Background: CDC48, a ubiquitin-dependent molecular chaperone, mediates a variety of degradative and regulatory processes.
Results: CDC48 deficiency produced lethal embryonic phenotypes, i.e. defects in neuronal outgrowth and neurodegeneration.
Conclusion: The abnormal phenotypes in the morphant were not rescued by the catalytically inactive CDC48 mutant that had two impaired ATPase domains.
Significance: CDC48 is essential for survival during neurodevelopment.
Keywords: Autophagy, Development, Molecular Chaperone, Neurodegeneration, Proteasome, Ubiquitination, Zebrafish, CDC48, VCP, p97
Abstract
Cell division cycle 48 (CDC48), a ubiquitin-dependent molecular chaperone, is thought to mediate a variety of degradative and regulatory processes and maintain cellular homoeostasis. To investigate the protective function of CDC48 against accumulated ubiquitinated proteins during neurodevelopment, we developed an in vivo bioassay technique that detects expression and accumulation of fluorescent proteins with a polyubiquitination signal at the N terminus. When we introduced CDC48 antisense morpholino oligonucleotides into zebrafish embryos, the morphant embryos were lethal and showed defects in neuronal outgrowth and neurodegeneration, and polyubiquitinated fluorescent proteins accumulated in the inner plexiform and ganglion cell layers, as well as the diencephalon and mesencephalon, indicating that the degradation of polyubiquitinated proteins by the ubiquitin-proteasome system was blocked. These abnormal phenotypes in the morphant were rescued by CDC48 or human valosin-containing protein overexpression. Therefore, the protective function of CDC48 is essential for neurodevelopment.
Introduction
Cell division cycle 48 (CDC48)2 was first identified in a cold-sensitive, cell division cycle mutant of yeast (1). CDC48/p97/valosin-containing protein (VCP) is involved in a wide variety of ATP-dependent cellular processes, such as cell cycle regulation, cell growth, cell death, membrane fusion, and transcriptional regulation (2). CDC48 is the major cold-inducible protein in fish cells and is thought to be responsible for a biochemical mechanism that compensates for reduced cellular processes, aiding survival in cold temperatures (3). A loss-of-function study using a mutant protein lacking a phosphorylation site at Tyr-805 in cultured zebrafish embryonic cells showed that CDC48 was essential for cell proliferation at a temperature of 15 °C (4). As this protein is highly expressed in the CNS in the zebrafish embryo (5), we focused on the significance of CDC48 in the neurodevelopment of the zebrafish embryo.
CDC48 is a regulator of the ubiquitin-proteasome system (UPS) that interacts with several components, including polyubiquitinated proteins, the 26 S proteasome subunits, and E3 ubiquitin ligases (6–8). RNAi depletion of CDC48 results in significant accumulation of polyubiquitinated proteins in Drosophila Schneider 2 and human HeLa cells (9). Thus, CDC48 is thought to act as a molecular chaperone in the UPS and enhance the degradation of unfolded proteins during endoplasmic reticulum (ER)-associated degradation (ERAD) (10–12). CDC48 forms a complex with p47, and the ubiquitin fusion degradation 1 (Ufd1)-nuclear protein localization 4 (Npl4) heterodimer (13) is involved in the binding of polyubiquitinated substrates at the cytoplasmic face of the ER membrane and their transfer to the 26 S proteasome (10, 14, 15). The activity of C-terminal ATPases associated with a variety of cellular activities (AAA) is required for ERAD in mammalian cells (16). Thus, CDC48 mediates the decomposition of N-end rule substrates through the ubiquitin-fusion degradation (UFD) pathway (17–24).
UPS substrates are misfolded proteins that fail ER quality control, such as N-end rule substrates and UFD substrates (25). To monitor the degradation pathways, YFP-based reporters, one ERAD substrate, and three cytosolic and nuclear reporters have been used in MelJuSo cells, HeLa cells, and primary mouse fibroblasts as well as in Drosophila (25, 26). However, these reporters have never been used in vertebrates in vivo.
The ter94 gene, which encodes the CDC48 Drosophila homolog, is a modifier of eye degeneration phenotypes induced by the expression of expanded-poly(Q) (27). Although CDC48 knock-out mice have been generated to reveal the function of this protein and its role in embryogenesis in mammals (28), the homozygous mutant is lethal to peri-implantation embryos; thus, the molecular functions of CDC48 during development have never been biochemically characterized.
CDC48 is highly expressed in the brain and other tissues (29), and CDC48 deficiency leads to cell death with accumulation of polyubiquitinated proteins and the formation of ER-derived vacuoles with ER stress (16, 29, 30). Undegraded proteins are thought to be intrinsically toxic to mice mutants (31). Hereditary inclusion body myopathy with Paget disease of the bone and frontotemporal dementia (IBMPFD) is a rare autosomal dominant disease caused by mutations in the CDC48 gene (32). Aggregated proteins such as β-amyloid, phosphorylated tau, and ubiquitin accumulate in patient tissues with IBMPFD (33). Studies of several transgenic mouse lines that carry the IBMPFD-causing CDC48 mutation have indicated that the accumulation of ubiquitinated proteins in CDC48 mutants induces neuropathy and muscle pathology at 6 months (31). All mutations causing IBMPFD have been identified in the N-terminal domain and D1 AAA domain, which are involved in substrate and cofactor association (32, 34). CDC48, containing the most prevalent IBMPFD-associated mutation, shows normal ATPase activity and a hexameric structure (35, 36). In the cells expressing mutants, ubiquitin-conjugated proteins increase and IBMPFD mutations in CDC48 disrupt the ERAD pathway in vivo (36). Although the IBMPFD mutant R155H retains ATPase activity, a disruption at the N and D1 domain may interfere with substrate release, leading to failure to efficiently release them into the UPS or aggresome (37). CDC48 ATPase activity is mainly mediated by the C-terminal AAA domain (D2) (38). D2 ATPase activity is required for ERAD and UFD (15), and D2 undergoes large conformational changes upon ATP hydrolysis, whereas D1 does not (38). Both the ERAD and UFD pathways are impaired by the expression of dominant negative (E305Q/E578Q) mutants. In contrast, expression of the IBMPFD mutation results in dysfunctional autophagosome accumulation, causing an autophagy defect in cultured human MelJuSo and U2OS TRex cells (39, 40), suggesting that CDC48 may be essential for clearing ubiquitinated proteins by autophagy (40). The molecular nature of CDC48 in the CNS during embryogenesis remains to be characterized with respect to UPS functions and neurodevelopment.
This study considered the in vivo functions of CDC48 in the UPS during zebrafish embryogenesis. Deficiency of CDC48 produced lethal embryonic phenotypes, i.e. defects in neuronal outgrowth and neurodegeneration, followed by the accumulation of polyubiquitinated proteins, the generation of reactive oxygen species (ROS), and extensive apoptosis in the CNS. Therefore, CDC48 appears to play a protective role by degrading ubiquitinated proteins during neurodevelopment in vivo.
EXPERIMENTAL PROCEDURES
Zebrafish Maintenance
Zebrafish (Danio rerio) were maintained under standard laboratory conditions of 28.5 °C and a 14-h light/10-h dark cycle (41). Embryos were staged in terms of hours postfertilization (hpf) at 28.5 °C based on morphological criteria (42).
