Background: The acyltransferase Porcupine (Porcn) is essential for active Wnt ligand production and is chemically tractable.
Results: Novel small molecules targeting Porcn enables interrogation of Wnt signaling in vitro and in vivo.
Conclusion: Porcn is highly druggable and supports diverse cellular responses in embryonic development and regeneration.
Significance: Porcn inhibitors represent versatile chemical probes for Wnt signaling in vivo and are potential anti-cancer therapeutic agents.
Keywords: Chemical Biology, Development, Protein Palmitoylation, Signal Transduction, Wnt Signaling
Abstract
Secreted Wnt proteins constitute one of the largest families of intercellular signaling molecules in vertebrates with essential roles in embryonic development and adult tissue homeostasis. The functional redundancy of Wnt genes and the many forms of cellular responses they elicit, including some utilizing the transcriptional co-activator β-catenin, has limited the ability of classical genetic strategies to uncover their roles in vivo. We had previously identified a chemical compound class termed Inhibitor of Wnt Production (or IWP) that targets Porcupine (Porcn), an acyltransferase catalyzing the addition of fatty acid adducts onto Wnt proteins. Here we demonstrate that diverse chemical structures are able to inhibit Porcn by targeting its putative active site. When deployed in concert with small molecules that modulate the activity of Tankyrase enzymes and glycogen synthase kinase 3 β (GSK3β), additional transducers of Wnt/β-catenin signaling, the IWP compounds reveal an essential role for Wnt protein fatty acylation in eliciting β-catenin-dependent and -independent forms of Wnt signaling during zebrafish development. This collection of small molecules facilitates rapid dissection of Wnt gene function in vivo by limiting the influence of redundant Wnt gene functions on phenotypic outcomes and enables temporal manipulation of Wnt-mediated signaling in vertebrates.
Introduction
The evolutionary elaboration of gene families in complex multicellular animals provides diverse instructive cellular cues based on single signaling modalities and safeguards against genetic insults. During development, members of the Wnt family of signaling molecules (19 in all) contribute to almost all aspects of vertebrate development through induction of unique and shared cellular responses (1, 2). The interrogation of such complex signaling systems in vivo frequently necessitates experimental strategies for tissue-specific gene targeting to deconvolute complex phenotypes, temporally controlled gene ablation to overcome embryonic lethality, or gene family analysis to circumvent genetic redundancy-related issues. Chemically based strategies are ideally suited for studying the molecular basis of complex biological phenomena given the potential of small molecules to overcome some of these limitations.
Previously, we had described two classes of small molecules that disengage Wnt-mediated signaling (3). The Inhibitor of Wnt Response (IWR)5 compounds target the Tankyrase (Tnks) enzymes that regulate Axin protein turnover, scaffolding molecules in the β-catenin destruction complex (3, 4). In the absence of Tnks activity, Axin proteins accumulate and accelerate the rate of β-catenin destruction thereby reducing the transcriptional activity of the TCF/LEF family of DNA-binding proteins. On the other hand, the Inhibitor of Wnt Production (IWP) compounds disrupt Wnt signaling by preventing Porcn-dependent lipidation of Wnt proteins. Porcn is the founding member of the membrane-bound O-acyltransferase (MBOAT) family that consists of 16 members (5). Several of these MBOAT proteins like Porcn have recognized protein substrates. Likely because of their limited bioavailability, the IWP compounds did not exhibit in vivo activity in contrast to the IWR compounds (3). Instead, the IWP compounds have been extensively used in a variety of in vitro settings for tissue engineering and stem cell biology (6–8).
To expand the utility of Porcn inhibitors to include in vivo studies we have identified additional Porcn compounds from screening a small collection of Wnt pathway inhibitors with no previously assigned target. We demonstrate that all of these compounds directly engage Porcn at its putative active site thus revealing Porcn to be a highly druggable enzyme. Using one of these novel Porcn inhibitors (IWP12) in concert with other Wnt pathway modulators, we provide evidence for Wnt protein lipidation in promoting diverse Wnt-mediated responses in development and tissue regeneration, and establish a chemical toolkit for interrogating Wnt signaling mechanisms in these contexts.
EXPERIMENTAL PROCEDURES
Reagents
Antibodies purchased from the following sources: Santa Cruz Biotechnology (Myc-9E10), Bethyl Laboratories (Human IgG-Fc), Cell Signaling Technology (Dvl2, Lrp6-C5C7, pJnk Thr183/Tyr185), and Sigma (Kif3a). The University of Texas Southwestern Medical Center chemical library is assembled from ChemDiv, ChemBridge, ComGenex, Prestwick, and TimT3k collections. C16 ω-alkynyl fatty acid (alkynyl-PA) was synthesized as previously described (9). Biotin-azide and buffers required for click chemistry were purchased from Invitrogen. Membrane fractionation buffer made from 10 mm HEPES, 10 mm KCl, 1.5 mm MgCl2, 1 mm Na-EDTA, and 250 mm sucrose in water, pH 7.4 Membrane solubilization buffer consisted of 100 mm MES, 20 mm NaCl, 1 mm DTT, 0.2 mm EDTA, 0.05% TX-100, 0.2% glycerol and 0.15% octylglucoside, pH 6.5. PL buffer contained 10 mm Tris-HCl, 150 mm NaCl, pH 7.5. pCMV-GLuc control plasmid from New England Biolabs. Hhat and Goat constructs were a generous gift from Mike Brown and Joe Goldstein. To generate Gaussia luciferase (GL) fusion proteins, GL lacking its signaling sequence was cloned into pcDNA3.1 and then cDNAs encoding various Wnt proteins subsequently ligated in-frame. PCR-based site-directed mutagenesis was used to generate Porcn H335D and H335L.
