Background: PTEN tumor suppressor is a master cellular regulator but was not previously implicated in pathogenic infection.
Results: PTEN expression or pharmacological inhibition of PI3K-Akt-mTOR pathway decreases mycobacterial infection.
Conclusion: The lipid phosphatase activity of PTEN suppresses mycobacterial infection by inhibiting PI3K signaling.
Significance: Understanding how the genetic context of cells influences their susceptibility to pathogens is important for cancer patient care and for treating pathogenic infection.
Keywords: Cancer, Mycobacteria, PI 3-Kinase (PI3K), Pten, Signal Transduction
Abstract
The tumor suppressor PTEN is a lipid phosphatase that is frequently mutated in various human cancers. PTEN suppresses tumor cell proliferation, survival, and growth mainly by inhibiting the PI3K-Akt signaling pathway through dephosphorylation of phosphatidylinositol 3,4,5-triphosphate. In addition to it role in tumor suppression, the PTEN-PI3K pathway controls many cellular functions, some of which may be important for cellular resistance to infection. Currently, the intersection between tumorigenic signaling pathways and cellular susceptibility to infection is not well defined. In this study we report that PTEN signaling regulates infection of both noncancerous and cancerous cells by multiple intracellular mycobacterial pathogens and that pharmacological modulation of PTEN signaling can affect mycobacterial infection. We found that PTEN deficiency renders multiple types of cells hyper-susceptible to infection by Mycoplasma and Mycobacterium bovis Bacillus Calmette-Guérin (BCG). The lipid phosphatase activity of PTEN is required for attenuating infection. Furthermore, we found mycobacterial infection activates host cell Akt phosphorylation, and pharmacological inhibition of Akt or PI3K activity reduced levels of intracellular infection. Intriguingly, inhibition of mTOR, one of the downstream components of the Akt signaling and a promising cancer therapeutic target, also lowered intracellular Bacillus Calmette-Guérin levels in mammary epithelial cancer MCF-7 cells. These findings demonstrate a critical role of PTEN-regulated pathways in pathogen infection. The relationship of PTEN-PI3K-Akt mTOR status and susceptibility to mycobacterial infection suggests that the interaction of mycobacterial pathogens with cancer cells may be influenced by genetic alterations in the tumor cells.
Introduction
The tumor suppressor PTEN plays critical roles in cell growth, migration, and death (1–5). PTEN is frequently mutated or deleted in various human cancers and is an important determinant of tumorigenesis (5–7). The PTEN protein is a phosphatase for the second messenger phosphatidylinositol 3,4,5-triphosphate, which is required for activation of the Akt/PKB kinase in the PI3K-Akt pathway (1, 5, 8). The PI3K-Akt pathway promotes cell metabolism, survival, and proliferation (9). One of the downstream effectors of the pathway, the mTOR-Raptor kinase complex, is the master regulator of cellular metabolism (10). As such, genetic mutation of PTEN and other components of this pathway have significant impact on pathogenesis of cancer and other diseases.
The PI3K-Akt pathway has also been implicated in infectious diseases and innate immunity. Mounting evidence supports the involvement of Akt in phagocytosis, intracellular bacterial infections, LPS tolerance, production of inflammatory cytokines and mediators, and migration during macrophage-mediated innate immunity, thus establishing a pivotal role for this enzyme in the functional activation of macrophages (11). Furthermore, it was found that intracellular survival and growth of certain phagocytosed bacteria in macrophages are controlled by a kinase network, of which Akt acts as a master regulator by controlling multiple host pathways (12).
Because the Akt pathway plays a role in both cancer development and interactions of pathogens with host cells, it is possible that these two events influence each other, especially during attempts to treat cancer with infectious biotherapies. One successful use of a bacterial pathogen to treat cancer is the use of an attenuated strain of Mycobacterium bovis, BCG,2 to treat superficial bladder cancer. Despite more than 30 years of clinical experience with intravesical BCG for bladder cancer, no markers exist to predict which patients will respond to therapy. This is due in part to the fact that its mechanism of action is not well understood. Direct and indirect immune mechanisms have been hypothesized to play a role (13, 14) as have direct cytotoxic effects of BCG (15). Whatever the mechanism of action, it does seem clear that BCG interaction with tumor cells, leading to internalization and processing of the mycobacterium, plays a crucial role in activation of BCG-mediated anti-tumor activity (16–19).
In this study we demonstrate that the activity of the PI3K-PTEN-Akt pathway determines the susceptibility of cells to infection by mycobacteria, including BCG, suggesting that tumor cell genetic characteristics may be important determinants of the BCG-tumor cell interaction.
EXPERIMENTAL PROCEDURES
Antibodies
Antibodies against S6 (5G10, #2217), S6K (49D7, #2708), p-S6K (Thr389) (108D2, #9234), Akt (#9272), p-Akt (Ser473) (#9271), and p-Akt (Thr308) (#2965) were from Cell Signaling Technology. PTEN antibody (clone 6H2.1) was from Cascade BioScience. Antibodies against PCAF (E-8), actin (C-2), and GFP (B-2) were from Santa Cruz Biotechnology, Inc. Tubulin antibody (DM1A) was from Calbiochem.