Microinjection of Morpholino Oligonucleotide (MO) and cDNA into Zebrafish Embryos
Morpholino antisense oligonucleotides (Gene Tools, Philomath, OR) were used to knock down the expression of the CDC48 gene. The stock solution was diluted to 250 μm for injections. MOs were injected at 1.6, 3.2, and 8.0 ng/embryo at the yolk-cytoplasm interface of one-cell stage embryos. The following CDC48 MO sequences were used: for CDC48-MO1, TTT TGG ATT CTC CAC CCG AAG CCA T; for CDC48-MO2, TAG TTG ATG GAA ATG AGT AGC TCT C; and for cont-MO, TAC CGA AGC CCA CCT CTT AGG TTT T. The complementary sequence of the putative CDC48 ATG start site is underlined. The gene constructs encoding CDC48 mutant proteins, i.e. zCDC48-WT, zCDC48-Y805A (4), hVCP-WT, hVCP-R155H, hVCP-A232E, and hVCP-DN (40), were used to rescue the CDC48 morphants. These constructs were injected into embryos.
Plasmids
The YFP-based reporter substrates Ub-R-YFP, YFP-CL1, UbG76V-YFP, and CD3δ-YFP (25) were purchased from Addgene (Cambridge, MA). The Myc-His-tagged wild-type CDC48 and mutant CDC48 constructs were used for the rescue experiments. zCDC48-WT and zCDC48-Y805A were subcloned into the multiple cloning site downstream from the human cytomegalovirus (CMV) promoter of pcDNA3.1/Myc-His vector (Invitrogen), and added Myc tag and His tag sequences after the C terminus. hVCP-WT, hVCP-R155H, hVCP-A232E, and hVCP-DN were subcloned into pEGFP-N1 (Clontech) to fuse a EGFP tag after the C terminus. The four-cell stage embryos were injected into the yolk-cytoplasm interface with 4 ng of zCDC48-MO1 or cont-MO and 100 pg of CDC48 or blank plasmid constructs.
Western Blot Analysis
Dechorionated embryos were lysed, and Western blotting was performed. Antibodies against zCDC48 (1:2500 dilution) (4), and polyubiquitinated proteins (1:1000; Biomol International, L.P., Plymouth Meeting, PA) were used. Positive signals were detected using an enhanced chemiluminescence kit (GE Healthcare).
Immunohistochemistry
Immunohistochemistry was performed as described previously (43). The following antibodies were used: anti-znp-1 (1:100; Developmental Studies Hybridoma Bank, University of Iowa), anti-ubiquitin (1:100), and anti-zCDC48 (1:100). Alexa Fluor 488- and Alexa Fluor 596-conjugated secondary antibodies were used for fluorescence detection. Cross-sections of embryos were made at 5–10-μm thickness using a cryostat (CM1850; Leica Microsystems, Tokyo, Japan).
Histological Observation
Embryos at 56–86 hpf were fixed overnight in 4% paraformaldehyde (PFA), and then embedded in paraffin. Sections (4 μm in thickness) were stained with H&E.
Detection of Neurodegeneration
Neurodegeneration was detected by Fluoro-Jade C (Chemicon International, Temecula, CA) staining of neural cells, as described previously (44). Briefly, embryos were fixed overnight in 4% PFA and then incubated overnight in 4% PFA with 20% sucrose. Then the embryos were sectioned at 25-μm thickness using a cryostat, and the slides were dried at 50 °C for 1 h. The slides were immersed in 1% sodium hydroxide, 50% ethanol for 5 min, 70% ethanol for 2 min, distilled water for 2 min, and 0.06% potassium permanganate for 10 min. After rinsing in distilled water for 20 min, 0.0001% Fluoro-Jade C in 0.1% acetic acid was added for 20 min. The slides were washed three times with distilled water for 1 min, dried at 50 °C for 5 min, and mounted. Fluorescence was detected by fluorescence microscopy (BX51; Olympus, Tokyo, Japan). Nuclei were counterstained with 100 ng/ml 4′,6-diamidino-2-phenylindole in PBS.
Whole Mount in Situ Hybridization of Embryos
Digoxigenin (DIG)-labeled antisense RNA probes were synthesized from the full-length zCDC48 cDNA using a DIG RNA labeling kit for in situ hybridization (Roche Applied Science). Sense RNA-labeled probes were synthesized as a control. In situ hybridization of whole mount zebrafish embryos was performed as described previously (45). Briefly, prior to hybridization, the embryos were fixed in 4% PFA in PBS at 4 °C for 15 h. Before staining, the embryos were rehydrated for 10 min in a PBS with Tween 20 (PBS-T) series that contained 75, 50, and 25% methanol, followed by washing for 5 min in PBS-T. Hybridization with labeled RNA probes was carried out at 68 °C overnight in hybridization solution (50% formamide, 5× SSC buffer, 0.5% yeast RNA, 5% heparin, and 0.1% Tween 20). Embryos were incubated for 30 min at room temperature with an anti-DIG antibody (1:2000 dilution; Roche Applied Science). Colorimetric detection was performed using BM Purple substrate (Roche Applied Science). The reaction was stopped by washing in PBS, and the embryos were examined under a light microscope (SZX12; Olympus).
TUNEL Staining
Embryos at 19–72 hpf were fixed overnight in 4% PFA and stored in 100% methanol at −20 °C. Samples were incubated in acetone at −20 °C for 20 min and in 0.5% Triton X-100 and 0.1% sodium citrate in PBS for 15 min, and then treated with 5–50 μg/ml proteinase K (Invitrogen) for 5–25 min depending on the embryonic stage. After fixation, the embryos were subjected to the TUNEL assay using the ApopTag Red in situ apoptosis detection kit (Chemicon International), according to the manufacturer's instructions.
ROS Assay
For in vivo ROS detection, live embryos (2–4 days postfertilization (dpf)) were incubated in 5 μm CM-H2DCFDA (Invitrogen) for 1 h at 28.5 °C and then washed three times for 5 min with embryonic water, as described previously (46). Fluorescence was observed by fluorescent microscopy (BX51; Olympus) with excitation at 488 nm.
RESULTS
Genomic Localization and CDC48 Expression in Zebrafish
Gene synteny analysis in the zebrafish genome showed that CDC48 was localized between DnaJ (heat shock protein 40 kDa, Hsp40) homolog subfamily B member 5 and the gene for Fanconi anemia complementation group G on chromosome 5 (supplemental Fig. S1A). This gene synteny was also well conserved in the human genome and is defined in a 0.3-Mb region on chromosome 9p25.0.
Northern blot analysis showed that CDC48 was weakly expressed at 0 and 6 hpf and subsequently increased after 12 hpf. Whole mount in situ hybridization with a DIG-labeled antisense RNA probe showed that CDC48 mRNA was maternally and ubiquitously expressed during embryogenesis (supplemental Fig. S1B). When a sense control RNA probe was used, no signal was detected at any developmental time point tested (supplemental Fig. S1C). CDC48 expression was observed at 2–6 hpf (blastula stage) and was weakly detected between 6 hpf (gastrula stage) and 12 hpf (6-somite stage). After 12 hpf, CDC48 expression increased dramatically, and strong expression was observed in the eye and brain, and weak expression was detected in the muscle cells of the trunk.