Luciferase Reporter Assays
Wnt-Gaussia luciferase secretion and SuperTopFlash assays were conducted as described using a Dual Luciferase kit (Promega) (3).
Flow Cytometry
The indicated constructs were introduced into COS1 or HEK293 cells via Fugene6 transfection (Roche), 6 well format, and expressed for 48 h. New media containing 100 nm IWP-Cy3 and an IWP (15 mm) or DMSO was added for 12 h. Following 3× PBS washes, cells were trypsinized, pelleted, resuspended in cold PBS, and kept on ice.
The gate for IWP-Cy3 cells was defined as the region excluding the bulk population of cells labeled in control DNA transfected cells. Cells diverging from the SSC/FSC primary population were excluded from analysis. Flow cytometry was carried out with a FACS Calibur (BD Biosciences) and data analyzed on Cell Quest Pro (BD Biosciences).
Click Chemistry
HEK293 cells transiently transfected with the Wnt3A-Fc DNA expression construct were treated with C16 ω-alkynyl fatty acid (see “Reagents”; 100 μm final concentration) for 6 h as previously described (9) in the presence or DMSO or various IWP compounds. C16 ω-alkynyl fatty acid-labeled Wnt3A-Fc protein isolated from cell lysate using Protein A-Sepharose was then subjected to a copper catalyzed alkyne-azide cycloaddition with biotin-azide with protein immobilized on the Sepharose. The biotinylated Wnt protein run on SDS-PAGE was detected using HRP-conjugated streptavidin.
Organotypic Kidney Culture
E11.5 urogenital systems were removed and bisected in sterile phosphate-buffered saline (PBS), and then the individual halves were cultured in 350 ml of media at the air-media interface on 24-well tissue culture treated, 6.5 mm diameter, 8.0 mm pore size Transwell filters (Corning, catalogue no. 3422). The media (DMEM with 10% fetal bovine serum (FBS) and Pen/Strep) was supplemented with either DMSO or IWP2 and replaced with fresh media every 12, 24, or 48 h. All treatments were repeated at least three times with a minimum of six individual kidneys from six distinct embryos each time.
Zebrafish Studies
All zebrafish experiments were performed in accordance to regulatory standards as accepted by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Southwestern Medical Center. To determine a comparable concentration of IWP12 and IWR1 in zebrafish, 7× TCF-siam:EGFP embryos at 4 h after fertilization, expressing EGFP under the control of seven TCF binding elements and a siamois minimal promoter were incubated with E3 medium (5 mm NaCl, 0.17 mm KCl, 0.33 mm CaCl2, 0.33 mm MgSO4) containing DMSO, IWR1, IWP12, and/or GSK3β inhibitor 1 (Calbiochem) for 20 h and subsequently EGFP signals quantified by measuring pixel density from the embryo pictures. Three different pictures of the embryos were taken and pixel numbers were measured by ImageJ software. For caudal fin regeneration assay, zebrafish larvae at 3 days after fertilization were anesthetized in 0.02% (v/v) Tricaine, and half of the fins resected using a razor blade. Subsequently, the larvae were reared at 28 °C in E3 medium containing DMSO or IWR1 (10 μm) or IWP-12 (50 μm) for an additional 4 days. Whole-mount in situ hybridization was performed at 10 h after fertilization with digoxigenin-labeled antisense RNA probes generated against dlx3b, ntl, and ctsl1b/hgg1. Whole-mount in situ hybridization was performed at 24 h after fertilization with eng1a. Primers used for generating in situ probes: dlx3b forward: 5′-CAACA GAGGGAGTGTGAGAAAGC dlx3b reverse: 5′-AACCTCGCCGTTCTTGTAAAGC ntl forward: 5′-GAATGAAGAGATTACCGCTCTG ntl reverse: 5′-CCAAGATCAAGTCCATAACTGC ctsl1b/(hgg1) forward: 5′-TGATGTTTGCTTTGCTCGTCAC ctsl1b/(hgg1) reverse: 5′-GAACTGTAGGGATTGATGTGATGC eng1a forward: 5′-GGAGGGCAGGACTGATCTCTG eng1a reverse: 5′-GCGTAATATAGGCTACAACACC.
