Background: The transmembrane domain of integrins plays a critical role in mediating receptor inside-out activation.
Results: Inside-out activation triggers the repartitioning of the intracellular border of αIIb but not the β3 transmembrane domain into the lipid bilayer in living cells.
Conclusion: Complex conformational changes occur in the transmembrane domain upon integrin αIIbβ3 inside-out activation.
Significance: Our findings represent a new mechanism for integrin inside-out activation.
Keywords: Membrane Proteins, Protein Chemical Modification, Protein Conformation, Receptor Structure-Function, Site-directed Mutagenesis
Abstract
Integrins are a family of heterodimeric adhesion receptors that transmit signals bi-directionally across the plasma membranes. The transmembrane domain (TM) of integrin plays a critical role in mediating transition of the receptor from the default inactive to the active state on the cell surfaces. In this study, we successfully applied the substituted cysteine scanning accessibility method to determine the intracellular border of the integrin αIIbβ3 TM in the inactive and active states in living cells. We examined the aqueous accessibility of 75 substituted cysteines comprising the C terminus of both αIIb and β3 TMs, the intracellular membrane-proximal regions, and the whole cytoplasmic tails, to the labeling of a membrane-permeable, cysteine-specific chemical biotin maleimide (BM). The active state of integrin αIIbβ3 heterodimer was generated by co-expression of activating partners with the cysteine-substituted constructs. Our data revealed that, in the inactive state, the intracellular lipid/aqueous border of αIIb TM was at Lys994 and β3 TM was at Phe727 respectively; in the active state, the border of αIIb TM shifted to Pro998, whereas the border of β3 TM remained unchanged, suggesting that complex conformational changes occurred in the TMs upon αIIbβ3 inside-out activation. On the basis of the results, we propose a new inside-out activation mechanism for integrin αIIbβ3 and by inference, all of the integrins in their native cellular environment.
Introduction
Integrins are a large family of adhesion receptors on the cell surfaces that mediate cell adhesion, migration, and extracellular matrix assembly (1, 2). 18 integrin α subunits and 8 β subunits form 24 heterodimers, of which each subunit consists of a large extracellular domain, a single transmembrane domain (TM),3 and a short unstructured cytoplasmic tail. Integrins exist in a default low affinity state on the cell surfaces. Upon stimulation by intracellular signals, integrins convert to the active state that permits extracellular ligands binding (inside-out activation), which in turn promotes interactions of intracellular proteins with the cytoplasmic tails (outside-in signaling) (3, 4). In this way, integrins transmit signals bi-directionally across plasma membranes.
Biochemical analyses (5, 6), electron microscopy (7, 8), and fluorescent energy transfer studies (9) have established that a constraint is present in the intracellular membrane-proximal (MP) region that restrains integrin αIIbβ3 in the inactive state. Intracellular signals interact with the β3 cytoplasmic tail that breaks the constraint to initiate a conformational change that traverses the TMs, which ultimately triggers activation of the extracellular domain. Two intracellular proteins, talin and kindlin, are reported to interact with the β3 cytoplasmic tail and work synergistically to activate αIIbβ3 (10–12). The extracellular domain of integrin αIIbβ3 was crystallized (13), and a low resolution electron cryomicroscopy structure (8) of the full heterodimer was also reported; however, attempts to obtain a crystal structure of the full-length receptor were not successful.
Two stretches of highly conserved amino acids in the intracellular MP region of integrin αIIbβ3 play pivotal roles in controlling the receptor inside-out activation (991GFFKR995 in αIIb; 717LLITIHD723 in β3) (5, 6, 14) (Fig. 1A). A salt bridge between αIIb Arg995 and β3 Asp723 was proposed to form a clasp constraining the receptor in the inactive state (15). Intense efforts including site mutagenesis scanning (15, 16), transmission electron microscopy (7), nuclear magnetic resonance (NMR) (17–20), chimeric swapping (21), and computational modeling (16, 22) have been invested to determine how the intracellular clasp is formed, which gave conflicting results. Recent studies using NMR on the structure of associated αIIbβ3 TM peptides in the lipid bicelles (20), αIIbβ3 TM and cytoplasmic tail complex in CD3CN/H2O (1:1) mixture (17), and by using disulfide constraints coupled with structural modeling (16) have yielded three different views on the intracellular clasp of αIIbβ3: the first proposed that the clasp is formed by the interaction between residues αIIb Phe992/Phe993 and β3 Tyr715, the second proposed that the clasp is formed by an inhibitory ligand binding in the MP region, and the third proposed that the clasp is formed between β3 Lys716 side chain and the peptide backbone of αIIb 991GFFKR995 motif.