Loss-of-function of CDC48: Developmental Retardation and Neurodegeneration Caused by CDC48 Deficiency
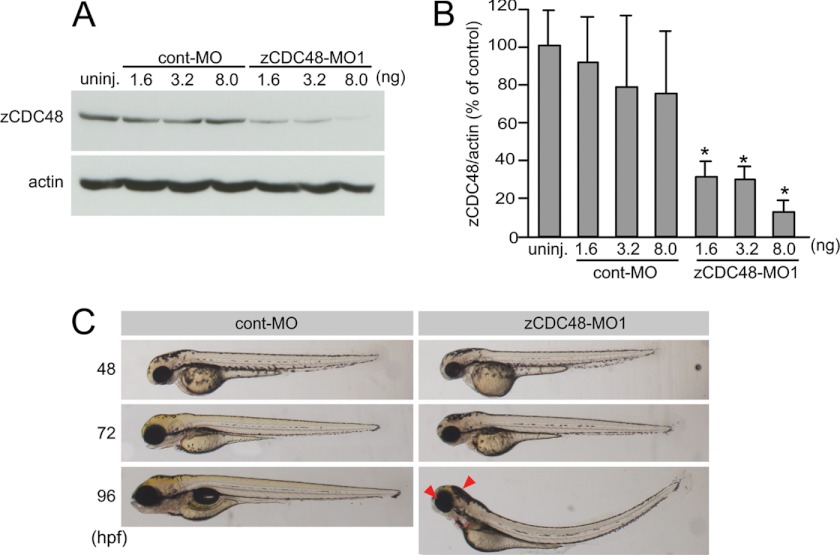
The MO knockdown technique was used, according to the methods of Nasevicius and Ekker (47), to induce CDC48 translational repression. When 1.6, 3.2, or 8.0 ng of control MO (cont-MO) or zebrafish CDC48 MO (zCDC48-MO1) was injected into embryos at the one- to four-cell stage, the latter reduced CDC48 levels (Fig. 1A) by 30.7, 29.3, and 12.3%, respectively, at 48 hpf compared with controls (Fig. 1B). To confirm the specificity of the observed MO-induced knockdown of CDC48 expression, we used an MO directed at the translational start site (zCDC48-MO2) of the gene. zCDC48-MO2 also reduced the CDC48 level by 23.4% of that of the control (supplemental Fig. S2A). In embryos that were injected with zCDC48-MO1 or zCDC48-MO2, embryogenesis was slightly delayed until 48 hpf, and their eyes and heads were smaller than those of the cont-MO-injected embryos after 72 hpf (Fig. 1C and supplemental Fig. S2B). At 96 hpf, 95.9% of them (n = 296) had small eyes and heads, and 81.4% exhibited ventral curvature, whereas none of the cont-MO-injected embryos (n = 300) showed these abnormal features (Figs. 1C and 4C). Embryos microinjected with 8 ng of zCDC48-MO2 (n = 48) also had small eyes/heads (87.5% of the embryos) and spinal abnormalities (22.9%). The abnormal phenotypes observed in zCDC48-MO1-injected embryos were rescued by overexpressing zCDC48 cDNA (zCDC48-WT) (see Fig. 4C): 13.9% had small eyes and heads, and 15.7% exhibited ventral curvature. The zCDC48-MO1-injected embryos died 4–5 dpf, indicating that CDC48 is essential for development.
FIGURE 1.
Knockdown of CDC48 protein expression during zebrafish development and the morphologic phenotypes of CDC48-deficient embryos. A, knockdown using antisense MOs was performed in zebrafish embryos. The embryos were injected with 0.5, 1.0, or 2.5 nm cont-MO and zCDC48-MO1 and sampled 2 dpf. The levels of zCDC48 protein were analyzed by Western blotting using an antibody against zebrafish CDC48. B, quantified data from the knockdown experiment for groups of 10 embryos in three different experiments. *, p < 0.001. Error bars, S.E. C, left, cont-MO-injected embryos; right, CDC48-deficient embryos at same magnification. Embryos 48, 72, and 96 hpf are presented (bright-field microscopy). The arrow indicates a smaller eye and head.
FIGURE 4.
Rescue of developmental abnormalities in zCDC48 morphants by overexpressing zebrafish CDC48 and human VCP. A and B, expression of zebrafish CDC48 (A) and human VCP (B) in zCDC48-deficient embryos. CDC48 and VCP were detected in injected embryos after Western blotting using antibodies against zCDC48, which bind human VCP, and anti-His tag antibodies. C, phenotypes of the zCDC48-deficient embryos assessed after injecting the indicated zebrafish CDC48 and human VCP constructs. The percentage of affected embryos was determined, and the embryos were categorized as normal or abnormal, according to head and eye sizes and ventral curvature. The number of embryos is shown at the top. D, phenotypes of the zCDC48-deficient embryos shown after injecting the indicated zebrafish CDC48 and human VCP constructs. Embryos at 96 hpf are presented (bright-field microscopy). The arrow indicates a smaller eye and head.
Severe Cell Death and Neurodegradation Resulting from CDC48 Deficiency
Embryogenesis was slightly delayed until 48 hpf in embryos that were injected with zCDC48-MO1 or zCDC48-MO2, and the eyes and heads were smaller than those of the cont-MO-injected embryos after 72 hpf (Fig. 1C and supplemental Fig. S2B). TUNEL-positive cells were detected in the head and trunk at 24 hpf (n = 20) (supplemental Fig. S3A), and the number of these cells decreased by 48 hpf (data not shown). However, numerous apoptotic cells were detected in the eyes and the diencephalon in the cross-sections of the 120-hpf embryos (supplemental Fig. S3B). In vivo staining of intracellular oxidants with CM-H2DCFDA revealed high levels of ROS in the head part and muscles of CDC48-deficient embryos at 3 dpf, specifically in the eye and brain (n = 30) (supplemental Fig. S3C).