Zebrafish embryonic cell cultures were initiated from embryos at the shield stage (6 hpf). The embryos were dissociated in trypsin/EDTA solution with gentle homogenization and pipetting. After centrifugation, the collected cells were resuspended in F12/L15/DMEM medium and placed into a 24-well tissue culture plate.
RESULTS
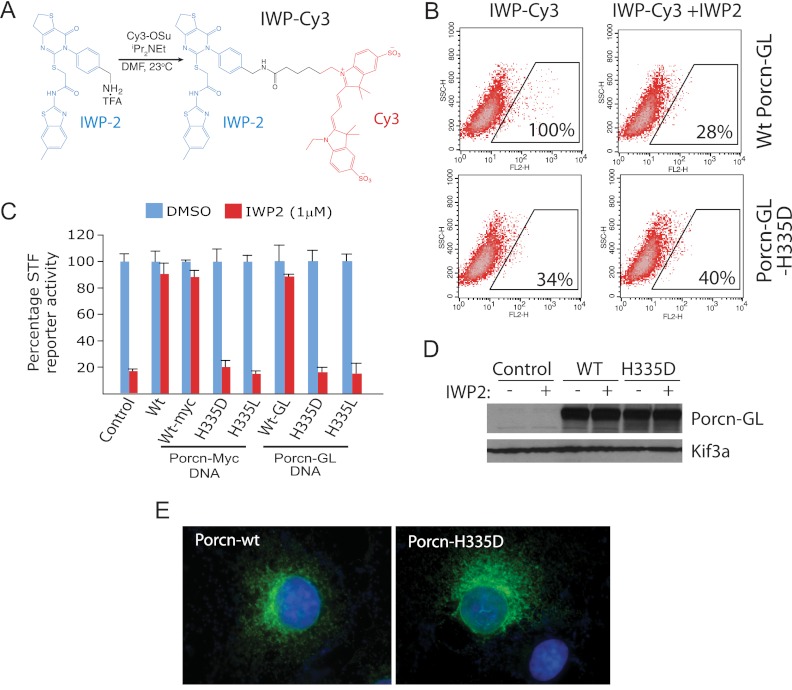
To better understand the interaction between IWP compounds and Porcn, we generated a fluorescently labeled reagent based on the IWP2 scaffold (IWP-Cy3; Fig. 1A) that enabled detection of IWP compound association with Porcn-transfected cells. Whereas wild-type Porcn expression correlated with IWP-Cy3 labeling, an inactive Porcn protein harboring a mutation in a highly conserved and presumed active site residue was unable to bind IWP-Cy3 (Fig. 1, B and C) (10, 11). Protein expression levels and intracellular localization patterns were nevertheless comparable for both proteins (Fig. 1, D and E).
FIGURE 1.
The IWP compounds directly attack Porcn. A, synthesis of a fluorescently labeled Porcn inhibitor. The IWP2 molecule was modified as shown with a linker and Cy3 adduct to generate an IWP-Cy3 fluorescently labeled probe. B, Porcn mutant with an altered putative active site residue (Porcn H335D) does not engage IWP-Cy3. Wild type Porcn or Porcn H335D sequence fused to Gaussia luciferase DNA (to stabilize the Porcn H335D protein) was transfected into COS1 cells. IWP binding to Porcn proteins was assessed by treating transfected cells with IWP-Cy3 and scoring the number of Cy3-positive cells in each experiment. Percentages shown are relative to control (wild type Porcn-GL-transfected cells). Competition with unlabeled IWP2 serves as a specificity control for IWP-Cy3 binding. C, Porcn fusion proteins exhibit comparable activity to unmodified Porcn protein. The ability of various Porcn proteins to counter the effects of IWP2 on Wnt/β-catenin pathway response was monitored using the SuperTopFlash (STF) reporter in HEK293 cells. Both wild-type Porcn and mutant proteins harboring a putative active site alteration (histidine 335) exhibited comparable activity in this assay regardless of whether GL or Myc epitope were appended to the Porcn C terminus. D, expression levels of wild-type and H335D Porcn are comparable. Western blot analysis of wt and H335D Porcn in COS1 cells in the presence or absence of IWP2. Kif3A serves as a loading control. E, Porcn and Porcn-H335D predominantly exhibit intracellular subcellular localization. Porcn-and Porcn-H335D-GL proteins exhibit a reticular expression pattern consistent with previous assignment of Porcn localization to the endoplasmic reticulum.
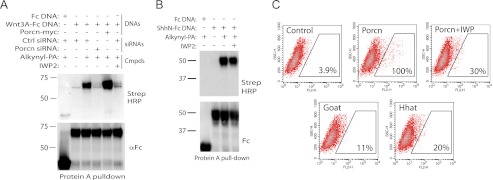
Using click chemistry technology, we confirmed that IWP2 disrupts Wnt protein acylation (Fig. 2A). We also demonstrated that IWP2 does not block fatty acylation of the related Hh signaling molecule as mediated by Hhat, another MBOAT family member (Fig. 2B). Consistent with the specificity of IWP2 action, cells expressing Porcn-related MBOAT family members (Goat or Hhat) were not labeled with IWP-Cy3 (Fig. 2C). Furthermore, we have previously demonstrated that IWP2 does not block general protein secretion or cellular responses mediated by the Hh and Notch proteins (3).