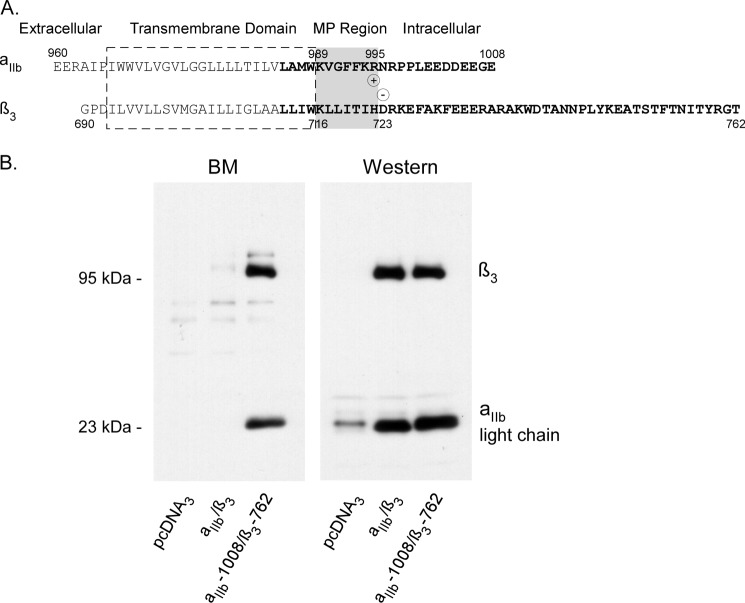
FIGURE 1.
A, amino acid sequences of the TMs and the cytoplasmic tails of integrin αIIbβ3. The proposed TMs are depicted in a box with broken lines, MP regions are colored in gray, and cysteine-substituted residues are highlighted in bold. The potential ionic interaction between αIIb Arg995 and β3 Asp723 is indicated as circled positive and negative symbols. B, BM labeling of wild-type and cysteine-substituted integrin αIIbβ3. HEK 293 cells transfected with cloning vector pcDNA3, wild-type αIIbβ3, and mutant αIIb E1008C/β3 T762C were collected and labeled with BM at room temperature for 20 min. Cells were lysed, and integrin αIIbβ3 heterodimer was immunoprecipitated, resolved on 10% SDS-PAGE, and transferred to a PVDF membrane. Incorporated biotin was detected by HRP-streptavidin and ECL. The blot was stripped and probed with an anti-αIIb light chain and an anti-β3 antibody simultaneously to detect the amount of protein in each sample.
All of the aforementioned studies have greatly improved our knowledge on the conformation of the TMs and the intracellular clasp in the MP region of integrin αIIbβ3; however, questions remain. It is known that the regulated association of αIIbβ3 TMs is driven by domains outside of the plasma membranes, whether the NMR structures of fragmented αIIbβ3 TM peptides correspond to any physiological states of the receptor is obscure. Questions such as how the TMs of integrin αIIbβ3 undergo spatial and conformational changes upon activation and how intracellular proteins interact with the cytoplasmic tails of αIIbβ3 to initiate inside-out activation can only be genuinely answered by the analyses of intact integrin protein in the cellular environment.
Here, we present a successful analysis of the cellular location of integrin αIIbβ3 TM intracellular borders in the receptor inactive and active states in the living cells by using the substituted cysteine scanning accessibility method (SCSAM). Our data revealed a new mechanism for integrin αIIbβ3 inside-out activation in cell membranes.
