/P cells impaired and re-expression of MTP restored their ability to induce endothelial cytokine/chemokine expression, p38 MAPK, or G protein activation. Cholera toxin blocked NS/P cell-induced endothelial responses, suggesting that the endothelial G protein activated by NS/P MTP is in the Gs subfamily. The addition of p38 MAPK inhibitor impaired NS/P cell-induced endothelial cytokine/chemokine expression. The known G protein-coupled receptor substrate of MTP, protease-activated receptor 2, was not involved in this system. These results revealed a novel signaling pathway in neural stem cell vascular niches that is mediated by neural MTP and endothelial Gs protein signaling at the cell-cell interface. This is the first report of direct cell-cell signaling between NS/P and bEND cells.
Introduction
Neurogenic stem cell niches in the central nervous system consist of various cell types including neural stem/progenitor (NS/P)2 cells, blood vessel cells, ependymal cells, astrocytes, microglia, and oligodendrocytes. A number of observations show that the vasculature is an important component of neural stem cell niches. In the developing CNS, nervous and vascular systems are both derived from the neural tube (1). Endothelial cells and neural stem cells appear at the same stages during development and are co-localized in the neural geminal zones (2). Invasion of spouts from the perineural vascular plexus into the ventral region of the neural tube are associated with the proliferation of neuroepithelium. In the adult brain, neural stem cells and their progeny in the subventricular zone (SVZ) of the lateral ventricle (LV) and in the subgranular zone of the dentate gyrus in the hippocampus lie close to the blood vessels (3). It has been shown that neurogenesis and angiogenesis are coupled processes in subgranular zone (4). In rostral migratory stream, blood vessels are tightly associated with the newborn SVZ neuroblasts and are involved in their migration (5). Similarly, in the olfactory bulb, blood vessels serve as scaffolding for radial migration of neural progenitor cells (6). After ischemic insult, neuroblasts of the SVZ are also found migrating along the sides of blood vessels toward the damaged sites (7). Early studies suggested that brain endothelial cells influenced NS/P cells through the action of their secreted factors. Indeed, in vitro studies have shown that diffusible factors from endothelial cells maintain and promote NS/P cell self-renewal (8) and migration (9).
It was recently demonstrated that neural stem cells and transit-amplifying cells in the LV-SVZ directly contact blood vessels at sites devoid of coverage by astrocyte endfeet and pericyte (5). LV-SVZ neurogenesis and injury-induced regeneration occur at these specialized neurovasculature contact sites (5, 10). An important regulatory mechanism for LV-SVZ neurogenesis may lie within the cell contact interface between the blood vessels and the NS/P cells.
Communication between endothelial cells and NS/P cells appears to be a two-way street, each cell type regulates the behavior of the other. It was shown that NS/P cell-derived nitric oxide induces the endothelial expression of VEGF and BDNF (11). BDNF and VEGF in turn activate brain endothelial cell angiogenesis. Nitric oxide also stimulates NS/P cell proliferation by activating endothelial NOS (11). This may represent one mechanism for reciprocal regulation between neurogenesis and angiogenesis. The cellular interaction mechanisms at NS/P cell-blood vessel direct contact sites are largely unexplored. A better understanding of the molecular signals that mediate interactions between NS/P cells and brain endothelial (bEND) cells would be important not only for the maintenance and differentiation of NS/P cells but also for blood vessel regulation. In the present studies we explored the interaction mechanisms between NS/P cells and bEND cells during direct cell contact. We found that NS/P cells induce an endothelial signaling pathway and lead to the production of cytokines/chemokines. Interestingly these endothelial responses were critically dependent on the expression of a type II transmembrane serine protease in NS/P cells and involve an endothelial Gs protein signal.
EXPERIMENTAL PROCEDURES
Cell Culture
NS/P cells were differentiated from the Sox1-GFP knock-in mouse ES cells (46C ES cells, obtained from Dr. Austin Smith at University of Edinburgh, UK (12)). Differentiation of NS/P cells was carried out by placing 46C ES cells on a gelatin-coated surface in neuronal differentiation medium (referred to as N2B27 medium) as described previously (13). GFP+ NS/P cells were collected on day 6 using an ARIA fluorescence-activated cell sorter (BD Biosciences) and were used in the co-culture experiments. For neurosphere culture, 46C ES cell-derived NS/P cells were cultured on an uncoated surface for 6 days. The Sox1-GFP-positive NS/P cell spheroids were then collected. The day 14 mouse embryonic neurocortex neurospheres were purchased from STEMCELL Technologies (Vancouver, Canada). Adult NS/P cells were isolated from SVZ of the LV from 8–12-week-old male FVB mouse as described previously (13); the mouse brain endothelial cell line bEnd.3 was purchased from the Bioscience Collection and Research Center (Hsinchu, Taiwan) and was routinely maintained in DMEM supplemented with 10% FBS.
For cell-cell contact co-culture, bEnd.3 cells were plated on 100-mm2 cell culture dishes the previous day to allow attachment. The medium was removed, the cells were washed and changed to N2B27 medium, and NS/P cells were then laid on the top of the attached bEnd.3 cells. Over 90% of NS/P cells attached to bEnd.3 cells in 2–3 h. Twenty-four hours later, NS/P cells were detached from bEnd.3 cells by repeated pipetting, which removed almost all the NS/P cells without detaching bEnd.3 cells as monitored microscopically and by GFP fluorescent of NS/P cells. Cell purity was examined further by RT-PCR for expression of endothelial marker FLK1 and the absence of neural stem/progenitor molecule nestin. After removing detached NS/P cells, bEnd.3 cells were detached from the culture dish with trypsin. All of the cells were then collected by centrifugation. For Fig. 2B, NS/P cells and bEnd.3 cells were mixed and seeded on a coverslip in endothelial cultural medium containing low serum (1%). After the cells attached (within 3 h), the medium was replaced with N2B27 medium and co-cultured for 24 h. For noncontact co-culture, bEnd.3 cells were allowed to attach overnight to the 6-well plate; Falcon Transwells with pore sizes of 8 μm (BD Labware, Franklin Lakes, NJ) were placed on each well, and Sox1-GFP+ NS/P cells were then added into the Transwell. Twenty-four hours later, the Transwell was removed, and bEnd.3 cells were collected as described above. Reagents for cell culture were all from Invitrogen. In cultures where toxin or inhibitor was tested, they were added directly to the medium. Pertussis toxin (PTX), cholera toxin (CTX), and SB203580 were from Sigma. The recombinant mouse MTP protein (purchased from R & D System) is the catalytic serine protease domain and has specific activity >4,000 pmol/min/μg supplied by the manufacturer. The recombinant MTP protein was either added directly to the medium or coated on the surface of culture dishes prior seeding bEnd.3 cells.
FIGURE 2.
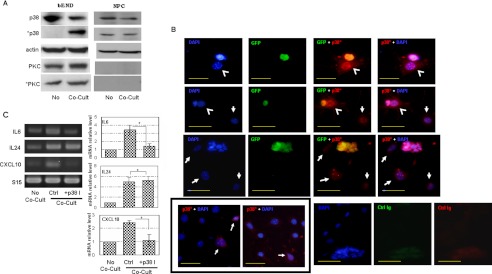
NS/P cells induce endothelial p38 MAPK activation for cytokine/chemokine production. A, cell lysates of bEND cells (bEND) and NS/P cells (NPC) that were either cultured separately (No) or under contact conditions (Co-Cult) were assayed by Western blot for the expression of the total p38 MAPK (p38), phosphorylated p38 MAPK (*p38), total PKCα (PKC) or phosphorylated PKCα (*PKC). Actin is the endogenous loading control. B, immunostaining of phosphorylated p38 MAPK (p38*, red fluorescence) in NS/P cells-bEND cells contact co-culture. Anti-GFP was used to show NS/P cells (GFP, green fluorescence). Cell nuclei were stained with DAPI (blue fluorescence). Arrowheads indicate bEND cells that are in contact with NS/P cells; arrows indicate bEND cells that are not in contact with NS/P cells. The two images in the box on the bottom left are bEND cells cultured either without NS/P cells (left panel) or co-cultured with NS/P cells in a Transwell (right panel). Arrows in these images indicate basal *p38 staining in bEND cells. The cells stained with control immunoglobins of the same subtype were also shown (control Ig). The scale bars are 50 μm. C, RT-PCR of IL6, IL24, or CXCL10 in bEND cells cultured without NS/P cells (No Co-Cult), in contact with NS/P cells (Co-Cult, Ctrl), or in contact with NS/P cells in the presence of SB203580 (+p38I). S15 is an internal loading control. The graph shows quantitation of RT-PCR by densitometry. The values of each molecule in co-culture were normalized to that of No Co-Cult that is set at 1. Comparisons were made between control and SB203580 (+p38I) co-culture. *, p ≦ 0.05.