Detection of Mycoplasmas with DAPI
Cells were fixed in 3.7% formaldehyde for 10 min, permeabilized with 0.5% Triton X-100 for 5 min, and stained with DAPI at 1 μg/ml. On adding DAPI to tissue culture cells, it is rapidly taken up into cellular DNA yielding highly fluorescent nuclei and no detectable cytoplasmic fluorescence. If the cells are contaminated with Mycoplasma, characteristic discrete fluorescent foci are readily detected over the cytoplasm and sometimes in intercellular spaces. Infection by mycoplasmas was further confirmed by detecting the presence of conserved mycoplasmal enzymes with a MycoAlertTM kit (Lonzabioscience).
A detailed analysis from the institutional facility indicates that the source of Mycoplasma that used in our experiments is a mixture mainly containing the following two species, Mycoplasma orale and Mycoplasma fermentans. M. orale was detected by PCR with primers of 5′-ACACCATGGGAGCTGGTAAT-3′ and 5′-CTTCTTCGACTTTCAGACCCAAGGCAT-3′, which gives a product of 423 bp in size. GAPDH was used as internal control with primers of 5′-ATTTGGCCGTATTGGGCGCCTG-3′ and 5′-CCCGGCCTTCTCCATGGTGG-3′ producing a 298-bp band.
Cell Lines
PC3, HeLa, MCF-7, UM-UC-3, and MGHU4 were obtained from the American Type Culture Collection (ATCC) and were cultured according to ATCC instructions. All cell lines were tested for their authenticity by cytogenetic analysis. Mouse epithelial fibroblasts (MEFs) of both pten+/+ and pten−/− were kindly provided by Dr. Pier Paolo Pandolfi and cultured and tested as previously described (2).
Transfection, PTEN RNAi Constructs, and Establishment of Stable Cell Lines
Cell culture transfection was performed using Lipofectamine 2000 from Invitrogen. The shRNA constructs against human PTEN generated using pLKO.1 vector were bought from Sigma. The PTEN DNA sequences used in the RNAi construct were 5′-AGGCGCTATGTGTATTATTAT-3′, 5′-CCACAGCTAGAACTTATCAAA-3′, 5′-CTAGAACTTATCAAACCCTTT-3′, 5′-CGTGCAGATAATGACAAGGAA-3′, and 5′-CCACAAATGAAGGGATATAAA-3′. Stable cell lines were established by infection of cells with pLKO.1 lentivirus. Puromycin selection at 0.6 μg/ml was carried out 24 h after removal of virus.
BCG and Infection
GFP-expressing BCG was created by transforming M. bovis Calmette-Guérin Pasteur strain with pYUB921 (expresses GFP and kanamycin resistance) (20). BCG-GFP was grown at 37 °C in Middlebrook 7H9 media supplemented with 10% albumin, dextrose, saline, 0.5% glycerol, and 0.05% Tween 80 and in the presence of 20 mg/ml kanamycin. To create a stock for infection, BCG-GFP was grown to mid-log phase (A600 0.4–0.6), washed twice in phosphate-buffered saline (PBS) with 0.05% Tween 80, resuspended in PBS with 25% glycerol, and stored at −80 °C. To measure final bacterial concentration, an aliquot was thawed, and serial dilutions were plated on 7H10 plates in the presence of 20 microgram/ml kanamycin. Cells were plated a day before infection in antibiotic-free media so as to reach 90% confluence on the day of infection. BCG-GFP was thawed and diluted in antibiotic-free media to achieve a multiplicity of infection of 10:1. Plates were incubated at 37 °C for the specified time period and then washed 3 times with PBS and 3 times with antibiotic-containing media (100 units/ml penicillin G and 100 μg/ml streptomycin). Cells were further incubated for indicated time before being imaged or detached using trypsin and resuspended in PBS for analysis by flow-cytometry.
Inhibitors
All inhibitors were used at concentrations reported to inhibit their kinase target. Wortmannin and rapamycin (Sigma) were used at a concentration of 100 nm to inhibit PI3K and mTOR, respectively. Akt was inhibited with Akti-1 (Calbiochem) at 10 μm or Akti-2 (Merck) at 2 μm. Inhibitors were added after infection and removal of extracellular BCG. To test if PI3K-Akt pathway is involved in BCG entry, cells were also pretreated with inhibitors 2 h before infection.
Immunostaining
To detect mitochondria, cells grown on glass coverslips in culture medium were first treated with 500 nm of MitoTracker Red CMXRos for 45 min before being fixed, permeabilized, and stained with DAPI. Polyribosome was stained with an antibody against S6 ribosomal protein.
Flow Cytometry
Samples were acquired on an LSR II flow cytometric device (BD Biosciences) using the FACS DiVa software (BD Biosciences) according to the manufacturer's instructions. Data analysis was performed with the FlowJo software package (Tree Star).
Nuclease Digestion
Cells were fixed in methanol for 2 min at −20 °C, rinsed in PBS and incubated in either DNase I (100 μg/ml in PBS and 5 mm MgCl2, RNase-free) or RNase A (100 μg/ml in PBS, DNase-free) for 2 h at 25 °C. After several washes in PBS, cells were stained with DAPI as described above.