EXPERIMENTAL PROCEDURES
Materials
Site-directed mutagenesis kits were from Strategene. Biotin maleimide, DMEM, and all cell culture reagents were from Invitrogen. Anti-integrin αIIb or β3 rabbit or mouse antibodies were from Santa Cruz Biotechnology. Protein A- and G-Sepharose, streptavidin/biotinylated-horseradish peroxidase complex (streptavidin-HRP), and goat anti-rabbit IgG-conjugated horseradish peroxidase were from GE Healthcare. Igepal was from Sigma. PVDF membrane was from Millipore.
Site-directed Mutagenesis
A wild-type human αIIb and a β3 cDNA were used as the templates for site-directed mutagenesis. Amino acids at the position of Leu985 to Glu1008 in αIIb and Leu712 to Thr762 in β3 were individually replaced with cysteines. Mutagenesis was performed using the Strategene site-directed mutagenesis kit following the manufacturer's instructions. The complete cDNA sequence of each mutant was verified by DNA sequencing.
Protein Expression
Cysteine-substituted αIIbβ3 was transiently expressed in the human embryonic kidney 293 cells (HEK 293) by using Lipofectamine 2000 (from Invitrogen) transfection following the manufacturer's instructions. Chinese hamster ovary (CHO) cells were also used in some experiments. Cells were grown at 37 °C in a 5% CO2 atmosphere and harvested 48 h after transfection.
Flow Cytometry
Flow cytometry assays for assessing the effects of cysteine substitutions on αIIbβ3 activation were performed as described previously (23). In brief, cysteine-substituted αIIb or β3 constructs were co-expressed with the wild-type partners in the CHO cells. 24 h after transfection, cells were stained with antibody D57, which measures receptor surface expression, and PAC1, which detects the active state of αIIbβ3, in the presence and absence of Ro43-5054 or anti-LIBS6, and then subjected to FACS scan. Ro43-5054 is a competitive antagonist of αIIbβ3, which was used to estimate nonspecific PAC1 binding (F0), and anti-LIBS6 is an αIIbβ3-activating antibody that was used to estimate maximal PAC1 binding (Fmax). The activation index was calculated as 100 × (F − F0)/(Fmax − F0), where F = mean fluorescence intensity of PAC1 staining under the test condition. For detailed methods, see Ref. 23.
Biotin Maleimide Labeling and Immunoprecipitation
Whole cell labeling with BM was performed as described previously (24). Briefly, transfected HEK 293 cells were collected and resuspended in PBSCM (PBS containing 0.1 mm CaCl2 and 1 mm MgCl2, pH 7.0) solution and subsequently labeled with BM (0.2 mm final) at room temperature for 20 min. Reactions were stopped by adding 5-fold glutathione in molar ratio. Cells were then washed with PBSCM and lysed in IPB buffer (150 mm NaCl, 1% (v/v) Igepal, 0.5% (w/v) sodium deoxycholate, 10 mm Tris-HCl, pH 7.5) containing 0.2% (w/v) BSA and protease inhibitors (from Roche Applied Science) on ice for 10 min. αIIbβ3 proteins were immunoprecipitated by a mouse anti-human αIIbβ3 monoclonal antibody (sc-21783 from Santa Cruz Biotechnology) and protein G beads for 4 h at 4 °C.
SDS-PAGE and Immunoblotting
Protein samples were resolved on 7.5% (for β3 subunit) and 10% (for αIIb light chain) SDS-polyacrylamide gels, respectively, and transferred to PVDF membranes. Biotinylated proteins were detected by incubation of blots with 1:10,000 diluted streptavidin-biotinylated horseradish peroxidase (GE Healthcare) in TBSTB buffer (TBST buffer (0.1% (v/v) Tween 20, 137 mm NaCl, 20 mm Tris, pH 7.5), containing 0.5% (w/v) BSA). Protein expression levels were determined by probing the blot with rabbit anti-αIIb or β3 polyclonal antibodies at 1:3,000 dilutions in TBSTM buffer (TBST buffer containing 5% (w/v) nonfat milk).