RNA Isolation and RT-PCR
Total RNA was prepared using RNeasy mini kit (Qiagen) following the manufacturer's protocol. RNA integrity was assessed by electrophoresis, and RNA concentration was measured with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA). First strand cDNA was synthesized from 1 μg of RNA using Superscript III reverse transcriptase and oligo(dT) primers (Invitrogen). The resulting cDNA was then used in PCRs. Gene-specific primers were as follows: S15 (mouse ribosomal protein S15): forward, 5′-TTCCGCAAGTTCACCTACC-3′, and reverse, 5′-TGCTTCACGGGTTTGTAGGT-3′; MTP: forward, 5′-CACTTCCATTATCGGAATGTGCG-3′, and reverse, 5′-GGATGTCGCCGGTCAGTATTGGTTATCA-3′; IL6: forward, 5′-GTTGTGCAATGGCAATTCTG-3′, and reverse, 5′-TGGTCTTGGTCCTTAGCCAC-3′; FLK1 (vascular endothelial growth factor receptor 2): forward, 5′-CC AAAAACCAATATGCCCTGAT-3′, and reverse, 5′-TCTC TCAGTACAATGCCTGAATCTTC-3′. IL24: forward, 5′-GATGACATCACAAGCATCCG-3′, and reverse, 5′-ATTTCTGCATCCAGGTCAGG-3′; CXC motif chemokine 10 (CXCL10): forward, 5′-ATGAACCCAAGTGCTGCC-3′, and reverse, 5′-TTCATCGTGGCAATGATCTC-3′; and nestin: forward, 5′-GGGAGCTCGGAGCCCAAGGA-3′, and reverse, 5′-GGGCATGGCTGCCCTCATGG-3′.
siRNA and Plasmid Transfection
Transfection of siRNA or mammalian expression plasmid was performed using Lipofectamine 2000 (Invitrogen) as described previously (13). MTP knockdown or overexpression was checked by RT-PCR or Western blot. The siRNA against mouse St14 and a control siRNA with no target (nonsilencing siRNA) were purchased from Qiagen. The MTP overexpression plasmid is a pSPORT6-CMV vector carrying the full-length mouse MTP cDNA.
Western Blot
Cell lysates were prepared in buffer containing 50 mm Tris-HCl (pH7.5), 1 mm EDTA, 1% Triton X-100, protease inhibitors, and phosphatase inhibitors. After 10 min of centrifugation at 13,000 × g, the supernatants were collected, and protein concentrations were determined by SuperSignal® West Pico protein assay kit (Pierce) using bovine serum albumin as a standard. An equal amount of protein was subjected to SDS-PAGE, followed by Western blot as described (14). Antibodies to p38 MAP kinase, phospho-p38 MAP kinase, PKCα, and phospho-PKCα were all from Cell Signaling Technology (Danvers, MA).
Immunofluorescence Staining of Cell Culture
Cell staining followed the procedure described previously (13). In brief, the cells were seeded on coverslips. After culture, they were fixed, permeabilized with 0.5% Triton X-100, and blocked with 4% horse serum. After incubation with primary antibody, the cells were then incubated with green or red fluorescence-conjugated secondary antibodies. The cell nuclei were counterstained with DAPI and analyzed using epifluorescence microscope (Olympus BX51).
ELISA
Cytokines released into the culture medium was assayed by ELISA according to the manufacturer's instructions. ELISA for mouse IL6, mouse IL24, and mouse CXCL10 were from Invitrogen, UCSN Life Science Inc. (Wuhan, PRC), and R & D Systems, respectively. Purified recombinant protein of each cytokine was used to obtain a standard curve for each assay to determine the concentration of each cytokine in the cell conditioned medium.
GTPase Activity Assay
GTPase activity of cell lysates was measured using a colorimetric assay kit (Innova Biosciences, Cambridge, UK) following the manufacturer's protocol. In brief, cell lysate or buffer was mixed with GTP substrate: the PiColorLock Gold reagent mix. The stabilizer was then added and incubated for 30 min. The absorbance was measured at 650-nm wavelength. A phosphate standard included in the package was used to calculate the specific GTPase activity in each sample of cell lysate.
Statistical Analysis
The data are presented as means ± S.D. derived from three to five independent experiments. Two-group t test was used for comparison between groups. For multiple-group comparisons, data were tested by one-way analysis of variance, and Bonferroni adjustment was used for within group comparisons.
NS/P Cell Differentiation with IL6 or Co-culture Conditioned Medium
NS/P cells were seeded on PDL/laminin-coated culture dishes and cultured in N2B27 medium in the absence or presence of recombinant IL6 protein or the conditioned medium before or after IL6 depletion. The medium was changed every other day and cells were harvested after 7 days of culture. The conditioned medium of NS/P cell-bEND cell contact co-culture were harvested after 24-hours and concentrated by Ultra-4 molecular cutoff at 5 kDa (Millipore-Amicon). For IL6 depletion, the concentrated conditioned medium was incubated with IL6 antibody, and the antibody-bond fraction was then removed by protein-A agarose beads. Depletion of IL6 was confirmed by ELISA.
RESULTS
NS/P Cell-induced Chemokine/Cytokine Production in bEND Cells by Direct Contact
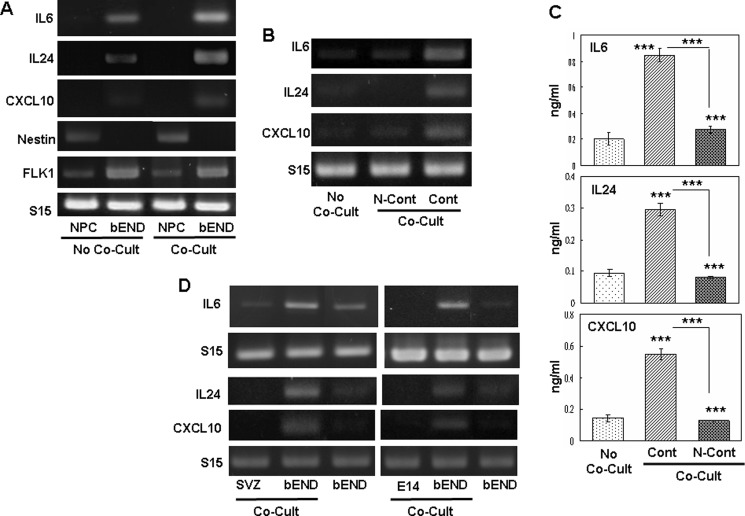
NS/P cells cultured directly on bEND cells attached to bEND cells after short incubation. Fig. 1A shows that expression of IL6, IL24, and CXCL10 in bEND cells was induced by the attached NS/P cells. Expression of endothelial molecule VEGF receptor 2 (FLK1) in bEND cells collected after contact co-culture with NS/P cells was similar to that in bEND cells without co-culture. The NS/P marker nestin was not detected in bEND cells after contact co-culture with NS/P cells. The expression of FLK1 and nestin indicated that the endothelial cells collected after contact co-culture were substantially free of NS/P cells. IL6, IL24, or CXCL10 were undetectable in NS/P cells (Fig. 1A, NPC) whether they were cultured without (Fig. 1A, No Co-Cult) or with (Fig. 1A, Co-Cult) bEND cells. Brain endothelial cells (Fig. 1A, bEND) cultured alone (Fig. 1, A, No Co-Cult, and B, No Co-Cult) expressed low or undetectable levels of IL6, IL24, or CXCL10. After co-culture (Fig. 1A, Co-Cult) with NS/P cells, IL6, IL24, and CXCL10 in bEND cells were significantly increased. In contrast to contact co-culture (Fig. 1, A and B, Cont, Co-Cult), IL6, IL24, and CXCL10 in bEND cells co-cultured with NS/P cells in a Transwell (Fig. 1B, N-Cont, Co-Cult), a noncontact co-culture condition, were not induced. Brain endothelial cells cultured with the conditioned medium of NS/P cell culture did not exhibit increased expression of these genes either.3 Fig. 1C shows that the secretion of IL6, IL24, and CXCL10 proteins after contact co-culture with NS/P cells (Fig. 1C, Cont, Co-Cult), but not in Transwell co-culture (Fig. 1C, No-Cont, Co-Cult), was three to four times greater than bEND cells without co-culture. Evidently, endothelial IL6, IL24, and CXCL10 induction occurs only in cells in direct contact with NS/P cells. The NS/P cells used in these cultures were differentiated from mouse embryonic stem cells. Identical results were observed in bEND cells in contact co-culture with NS/P cells isolated either from SVZ of the adult mouse brain LV (Fig. 1D, SVZ) or from neural cortex of 14-day mouse embryo (Fig. 1D, E14), clearly demonstrating that induction of endothelial cytokine/chemokine is a general feature of NS/P cells. These data demonstrate that endothelial expression of cytokines/chemokines is induced by cell contact between NS/P and bEND cells.