FIGURE 2.
The IWP compounds specifically inhibit Porcn acyltransferase activity. A, IWP2 inhibits Wnt fatty acylation. Cells transfected with an expression construct expressing either a fusion molecule consisting of Wnt3A and the Fc region of human IgG (Wnt3A-Fc) or IgG-Fc (Fc) alone are treated with C16 ω-alkynyl fatty acid (alkynyl-PA). Purified alkynyl-PA-labeled fusion protein bound to protein A-Sepharose is treated with biotin-azide reagent which enables protein detection using streptavidin-HRP. RNAi-mediated knock-down or overexpression of Porcn respectively results in loss or increase in Wnt3A-Fc protein labeling with alkynyl-PA. IWP2 is able to block the labeling of Wnt3A. B, IWP2 does not inhibit Hh fatty acylation. The same click chemistry strategy is used to monitor fatty acylation of Hh protein. C, IWP-Cy3 specifically binds to Porcn. COS-1 cells overexpressing Porcn or other members of the MBOAT family with recognized protein substrates (HHAT and GOAT) were treated with IWP-Cy3 and then gated for Cy3 staining. The number of IWP-Cy3-associated cells was scored as before.
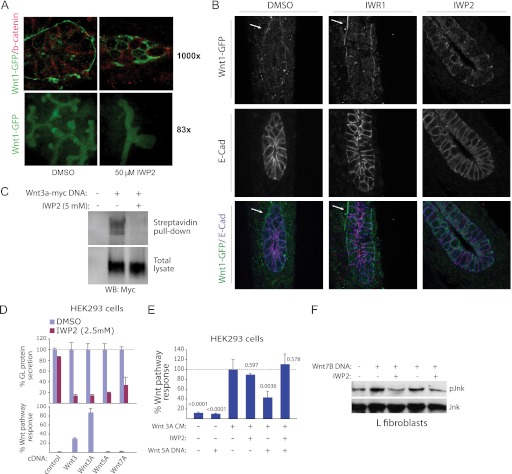
The transport of Wnt proteins through the secretory pathway relies upon the chaperone protein Wntless (Wls), which binds only to Wnt proteins lipidated on a conserved serine residue (12, 13). Using in vitro cultured embryonic kidney tissue derived from Wnt1-GFP expressing transgenic mice, we demonstrated that IWP2 can block the accumulation of Wnt1 on the cell surface in contrast to tissue treated with DMSO or another class of Wnt pathway inhibitors targeting the Tnks enzymes (IWR compounds) (Fig. 3, A and B). IWP2 also disrupted tubule induction, a Wnt/β-catenin-dependent process (14) (see Fig. 3A). Cell surface accumulation of another Wnt protein (Wnt3a) is also decreased in cultured cells treated with IWP2 (Fig. 3C).
FIGURE 3.
The IWP compounds target both β-catenin-dependent and -independent Wnt pathway responses. A, IWP2 inhibits the secretion of Wnt1 protein in embryonic kidneys. Urogenital systems from E11.5 mice expressing Wnt1-GFP were removed, bisected, and treated with either DMSO or IWP2 in vitro for 24 h. A cross section of a ureteric bud was analyzed for Wnt1-GFP expression (top panels). The effect of IWP2 on branching morphogenesis in the kidney was assayed using Wnt1-GFP protein to outline the ureteric buds (bottom panels). IWP2 markedly inhibited branching morphogenesis. B, similar experiment as in A except cellular boundaries were revealed by E-cadherin staining. White arrows indicate representative Wnt1-GFP cell surface staining. C, IWP inhibits the accumulation of Wnt3A on the cell surface in cultured cells. COS1 cells transiently expressing Wnt3A-myc were treated for 48 h in the presence or absence of IWP prior to cell surface biotinylation using a cell-impermeable labeling agent. Amounts of cell surface Wnt3A-myc protein was then determined by comparing total and streptavidin agarose-precipitated Wnt3A protein by Western blot analysis. D, IWP2 inhibits the secretion of Wnt proteins regardless of their ability to induce transcriptional responses in HEK293 cells. The secretion of several Wnt-GL fusion proteins introduced by DNA transfection into HEK293 cells was tested for sensitivity to IWP2 (top). In parallel, the ability of the same Wnt molecules lacking GL to activate Wnt/β-catenin pathway response as measured using the STF reporter was determined (bottom). Data are mean ± S.E. from three measurements. E, evidence that IWP2 inhibits the production of a non-canonical Wnt (Wnt5A). Antagonism of Wnt/β-catenin signaling by expression of Wnt5A in the presence or absence of IWP compound was determined in HEK293 cells transfected with the STF reporter and treated with Wnt3A-containing conditioned medium. STF activity was normalized to the activity of a co-transfected control reporter. Data are mean ± S.E. from three measurements. p values for change from control response are indicated. F, IWP inhibits Wnt-dependent activation of Jnk. Mouse L fibroblasts transfected with Wnt7B DNA induce IWP-sensitive phosphorylation of Jnk, a target of multiple β-catenin-independent Wnt pathways.