Spinal Motor Axon Defects and Neurodegeneration due to CDC48 Deficiency
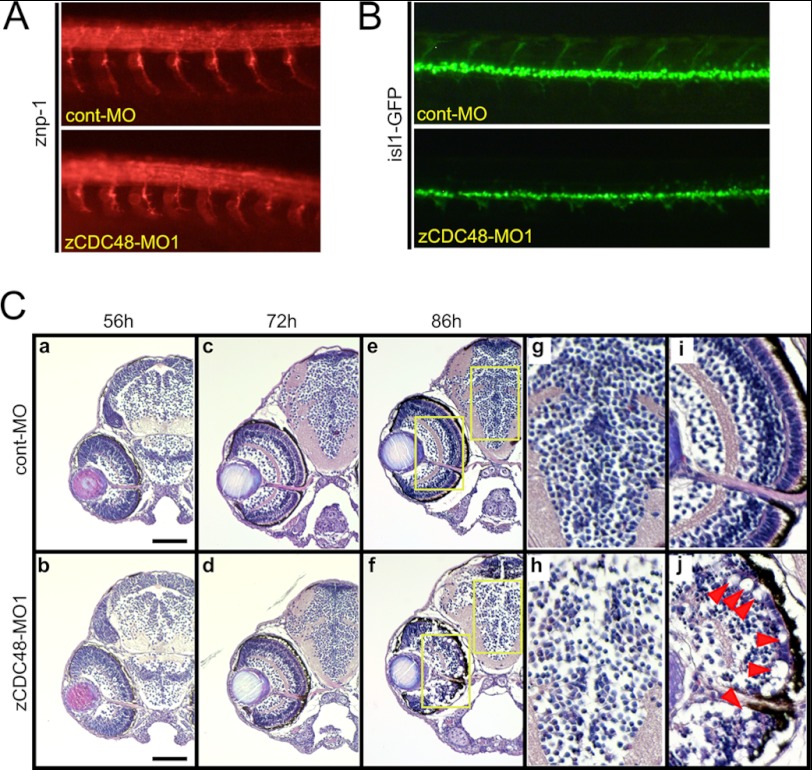
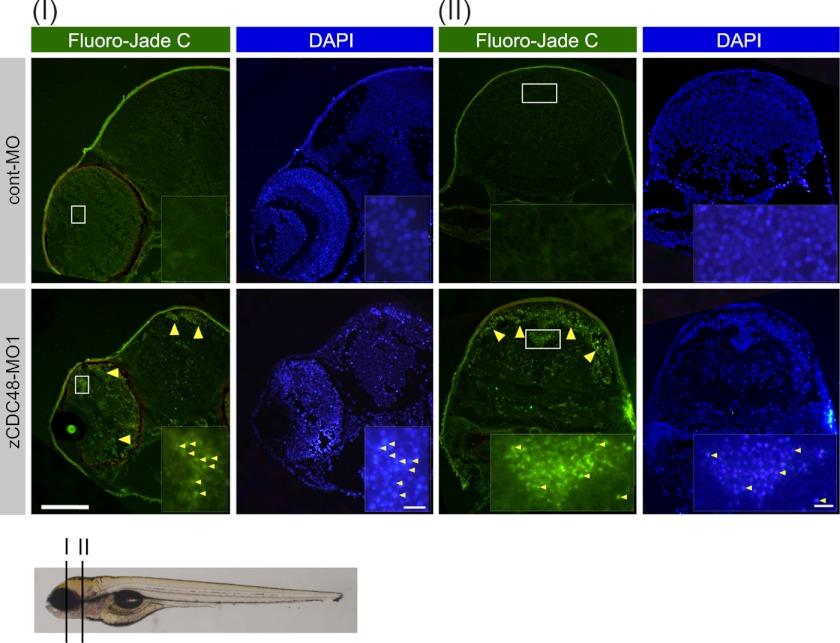
The zCDC48-MO1-injected embryos showed motor axon defects (Fig. 2A). Whole mount immunostaining with an anti-znp-1 antibody visualized the primary and secondary motor axons in zebrafish embryos. By 36 hpf, secondary motor axons extended into the ventral muscle and fasciculated to form a nerve. The zCDC48-MO1-injected embryos had truncated and branched axons compared with those in cont-MO-injected embryos (Fig. 2A). The dorsal projecting motor nerves did not extend in the islet1-GFP transgenic zebrafish (48) that were injected with 4 of ng cont-MO or zCDC48-MO1 (Fig. 2B). Histological examination by H&E staining revealed abnormal tissue degeneration in the eye and brain at 86 hpf. The diencephalon cell number in CDC48-deficient embryos decreased significantly to 58.2% of the cont-MO-injected embryos (Fig. 2C, g and h). The structures of the inner plexiform and ganglion cell layers were almost completely decomposed, the rods and cones had disappeared (Fig. 2Cj, arrowhead), and the optic nerve had narrowed (Fig. 2Cj). Neurodegeneration was detected in cross-sections (I and II) stained with the neuronal aging marker Fluoro-Jade C. Unlike control embryos, zCDC48-MO1-injected embryos showed neurodegradation of the inner plexiform and ganglion cell layers as well as the diencephalon (Fig. 3). Many nuclei were aggregated in the diencephalon, cerebellum, mesencephalon, and eye, and colocalized with Fluoro-Jade C-positive cells.
FIGURE 2.
Spinal motor axon defects and decreased numbers of neural cells in the eyes and brains of CDC48-deficient embryos. A, primary and secondary motor axons in zebrafish embryos were visualized by immunostaining with the anti-znp-1 (anti-synaptotagmin 2) antibody. By 36 hpf, secondary motor axons had extended into the ventral muscle and fasciculate to form a nerve. The zCDC48-MO-injected embryos had truncated and branched axons, compared with those of the control. B, primary and secondary motor axons in isl1-GFP-transgenic zebrafish are visualized. C, H&E staining revealed almost no changes in histology and normal development of the eye and brain until 72 hpf. After 86 hpf, the number of cells in the diencephalon decreased significantly; e is magnified to g (diencephalon) and i (eye), and f are magnified to h (diencephalon) and j (eye). The structures of the inner plexiform layer and ganglion cell layer are almost decomposed, the rods and cones have disappeared (j, red arrowhead), and the optic nerve has narrowed (j).
FIGURE 3.
Neurodegeneration in zCDC48-deficient embryos. Staining with Fluoro-Jade C, used as a histological marker of neurodegeneration, was performed. Neurodegeneration was detected in the eyes and brains of zCDC48-injected embryos (yellow arrowhead). The white boxes are magnified. I and II denote the cutting position, as described below. Sections were counterstained with DAPI. Scale bars, 100 μm in each panel and 10 μm in magnified panels.
Rescue of CDC48-deficient Abnormal Phenotypes by Introducing CDC48 Mutant
We performed rescue experiments with mutated zCDC48 and hVCP to further investigate the CDC48 functions involved in the neural defects seen in CDC48-deficient zebrafish. A catalytically inactive, dominant negative mutant of hVCP was generated by introducing mutations that impaired both ATPase domains (E305Q/E578Q) according to a previous study (36). The expression of dominant negative VCP (hVCP-DN) strongly impaired the ERAD pathway. In contrast, no impairment of the ERAD pathway was observed in cells that expressed the disease-associated mutants hVCP-R155H and hVCP-A232E (40). A tyrosine residue near the C terminus regulates cell cycle-dependent phosphorylation (49). Under low temperature conditions, the expression of a mutant molecule with a Tyr-805 to alanine substitution at the C-terminal phosphorylation site inhibits cell proliferation and induces apoptosis (4). In the present study, overexpression of mutated zCDC48 and hVCP was detected by Western blotting using anti-VCP antibody, anti-His tag antibody, and anti-GFP antibody (Fig. 4, A and B). Exogenous zCDC48-WT and zCDC48-Y805A were detected by anti-His tag antibody, and hVCP-WT, hVCP-R155H, hVCP-A232E, and hVCP-DN were detected by anti-GFP antibody. The expression of both endogenous CDC48 and exogenous overexpressed CDC48/VCP proteins was confirmed by Western blotting with anti-VCP antibody. CDC48 expression was recovered by the overexpressing wild-type and mutated zCDC48 or hVCP (Fig. 4, A and B). GFP-tagged hVCP and mutants had high molecular mass with EGFP protein compared with endogenous zCDC48. The abnormal phenotypes of the zCDC48-MO2-injected embryos were dramatically rescued by overexpressing the zCDC48-WT, zCDC48-Y805A, hVCP-WT, hVCP-R155H, and hVCP-A232E proteins (Fig. 4, C and D). There were not significant differences between each embryo overexpressing the hVCP-WT, hVCP-R155H, and hVCP-A232E constructs from 10 to 100 μg/ml (supplemental Fig. S4). Overexpression of hVCP-DN caused an abnormal phenotype, and 56.5% of these embryos had small eyes and heads and ventral curvature even in cont-MO injected embryos (Fig. 4B). We could not confirm whether the abnormal phenotypes of the morphant were rescued by overexpressing hVCP-DN. These findings indicate that CDC48 is essential for normal embryonic development.