FIGURE 1.
NS/P cells induced cytokine/chemokine expression in bEND cells through cell contact. A, RT-PCR assay of IL6, IL24, and CXCL10 mRNA in NS/P cells (NPC) or bEND cells (bEND) cultured either separately (No Co-Cult) or co-cultured under direct contact conditions (Co-Cult). NS/P marker nestin (Nestin) and endothelial marker FLK1 indicate sufficient purity of bEND cells isolated from co-culture. B, RT-PCR assay of IL6, IL24, and CXCL10 mRNA. C, ELISA of IL6, IL24, and CXCL10 proteins released by bEND cells cultured alone (No Co-Cult) or co-cultured (Co-Cult) with NS/P cells under direct contact conditions (Cont) or in a Transwell (N-Cont). The asterisks above the bars indicate a significant difference of each co-cultured condition compared with No Co-Cult. Comparison was also made between two co-cultured conditions. ***, p ≦ 0.001. D, RT-PCR of IL6, IL24, and CXCL10 in bEND cells, in NS/P cells isolated from the SVZ of adult mouse brain lateral ventricle (SVZ) and in NS/P cells of neural cortex from 14-day mouse embryo (E14) after contact co-culture (Co-Cult). Expression of IL6, IL24, and CXCL10 in bEND cells cultured without NS/P cells is also shown. Mouse small ribosome protein 15 (S15) in each figure serves as an internal loading control for RT-PCR. The same results for RT-PCR were observed in at least three independent experiments.
Endothelial p38 MAPK Is Activated by Contact with NS/P Cells in Culture
Our data suggest that signaling between NS/P and bEND cells takes place during cell-cell contact interaction and results in induction of endothelial cytokine/chemokine expression. We investigated whether p38 MAPK or PKCα was activated in bEND cells by contact with NS/P cells. We found that p38 MAPK protein (Fig. 2A, p38) was expressed in both NS/P (Fig. 2A, NPC) and bEND cells (Fig. 2A, bEND). In NS/P cells, p38 MAPK was present in the active, phosphorylated state (Fig. 2A, *p38), and it was not altered by co-culture with bEND cells (Fig. 2A, *p38 in NPC under Co-Cult). p38 MAPK proteins in bEND cells, on the other hand, were not phosphorylated until after contact co-culture with NS/P cells (Fig. 2A, bEND, Co-Cult). PKCα was not detected in NS/P cells; nor was its expression induced by co-culture with bEND cells (Fig. 2A, PKC in NPC). PKCα was present in bEND cells, but it was in a phosphorylated state; neither its expression nor its phosphorylation was affected by co-culture with NS/P cells (Fig. 2A, *PKC in bEND). These observations show that endothelial p38 MAPK, not PKCα, is activated by NS/P cells during cell contact.
Cell contact induction of endothelial p38 MAPK phosphorylation was checked by immunofluorescence cell staining. Fig. 2B shows that bEND cells in contact with GFP-positive NS/P cells (Fig. 2B, green fluorescence) were highly stained by antibody to the phosphorylated p38 MAPK (Fig. 2B, red fluorescence, arrowheads). Cells that are not in contact with NS/P cells, on the other hand, were either negative or weakly stained (Fig. 2B, arrows). Weak staining of anti-phosphorylated p38 MAPK was observed occasionally in bEND cells without co-culture (Fig. 2B, left panel in the box) or in bEND cells co-cultured with NS/P cell in Transwell (Fig. 2B, right panel in the box), suggesting basal staining of phosphorylate p38MAP in uninduced bEND cells. Same as that shown in Fig. 2A, p38 MAPK are phosphorylated in NS/P cells whether or not they were in contact with bEND cells (Fig. 2B co-stain of GFP and p38*). Cell staining further confirmed that p38 MAPK activation in bEND cells is promoted by contact with NS/P cells.
To investigate whether p38 MAPK signaling is responsible for NS/P cell-induced endothelial IL6, IL24 or CXCL10 expression, co-cultured bEND-NS/P cells were treated with p38 MAPK inhibitor SB203580. In the presence of SB203580, NS/P cells failed to induce endothelial IL6 and CXCL10 expression, showing that induction of these endothelial molecules is mediated by p38 MAPK activation induced during co-culture. IL24 expression induced by NS/P cells, however, was not affected much by SB203580 (Fig. 2C, +p38I, Co-Cult), suggesting that p38 MAPK signaling is not the main responsive pathway for IL24 induction. These data demonstrate that NS/P cells in contact with bEND cells activate endothelial p38 MAPK, which can lead to overproduction of some (if not all) endothelial cytokines/chemokines.
Endogenous Expression of MTP in NS/P Cells Is Critical for Endothelial p38 MAPK Activation and Endothelial Chemokine/Cytokine Induction during Co-culture
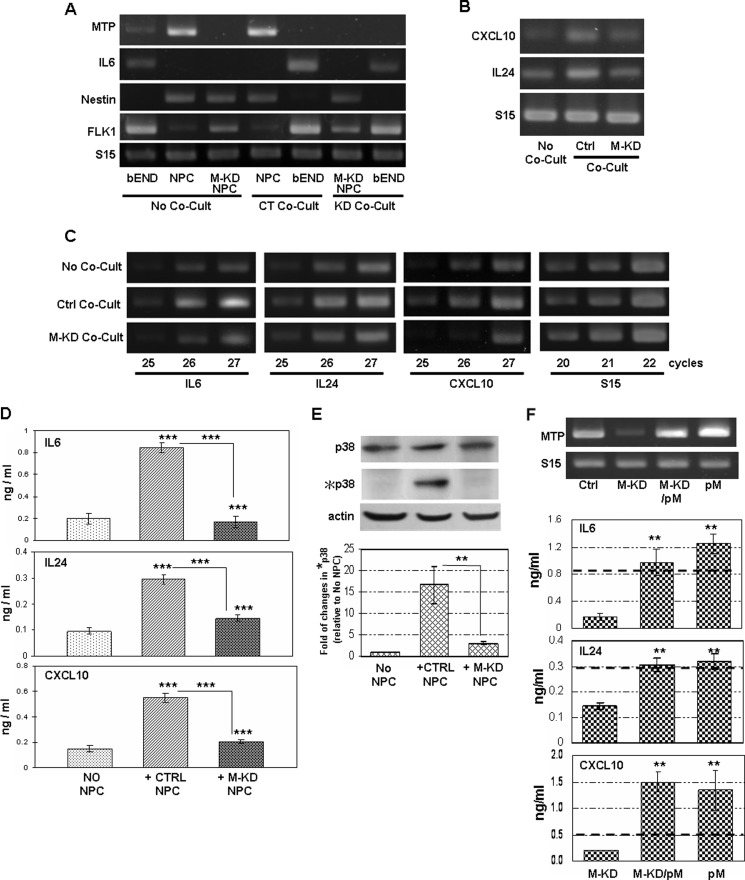
The observations described above suggested that cell to cell signaling systems exist at the cell contact interface between NS/P cells and bEND cells. We recently showed that the type II transmembrane serine protease matriptase (MTP) is expressed in NS/P cells (13). Considering its cell surface locality and its substrate diversity, MTP represents a candidate NS/P cell membrane activator protein for inducing endothelial p38 MAPK signaling and the subsequent cytokine/chemokine expression. We tested this possibility by investigating the effect of knocking down the MTP expression in NS/P cells. MTP is highly expressed in NS/P cells; only a small amount of expression was detected in bEND cells (Fig. 3A, No Co-Cult) (13). Consistent with the results in Fig. 1, contact co-culture with NS/P cells induced endothelial IL6 mRNA expression (Fig. 3A, CT Co-Cult). Contact of NS/P cells and bEND cells did not change the expression of MTP in either cell type (Fig. 3A, CT Co-Cult). MTP mRNA expression in NS/P cells was reduced by specific siRNA (Fig. 3A, M-KD NPC), and it remained knocked down during the course of co-culture with bEND cells (Fig. 3A, KD Co-Cult). These MTP knockdown cells were unable to induce endothelial IL6 mRNA during contact co-culture (Fig. 3A, KD Co-Cult). The ability of NS/P cells to induce endothelial CXCL10 in contact was also impaired by MTP knockdown (Fig. 3B). Interestingly, although IL24 induction was not affected by p38 MAPK inhibitor (Fig. 2), it was impaired by MTP knockdown (Fig. 3B), suggesting that MTP can activate additional endothelial signaling pathways. Semiquantitative RT-PCR is shown in Fig. 3C. Exponential amplification for IL6, IL24, and CXCL10 in bEND cells without co-culture (Fig. 3C, No Co-Cult) or co-cultured with MTP knockdown NS/P cells (Fig. 3C, M-KD Co-Cult) occurred between PCR cycles 25–27. After co-culture with NS/P cells, however, expression of these three molecules reached plateau before or around PCR cycle number 26 (Fig. 3C, Ctrl Co-Cult), confirming that induction of these transcripts in bEND cells by the NS/P cells they directly contact to is dependent on MTP. At the protein level, secretion of all three proteins was induced three or four times after contact with NS/P cells (Fig. 3D, +CTRL NPC). Cells in contact with MTP knockdown NS/P cells (Fig. 3D, +M-KD NPC), on the other hand, expressed all three proteins approximately the same levels as that in cells without co-culture (Fig. 3D, No NPC). Consistent with their mRNA expression, induction of endothelial IL6, IL24, and CXCL10 proteins was lost in MTP knockdown NS/P cell. MTP knockdown NS/P cells also lost their ability to induce endothelial p38 MAPK activation in co-culture (Fig. 3E, +M-KD NPC).