The addition of IWP2 to cells expressing one of several Wnt proteins, including those unable to elicit Wnt/β-catenin pathway response, abrogated their accumulation in the cell culture medium consistent with a general role for Porcn in the production of Wnt proteins (Fig. 3D). We demonstrate that this blockade in protein maturation correlates with loss of non-canonical Wnt activity using an assay that measures Wnt5a-dependent antagonism of canonical Wnt pathway response (15) (Fig. 3E). Additionally, activation of the non-canonical Wnt pathway effector Jnk in fibroblasts is disrupted by IWP2 (Fig. 3F). Taken together, these observations support a general role for lipidation in the maturation of Wnt family members and the utility of IWP2 for interrogating diverse forms of Wnt-mediated cellular responses.
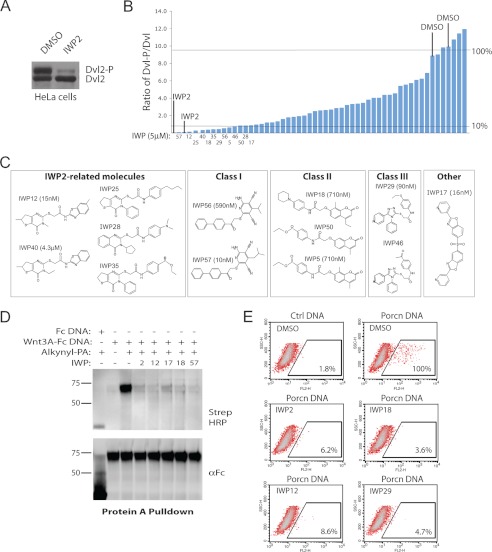
The same chemical library screen that yielded IWP2 also uncovered ∼50 other molecules with potential activity against Wnt protein production (3). In addition to previous studies demonstrating that all of these compounds exhibit activity for Wnt- but not Hh- or Notch-dependent signaling (3), we biochemically validated the Wnt-inhibitory activity of these chemicals in HeLa cells that exhibit elevated levels of cell autonomous Wnt signaling (16) (Fig. 4A). With the exception of five compounds, all other putative Wnt inhibitors blocked Wnt-induced phosphorylation of Dvl, a signaling molecule directly activated by the Frizzled family of Wnt receptors (17) (Fig. 4B).
FIGURE 4.
Diverse chemical scaffolds support Porcn inhibition by targeting the putative active site. A, Dvl2 phosphorylation status in HeLa cells reflects Porcn activity. IWP2 inhibits Dvl2 phosphorylation in HeLa cells indicating cell-autonomous Wnt-mediated signaling in these cells as previously described. B, identification of additional Porcn inhibitors. The IWP compound collection of Wnt/β-catenin pathway inhibitors was tested for their ability to inhibit Dvl2 phosphorylation in HeLa cells. The ratio of phosphorylated to unphosphorylated Dvl protein in cells treated with each IWP compound was determined by densitometric analysis of Western blot results as shown in A. Compounds inhibiting 90% or more of Dvl phosphorylation are labeled. C, shared chemical scaffolds yielding the most active IWP molecules. Compounds are clustered based on their similarity to IWP2 or shared chemical structures. IC50 against Wnt/β-catenin pathway response as measured by STF is provided for at least one representative compound from each class. D, novel IWP compounds disrupt Wnt protein acylation. Wnt3A-Fc protein from cells treated with alkynyl-PA in the presence of indicated IWP compound or DMSO was subjected to an alkyne cycloaddition reaction to label fatty acylated Wnt3A with biotin. Biotinylated protein separated on SDS-PAGE was visualized with streptavidin HRP. E, novel Porcn inhibitors likely bind directly to Porcn. The ability of indicated IWP compounds to compete for IWP-Cy3 binding to Porcn was determined as before.
Organizing the top twelve compounds based upon their similarity to IWP2 (or otherwise shared chemical scaffolds) revealed four distinct chemical classes capable of specifically inhibiting Wnt/β-catenin transcriptional response (see Ref. 3) by targeting a component functioning upstream of Dvl, presumably at the level of Wnt protein production (Fig. 4C). Representative molecules from the different classes, which are structurally distinct from IWP2 class compounds, likely function as Porcn inhibitors given their ability to inhibit Wnt fatty acylation as determined using the click chemistry strategy, and to compete with IWP-Cy3 binding for Porcn (Fig. 4, D and E). Thus, these diverse chemical structures likely engage the same protein pocket in Porcn to disrupt its activity.