Accumulation of Polyubiquitinated Proteins in Relation to Neurodegeneration Associated with CDC48 Deficiency
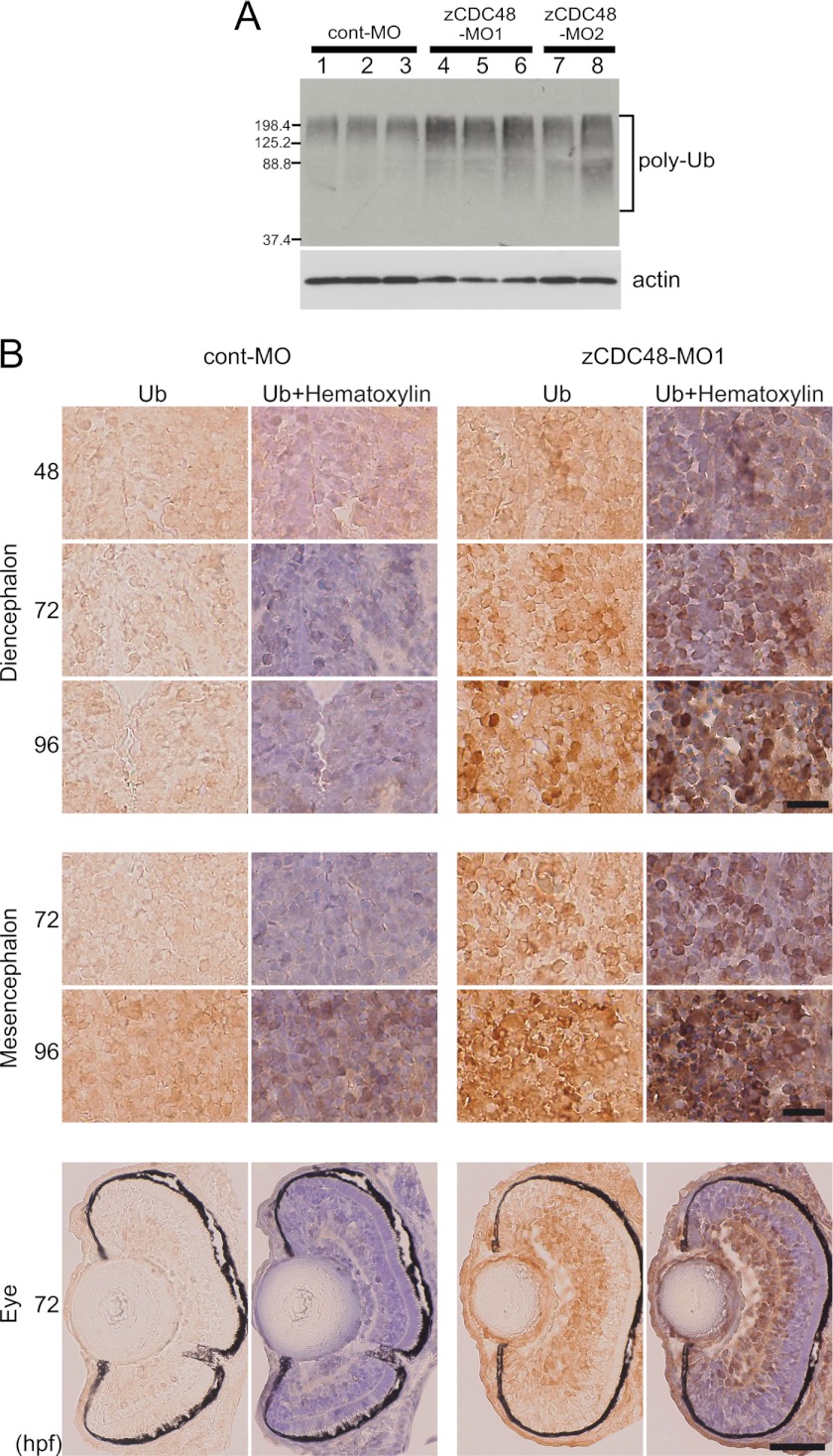
In embryos that were injected with zCDC48-MO1 (8 ng) or zCDC48-MO2 (8 ng), polyubiquitinated proteins increased 2.2-fold and 2.3-fold, respectively, compared with the levels in embryos injected with cont-MO (Fig. 5A). Polyubiquitinated proteins were detected in the eyes, diencephalon, and mesencephalon by immunohistochemical staining of embryo cross-sections with an antibody against ubiquitinated proteins (Fig. 5B). In the cross-sections of the 72-hpf embryos, many of the cells in the eyes and the diencephalon showed significant accumulation of polyubiquitinated proteins (Fig. 5B). In the cont-MO-injected cells, 1.9 and 3.3% positive cells were detected in the diencephalon and eye, respectively, and 61.7 and 60.8% positive cells were detected in the diencephalon and eyes of fish injected with zCDC48-MO cells of 96 hpf, respectively. Accumulation of polyubiquitinated proteins increased in the brain from 48 to 96 hpf (Fig. 5B).
FIGURE 5.
Accumulation of polyubiquitinated proteins in zCDC48-deficient embryos. A, accumulation of polyubiquitinated proteins was detected by Western blotting. Triplicate samples of cont-MO- and zCDC48-MO1-injected embryos (10 embryos) and duplicate samples of zCDC48-MO2-injected embryos were loaded. B, cryosections were stained with an antibody that detects ubiquitinated proteins in the eyes and brain. Ubiquitinated protein-positive cells were detected using DAB substrate. Immunohistochemistry of zCDC48-MO1-injected embryos at different developmental stages (48, 72, and 96 hpf). Nucleus was counterstained by hematoxylin in right panel. Scale bars, 20 μm in brain and 50 μm in eye.