FIGURE 3.
Endogenous expression of MTP in NS/P cells mediates cell-contact induced endothelial p38 MAPK activation and cytokine/chemokine expression. A, RT-PCR assay of MTP and IL6 mRNAs in NS/P cells (NPC), bEND cells (bEND), and MTP knockdown NS/P cells (M-KD NPC) without co-culture (No Co-Cult). MTP and IL6 mRNAs in bEND cells in contact with NS/P cells transfected with control siRNA (CT Co-Cult) or with MTP siRNA (KD Co-Cult) were compared. Endothelial FLK1 and NPC marker nestin are used as monitors for bEND cells and their purity after co-culture. B, expression of CXCL10 or IL24 in bEND cells cultured without NS/P cells (No Co-Cult), after direct contact co-culture (Co-Cult) with control siRNA transfected (Ctrl) or with MTP siRNA transfected (M-KD) NS/P cells. C, semiquantitative PCR for IL6, IL24, and CXCL10 in bEND cells cultured without NS/P cells (No Co-Cult), after direct contact co-culture with control siRNA transfected NS/P cells (Ctrl Co-Cult) or with MTP siRNA transfected NS/P cells (M-KD Co-Cult). PCR product after 25, 26, and 27 cycles of amplification were shown. The PCR product of internal control S15 was taken after 20, 21, and 22 cycles of amplification. A–C show the representative data of at least three independent experiments. S15 is loading control for RT-PCR. D, ELISA of IL6, IL24, and CXCL10 proteins in conditioned medium of bEND cells cultured without NS/P cells (No NPC), bEND cells after contact with control siRNA transfected (+Ctrl NPC), or with MTP knockdown (+M-KD NPC) NS/P cells. Asterisks above the bars indicate significant difference compared with No NPC. Comparisons were also made between +CTRL NPC and +M-KD NPC. E, total and phosphorylated p38 MAPK protein (p38 and *p38, respectively) in bEND cells cultured in conditions described in D. Actin is the endogenous loading control for Western blot. The graph shows quantitation of chemiluminescence by densitometry. The value of phosphorylated p38 was normalized to that of the total p38 for each sample; the resulting value was then normalized to that of bEND cells without co-culture that is set at 1. Comparisons were made between +CTRL NPC and +M-KD NPC. F, image on the left shows RT-PCR of MTP in NS/P cells (Ctrl), in MTP knockdown NS/P cells (M-KD), and in MTP knockdown or control NS/P cells transfected with MTP expression vector (M-KD/pM and pM, respectively). The graphs on the right show ELISA of IL6, IL24, and CXCL10 proteins in conditioned medium of bEND cells cultured with MTP knockdown (M-KD) cells and with MTP knockdown or control NS/P cells carrying MTP expression vector (M-KD/pM and pM, respectively). The dark dotted line across each graph shows the amount of each molecule induced by the control NS/P cells. Cultures with M-KD/pM or pM were compared with cultures with M-KD. **, p ≦ 0.01.
Transfection of the MTP expression plasmid into MTP knockdown NS/P cells (Fig. 3F, M-KD/pM) restored MTP expression (Fig. 3F, gel image). These cells regained their ability to induce endothelial IL6, IL24, and CXCL10 in contact co-culture (Fig. 3F, graph, M-KD/pM). These data confirmed that NS/P cells induction of endothelial IL6, IL24, and CXCL10 is a specific effect of MTP. Transfection of MTP expression vector into control NS/P cells induced approximately the same amount of IL6, IL24, or CXCL10 (Fig. 3F, graph, pM). It is obvious that NS/P cell induction of endothelial IL6 and IL24 have reached the maximal level because overexpression of MTP, whether in the control or in MTP knockdown NS/P cells, did not induce these molecules much higher than the control NS/P cells (Fig. 3, D, and F, dark dotted lines).
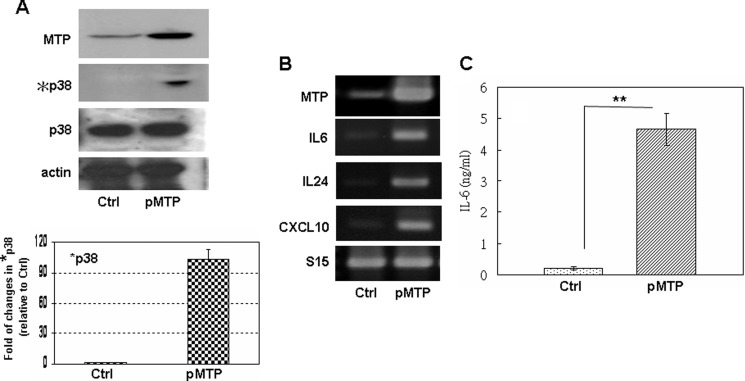
The influence of MTP in these endothelial cell effects was further examined by overexpressing MTP in bEND cells. As shown in Fig. 4, MTP expression is elevated in bEND cells carrying the MTP-expressing plasmid (pMTP) (mRNA in Fig. 4A; protein in Fig. 4C). Ecotopic expression of MTP (Fig. 4A, pMTP) in bEND cells increased the p38 MAPK phosphorylation (Fig. 4A, *p38) that is accompanied by elevated IL6, IL24, and CXCL10 mRNA (Fig. 4B). The secreted IL6 protein was increased over 9-fold in MTP-overexpressing bEND cells (Fig. 4C). These data clearly demonstrate that MTP is a crucial signaling activator for brain endothelial p38 MAPK activation and cytokine/chemokine production.
FIGURE 4.
Ecotopic expression of MTP in bEND cells induces their p38 MAPK activation and cytokine/chemokine expression. A, Western immunoblot assay of MTP protein, phosphorylated (*p38), and total (p38) p38 MAPK protein in control bEND cells transfected with an empty vector (Ctrl) or bEND cells transfected with MTP-expressing plasmid (pMTP). The graph shows quantitation of chemiluminescence by densitometry. The value of phosphorylated p38 was normalized to that of the total p38 for each sample; the resulting value was then normalized to that of control bEND cells (set at 1). B, RT-PCR of MTP, IL6, IL24, and CXCL10 mRNA in bEND cells collected in A. S15 and actin are loading controls for RT-PCR and Western blot, respectively. The same results were observed in at least three independent experiments. C, ELISA of the secreted IL6 protein in bEND cells collected in A. **, p ≦ 0.01.