Membrane Isolation and Na2CO3 Treatment
Membrane treatment with Na2CO3 was performed as described previously (25). Briefly, transfected cells (10-cm plate) were collected, washed (TBS buffer: 140 mm NaCl, 10 mm Tris, pH 7.4), and incubated with the homogenization buffer (10 mm Tris, pH 7.4, with Roche protease inhibitors) for 30 min on ice. Cells were then lysed by Dounce homogenization. Cell debris were removed by low speed centrifugation (4,000 × g, 5 min, 4 °C), and the membrane fractions were collected by high speed centrifugation (35,000 × g, 30 min, 4 °C). Membrane pellets were first resuspended in 100 μl of 0.3 m sucrose and then mixed with 2 ml of ice-cold 0.1 m or 3 m Na2CO3, pH 11.5, and incubated for 30 min on a rotating shaker at 4 °C. Membranes were then collected by centrifugation, washed with PBSCM, and resuspended in 1.0 ml of PBSCM followed by BM labeling. In some experiments, the protein levels of the stripped and unstripped membranes were determined by the BCA method (from Thermo Scientific). Equal amounts of protein samples were resolved on SDS-PAGE and stained with Coomassie Blue.
Image and Data Analysis
Films from immunoblots and biotinylation blots were scanned with a Hewlett-Packard Scanjet 5590. Scanned images were quantified with UN-SCAN-IT gelTM version 6.1 software. Biotinylation levels were calculated according to Ref. 24.
Statistical Analysis
Means ± S.E. were calculated with SigmaPlot 10 software. Statistical analysis was performed using SigmaPlot 10 software.
RESULTS
Substituted Cysteine Scanning Accessibility Method for Integrin αIIbβ3 Conformational Analysis
SCSAM uses sulfhydryl reactive chemical probes to determine the location of the introduced cysteines in a target membrane protein that is free of endogenous reactive cysteines (26, 27). BM, a membrane-permeable sulfhydryl-specific reagent, has been used successfully for the topological analysis of transmembrane proteins (24, 28). SCSAM is based on the observation that the chemical reaction only occurs in the aqueous environment. Cysteines residing in the aqueous medium can react with BM, whereas cysteines residing in the lipid bilayer, the protein interior, or forming disulfide bonds cannot. BM minimally labels the endoplasmic reticulum-retained membrane proteins (24), which is probably due to the high content of glutathione in the cytosol. Integrin αIIbβ3 is a cysteine-rich membrane receptor that contains 20 endogenous cysteines in αIIb and 56 in the β3 subunit. The recent crystal structure of the extracellular domain of αIIbβ3 showed that all of the cysteines in αIIbβ3 form disulfide bonds (13); however, conflicting reports also suggested that the receptor may have endogenous thiol isomerase activity that exposes free cysteines (29). To determine whether free reactive cysteines are present in the wild-type αIIbβ3, we tested wild-type integrin αIIbβ3 for labeling with BM. Wild-type αIIbβ3 and mutant constructs αIIb-E1008C/β3-T762C were expressed in the HEK 293 cells and subjected to the whole cell labeling. Fig. 1B shows that the wild-type αIIb or β3 has no detectable BM labeling, whereas the light chain of αIIb with a single cysteine substitution (E1008C) at the C terminus or β3 with a cysteine substitution at Thr762 (residue at the C terminus of β3) was strongly labeled, suggesting that no free endogenous cysteines are present in wild-type αIIb or β3 that are available for BM labeling. Western blots verified that αIIb (sc-6602, from Santa Cruz Biotechnology) and β3 (sc-6627, from Santa Cruz Biotechnology) are both well expressed. Based on the results, we individually substituted single amino acids (between Leu985 and Glu1008) with cysteines in the C-terminal region of αIIb that covers the proposed C terminus of the TM, the intracellular MP region, and the whole intracellular tail, and the positions between Leu712 and Phe730 in β3 covering the proposed MP region of β3 TM (Fig. 1A).