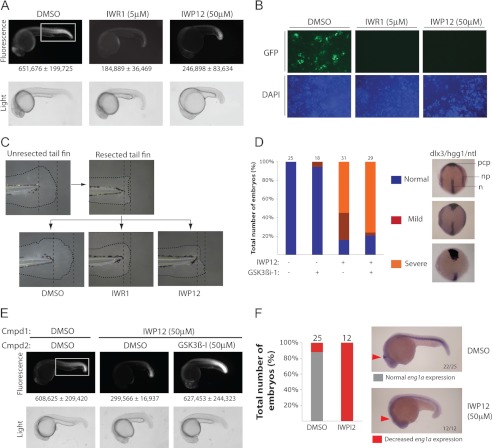
Despite earlier successes in achieving chemical targeting of Wnt/β-catenin signaling in zebrafish using the IWR class of Tnks inhibitors (3, 18), we were previously unable to demonstrate similar activity using Porcn inhibitors, possibly as a result of poor bioavailability. Evaluating the ability of several new IWP compounds to inhibit in vivo Wnt-mediated response using a transgenic zebrafish line harboring a Wnt-responsive GFP reporter (7XTCF-siam:EGFP) (19), we uncovered a loss of Wnt signaling activity in animals treated with IWP12 (Fig. 5A). Cultured embryonic fibroblasts from the same transgenic line also revealed loss of Wnt/β-catenin pathway responses when treated with an IWR compound or IWP12 (Fig. 5B). Accordingly, IWP12 was able to block juvenile fish tailfin regeneration following resection, a Wnt/β-catenin pathway-dependent process (3, 20) (Fig. 5C). The weaker Wnt/β-catenin signaling inhibitory activity observed with IWP12 as compared with IWR1 was nevertheless associated with a severe effect on posterior body morphogenesis, possibly signifying additional effects of Porcn disruption on non-canonical Wnt signaling (21) (see Fig. 5A).
FIGURE 5.
Concerted deployment of IWP and IWR compounds distinguishes β-catenin-dependent and -independent responses in vivo. A, identification of an IWP compound with in vivo activity in zebrafish. IWP12 inhibits the expression of an EGFP fluorescent protein reporter driven by synthetic TCF-binding elements in a transgenic line (Tg(7xTCF-Xla.Siam:GFP)ia4]. A ∼10-fold excess of IWP12 is equivalent in activity to IWR1 compound. Fluorescence intensity was quantified (below) in an area that covers most of the posterior region (box). Data are mean ± S.E. from three animals. B, IWR and IWP compounds inhibit Wnt signaling in zebrafish primary embryonic fibroblasts. Embryonic fibroblasts isolated from 6 hpf Tg(7xTCF-Xla.Siam:GFP)ia4 embryos were cultured in the presence or absence of indicated compound. GFP expression was visualized 20 h later. C, IWP compounds inhibit tailfin regeneration, a Wnt-dependent process. Tailfins of zebrafish larvae at 3 days post fertilization were resected and the larvae subsequently reared in medium containing DMSO, IWR1 (10 μm), or IWP12 (50 μm) for an additional 4 days. D, IWP12 inhibits embryonic convergent extension by targeting β-catenin-independent Wnt signaling. Zebrafish embryos were treated with GSK3β inhibitor (a Wnt/β-catenin pathway activator), IWP12 compound, or both starting 4 hpf followed by whole mount in situ analysis at 24 hpf with probes and the respective developmental structures they label indicated: hgg1 (ctsl1b) [prechordal plate (pcp)], ntl [prospective notochord (n) and germ ring blastopore margin], and dlx3b [anterior edge of the neural plate (np)]. Changes in the distance between the neural plates and prechordal plates, as well as the notochord were quantified based upon the severity of the phenotype as represented. Number of animals examined under each condition is indicated above each plot. E, inactivation of GSK3β rescues Wnt/β-catenin pathway activity in animals treated with IWP12. F, engrailed expression in the midbrain-hindbrain boundary (MHB) is suppressed by chemical inhibition of Porcn. Zebrafish embryos (4 hpf) treated with IWP12 for 20 h were subjected to in situ analysis with a probe for eng1a. Number of animals examined in each condition is indicated within each plot.
Whereas the role of Wnt lipidation during Wnt/β-catenin signaling is well validated, its contribution to β-catenin-independent Wnt cellular responses is unclear (22). Based on our in vitro and in vivo results, we anticipated that IWP12 may be useful for studying these other forms of Wnt signaling in vivo. Indeed, IWP12 was able to block convergence and extension gastrulation movements, a process dependent upon Wnt-planar cell polarity (Wnt/PCP) signaling (23, 24) (Fig. 5D). This defect was not rescued by the addition of a GSK3β inhibitor (GSK3β inhibitor 1 or GSK3βi-1), a molecule that blocks β-catenin destruction and reverses the effects of IWP12 on Wnt/β-catenin pathway activity (Fig. 5E). These observations taken together are consistent with a biosynthetic role for Wnt protein lipidation and β-catenin-independent Wnt-mediated development processes.