Brain Endothelial Guanine Nucleotide-binding Protein (G Protein) Is Signaling Target of Neural MTP
To determine the endothelial surface component that receives signaling from NS/P cell surface MTP, we looked into cell surface signaling molecules that could be activated by MTP. Protease-activated receptors (PARs) are members of the G protein-coupled receptor (GPCR) family. There are currently four members in the family: PAR1, PAR2, PAR3, and PAR4 (15). PAR2 has been reported to be activated by MTP, whereas PAR1, PAR3, and PAR4 are poor substrates of MTP (16). It is conceivable that PAR2 may serve as the brain endothelial surface target for NS/P MTP. However, we did not detect PAR2 in bEND cells (Fig. 5A, PAR2). To verify the involvement of the PAR family in the present cell system, we checked the effect of another PAR protein: PAR1, which we detected in bEND cells (Fig. 5B). PAR1-specific siRNA significantly reduced PAR1 expression in bEND cells (Fig. 5B, siPAR1), but it did not affect IL6, IL24, or CXCL10 induction by NS/P cells in co-culture (Fig. 5B). The PAR family apparently was not involved in NS/P-bEND cell signaling.
FIGURE 5.
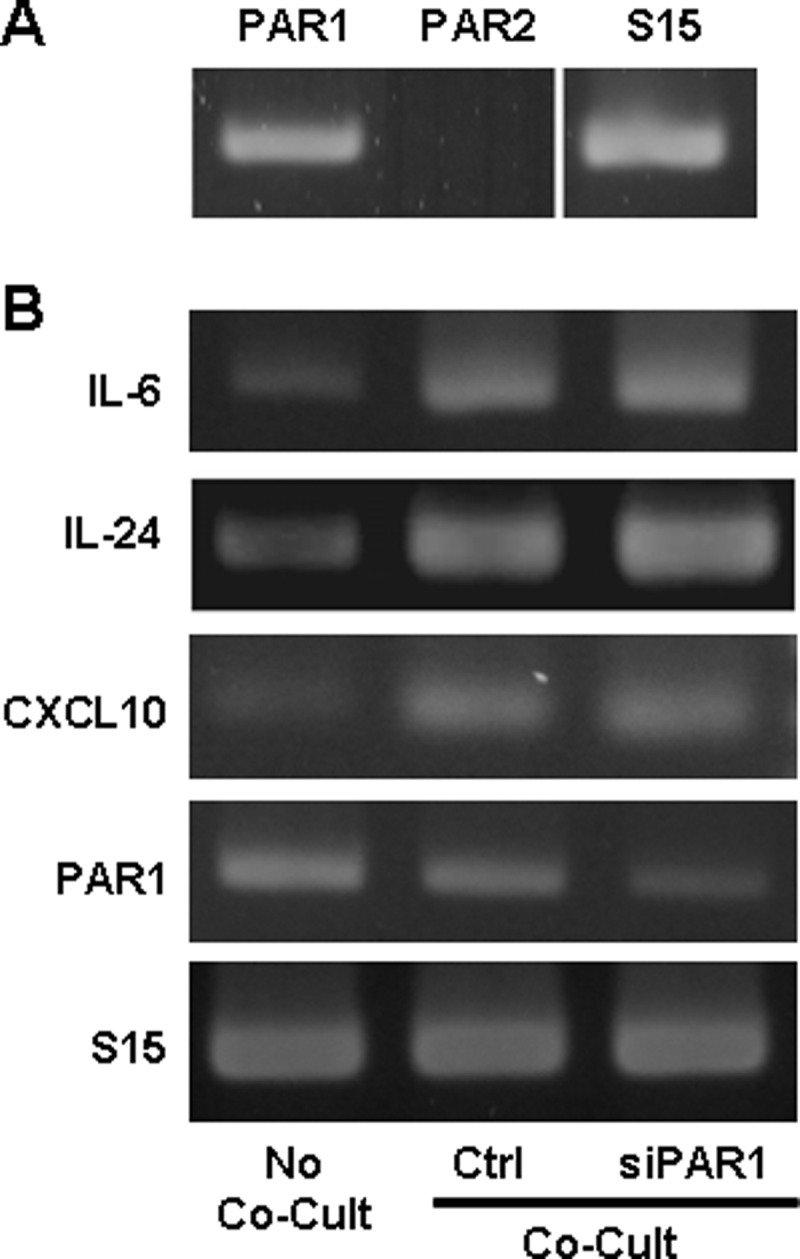
PAR1 or PAR2 is not involved in NS/P-bEND cell contact induced cytokine/chemokine production. A, RT-PCR of PAR1 and PAR2 in bEND cells. B, RT-PCR of IL6, IL24, and CXCL10 mRNA in bEND cells cultured without NS/P cells (No Co-Cult), in bEND cells (Ctrl), or PAR1 knockdown bEND cells (siPAR1) after contact with NS/P cells (Co-Cult). RT-PCR of PAR1 shows the knockdown efficiency. S15 is an internal loading control. Shown here are representative data of three independent experiments.
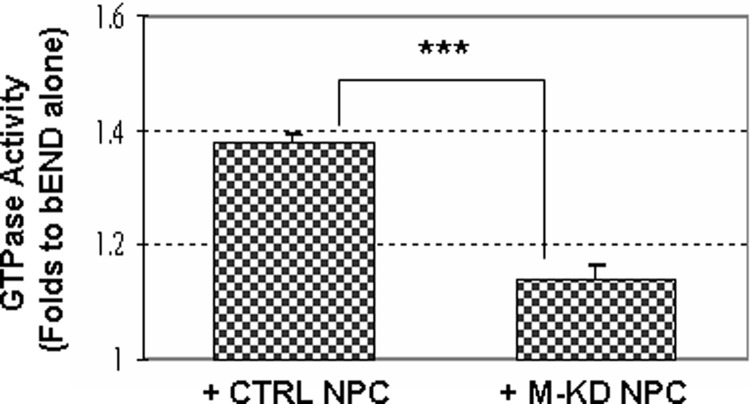
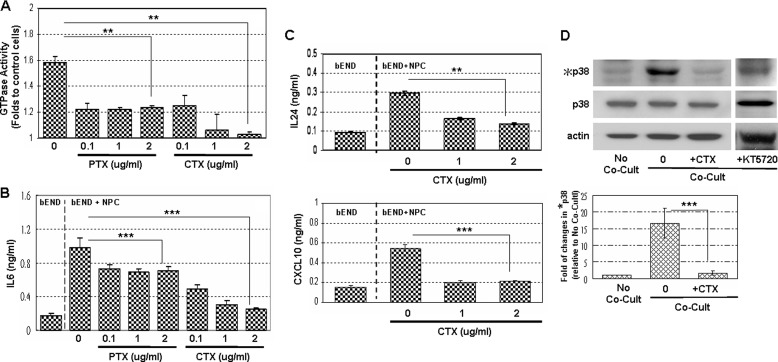
During NS/P cell-bEND cell contact co-culture, brain endothelial GTPase activity was activated (Fig. 6, +CTRL NPC). Knocking down of MTP in NS/P cells significantly impaired their ability to promote endothelial GTPase activation (Fig. 6, +M-KD NPC). To investigate whether MTP-mediated activation of brain endothelial GTPase resulted from G protein activation, the effect of PTX and CTX was examined. PTX is an inhibitor of Gi and Go proteins, and CTX is an inhibitor of Gs protein. PTX or CTX did not affect the overall growth of NS/P cells or bEND cells.3 PTX and CTX both achieved ∼75% inhibition of the NS/P cell-induced endothelial GTPase activity at 0.1 ng/ml of concentration (Fig. 7A). Increasing the concentration of CTX but not of PTX further reduced GTPase activation and completely inhibited NS/P cells induced endothelial GTPase activation at 2 μg/ml (Fig. 7A). NS/P cell-induced endothelial IL6 production was impaired ∼25% by PTX at all tested concentrations (Fig. 7B, PTX). Using the same range of concentrations, CTX inhibited endothelial IL6 induction profoundly and returned it to ground level (Fig. 7B, CTX in bEND+NPC). Similarly, NS/P cell-induced endothelial IL24 and CXCL10 were both returned to ground level by CTX (Fig, 7C, CTX in bEND+NPC). p38 MAPK activation in bEND cells could not be induced by NS/P cells in the presence of CTX (Fig. 7D). Protein kinase A inhibitor K5739 did not influence the p38 MAPK activation induced by NS/P cells (Fig. 7D), ruling out the involvement of adenyl cyclase in this cell system. Altogether, these data showed that during NS/P and bEND cell-cell communication, MTP in NS/P cells induces activation of the CTX-sensitive Gs protein in bEND cells, probably through activation of GPCR. The known MTP activating receptor PAR2, however, was not the responsive endothelial GPCR in CNS neurovascular niches.