Whole Cell Labeling of Cysteine-substituted Integrin αIIbβ3 with BM
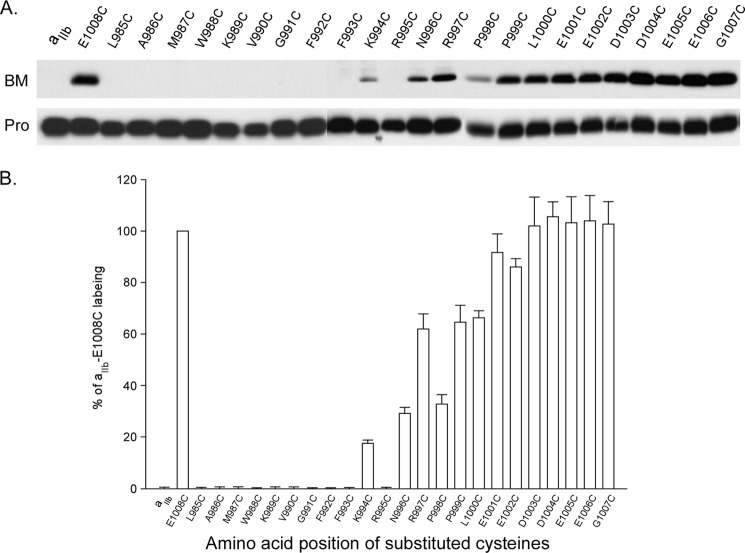
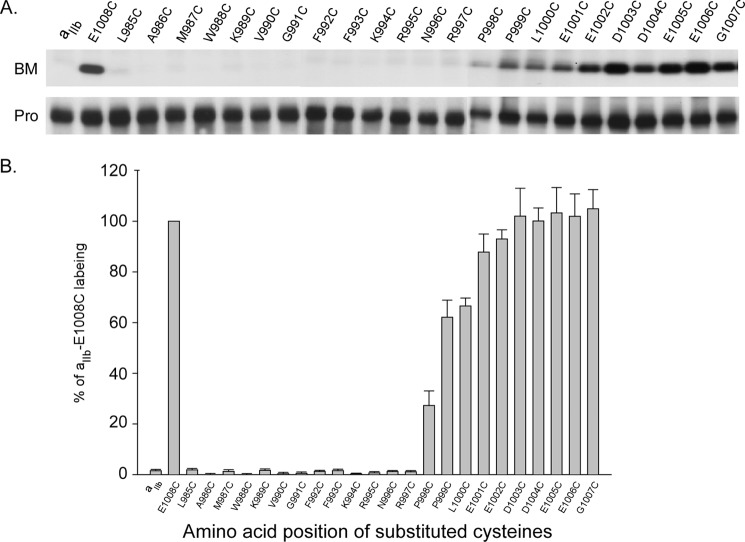
Integrin αIIb carrying substituted cysteines was co-expressed with the wild-type β3 in the HEK 293 cells using Lipofectamine 2000 transfection. 48 h after transfection, cells were collected and subjected to BM labeling. Fig. 2 shows that the positive control, αIIb E1008C was strongly labeled, whereas the negative control, wild-type αIIb, had no labeling. Amino acids between Leu985 and Phe993 were not labeled, suggesting that they are embedded in the lipid bilayer; amino acids between Asn996 and Glu1008 were increasingly labeled, indicating the C-terminal tail is free of any cytosolic protein interactions. Interestingly, K994C was weakly labeled, whereas the adjacent residue, R995C was unlabeled compared with the controls. These data suggest that in the intact αIIbβ3 complex in living cells, the intracellular border of αIIb TM is at Lys994, which leaves the proposed salt bridge αIIb Arg995/β3 Asp723 outside of the plasma membrane at the lipid/aqueous interface in the cytosol.
FIGURE 2.