RESULTS
Absence of PTEN Expression Confers Susceptibility to Mycoplasma Infection
This work originated from a serendipitous observation in the course of our investigation of the PTEN pathway. With fluorescent microscopy, we observed that MEFs deficient in PTEN had unusually high levels of DAPI-stained structures in the cytoplasm (Fig. 1A). These cytosolic DAPI-positive particles did not co-localize with mitochondrial or ribosomal stains, indicating that they are not clusters of mitochondria or polyribosomes, the two major cytosolic nucleic acid-containing structures (Fig. 1, B and C). The DAPI stained structures are sensitive to DNase but not RNase digestion, indicating that the DAPI signal is from cytosolic DNA (Fig. 1D). We subsequently found that these cytosolic DAPI-positive structures were infected Mycoplasma (the laboratory had just suffered a Mycoplasma infection then).
FIGURE 1.
Cytosolic DAPI signal detected in pten−/− MEFs. A, DAPI-positive signal was detected in the cytoplasm of pten−/− MEFs but not wild-type MEFs. Scale bar = 20 μm. B, cytosolic DAPI signal was not from mitochondria. Mitochondria were detected by MitoTracker Red (Mito) as described under “Experimental Procedures.“ Scale bar = 10 μm. C, the cytosolic DAPI signal was not from polyribosomes. Polyribosomes were stained with a primary antibody against S6 ribosomal protein and then a Cy3 conjugated secondary antibody. Scale bar = 10 μm. D, the cytosolic DAPI signal can be removed by DNase but not RNase. Cytosolic DAPI-positive pten−/− MEFs were treated with either DNase or RNase as described under “Experimental Procedures” and then subjected to DAPI staining. Scale bar = 20 μm.
The tight correlation of the Mycoplasma with the PTEN status of cells prompted us to further investigate the potential relationship between PTEN function and Mycoplasma infection. First, we infected a battery of cell lines with culture medium containing Mycoplasma. These cell lines include wild-type MEFs and HeLa cells (both PTEN-positive) as well as PTEN-null MEFs and human prostate cancer cells PC3 (both PTEN-negative). Indeed, compared with PTEN-positive cells, PTEN-negative cells were hypersusceptible to infection with Mycoplasma (Fig. 2A). Second, in a time-course experiment, we subjected both wild-type MEFs and PTEN-null MEFs to Mycoplasma infection and monitored intracellular Mycoplasma using DAPI-staining at different time points. PTEN-null MEFs showed detectable intracellular Mycoplasma after 8 h, whereas wild-type MEFs showed similar levels of infection only after more than 24 h (Fig. 2B). This was confirmed by PCR with primers specific to M. orale (Fig. 2C). To further validate that PTEN status influences susceptibility to Mycoplasma infection, we knocked down PTEN by shRNA in HeLa cells and measured Mycoplasma infection (Fig. 2, D and E). As expected, HeLa cells with PTEN knockdown showed hypersusceptibility to Mycoplasma infection compared with cells that had received a LacZ shRNA control. Taken together, these results indicate that defective PTEN function results in higher susceptibility of host cells to Mycoplasma infection.
FIGURE 2.
Absence of PTEN expression confers susceptibility of cells to Mycoplasma infection. A, PTEN-null cells are prone to infection by Mycoplasma. PTEN-positive cells (wild-type MEFs and HeLa cells) and PTEN-null cells (pten−/− MEFs and PC3 cells) were infected with culture medium containing Mycoplasma and then tested by DAPI staining as described under “Experimental Procedures.“ B, pten−/− MEF cells were infected by Mycoplasma more quickly than the wild-type counterpart. Both pten−/− and wild-type MEFs were infected with Mycoplasma. Infection was followed by DAPI staining at the indicated time points. C, infection was also detected by PCR using primers specific for M. orale. D, Western blot shows the effect of knockdown of PTEN by shRNA (iPTEN) in HeLa cells. LacZ shRNA (iLacZ) was used as negative control. A PCAF blot serves as the loading control. E, PTEN knockdown but not control knockdown makes HeLa Mycoplasma infection-prone. Mycoplasma infection was monitored by DAPI staining. Scale bar = 20 μm.