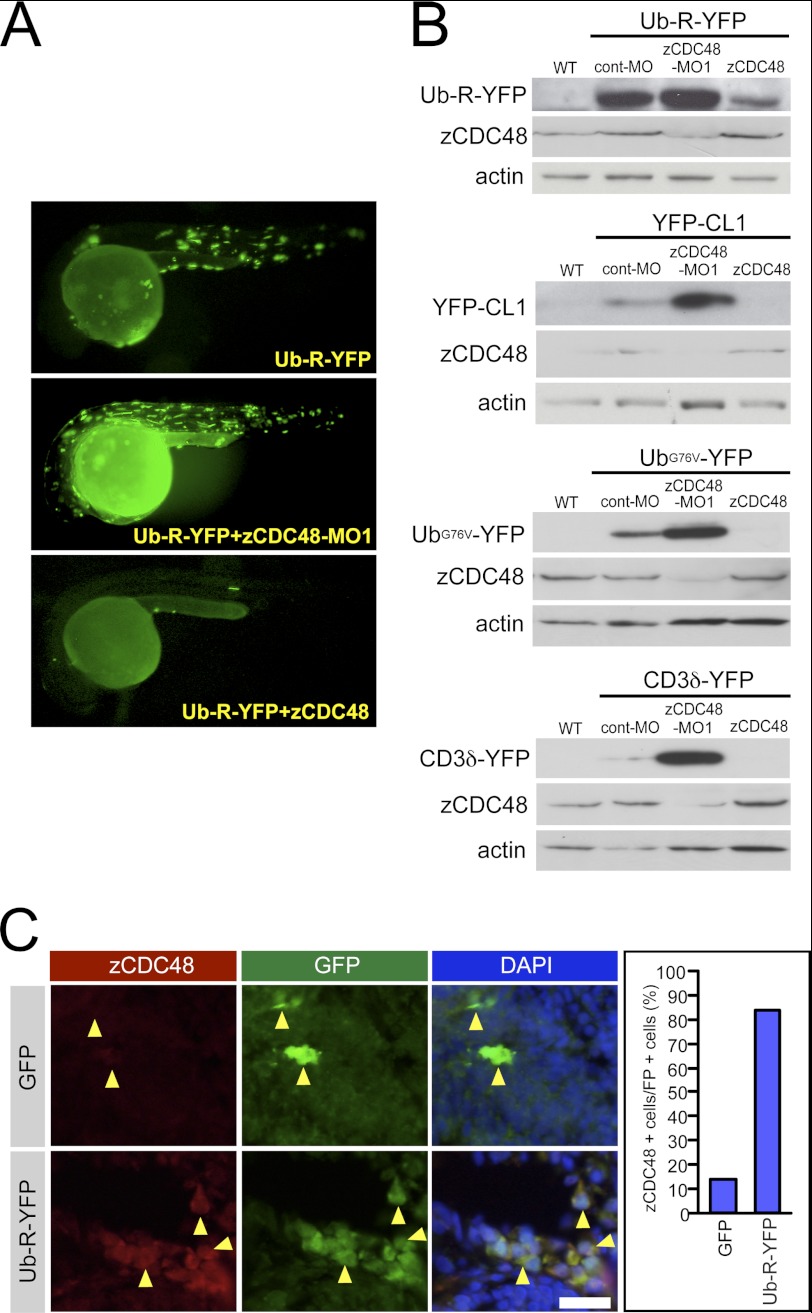
As the degradation of polyubiquitinated proteins depends on UPS (6), impairment of UPS was characterized in terms of CDC48 deficiency. A stable, YFP fusion protein, Ub-R-YFP, which introduces a polyubiquitination signal at the N terminus, is rapidly degraded by UPS (25). Our attempts to generate stable lines expressing Ub-R-YFP were unsuccessful, as Ub-R-YFP may be toxic, and viable offspring could not be obtained. Ub-R-YFP was degraded in the body, whereas a few strong fluorescent signals were detected in the muscles and the skin of cont-MO-injected embryos (n = 43) (Fig. 6A); stronger fluorescent signals were detected in the muscles, skin, and yolk sac (n = 49) of zCDC48-MO1-injected embryos. In contrast, overexpression of CDC48 suppressed Ub-R-YFP expression, and these fluorescent signals depended on CDC48 levels (n = 45) (Fig. 6A). The N-end rule substrate YFP-CL1, the ubiquitin fusion degradation substrates sUb-R-YFP and UbG76V-YFP, and the ERAD substrate CD3δ-YFP (25) were used to confirm the effect of CDC48 deficiency on UPS function. YFP-CL1, Ub-R-YFP, UbG76V-YFP, and CD3δ-YFP were hydrolyzed in a CDC48-dependent manner (data not shown). Fluorescent proteins were detected by Western blotting using an anti-GFP antibody. Ub-R-YFP, YFP-CL1, UbG76V-YFP, and CD3δ-YFP accumulated in response to CDC48 deficiency. The levels of Ub-R-YFP, YFP-CL1, UbG76V-YFP, and CD3δ-YFP in CDC48-MO-injected embryos were higher than those in wild-type embryos, cont-MO-injected control embryos, and CDC48-overexpressing embryos (Fig. 6B). To confirm specific degradation of these reporters by proteasomal function, the embryos were treated with proteasome inhibitor, MG-132. The reporter fluorescence substrates were accumulated in the MG-132-treated embryos (supplemental Fig. S5). Furthermore, to block the lysosomal function, the embryos were treated with bafilomycin A1 and chloroquine. The signals in embryos treated with bafilomycin A1 and chloroquine were also higher than those in wild-type embryos. These findings indicate that degradation of polyubiquitinated proteins depends on CDC48 levels via both UPS and lysosomal pathways. Ub-R-YFP was colocalized with zCDC48 in the cytoplasm in Ub-R-YFP-injected embryos (Fig. 6C). Almost all Ub-R-YFP-positive cells (83% of all cells) were colocalized with zCDC48.
FIGURE 6.
Monitoring UPS function in zebrafish embryos. A, we created a system involving transient expression of a UPS fluorescent reporter. A polyubiquitination signal was joined to an otherwise stable YFP to create the Ub-R-YFP fusion protein. Ub-R-YFP was left undegraded after CDC48 knockdown and was degraded after CDC48 overexpression. B, fluorescent proteins were detected by Western blotting using the anti-GFP antibody. Ub-R-YFP, YFP-CL1, UbG76V-YFP, and CD3δ-YFP were introduced in the presence of the cont-MO, zCDC48-MO, or zCDC48 constructs. C, immunohistochemistry of ubiquitinated fluorescent proteins and zCDC48 in brain is shown. GFP and Ub-R-YFP were injected and stained by anti-GFP and CDC48. The numbers of zCDC48- and GFP-positive cells were counted.
Accumulation of Polyubiquitinated Proteins Was Associated with Neurodegeneration
Polyubiquitinated proteins accumulated in the cells and were associated with neurodegeneration in zCDC48-MO-injected embryos (Fig. 7). Cross-sections of the embryos were stained with an anti-polyubiquitinated protein antibody and Fluoro-Jade C (a marker of neurodegeneration). The cells that contained polyubiquitinated proteins colocalized with the Fluoro-Jade C-positive cells in the cerebellum, diencephalon, and eye (Fig. 7).
FIGURE 7.
Accumulation of polyubiquitinated proteins in the zCDC48 morphant. Cross-sections of embryos were stained with the anti-polyubiquitinated protein antibody and Fluoro-Jade C (a marker of neurodegeneration). The cells that contained polyubiquitinated proteins colocalized with the Fluoro-Jade C-positive cells in the diencephalon and eye. The sections in white boxes are magnified. Sections were counterstained with DAPI. Scale bar, 50 μm.
DISCUSSION
CDC48-deficient embryos showed an embryonic lethal phenotype, indicating that CDC48 is essential for zebrafish development with respect to neurodegeneration. Development of CDC48-deficient embryos was normal until 48 hpf, with neurodegeneration occurring after 72 hpf. CDC48 deficiency induced significant accumulation of polyubiquitinated proteins due to reduced UPS function. Immunohistochemical analysis detected ubiquitinated proteins in the eyes and brain during development. We used reporter YFPs, N-end rule substrate YFP-CL1, and the ubiquitin fusion degradation substrates Ub-R-YFP, UbG76V-YFP, and CD3δ-YFP (25). CDC48 knockdown or overexpression affected the fluorescent intensities of both reporters in the embryos (Fig. 6). UPS function was suppressed in CDC48-deficient embryos (Fig. 6, A and B). Both ERAD and UFD are impaired by the expression of dominant negative VCP (hVCP-DN), which is a catalytically dead mutant (E305Q and E578Q) (50). This study confirmed that the CDC48 morphant could be rescued by disease-associated mutants (hVCP-R155H or hVCP-A232E).
Polyubiquitinated protein was accumulated in neural tissues of zebrafish embryos (Fig. 7), and colocalized with CDC48 in the cytosol (Fig. 6). CDC48 has been implicated in the turnover of neural proteins, such as LIM kinase 1 (51), Epsin 1 (52), GKAP (53), Liprin-α (54), and β-catenin (55). In this study, CDC48 deficiency induced polyubiquitinated neural proteins in the CNS and developmental abnormalities in zebrafish embryos. CDC48 deficiency might alter the levels of transcription factors that lead to neurodegeneration in parallel with the appearance of polyubiquitinated proteins, as pointed out previously (2, 27).