BM labeling of cysteine-substituted αIIb co-expressed with wild-type β3. A, representative results of BM labeling on integrin αIIb-substituted cysteines. 24 amino acids in the C terminus of αIIb were individually substituted with cysteines and labeled with BM as described under “Experimental Procedures.” B, summary of BM labeling of integrin αIIb-substituted cysteines. The level of biotin incorporation into each sample was quantified by densitometry, and the signal was normalized to the amount of integrin αIIb light chain present in the sample. In each experiment, the level of biotinylation was compared with that of the αIIb E1008C, whose labeling was set to 100%. Data represent mean of 3–5 experiments ± S.E. (error bars).
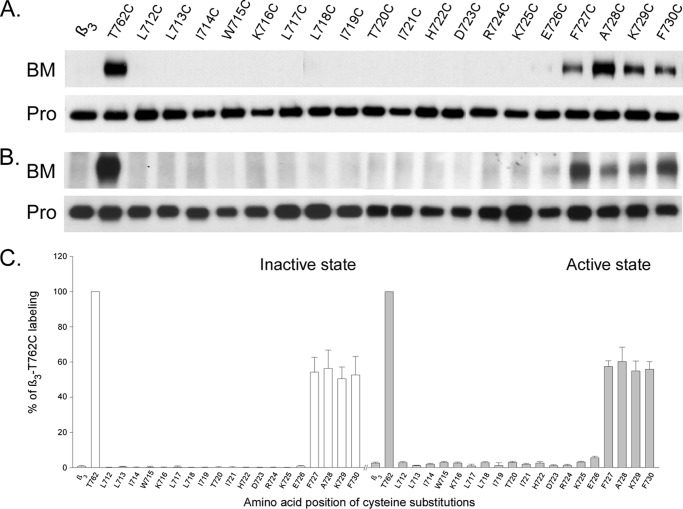
We then performed whole cell labeling on the cysteine-substituted integrin β3 constructs co-expressed with wild-type αIIb in HEK 293 cells. Fig. 3, A and C, shows that none of the substituted cysteines in the region between Leu712 and Glu726 was labeled with BM, and the first BM labeled residue was at Phe727 marking the intracellular border of β3 TM. The intensity of BM labeling on the cysteine substitutions from F727C to F730C was similar suggesting the peptide after Phe727 enters the aqueous medium abruptly.
FIGURE 3.
BM labeling on integrin β3-substituted cysteines. Amino acids between Leu712 and Phe730 in the β3 subunit were individually substituted with cysteines and labeled with BM. A, BM labeling of cysteine-substituted β3 co-expressed with wild-type αIIb. B, BM labeling of cysteine-substituted β3 co-expressed with mutant αIIb F992A/F993A. Similar results were obtained with co-expression of αIIb Gly991 truncation. C, summary of BM labeling of cysteine-substituted β3. Biotin incorporation into each sample was quantified as described in Fig. 2 and was compared with β3-T762C, whose labeling was set to 100%. Data represent mean of 3–6 experiments ± S.E. (error bars).