FIGURE 6.
NS/P cell-induced endothelial GTPase activity in contact co-culture is dependent on endogenous expression of MTP in NS/P cells. GTPase activity in bEND cells after contact with control siRNA transfected (CTRL) or with MTP siRNA transfected NS/P cells (M-KD) were normalized to that in bEND cells cultured without NS/P cells (set at 1). ***, p ≦ 0.001.
FIGURE 7.
CTX-sensitive endothelial G protein is responsible for NS/P cell-induced endothelial IL6, IL24, and CXCL10 production and p38 MAPK activation. A, endothelial GTPase activity in bEND cells co-cultured with NS/P cells in the absence (0) or presence of various concentrations (0.1, 1, and 2 μg/ml) of PTX or CTX. The data were normalized to bEND cells cultured without NS/P cells (set at 1). B and C, ELISA of IL6 protein (B) and ELISA of IL24 and CXCL10 in bEND cells (C) cultured without NS/P cells (bEND) or in contact with NS/P cells (bEND+NPC) in the absence (0) or presence of various concentrations (0.1, 1, and 2 μg/ml) of PTX or CTX. D, Western immunoblot of p38 MAPK in bEND cells cultured alone (No Co-Cult) or with NS/P cells (Co-Cult) in the absence (0) or presence of 1 ng/ml CTX (+CTX) or KT5720 (+KT5720). Actin is the internal loading control for Western blot assay. The graph shows quantitation of chemiluminescence by densitometry. The value of phosphorylated p38 was normalized to that of the total p38 for each sample; the resulting value was then normalized to that of bEND cells without co-culture that is set at 1. Toxin-treated cultures were compared with zero-toxin-treated cultures. **, p ≦ 0.01; ***, p ≦ 0.001.
Recombinant MTP Protease Domain Has Little Effect on Brain Endothelial Responses
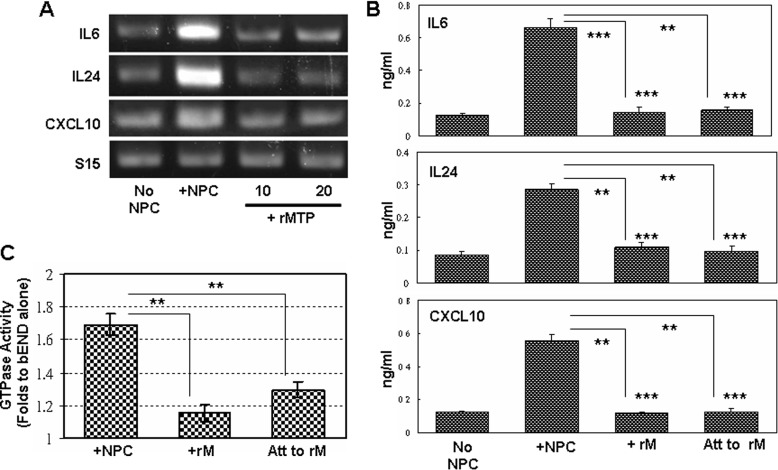
There are released active forms of full-size MTP in NS/P cell culture.4 However, NS/P cells co-cultured with bEND cells in a Transwell (Fig. 1) or the conditioned medium of NS/P cells3 failed to induce endothelial cytokine/chemokine expression. It might be that there is not sufficient amount of active MTP released from NS/P cells to achieve their effects on bEND cells. To evaluate whether this is the case, we used exogenous recombinant protein in bEND cell culture. The addition of MTP recombinant protein to bEND cell culture did not induce IL6, IL24, or CXCL10 mRNA (Fig. 8A, +rMTP) or protein (Fig. 8B, +rM). Endothelial GTPase was not activated by the added recombinant protein either (Fig. 8C, +rM). To make sure that the added recombinant MTP protein is in contact with bEND cells, we also coated the protein on the culture dishes. IL6, IL24, or CXCL10 in bEND cells cultured on recombinant MTP-coated surface, however, were not induced (Fig. 8B, Att to rM). GTPase activation in these cultures were not induced much either (Fig. 8C, Att to rM). The recombinant protein used in these studies is the active protease domain of MTP. These results demonstrated that MTP protease activity by itself is not sufficient to induce brain endothelial responses, even if it is in close vicinity to bEND cells. Evidently, NS/P cell-associated full-length MTP protein is essential to induce brain endothelial responses.
FIGURE 8.
Recombinant MTP protease fails to induce brain endothelial responses. A, RT-PCR of IL6, IL24, and CXCL10 mRNA in bEND cells cultured without NS/P cells (No Co-Cult), in contact with NS/P cells (Co-Cult), or in medium containing recombinant protein of MTP protease domain (+rMTP, 10 or 20 nm). Shown here are representative data of three independent experiments. B, ELISA of IL6, IL24, and CXCL10 proteins in conditioned medium of bEND cells cultured without NS/P cells (No NPC), after contact with NS/P cells (+NPC), cultured in medium containing recombinant protein of MTP protease domain (+rM), or cultured on dishes coated with recombinant protein of MTP protease domain (Att to rM). Recombinant protein-treated cultures (+rM and Att to rM) were compared with No NPC cultures (asterisks above bars) and to +NPC cultures. C, GTPase activity in bEND cells after contact with NS/P cells (+NPC), cultured in medium containing recombinant protein of MTP protease domain (+rM), or cultured on dishes coated with recombinant protein of MTP protease domain (Att to rM). The data were normalized to bEND cells cultured without NPC (set at 1). The cells cultured with recombinant protein (+rM or Att to rM) were compared with cells cultured with NS/P cells (+NPC). **, p ≦ 0.01; ***, p ≦ 0.001.
Endothelial IL6 in Contact Co-culture Promotes NS/P Cell Differentiation
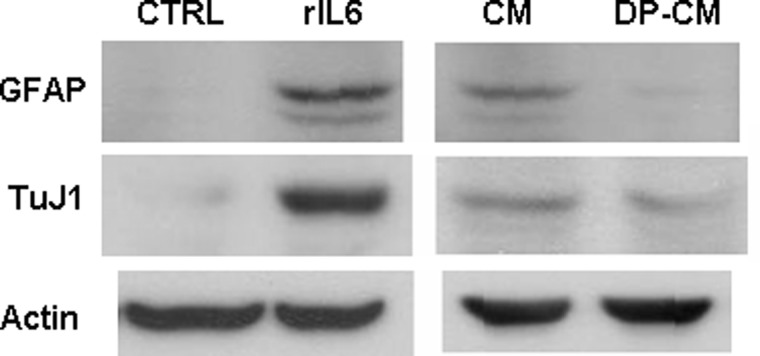
Because IL6 promotes both gliogenesis and neurogenesis of NS/P cells, we decided to investigate the significance of NS/P cell-induced endothelial IL6. We examined whether the endothelial IL6 induced by NS/P cells affects NS/P cell differentiation. Under differentiation culture conditions, recombinant IL6 protein promoted NS/P cell differentiation to astrocyte and neuron as measured by expression of glial protein GFAP and neuron protein β-tubulin III (TuJ1) (Fig. 9, rIL6) confirming the presence of IL6 responsive components in the culture. NS/P cells cultured with conditioned medium collected from NS/P cell-bEND cell co-culture (Fig. 9, CM) produced higher levels of GFAP and β-tubulin III proteins than NS/P cells cultured in their regular differentiation medium (Fig. 9, CTRL), indicating enhanced glial and neuronal differentiation by the co-culture conditioned medium. Removal of IL6 protein from the co-culture conditioned medium (Fig. 9, DP-CM) reduced the ability to promote astrocyte and neuron differentiation as shown with reduced expression of GFAP and β-tubulin III.
FIGURE 9.
NS/P cell-induced endothelial IL6 promotes glial and neural differentiation of NS/P cells. Expression of glial protein GFAP and early neuron protein TuJ1 in NS/P cells cultured under differentiation conditions in the absence (CTRL) or presence of recombinant IL6 protein (rIL6) or cultured with conditioned medium collected of NS/P-bEND cells in contact co-culture before (CM) or after (DP-CM) removal of IL6 protein by specific antibody. Actin is the internal loading control for Western blot assay.