Suppression of Intracellular Mycoplasmas Depends on Lipid Phosphatase Activity of PTEN
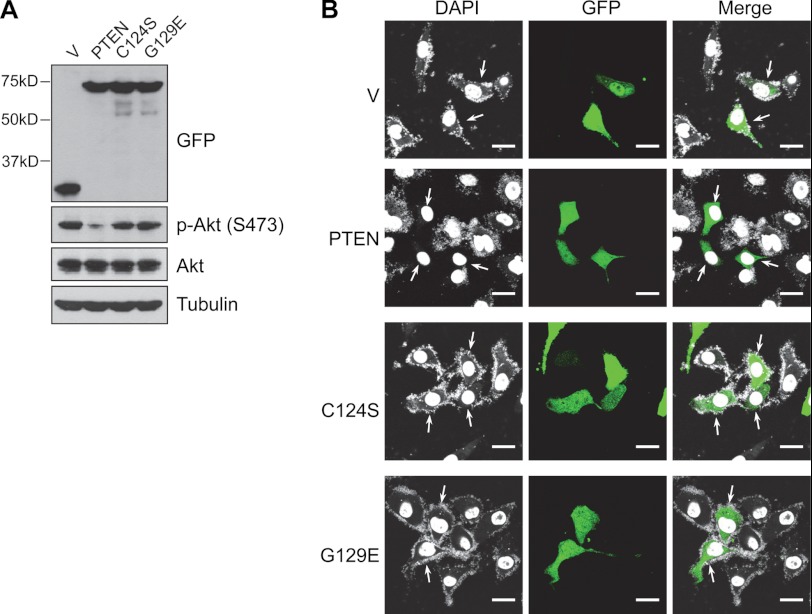
Next, we tested whether overexpression of PTEN can reduce intracellular Mycoplasma levels and whether the enzymatic activity of PTEN is required. Mycoplasma-infected PTEN-deficient PC3 cells were transfected with either GFP-tagged wild-type PTEN or lipid phosphatase inactive mutants (C124S or G129E mutant). As expected, in the PTEN-negative PC3 cells, Akt is constitutively activated, shown by high levels of phosphorylation at serine 473 (Fig. 3A). Overexpression of wild-type PTEN, but not C124S or G129E PTEN, suppressed Akt activation (Fig. 3A). Importantly, we found that restoration of PTEN expression eliminated intracellular Mycoplasma and that this role was dependent on PTEN lipid phosphatase activity, as both the C124S and G129E mutants failed to do so (Fig. 3B). Recent evidence suggests that PTEN can also regulate various cellular processes with its protein phosphatase activity, although it is not clear what its physiological protein substrates are (3, 4, 21–23). Because the G129E mutant is still functional as a protein phosphatase (3), failure of the G129E mutant to suppress intracellular Mycoplasma indicates that the protein phosphatase activity of PTEN is not sufficient for this function.
FIGURE 3.
The lipid phosphatase activity of PTEN is required for suppression of intracellular Mycoplasma. PC3 were infected with Mycoplasma and transiently transfected with empty GFP vector (V) or GFP tagged PTEN, PTEN C124S, or PTEN G129E. Intracellular mycoplasmas were evaluated 3 days after by DAPI staining. A, a Western blot shows equal levels of expression of PTEN and its derivatives and their ability to inhibit Akt phosphorylation. B, wild-type PTEN, but not lipid phosphatase-dead mutants, suppressed intracellular Mycoplasma (indicated by arrows). Scale bar = 20 μm.
PTEN Inhibits Intracellular BCG Growth in MCF-7 Cells
Mycoplasmas are cell wall deficient bacteria that can exist either extracellularly or within the cytoplasm of host cells (24). We sought to understand whether PTEN deficiency confers susceptibility to other intracellular bacterial pathogens. For this purpose, we used M. bovis BCG, an attenuated strain of M. bovis that was derived by prolonged in vitro passage. Pathogenic mycobacteria are intracellular pathogens that replicate and survive within host cells, usually professional phagocytes such as macrophages and dendritic cells.
We first infected MCF-7 mammary epithelial cancer cells with BCG carrying an episomal plasmid expressing green fluorescent protein (GFP). This fluorescent marker allowed easy quantitation of intracellular BCG by fluorescent microscopy and flow cytometry. We found that shRNA knockdown of PTEN (Fig. 4A) enhanced BCG infection in MCF-7 cells, as reflected by the number and intensity of GFP foci inside MCF-7 cells determined by fluorescence microscopy and flow cytometry (Fig. 4, B and C).
FIGURE 4.
PTEN inhibits BCG infection in MCF-7 cells. MCF-7 cells, harboring either scrambled shRNA knockdown (SC) or PTEN knockdown (iPTEN) (A) were infected with GFP-BCG at multiplicity of infection of 10 for 5 h. Extracellular bacteria were washed away, and cells were incubated for 72 h before intracellular bacterial levels were determined by confocal microscopy (B) or flow cytometry (C). Scale bar = 50 μm. FSC, forward scatter.