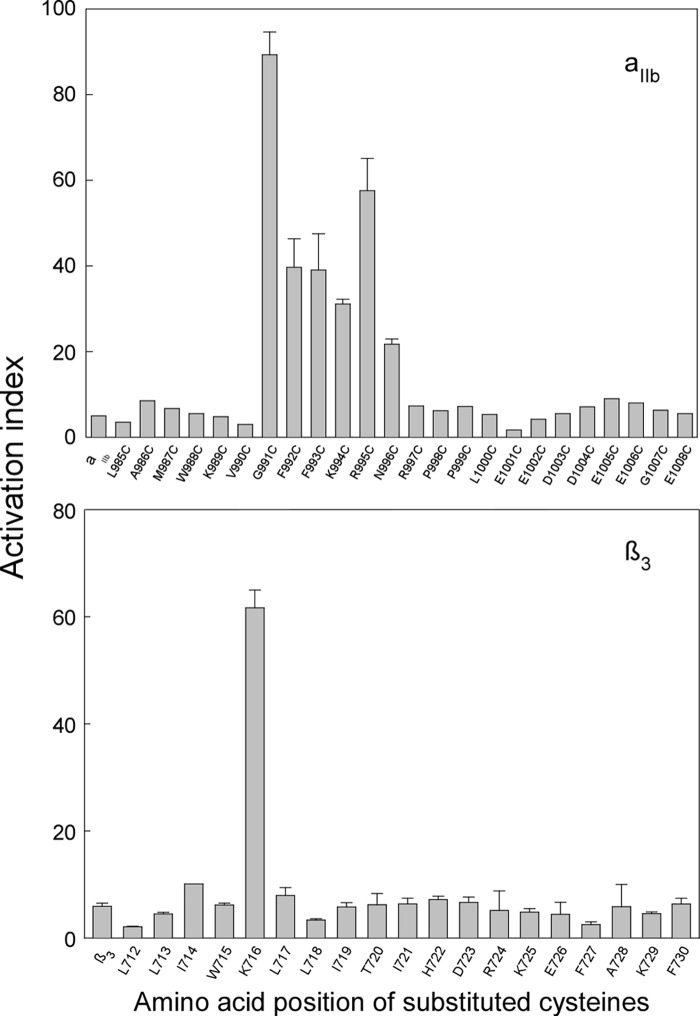
Functional Analysis of Cysteine-substituted Integrin αIIbβ3
Functional effects of cysteine substitutions in the TM and the MP regions of integrin αIIbβ3 have recently been reported (16), in that cysteine substitution of any of the residues in the 991GFFKRN996 region of αIIb activated the receptor to various degrees, whereas substitution in the remaining portion of the αIIb intracellular tail had no functional effect. Surprisingly, in the MP region of β3, only one cysteine substitution at amino acid Lys716 substantially activated integrin αIIbβ3. Because most of the functional studies of integrin αIIbβ3 were performed in the CHO cells, we expressed the cysteine-substituted constructs in the CHO cells and subjected them to FACS analysis with PAC1 antibody that recognizes the active state of integrin αIIbβ3. Fig. 4 shows that, indeed, our findings were in agreement with the previous report.
FIGURE 4.
Effect of cysteine substitutions in αIIb or β3 subunit on integrin αIIbβ3 activation. Upper, activation index of cysteine-substituted αIIb co-expressed with wild-type β3. Lower, activation index of cysteine-substituted β3 co-expressed with wild-type αIIb. Cysteine-substituted αIIb or β3 were co-expressed with the respective wild-type partners in the CHO cells. 24 h after transfection, cells were stained with PAC1 antibody to measure activation and with D57 antibody to measure surface expression. Data represent mean of 1–3 experiments ± S.E. (error bars). Details are described under “Experimental Procedures.”
Intracellular Border of Integrin αIIb TM in Fully Active State
Functional results indicated that the intracellular border of integrin αIIb TM represented mixed states of active/inactive receptors. Substitution of β3 Lys716 with Cys or Pro was shown to activate integrin αIIbβ3 close to the full activation level (16) in the HEK 293 cells. To obtain the intracellular border of αIIb TM in the active state, we co-expressed αIIb cysteine-substituted constructs with β3 K716P and subjected them to whole cell labeling with BM. Fig. 5 shows that activation of integrin αIIbβ3 had no effect on the labeling in the region of Pro998 to Glu1008; however, the region of Lys994-Arg997 was no longer labeled with BM, and the first labeled residues started with Pro998, which differs significantly from the previous results obtained in the mixed active/inactive states of the receptor. We further tested BM labeling on the αIIb cysteine-substituted constructs co-expressed with a truncated form of β3 at Lys716, which also produces a highly active state of αIIbβ3 (14). The same results were observed (data not shown). To test whether breaking of the predicted outer membrane clasp (highly activates integrin αIIbβ3) affects conformation of the region, we whole cell labeled the αIIb cysteine-substituted constructs co-expressed with β3 G708I (20). Again, we observed a similar pattern of labeling (data not shown), indicating that this conformational change in αIIb is irrespective to the breaking of either the inner or the outer membrane clasp.
FIGURE 5.