DISCUSSION
NS/P cells and microvasculature are thought to be regulated reciprocally. Most studies on NS/P cell vasculature have focused on how endothelial cells and their diffusible endothelial factors regulate NS/P cell properties. Our studies provided evidence that direct cell contact signaling exists between NS/P cells and bEND cells. This direct cell interaction allowed NS/P cells to activate brain endothelial signaling and gene expression. We demonstrated that the brain endothelial cytokines/chemokines induced by NS/P cells during direct cell contact are critically dependent on the NS/P cell-derived type II transmembrane serine protease MTP. MTP on the surface of NS/P cells caused activation of brain endothelial Gs protein, which in turn led to activation of endothelial p38 MAPK and resulted in enhanced expression of some cytokines/chemokines.
Recombinant protein of MTP protease domain added to brain endothelial culture or immobilized to the culture dishes in contact with bEND cells could not induce brain endothelial signaling activation or cytokine/chemokine expression. Full-length active forms of MTP released in NS/P cell culture failed to induce brain endothelial responses either. Only when NS/P cells and bEND cells are in direct contact and only when MTP is present in NS/P cells can brain endothelial cell signaling activation and cytokine/chemokine expression be stimulated. This differs from human umbilical vein endothelial cells that soluble protein of MTP protease domain is sufficient to induce their cellular response (20). Although our observations did not rule out the importance of MTP protease activity in brain endothelium, these data demonstrated that MTP at the cell contact sites is crucial for NS/P-bEND signaling interaction. The observation that bEND cells cultured on top of the immobilized recombinant MTP had little response implied that other parts of MTP protein may be important on NS/P-bEND communication as well. It is possible that additional factors at the cell contact sites are required for MTP to achieve its action. Our studies did not exclude effects from other factors in NS/P-bEND cell interaction. The extracellular part of MTP contains multiple CUB (Cls/Clr urichin embryonic growth factor, bone morphogenic protein-1) domains and low density lipoprotein receptor class A domains that are thought to be involved in protein-protein interaction. It is conceivable that these domains bring additional factors to the cell contact sites for proper action of MTP.
Recently, it is shown that in the neurogenic niches of LV in brain, the slowly dividing neural stem cells, and their rapidly dividing progeny are frequently in direct contact with blood vessels (5, 10). These direct cell contact sites are also the cell proliferation “hot spot” (5, 10). Contact of NS/P cells with blood vessels would allow direct access of vascular-derived signals. It would allow NS/P cells to directly affect the behavior of endothelial cells as well. The primitive neural stem cell subpopulation B1 actually contacts both the ventriclar surface and the blood vessel, which allow access and transfer information between the compartments (17). Brain endothelial cytokine/chemokine induced by NS/P cells could provide feedback regulation to NS/P cell properties such as differentiation or migration. Endothelial factors induced by the NS/P cells could also affect other cell types in the LV neurogenic niches such as astrocytes and microglia to influence NS/P cell functions. Our studies provided evidence that NS/P cell-derived MTP is a control molecule in the cell-cell direct communication of neurovascular niche. Activation of the endothelial Gs protein is one mechanism through which NS/P cells regulate brain endothelial responses. NS/P cell-derived MTP and endothelial Gs protein activation could assure tight and highly localized regulation at cell-cell contact sites, which is clearly vital for the specialized neurovascular niches in brain neurogenic LV.
The heterotrimeric G proteins consist of α, β, and γ subunits. They function to transduce signals from receptors to intracellular effectors. In the absence of stimulation, α subunit of the G protein is GDP-bound and is associated with β and γ subunits. Agonist binding of the receptor promotes association of the intracellular domain to the heterotrimeric G protein and induces exchange of GTP for GDP binding to Gα subunit. The GTP-bound Gα subunit dissociates from the Gβγ complex, and each of these subunits activates downstream responsive effectors. The intrinsic GTPase activity of the Gα subunit hydrolyzes the bound GTP to GDP, allowing reassociation of Gα to Gβγ, and returns the heterotrimer to its inactive state until activation by agonist binding (18). Although our GTPase assays did not exclude activity coming from non-G protein molecules, the effect of CTX argues that endothelial Gs protein is the main source of GTPase activity resulting from activation of an endothelial GPCR by the NS/P MTP.
GPCR is the largest family of transmembrane signaling molecules that induce G protein activation. The PAR family of GPCR is activated by an unusual irreversible proteolytic mechanism. Cleavage of the amino-terminal exodomain of PARs generates a new amino terminus functioning as a ligand that binds and activates the receptors (19). Presently, PAR2 is the only GPCR identified to be activated by MTP. MTP activation of PAR2 was recently shown to induce IL6 expression in human umbilical vein endothelial cells (20). In bEND cells, however, PAR2 was not present. PAR members are capable of activating Gq/11, Gi/o/z, and G12/13 signaling responses (15). In bEND cells, however, Gs protein was the major brain endothelial G protein activated by NS/P MTP. Together these data suggest that the PAR family of GPCR is not involved in NS/P-bEND cell communication and that other brain endothelial GPCRs exist to serve as the target molecule of NS/P cell-derived MTP. Our data, however, could not determine definitively whether this is a direct proteolytic activation by MTP or an indirect activation mediated by MTP.
IL6 is a multifunctional neurotrophin and cytokine. Besides its function in immune and inflammatory responses, IL6 plays an important role in CNS vascular development and vascularization, under both normal conditions and during the healing of brain injuries (21). IL6 may function as a neuron-protective agent within the brain to protect neurons from oxidative damage. It promotes neurite outgrowth and neuronal survival (22), and it supports axonal regrowth and network repair in CNS following lesions (23). Importantly, it promotes gliogenesis and neurogenesis of NS/P cells (24, 25). Chemotactic activity has been attributed to CXCL10 for T cells, NK cells, and dendritic cells. Its expression is linked with many T cell-mediated inflammatory diseases (26). In the CNS, CXCL10 was shown to associate with viral infection-promoted neuronal apoptosis. Therapeutic neutralization of CXCL10 in a model of spinal cord injury reduces neuroinflammation, apoptosis, and neuronal loss (27). On stem or progenitor cells, CXCL10 has been shown recently to be essential for the mobilization of rat neural progenitor cells (28), human hematopoietic stem cells (29), and mesenchymal stem cells (30). In the NS/P cell vascular niches, endothelial CXCL10 might act on NS/P cells as a mobilization factor. IL24 is the protein product of melanoma differentiation-associated gene 7. IL24 is classified as a cytokine of the IL10 family because of its chromosomal localization, sequence homology, and functional properties. Originally identified as a tumor suppressor molecule, IL24 has anti-cancer properties for a broad range of tumors (31–35). Little is known about its function in brain or in NS/P cells. In skin, IL24 is involved in the “resolution phase” of wound healing when the keratinocytes revert to their normal, nonproliferating, differentiated state (36). It is possible that IL24 participates in a similar healing process during brain injury.
In summary, our studies revealed a novel cell communication in neural stem cell vascular niche that involves direct interaction between the NS/P membrane protease and the bEND Gs protein. Our findings advanced our understanding of cell contact communication in neurovascular niches. Our studies further unveiled the functional and regulatory complexity of MTP.
This work was supported by National Health Research Institutes Grant 00A1-CSPP13-014 (to S.-L. L.) Taiwan, Republic of China.
H.-H. Tung and S.-L. Lee, unpublished observation.
H.-C. Chou and S.-L. Lee, unpublished observation.
- NS/P
- neural stem/progenitor
- bEND
- brain endothelial
- MTP
- matriptase
- SVZ
- subventricular zone
- LV
- lateral ventricle
- PTX
- pertussis toxin
- CTX
- cholera toxin
- CXCL10
- CXC motif chemokine 10
- PAR
- protease-activated receptor
- GPCR
- G protein-coupled receptor
- GFAP
- glial fibrillary acidic protein.