PTEN Inhibits Intracellular Pathogen Growth in MCF-7 Cells through Suppressing PI3K-Akt-mTOR Pathway
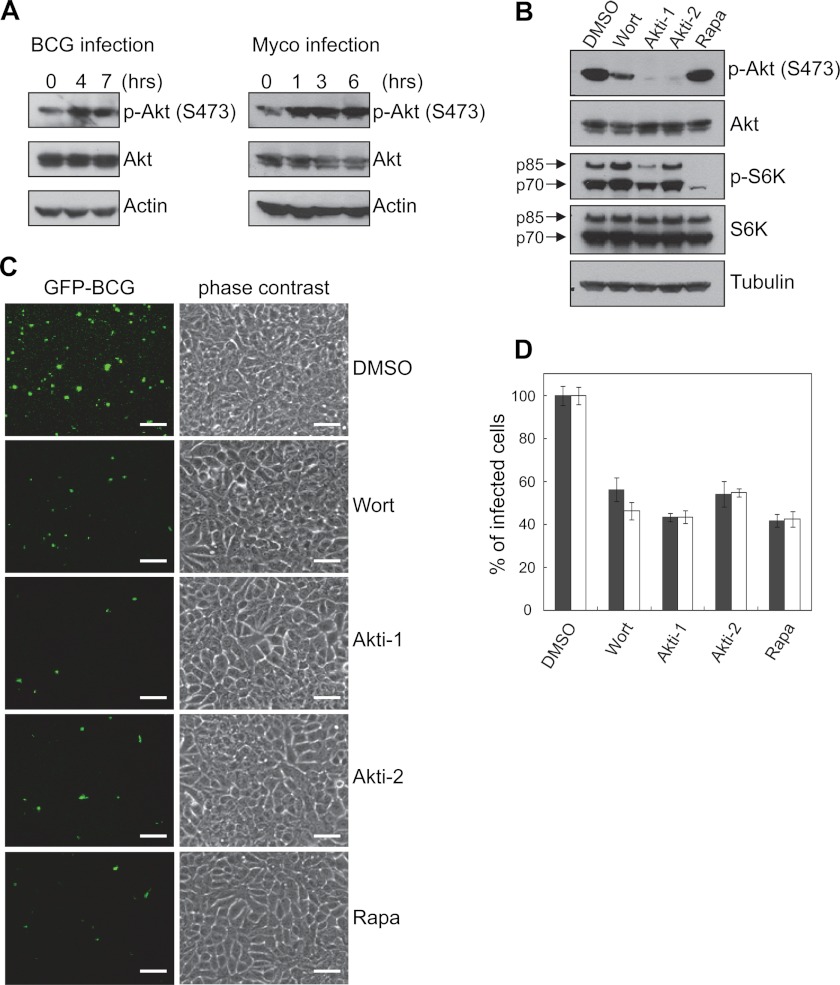
PTEN is a predominant negative regulator of the PI3K-Akt pathway. To test whether the effect of PTEN knockdown on intracellular growth is mediated by activation of the PI3K-Akt pathway, we examined the effect of mycobacterial infection on host cell Akt activation in MCF-7 cells. As shown in Fig. 5, both BCG and Mycoplasma activated Akt as early as 1 h after infection (Fig. 5A). To test whether Akt activation is required for mycobacterial infection, we treated infected cells with small molecule inhibitors of Akt, PI3K, and mTOR. The efficacy of these inhibitors was verified by measuring their effect on inhibition of Akt and S6K phosphorylation (Fig. 5B). Indeed, inhibition of Akt phosphorylation and PI3K (wortmannin) reduced BCG infection in infected cells, as manifested by reduced number and size of intracellular GFP-BCG foci (Fig. 5, C and D). Surprisingly, we found that inhibition of mTOR (rapamycin), one of the downstream components of the Akt signaling and a promising cancer therapeutic target, also conferred resistance to BCG infection (Fig. 5, C and D). This function of mTOR is apparently distinct from the proposed role of the Akt in pathogenic infection, because 1) the endocytic and phagocytic function of Akt does not require mTOR, and 2) inhibition of mTOR did not ablate Akt activation in MCF-7 cells (Fig. 5B) and in many cell types even leads to a strong feedback activation of Akt, as demonstrated previously (25).
FIGURE 5.
PTEN inhibits pathogen infection in MCF-7 cells through suppressing PI3K-Akt-mTOR pathway. A, infection with both Mycoplasma and BCG leads to Akt activation in MCF-7 cells. MCF-7 cells were infected with either BCG or Mycoplasma (Myco) and harvested at different time points. Level of p-Akt at serine 473 was measured by immunoblot. B, Western blotting shows the efficacy of PI3K-Akt-mTOR pathway inhibition by different inhibitors. MCF-7 cells were infected with GFP-BCG. Extracellular bacteria were removed, and cells were further treated with different inhibitors for 72 h before harvest. The efficacy of inhibition was evaluated by suppression of phosphorylation of Akt and S6K. C and D, inhibition of PI3K-Akt-mTOR pathway blocks survival and/or proliferation but not entry of BCG in MCF-7. Inhibitors were added to cells either after (black column) or before (white column) infection. Intracellular BCG levels were determined by fluorescent microscopy (C) and flow cytometry (D). Results are averaged from three independent experiments, and infected cells in DMSO control was considered as 100%. Scale bar = 50 μm.
To address whether the PI3K-Akt pathway also promotes BCG entry, we compared the degree of BCG infection by treating MCF-7 cells with the inhibitors either before or after BCG infection. We did not observe any obvious change in percentages of infected cells (Fig. 5D), indicating phosphatidylinositol-3,4,5-triphosphate signal is not involved in pathogen entry under this condition.