In addition to the complexities of phenotypic analysis associated with overlapping roles of various genes in β-catenin-dependent and -independent Wnt signaling, the presence of multiple Wnt proteins in vertebrates has limited our ability to recognize Wnt functions in developmental processes. We demonstrated the utility of a chemically based approach to reveal a role for Wnt-dependent signaling in midbrain-hindbrain boundary (MHB) formation, a process previously shown to be coordinated by three Wnt proteins with overlapping functions: Wnt1, Wnt3A, and Wnt10B (25). Similar to animals lacking all three genes that fail to develop the MHB constriction, embryos treated with IWP12 exhibited decreased expression of the MHB marker Engrailed (eng1a) (26) (Fig. 5F). We anticipate that additional functions of this large family of signaling molecules in vertebrate development could be readily uncovered by limiting the influence of gene redundancy on phenotypic outcomes using this chemically based strategy.
DISCUSSION
Our study reveals Porcn to be a chemical vulnerability in multiple Wnt signaling processes including those governing β-catenin-independent events such as Wnt/PCP signaling. This vulnerability forms the basis of a chemical strategy described herein for probing the participation of different forms of Wnt signaling in vivo. These Porcn inhibitors combined with Tnks and GSK3β antagonists should facilitate the systematic identification of Wnt-dependent cellular processes in vertebrate embryogenesis and tissue regeneration not readily achievable with classical genetic approaches.
The shared sensitivity of Wnt proteins that mediate different cellular responses to Porcn inhibitors are consistent with previous findings that implicate Porcn activity to be essential to the production of most if not all Wnt proteins (27, 28). Furthermore, this observation suggests that the Wnt chaperone Wls, which binds to fatty acylated Wnt proteins, is similarly required for the production of most if not all Wnt proteins. Yet, the dependence of individual Wnt activities upon Porcn may vary as a consequence of differences in: (a) the ligand dose required to engage cellular responses, (b) the determinants that promote ER retention of non-acylated Wnt proteins, and (c) the participation of other acyltransferases that may modify Wnt proteins. These possibilities could in part contribute to previously observed differences in assignment of Porcn function to various Wnt-dependent processes (10, 22, 29). Evidence that at least some Wnt proteins may also harbor monounsaturated fatty acid modifications suggests that specific Wnt functions may also be dictated by a complex fatty acyl code that could be better understood with the aid of the chemical tools described here (30, 31).
Porcn exhibits an ability to accommodate diverse chemical inhibitors, potentially indicating an abundance of opportunities for the refinement of IWP compounds as chemical probes and therapeutic agents. Given that crystallographically guided clinical development of small molecules will not likely be forthcoming for Porcn inhibitors due to the polytopic nature of Porcn, the chemical portfolio described here should improve our understanding of how these molecules achieve Porcn inhibitory activity and how they can be evolved for clinical use. Porcn is a founding member of a large protein family with roles in the production of other important signaling molecules such as the cell-fate determination molecule Hedgehog and the appetite-controlling hormone Ghrelin (5, 32). Thus, our findings should also facilitate the development of small molecules targeting other important signaling processes relevant to disease.
Acknowledgments
We thank Mike Brown and Joe Goldstein (UT Southwestern Medical Center, Dallas, TX) for reagents.
This work was supported, in whole or in part, by National Institutes of Health Grants 5R21HD061303 (to J. F. A., C. C., and L. L.), R01DK080004 & P30DK079328 (to T. C.), Cancer Prevention and Research Institute of Texas (RP100119; to L. L. and C. C.), and the Welch Foundation (I-1665; to L. L.), a CARIPARO grant (to F. A.), the Ministry of Health (Grant GR-2008-1139743; to E. M.), and the Mary Kay Ash Foundation.
- IWR
- Inhibitor of Wnt Response
- Tnk
- Tankyrase
- IWP
- Inhibitor of Wnt Production
- Porcn
- Porcupine
- MBOAT
- membrane-bound O-acyltransferase
- GL
- Gaussia luciferase
- MHB
- midbrain-hindbrain boundary.