Ju et al. (39) showed that CDC48 deficiency or dominant negative CDC48 overexpression results in significant accumulation of immature autophagic vesicles and ubiquitin-dependent proteasomal degradation. Furthermore, R155H and A232E mutations also cause autophagy defects (39, 40). These findings suggest that both ATPase domains with Glu-305 and Glu-578 are important for regulating autophagy, probably with respect to autophagosome trafficking or autophagosome-lysosome fusion. In this study, the dominant negative CDC48 mutant overexpression and CDC48 deficiency showed developmental abnormalities (Fig. 4, B and C). Although CDC48 deficiency induced macroautophagy and caused the accumulation of the LC3-II form in the brain of GFP-LC3 transgenic zebrafish, lysosomal degradation was impaired.3 Therefore, CDC48 ATPase activity may be responsible for the selective degradation of ubiquitinated proteins in zebrafish embryos.
We previously showed that CDC48 is induced during cold acclimation in cultured trout and zebrafish cells and is processed into a protein with a molecular mass of about 70 kDa (3). CDC48 promotes cellular proliferation and prevents the induction of apoptosis under cold conditions (4). Mutation of the phosphorylation site at Tyr-805 in CDC48 results in severe apoptosis. These findings indicate that CDC48 degrades accumulated proteins under cold conditions and promotes cell viability in response to both the proteolytic processing of the 70-kDa peptide and Tyr-805 phosphorylation. CDC48 enhances proteolysis of ubiquitinated proteins to advance the development of the zebrafish embryo. The abnormal phenotypes of CDC48-deficient embryos were rescued by overexpressing zCDC48-Y805A as well as by disease-associated mutants at the ubiquitination sites hVCP-R155H and hVCP-A232E but not the catalytically inactive CDC48 mutant that was impaired in two ATPase domains. Therefore, phosphorylation at Tyr-805 and ubiquitination in the N-terminal region are important for cell growth and cell death mechanisms, whereas the ATPase domains are essential to molecular chaperone function in the UPS during neurodevelopment.
Supplementary Material
This work was supported by Grants-in-aid for Scientific Research 22780195 and 20880045 (to S. I.).

This article contains supplemental Figs. S1–S5.
S. Imamura, T. Yabu, and M. Yamashita, unpublished data.
- CDC48
- cell division cycle 48
- AAA
- ATPases associated with a variety of cellular activities
- CM-H2DCFDA
- 5-(and-6)-chloromethyl-2′,7′-dichloro-dihydro-fluorescein diacetate acetyl ester
- DIG
- digoxigenin
- DN
- dominant negative
- dpf
- days postfertilization
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- hpf
- hours postfertilization
- IBMPFD
- inclusion body myopathy with Paget disease of bone and frontotemporal dementia
- MO
- morpholino oligonucleotide
- PFA
- paraformaldehyde
- ROS
- reactive oxygen species
- Ub
- ubiquitin
- UFD
- ubiquitin-fusion degradation
- UPS
- ubiquitin-proteasome system
- VCP
- valosin-containing protein.
REFERENCES
- 1. Moir D., Stewart S. E., Osmond B. C., Botstein D. (1982) Cold-sensitive cell division cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics 100, 547–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Q., Song C., Li C. C. (2004) Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J. Struct. Biol. 146, 44–57 [DOI] [PubMed] [Google Scholar]
- 3. Yamashita M., Ojima N., Sakamoto T. (1996) Induction of proteins in response to cold acclimation of rainbow trout cells. FEBS Lett. 382, 261–264 [DOI] [PubMed] [Google Scholar]
- 4. Imamura S., Ojima N., Yamashita M. (2003) Cold-inducible expression of the cell division cycle gene CDC48 and its promotion of cell proliferation during cold acclimation in zebrafish cells. FEBS Lett. 549, 14–20 [DOI] [PubMed] [Google Scholar]
- 5. Imamura S., Ojima N., Yamashita M. (2004) Mar. Biotech. 6, S1–7 [Google Scholar]
- 6. Dai R. M., Li C. C. (2001) Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat. Cell Biol. 3, 740–744 [DOI] [PubMed] [Google Scholar]
- 7. Hatakeyama S., Matsumoto M., Yada M., Nakayama K. I. (2004) Interaction of U-box-type ubiquitin-protein ligases (E3s) with molecular chaperones. Genes Cells 9, 533–548 [DOI] [PubMed] [Google Scholar]
- 8. Zhong X., Shen Y., Ballar P., Apostolou A., Agami R., Fang S. (2004) AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation. J. Biol. Chem. 279, 45676–45684 [DOI] [PubMed] [Google Scholar]
- 9. Wójcik C., Yano M., DeMartino G. N. (2004) RNA interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J. Cell Sci. 117, 281–292 [DOI] [PubMed] [Google Scholar]
- 10. Bays N. W., Wilhovsky S. K., Goradia A., Hodgkiss-Harlow K., Hampton R. Y. (2001) HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol. Biol. Cell 12, 4114–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braun S., Matuschewski K., Rape M., Thoms S., Jentsch S. (2002) Role of the ubiquitin-selective CDC48(UFD1/NPL4 )chaperone (segregase) in ERAD of OLE1 and other substrates. EMBO J. 21, 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ye Y., Meyer H. H., Rapoport T. A. (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414, 652–656 [DOI] [PubMed] [Google Scholar]
- 13. Tran J. R., Tomsic L. R., Brodsky J. L. (2011) A Cdc48p-associated factor modulates endoplasmic reticulum-associated degradation, cell stress, and ubiquitinated protein homeostasis. J. Biol. Chem. 286, 5744–5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabinovich E., Kerem A., Fröhlich K. U., Diamant N., Bar-Nun S. (2002) AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell. Biol. 22, 626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye Y., Meyer H. H., Rapoport T. A. (2003) Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J. Cell Biol. 162, 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi T., Tanaka K., Inoue K., Kakizuka A. (2002) Functional ATPase activity of p97/valosin-containing protein (VCP) is required for the quality control of endoplasmic reticulum in neuronally differentiated mammalian PC12 cells. J. Biol. Chem. 277, 47358–47365 [DOI] [PubMed] [Google Scholar]
- 17. Ghislain M., Dohmen R. J., Levy F., Varshavsky A. (1996) Cdc48p interacts with Ufd3p, a WD repeat protein required for ubiquitin-mediated proteolysis in Saccharomyces cerevisiae. EMBO J. 15, 4884–4899 [PMC free article] [PubMed] [Google Scholar]
- 18. Alexandru G., Graumann J., Smith G. T., Kolawa N. J., Fang R., Deshaies R. J. (2008) UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1α turnover. Cell 134, 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao K., Nakajima R., Meyer H. H., Zheng Y. (2003) The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis. Cell 115, 355–367 [DOI] [PubMed] [Google Scholar]
- 20. Fu X., Ng C., Feng D., Liang C. (2003) Cdc48p is required for the cell cycle commitment point at Start via degradation of the G1-CDK inhibitor Far1p. J. Cell Biol. 163, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halawani D., Latterich M. (2006) p97: The cell's molecular purgatory? Mol. Cell 22, 713–717 [DOI] [PubMed] [Google Scholar]