Effect of PTEN Deficiency on BCG Infection in Bladder Cancer Cells
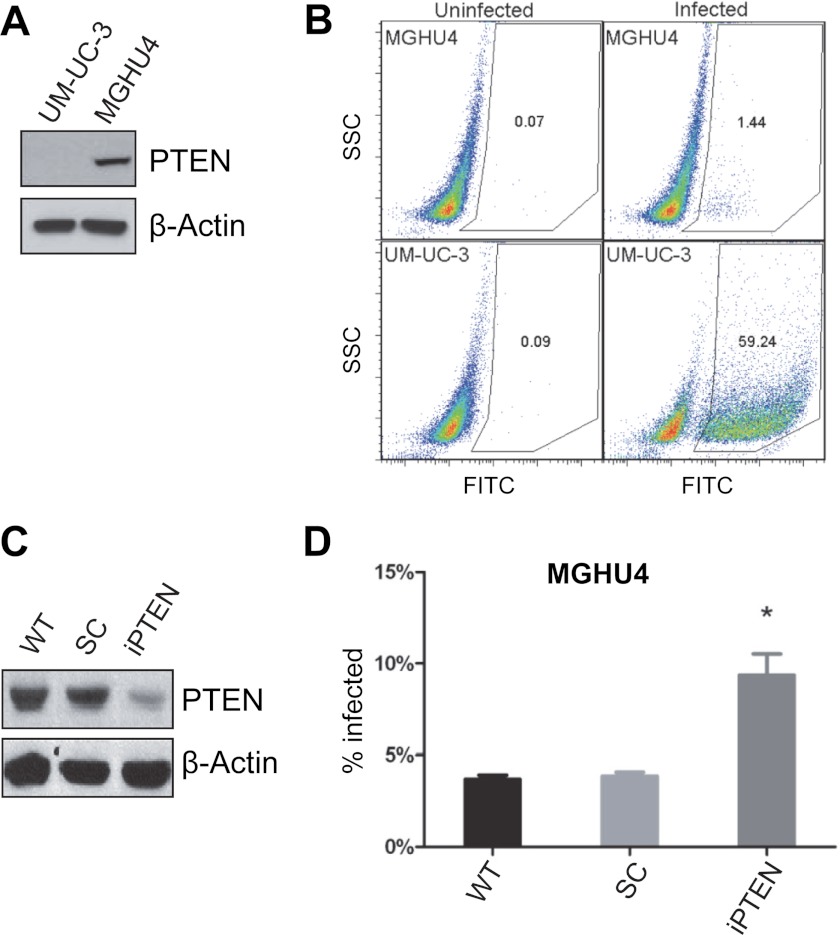
To extend our findings to a tumor cell type that may encounter BCG during the course of cancer therapy, we also determined the susceptibility of bladder cancer cells with altered PTEN levels to infection with BCG. We infected bladder cancer cell lines UM-UC-3 and MGHU4 with GFP-expressing BCG and measured the proportion of infected (GFP-positive) cells by flow cytometry. Western blots revealed that UM-UC-3 cells lack PTEN expression, whereas MGHU4 cells possess readily detectable PTEN expression (Fig. 6A). After 22 h of infection there was a dramatic difference between the two cell lines in their susceptibility to BCG infection. MGHU4 was highly resistant to infection, with less than 2% of the population being GFP-positive, whereas UM-UC-3 was susceptible to infection with more than 50% of the cells being GFP-positive (Fig. 6B). To assess whether loss of PTEN is sufficient to render MGHU4 cells susceptible to BCG infection, we knocked down PTEN in MGHU4 by shRNA (Fig. 6C). PTEN knockdown increased the fraction of infected cells by ∼2-fold compared with control shRNA (Fig. 6D). It should be noted that the absolute level of infection in MGHU4 cells upon PTEN knockdown did not approach that observed in UM-UC-3 cells. This might be due to incomplete PTEN knockdown (Fig. 6C), and it is also likely that other factors might contribute to the difference of these two cell lines in susceptibility to BCG infection. Taken together, our results indicate a causal relationship between the PTEN status of epithelial cells and their permissiveness for intracellular pathogens.
FIGURE 6.
PTEN inhibits BCG infection in bladder cancer cells. A, a Western blot shows the levels of PTEN and actin in UM-UC-3 and MGHU4. B, shown are flow cytometry plots of UM-UC-3 (top panels) or MGHU4 cells (bottom panels) either uninfected (left panels) or infected with BCG-GFP (right panels) for 22 h. The x axis plots GFP fluorescence intensity on a log scale and the numbers within each quadrant are the percent of GFP-positive cells within the indicated gate. C and D, PTEN shRNA increases infection in MGHU4 cells when compared with wild-type or non-targeting shRNA transfected cells. Knockdown of PTEN in MGHU4 was confirmed by Western blot (C). SC, scramble shRNA; iPTEN, PTEN shRNA. *, p < 0.05, compared with scramble shRNA. SSC, side scatter.
DISCUSSION
Loss of PTEN Confers Susceptibility to Intracellular Infection Across Pathogen and Cell Types
This study indicates that a competent PTEN protein is not only critical for tumor suppression but also for resisting infection of mammalian cells by BCG and Mycoplasma, two pathogens with distinct pathogenic strategies. Our data also indicate that infection with BCG or Mycoplasma activates Akt, indicating that loss of PTEN may promote infection by mimicking the natural pathogenic strategy used by these organisms. The mechanisms that mediate the effect of PTEN loss on bacterial infection remain to be defined and could be the consequence of enhanced bacterial intracellular proliferation, intracellular survival, or a combination.