REFERENCES
- 1. Angers S., Moon R. T. (2009) Proximal events in Wnt signal transduction. Nature Reviews 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 2. van Amerongen R., Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 [DOI] [PubMed] [Google Scholar]
- 3. Chen B., Dodge M. E., Tang W., Lu J., Ma Z., Fan C. W., Wei S., Hao W., Kilgore J., Williams N. S., Roth M. G., Amatruda J. F., Chen C., Lum L. (2009) Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang H., He X. (2008) Wnt/beta-catein signaling: new (and old) players and new insights. Curr. Opin. Cell Biol. 20, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang J., Brown M. S., Liang G., Grishin N. V., Goldstein J. L. (2008) Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132, 387–396 [DOI] [PubMed] [Google Scholar]
- 6. Ren Y., Lee M. Y., Schliffke S., Paavola J., Amos P. J., Ge X., Ye M., Zhu S., Senyei G., Lum L., Ehrlich B. E., Qyang Y. (2011) Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J. Mol. Cell Cardiol. 51, 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M., Clevers H. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ten Berge D., Kurek D., Blauwkamp T., Koole W., Maas A., Eroglu E., Siu R. K., Nusse R. (2011) Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 13, 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao X., Arenas-Ramirez N., Scales S. J., Hannoush R. N. (2011) Membrane targeting of palmitoylated Wnt and Hedgehog revealed by chemical probes. FEBS Lett. 585, 2501–2506 [DOI] [PubMed] [Google Scholar]
- 10. Barrott J. J., Cash G. M., Smith A. P., Barrow J. R., Murtaugh L. C. (2011) Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc. Natl. Acad. Sci. U.S.A. 108, 12752–12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galli L. M., Barnes T. L., Secrest S. S., Kadowaki T., Burrus L. W. (2007) Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development 134, 3339–3348 [DOI] [PubMed] [Google Scholar]
- 12. Coombs G. S., Yu J., Canning C. A., Veltri C. A., Covey T. M., Cheong J. K., Utomo V., Banerjee N., Zhang Z. H., Jadulco R. C., Concepcion G. P., Bugni T. S., Harper M. K., Mihalek I., Jones C. M., Ireland C. M., Virshup D. M. (2010) WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J. Cell Science 123, 3357–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herr P., Basler K. (2012) Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev. Biol. 361, 392–402 [DOI] [PubMed] [Google Scholar]
- 14. Merkel C. E., Karner C. M., Carroll T. J. (2007) Molecular regulation of kidney development: is the answer blowing in the Wnt? Pediatric Nephrology 22, 1825–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J. M., Kim I. S., Kim H., Lee J. S., Kim K., Yim H. Y., Jeong J., Kim J. H., Kim J. Y., Lee H., Seo S. B., Rosenfeld M. G., Kim K. I., Baek S. H. (2010) RORα attenuates Wnt/β-catenin signaling by PKCα-dependent phosphorylation in colon cancer. Mol. Cell 37, 183–195 [DOI] [PubMed] [Google Scholar]
- 16. Jacob L. S., Wu X., Dodge M. E., Fan C. W., Kulak O., Chen B., Tang W., Wang B., Amatruda J. F., Lum L. (2011) Genome-wide RNAi screen reveals disease-associated genes that are common to Hedgehog and Wnt signaling. Science Signaling 4, ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao C., Chen Y. G. (2010) Dishevelled: The hub of Wnt signaling. Cell Signal 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 18. Huang S. M., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C. J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M. W., Lengauer C., Finan P. M., Tallarico J. A., Bouwmeester T., Porter J. A., Bauer A., Cong F. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signaling. Nature 461, 614–620 [DOI] [PubMed] [Google Scholar]
- 19. Moro E., Ozhan-Kizil G., Mongera A., Beis D., Wierzbicki C., Young R. M., Bournele D., Domenichini A., Valdivia L. E., Lum L., Chen C., Amatruda J. F., Tiso N., Weidinger G., Argenton F. (2012) In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev. Biol. 366, 327–340 [DOI] [PubMed] [Google Scholar]
- 20. Stoick-Cooper C. L., Weidinger G., Riehle K. J., Hubbert C., Major M. B., Fausto N., Moon R. T. (2007) Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134, 479–489 [DOI] [PubMed] [Google Scholar]
- 21. Marlow F., Gonzalez E. M., Yin C., Rojo C., Solnica-Krezel L. (2004) No tail co-operates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development 131, 203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biechele S., Cox B. J., Rossant J. (2011) Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev. Biol. 355, 275–285 [DOI] [PubMed] [Google Scholar]
- 23. Roszko I., Sawada A., Solnica-Krezel L. (2009) Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Seminars Cell Dev. Biol. 20, 986–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sepich D. S., Usmani M., Pawlicki S., Solnica-Krezel L. (2011) Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development 138, 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buckles G. R., Thorpe C. J., Ramel M. C., Lekven A. C. (2004) Combinatorial Wnt control of zebrafish midbrain-hindbrain boundary formation. Mech. Dev. 121, 437–447 [DOI] [PubMed] [Google Scholar]
- 26. Ekker M., Wegner J., Akimenko M. A., Westerfield M. (1992) Coordinate embryonic expression of three zebrafish engrailed genes. Development 116, 1001–1010 [DOI] [PubMed] [Google Scholar]
- 27. Bartscherer K., Boutros M. (2008) Regulation of Wnt protein secretion and its role in gradient formation. EMBO Rep. 9, 977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Port F., Basler K. (2010) Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic 11, 1265–1271 [DOI] [PubMed] [Google Scholar]
- 29. Chen Q., Takada R., Takada S. (2012) Deficiency of Porcupine, an O-acyltransferase gene, impairs convergent extension during gastrulation in zebrafish embryos and does not affect equivalently the trafficking of different Wnt proteins. J. Cell Sci., in press [Google Scholar]
- 30. Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. (2006) Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell 11, 791–801 [DOI] [PubMed] [Google Scholar]
- 31. Mulligan K. A., Fuerer C., Ching W., Fish M., Willert K., Nusse R. (2012) Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility. Proc. Natl. Acad. Sci. U.S.A. 109, 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buglino J. A., Resh M. D. (2008) Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J. Biol. Chem. 283, 22076–22088 [DOI] [PMC free article] [PubMed] [Google Scholar]