- 22. Janiesch P. C., Kim J., Mouysset J., Barikbin R., Lochmüller H., Cassata G., Krause S., Hoppe T. (2007) The ubiquitin-selective chaperone CDC-48/p97 links myosin assembly to human myopathy. Nat. Cell Biol. 9, 379–390 [DOI] [PubMed] [Google Scholar]
- 23. Sasagawa Y., Otani M., Higashitani N., Higashitani A., Sato K., Ogura T., Yamanaka K. (2009) Caenorhabditis elegans p97 controls germ line-specific sex determination by controlling the TRA-1 level in a CUL-2-dependent manner. J. Cell Sci. 122, 3663–3672 [DOI] [PubMed] [Google Scholar]
- 24. Yi J. J., Ehlers M. D. (2007) Emerging roles for ubiquitin and protein degradation in neuronal function. Pharm. Rev. 59, 14–39 [DOI] [PubMed] [Google Scholar]
- 25. Menéndez-Benito V., Verhoef L. G., Masucci M. G., Dantuma N. P. (2005) Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum. Mol. Genet. 14, 2787–2799 [DOI] [PubMed] [Google Scholar]
- 26. Pandey U. B., Nie Z., Batlevi Y., McCray B. A., Ritson G. P., Nedelsky N. B., Schwartz S. L., DiProspero N. A., Knight M. A., Schuldiner O., Padmanabhan R., Hild M., Berry D. L., Garza D., Hubbert C. C., Yao T. P., Baehrecke E. H., Taylor J. P. (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 [DOI] [PubMed] [Google Scholar]
- 27. Higashiyama H., Hirose F., Yamaguchi M., Inoue Y. H., Fujikake N., Matsukage A., Kakizuka A. (2002) Identification of ter94, Drosophila VCP, as a modulator of polyglutamine-induced neurodegeneration. Cell Death Differ. 9, 264–273 [DOI] [PubMed] [Google Scholar]
- 28. Müller J. M., Deinhardt K., Rosewell I., Warren G., Shima D. T. (2007) Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem. Biophys. Res. Commun. 354, 459–465 [DOI] [PubMed] [Google Scholar]
- 29. Hirabayashi M., Inoue K., Tanaka K., Nakadate K., Ohsawa Y., Kamei Y., Popiel A. H., Sinohara A., Iwamatsu A., Kimura Y., Uchiyama Y., Hori S., Kakizuka A. (2001) VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 8, 977–984 [DOI] [PubMed] [Google Scholar]
- 30. Kakizuka A. (2008) Roles of VCP in human neurodegenerative disorders. Biochem. Soc. Trans. 36, 105–108 [DOI] [PubMed] [Google Scholar]
- 31. Weihl C. C., Miller S. E., Hanson P. I., Pestronk A. (2007) Transgenic expression of inclusion body myopathy-associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum. Mol. Genet. 16, 919–928 [DOI] [PubMed] [Google Scholar]
- 32. Watts G. D., Wymer J., Kovach M. J., Mehta S. G., Mumm S., Darvish D., Pestronk A., Whyte M. P., Kimonis V. E. (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381 [DOI] [PubMed] [Google Scholar]
- 33. Askanas V., Engel W. K. (2005) Molecular pathology and pathogenesis of inclusion-body myositis. Microsc. Res. Tech. 67, 114–120 [DOI] [PubMed] [Google Scholar]
- 34. Weihl C. C., Pestronk A., Kimonis V. E. (2009) Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia. Neuromuscul. Disord. 19, 308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Esaki M., Ogura T. (2010) ATP-bound form of the D1 AAA domain inhibits an essential function of Cdc48p/p97. Biochem. Cell Biol. 88, 109–117 [DOI] [PubMed] [Google Scholar]
- 36. Weihl C. C., Dalal S., Pestronk A., Hanson P. I. (2006) Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum. Mol. Genet. 15, 189–199 [DOI] [PubMed] [Google Scholar]
- 37. Ju J. S., Miller S. E., Hanson P. I., Weihl C. C. (2008) Impaired protein aggregate handling and clearance underlie the pathogenesis of p97/VCP-associated disease. J. Biol. Chem. 283, 30289–30299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Q., Song C., Yang X., Li C. C. (2003) D1 ring is stable and nucleotide-independent, whereas D2 ring undergoes major conformational changes during the ATPase cycle of p97-VCP. J. Biol. Chem. 278, 32784–32793 [DOI] [PubMed] [Google Scholar]
- 39. Ju J. S., Fuentealba R. A., Miller S. E., Jackson E., Piwnica-Worms D., Baloh R. H., Weihl C. C. (2009) Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J. Cell Biol. 187, 875–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tresse E., Salomons F. A., Vesa J., Bott L. C., Kimonis V., Yao T. P., Dantuma N. P., Taylor J. P. (2010) VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes, and this function is impaired by mutations that cause IBMPFD. Autophagy 6, 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Westerfield M. (1995) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd Ed., University of Oregon Press, Eugene, OR [Google Scholar]
- 42. Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 43. McWhorter M. L., Monani U. R., Burghes A. H., Beattie C. E. (2003) Knockdown of the survival motor neuron (Smn) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. J. Cell Biol. 162, 919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valenzano D. R., Terzibasi E., Genade T., Cattaneo A., Domenici L., Cellerino A. (2006) Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 16, 296–300 [DOI] [PubMed] [Google Scholar]
- 45. Imamura S., Uchiyama J., Koshimizu E., Hanai J., Raftopoulou C., Murphey R. D., Bayliss P. E., Imai Y., Burns C. E., Masutomi K., Gagos S., Zon L. I., Roberts T. M., Kishi S. (2008) A non-canonical function of zebrafish telomerase reverse transcriptase is required for developmental hematopoiesis. PLoS One 3, e3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kishi S., Bayliss P. E., Uchiyama J., Koshimizu E., Qi J., Nanjappa P., Imamura S., Islam A., Neuberg D., Amsterdam A., Roberts T. M. (2008) The identification of zebrafish mutants showing alterations in senescence-associated biomarkers. PLoS Genet. 4, e1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nasevicius A., Ekker S. C. (2000) Effective targeted gene “knockdown” in zebrafish. Nat. Genet. 26, 216–220 [DOI] [PubMed] [Google Scholar]
- 48. Higashijima S., Hotta Y., Okamoto H. (2000) Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J. Neurosci. 20, 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Madeo F., Schlauer J., Zischka H., Mecke D., Fröhlich K. U. (1998) Tyrosine phosphorylation regulates cell cycle-dependent nuclear localization of Cdc48p. Mol. Biol. Cell 9, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dalal S., Rosser M. F., Cyr D. M., Hanson P. I. (2004) Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell 15, 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tursun B., Schlüter A., Peters M. A., Viehweger B., Ostendorff H. P., Soosairajah J., Drung A., Bossenz M., Johnsen S. A., Schweizer M., Bernard O., Bach I. (2005) The ubiquitin ligase Rnf6 regulates local LIM kinase 1 levels in axonal growth cones. Genes Dev. 19, 2307–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen H., Polo S., Di Fiore P. P., De Camilli P. V. (2003) Rapid Ca2+-dependent decrease of protein ubiquitination at synapses. Proc. Natl. Acad. Sci. U.S.A. 100, 14908–14913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ehlers M. D. (2003) Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 6, 231–242 [DOI] [PubMed] [Google Scholar]
- 54. Kaufmann N., DeProto J., Ranjan R., Wan H., Van Vactor D. (2002) Drosophila liprin-α and the receptor phosphatase Dlar control synapse morphogenesis. Neuron 34, 27–38 [DOI] [PubMed] [Google Scholar]
- 55. Dreier L., Burbea M., Kaplan J. M. (2005) LIN-23-mediated degradation of β-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Neuron 46, 51–64 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.