Previous reports suggested a role of Akt in mediating infection of epithelial cells and macrophages by mycobacteria and Salmonella (12, 26, 27). Although the mechanisms by which Akt functions to promote bacterial entry, intracellular proliferation, or survival has not been defined, hypotheses have been raised to explain this phenomenon. By activating p21-activated kinase 4 (PAK4) and downstream small G protein RAC1, Akt modulates actin cytoskeleton and phagocytosis, thus possibly affecting bacterial entry (28, 29); by accelerating cycling of RAB14 small G protein, Akt delays phagosomal-lysosomal fusion, thus likely enhancing intracellular survival of bacteria (30, 31). All these functions of Akt do not involve mTOR, making our finding that rapamycin can suppress BCG infection in MCF-7 cells highly intriguing. Inhibition of mTOR by rapamycin can promote autophagy. To test if autophagy contributes to the effect of rapamycin on BCG infection, we knocked down the autophagy-essential gene ATG7 in MCF-7 to abrogate autophagy. However, ATG7 deficiency does not affect BCG infection in MCF-7 (data not shown), suggesting autophagy is not responsible for our observation at least under this context.
Pathogenic Infection and Cancer
The fact that PTEN-deficient cells are broadly sensitive to bacterial infection has potential implications for clinical oncology. This study suggests that certain tumor cells, especially those with PTEN mutation or PI3K/Akt activation, might be more susceptible to infection by certain intracellular bacteria. To date, whether cancer cells are in general more vulnerable to pathogenic infection and whether pathogenic infection could affect cancer development are underexplored areas that have potential clinical significance.
The PTEN-PI3K pathway is a commonly mutated pathway in human cancer. Consequently, the kinases in this and related pathways are being aggressively pursued as targets for therapeutic intervention. Our finding that loss of PTEN with consequent activation of the PI3K pathway confers intrinsic susceptibility to infection suggests that these inhibitors may have complex effects on infectious risk in patients. Inhibitors of mTOR such as rapamycin and sirolimus are potent immunosuppressants due to their effects on T cell proliferation. However, our results indicate that small molecule inhibitors of the PI3K pathway may actually promote resistance to infection in epithelial cells and possibly macrophages. Our results also have potential implications for BCG therapy of Carcinoma in situ of the bladder. Our results demonstrate that the genetic background of a bladder cancer cell may affect its susceptibility to BCG infection, a variable that could determine response to BCG therapy. Further elucidation of the molecular determinants of bladder cancer cell susceptibility to BCG infection has the potential to reveal predictors of BCG response, none of which are presently available for clinical use.
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA113890 (to X. J.). This work was also supported by the Geoffrey Beene Cancer Research fund (to M. S. G. and X. J.).
- BCG
- Bacillus Calmette-Guérin
- MEF
- mouse embryonic fibroblasts.
REFERENCES
- 1. Stambolic V., Suzuki A., de la Pompa J. L., Brothers G. M., Mirtsos C., Sasaki T., Ruland J., Penninger J. M., Siderovski D. P., Mak T. W. (1998) Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 [DOI] [PubMed] [Google Scholar]
- 2. Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P. P. (1998) Pten is essential for embryonic development and tumor suppression. Nat. Genet. 19, 348–355 [DOI] [PubMed] [Google Scholar]
- 3. Tamura M., Gu J., Matsumoto K., Aota S., Parsons R., Yamada K. M. (1998) Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617 [DOI] [PubMed] [Google Scholar]
- 4. Raftopoulou M., Etienne-Manneville S., Self A., Nicholls S., Hall A. (2004) Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303, 1179–1181 [DOI] [PubMed] [Google Scholar]
- 5. Sansal I., Sellers W. R. (2004) The biology and clinical relevance of the PTEN tumor suppressor pathway. J. Clin. Oncol. 22, 2954–2963 [DOI] [PubMed] [Google Scholar]
- 6. Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., Bigner S. H., Giovanella B. C., Ittmann M., Tycko B., Hibshoosh H., Wigler M. H., Parsons R. (1997) PTEN, a putative protein-tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 [DOI] [PubMed] [Google Scholar]
- 7. Steck P. A., Pershouse M. A., Jasser S. A., Yung W. K., Lin H., Ligon A. H., Langford L. A., Baumgard M. L., Hattier T., Davis T., Frye C., Hu R., Swedlund B., Teng D. H., Tavtigian S. V. (1997) Identification of a candidate tumor suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 [DOI] [PubMed] [Google Scholar]
- 8. Maehama T., Taylor G. S., Dixon J. E. (2001) PTEN and myotubularin. Novel phosphoinositide phosphatases. Annu. Rev. Biochem. 70, 247–279 [DOI] [PubMed] [Google Scholar]