REFERENCES
- 1. Temple S. (2001) The development of neural stem cells. Nature 414, 112–117 [DOI] [PubMed] [Google Scholar]
- 2. Zerlin M., Goldman J. E. (1997) Interactions between glial progenitors and blood vessels during early postnatal corticogenesis. Blood vessel contact represents an early stage of astrocyte differentiation. J. Comp. Neurol. 387, 537–546 [DOI] [PubMed] [Google Scholar]
- 3. Alvarez-Buylla A., Lim D. A. (2004) For the long run. Maintaining germinal niches in the adult brain. Neuron 41, 683–686 [DOI] [PubMed] [Google Scholar]
- 4. Palmer T. D., Willhoite A. R., Gage F. H. (2000) Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479–494 [DOI] [PubMed] [Google Scholar]
- 5. Tavazoie M., Van der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J. M., Doetsch F. (2008) A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bovetti S., Hsieh Y. C., Bovolin P., Perroteau I., Kazunori T., Puche A. C. (2007) Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 27, 5976–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamashita T., Ninomiya M., Hernández Acosta P., García-Verdugo J. M., Sunabori T., Sakaguchi M., Adachi K., Kojima T., Hirota Y., Kawase T., Araki N., Abe K., Okano H., Sawamoto K. (2006) Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci. 26, 6627–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen Q., Goderie S. K., Jin L., Karanth N., Sun Y., Abramova N., Vincent P., Pumiglia K., Temple S. (2004) Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 [DOI] [PubMed] [Google Scholar]
- 9. Wang L., Zhang Z. G., Zhang R. L., Gregg S. R., Hozeska-Solgot A., LeTourneau Y., Wang Y., Chopp M. (2006) Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J. Neurosci. 26, 5996–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen Q., Wang Y., Kokovay E., Lin G., Chuang S. M., Goderie S. K., Roysam B., Temple S. (2008) Adult SVZ stem cells lie in a vascular niche. A quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Q., Ford M. C., Lavik E. B., Madri J. A. (2006) Modeling the neurovascular niche. VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells. An in vitro study. J. Neurosci. Res. 84, 1656–1668 [DOI] [PubMed] [Google Scholar]
- 12. Ying Q. L., Stavridis M., Griffiths D., Li M., Smith A. (2003) Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 [DOI] [PubMed] [Google Scholar]
- 13. Fang J. D., Chou H. C., Tung H. H., Huang P. Y., Lee S. L. (2011) Endogenous expression of matriptase in neural progenitor cells promotes cell migration and neuron differentiation. J. Biol. Chem. 286, 5667–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee S. L., Dickson R. B., Lin C. Y. (2000) Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J. Biol. Chem. 275, 36720–36725 [DOI] [PubMed] [Google Scholar]
- 15. Trejo J. (2003) Protease-activated receptors. New concepts in regulation of G protein-coupled receptor signaling and trafficking. J. Pharmacol. Exp. Ther. 307, 437–442 [DOI] [PubMed] [Google Scholar]
- 16. Takeuchi T., Harris J. L., Huang W., Yan K. W., Coughlin S. R., Craik C. S. (2000) Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J. Biol. Chem. 275, 26333–26342 [DOI] [PubMed] [Google Scholar]
- 17. Mirzadeh Z., Merkle F. T., Soriano-Navarro M., Garcia-Verdugo J. M., Alvarez-Buylla A. (2008) Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milligan G., Kostenis E. (2006) Heterotrimeric G-proteins: a short history. Br. J. Pharmacol. 147, S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hollenberg M. D., Compton S. J. (2002) International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol. Rev. 54, 203–217 [DOI] [PubMed] [Google Scholar]
- 20. Seitz I., Hess S., Schulz H., Eckl R., Busch G., Montens H. P., Brandl R., Seidl S., Schömig A., Ott I. (2007) Membrane-type serine protease-1/matriptase induces interleukin-6 and -8 in endothelial cells by activation of protease-activated receptor-2. Potential implications in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 769–775 [DOI] [PubMed] [Google Scholar]
- 21. Fee D., Grzybicki D., Dobbs M., Ihyer S., Clotfelter J., Macvilay S., Hart M. N., Sandor M., Fabry Z. (2000) Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine 12, 655–665 [DOI] [PubMed] [Google Scholar]
- 22. Schäfer K. H., Mestres P., März P., Rose-John S. (1999) The IL-6/sIL-6R fusion protein hyper-IL-6 promotes neurite outgrowth and neuron survival in cultured enteric neurons. J. Interferon Cytokine Res. 19, 527–532 [DOI] [PubMed] [Google Scholar]
- 23. Hakkoum D., Stoppini L., Muller D. (2007) Interleukin-6 promotes sprouting and functional recovery in lesioned organotypic hippocampal slice cultures. J. Neurochem. 100, 747–757 [DOI] [PubMed] [Google Scholar]
- 24. Nakanishi M., Niidome T., Matsuda S., Akaike A., Kihara T., Sugimoto H. (2007) Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur. J. Neurosci. 25, 649–658 [DOI] [PubMed] [Google Scholar]
- 25. Islam O., Gong X., Rose-John S., Heese K. (2009) Interleukin-6 and neural stem cells. More than gliogenesis. Mol. Biol. Cell 20, 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lacotte S., Brun S., Muller S., Dumortier H. (2009) CXCR3, inflammation, and autoimmune diseases. Ann. N.Y. Acad. Sci. 1173, 310–317 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez R., Hickey M. J., Espinosa J. M., Nistor G., Lane T. E., Keirstead H. S. (2007) Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen. Med. 2, 771–783 [DOI] [PubMed] [Google Scholar]
- 28. Honeth G., Staflin K., Kalliomäki S., Lindvall M., Kjellman C. (2006) Chemokine-directed migration of tumor-inhibitory neural progenitor cells towards an intracranially growing glioma. Exp. Cell Res. 312, 1265–1276 [DOI] [PubMed] [Google Scholar]
- 29. Jinquan T., Anting L., Jacobi H. H., Glue C., Jing C., Ryder L. P., Madsen H. O., Svejgaard A., Skov P. S., Malling H. J., Poulsen L. K. (2001) CXCR3 expression on CD34+ hemopoietic progenitors induced by granulocyte-macrophage colony-stimulating factor. II. Signaling pathways involved. J. Immunol. 167, 4405–4413 [DOI] [PubMed] [Google Scholar]
- 30. Kalwitz G., Andreas K., Endres M., Neumann K., Notter M., Ringe J., Sittinger M., Kaps C. (2010) Chemokine profile of human serum from whole blood. Migratory effects of CXCL-10 and CXCL-11 on human mesenchymal stem cells. Connect. Tissue Res. 51, 113–122 [DOI] [PubMed] [Google Scholar]
- 31. Dong C. Y., Zhang F., Duan Y. J., Yang B. X., Lin Y. M., Ma X. T. (2008) mda-7/IL-24 inhibits the proliferation of hematopoietic malignancies in vitro and in vivo. Exp. Hematol. 36, 938–946 [DOI] [PubMed] [Google Scholar]
- 32. Kaliberova L. N., Krendelchtchikova V., Harmon D. K., Stockard C. R., Petersen A. S., Markert J. M., Gillespie G. Y., Grizzle W. E., Buchsbaum D. J., Kaliberov S. A. (2009) CRAdRGDflt-IL24 virotherapy in combination with chemotherapy of experimental glioma. Cancer Gene Ther. 16, 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta P., Emdad L., Lebedeva I. V., Sarkar D., Dent P., Curiel D. T., Settleman J., Fisher P. B. (2008) Targeted combinatorial therapy of non-small cell lung carcinoma using a GST-fusion protein of full-length or truncated MDA-7/IL-24 with Tarceva. J. Cell. Physiol. 215, 827–836 [DOI] [PubMed] [Google Scholar]
- 34. Rahmani M., Mayo M., Dash R., Sokhi U. K., Dmitriev I. P., Sarkar D., Dent P., Curiel D. T., Fisher P. B., Grant S. (2010) Melanoma differentiation associated gene-7/interleukin-24 potently induces apoptosis in human myeloid leukemia cells through a process regulated by endoplasmic reticulum stress. Mol. Pharmacol. 78, 1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sauane M., Gopalkrishnan R. V., Sarkar D., Su Z. Z., Lebedeva I. V., Dent P., Pestka S., Fisher P. B. (2003) MDA-7/IL-24. Novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 14, 35–51 [DOI] [PubMed] [Google Scholar]
- 36. Poindexter N. J., Williams R. R., Powis G., Jen E., Caudle A. S., Chada S., Grimm E. A. (2010) IL-24 is expressed during wound repair and inhibits TGFα-induced migration and proliferation of keratinocytes. Exp. Dermatol. 19, 714–722 [DOI] [PMC free article] [PubMed] [Google Scholar]