- 9. Luo J., Manning B. D., Cantley L. C. (2003) Targeting the PI3K-Akt pathway in human cancer. Rationale and promise. Cancer cell 4, 257–262 [DOI] [PubMed] [Google Scholar]
- 10. Kim D. H., Sabatini D. M. (2004) Raptor and mTOR. Subunits of a nutrient-sensitive complex. Curr. Top. Microbiol. Immunol. 279, 259–270 [DOI] [PubMed] [Google Scholar]
- 11. Lee Y. G., Lee J., Byeon S. E., Yoo D. S., Kim M. H., Lee S. Y., Cho J. Y. (2011) Functional role of Akt in macrophage-mediated innate immunity. Front. Biosci. 16, 517–530 [DOI] [PubMed] [Google Scholar]
- 12. Kuijl C., Savage N. D., Marsman M., Tuin A. W., Janssen L., Egan D. A., Ketema M., van den Nieuwendijk R., van den Eeden S. J., Geluk A., Poot A., van der Marel G., Beijersbergen R. L., Overkleeft H., Ottenhoff T. H., Neefjes J. (2007) Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450, 725–730 [DOI] [PubMed] [Google Scholar]
- 13. Loskog A., Ninalga C., Paul-Wetterberg G., de la Torre M., Malmström P. U., Tötterman T. H. (2007) Human bladder carcinoma is dominated by T-regulatory cells and Th1 inhibitory cytokines. J. Urol. 177, 353–358 [DOI] [PubMed] [Google Scholar]
- 14. Luo Y., Yamada H., Evanoff D. P., Chen X. (2006) Role of Th1-stimulating cytokines in bacillus Calmette-Guérin (BCG)-induced macrophage cytotoxicity against mouse bladder cancer MBT-2 cells. Clin. Exp. Immunol. 146, 181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pook S. H., Rahmat J. N., Esuvaranathan K., Mahendran R. (2007) Internalization of Mycobacterium bovis, Bacillus Calmette Guerin, by bladder cancer cells is cytotoxic. Oncol. Rep. 18, 1315–1320 [PubMed] [Google Scholar]
- 16. Hudson M. A., Ritchey J. K., Catalona W. J., Brown E. J., Ratliff T. L. (1990) Comparison of the fibronectin-binding ability and antitumor efficacy of various mycobacteria. Cancer Res. 50, 3843–3847 [PubMed] [Google Scholar]
- 17. Ikeda N., Toida I., Iwasaki A., Kawai K., Akaza H. (2002) Surface antigen expression on bladder tumor cells induced by bacillus Calmette-Guérin (BCG). A role of BCG internalization into tumor cells. Int. J. Urol. 9, 29–35 [DOI] [PubMed] [Google Scholar]
- 18. Ratliff T. L., Kavoussi L. R., Catalona W. J. (1988) Role of fibronectin in intravesical BCG therapy for superficial bladder cancer. J. Urol. 139, 410–414 [DOI] [PubMed] [Google Scholar]
- 19. Zhao W., Schorey J. S., Bong-Mastek M., Ritchey J., Brown E. J., Ratliff T. L. (2000) Role of a bacillus Calmette-Guérin fibronectin attachment protein in BCG-induced antitumor activity. Int. J. Cancer 86, 83–88 [DOI] [PubMed] [Google Scholar]
- 20. Teitelbaum R., Cammer M., Maitland M. L., Freitag N. E., Condeelis J., Bloom B. R. (1999) Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc. Natl. Acad. Sci. U.S.A. 96, 15190–15195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hlobilkova A., Guldberg P., Thullberg M., Zeuthen J., Lukas J., Bartek J. (2000) Cell cycle arrest by the PTEN tumor suppressor is target cell specific and may require protein phosphatase activity. Exp. Cell Res. 256, 571–577 [DOI] [PubMed] [Google Scholar]
- 22. Shen W. H., Balajee A. S., Wang J., Wu H., Eng C., Pandolfi P. P., Yin Y. (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128, 157–170 [DOI] [PubMed] [Google Scholar]
- 23. Weng L., Brown J., Eng C. (2001) PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum. Mol. Genet. 10, 237–242 [DOI] [PubMed] [Google Scholar]
- 24. Razin S., Yogev D., Naot Y. (1998) Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62, 1094–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Reilly K. E., Rojo F., She Q. B., Solit D., Mills G. B., Smith D., Lane H., Hofmann F., Hicklin D. J., Ludwig D. L., Baselga J., Rosen N. (2006) mTOR inhibition induces upstream receptor-tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knodler L. A., Finlay B. B., Steele-Mortimer O. (2005) The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 280, 9058–9064 [DOI] [PubMed] [Google Scholar]
- 27. Chiu H. C., Kulp S. K., Soni S., Wang D., Gunn J. S., Schlesinger L. S., Chen C. S. (2009) Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob. Agents Chemother. 53, 5236–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scott C. C., Dobson W., Botelho R. J., Coady-Osberg N., Chavrier P., Knecht D. A., Heath C., Stahl P., Grinstein S. (2005) Phosphatidylinositol 4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J. Cell Biol. 169, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wells C. M., Abo A., Ridley A. J. (2002) PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J. Cell Sci. 115, 3947–3956 [DOI] [PubMed] [Google Scholar]
- 30. Smith A. C., Heo W. D., Braun V., Jiang X., Macrae C., Casanova J. E., Scidmore M. A., Grinstein S., Meyer T., Brumell J. H. (2007) A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J. Cell Biol. 176, 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kyei G. B., Vergne I., Chua J., Roberts E., Harris J., Junutula J. R., Deretic V. (2006) Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. EMBO J. 25, 5250–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]