BM labeling of cysteine-substituted αIIb co-expressed with β3 K716P. A, representative results of BM labeling on integrin αIIb-substituted cysteines. The experiments were performed as described in the Fig. 3. B, summary of BM labeling of integrin αIIb-substituted cysteines. Results were analyzed same as described in Fig. 3. Data represent mean of 3–5 experiments ± S.E. (error bars). Similar labeling results were obtained on cysteine-substituted αIIb co-expressed with β3 Lys716 truncation or β3 G708I mutation.
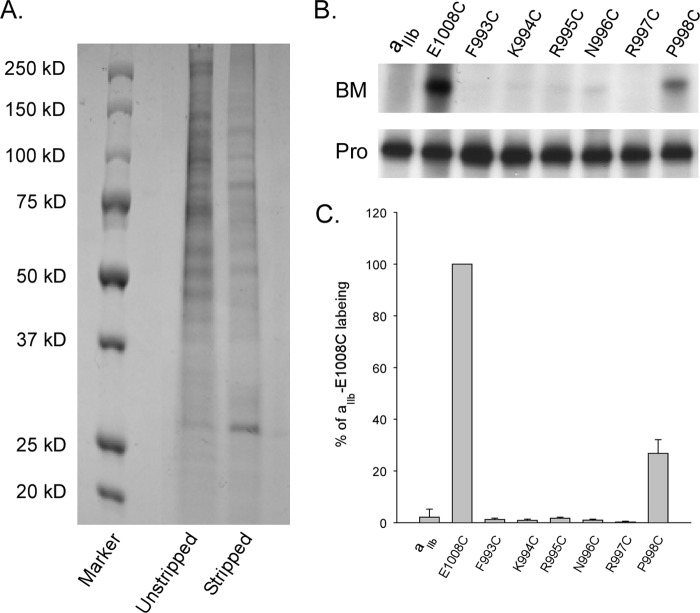
It was previously reported that the GFFKR region of αIIb may associate with intracellular peripheral proteins such as calcium- and integrin-binding protein (30) which potentially shield the substituted cysteines from being labeled. To test this, we isolated the plasma membranes from cells expressing the αIIb cysteine-substituted constructs (Phe993-Phe998) with β3 K716P and subjected to chemical stripping with 0.1 m Na2CO3 prior to BM labeling. Compared with the unstripped plasma membranes, Na2CO3 treatment effectively removed multiple protein bands from the membrane samples as shown on the Coomassie Blue-stained SDS-PAGE (Fig. 6A). Fig. 6, B and C, shows no differences in BM labeling were detected compared with the fully active state of αIIbβ3. We then tested BM labeling on the membranes stripped with 3 m Na2CO3, a stringent condition inducing both counter-ion and ionic strength that could effectively expose a deeply buried residue in the sodium bicarbonate co-transporter 1 (25). Again, we did not observe any labeling on the substituted cysteines in Phe993-Arg997 (data not shown). Interestingly, the Na2CO3-treated αIIbβ3 protein was no longer immunoprecipitated by the antibody recognizing the protein complex, but instead, by an antibody that recognizes αIIb, suggesting that a conformational change in the extracellular domain of the receptor must have been induced by the Na2CO3 treatment. Our data indicated that activation of αIIbβ3 triggers repartition of the αIIb MP region from aqueous into the lipid bilayer.
FIGURE 6.
BM labeling of cysteine-substituted αIIb co-expressed with β3-K716P after Na2CO3 stripping. A, comparison of protein samples from membranes without and with Na2CO3 stripping. Half-fraction of the isolated cell membranes was treated with 0.1 m Na2CO3 for 30 min on ice. Membranes were then pelleted by centrifugation and washed once with PBS. Membrane pellets were lysed in IPB buffer, and protein levels were determined by the BCA method. Equal amounts of protein samples were loaded on the 4–20% SDS-polyacrylamide gel. B, representative BM labeling of cysteine-substituted αIIb Phe993-Pro998 co-expressed with β3 K716P after 0.1 m Na2CO3 treatment. Similar labeling results were obtained after 3 m Na2CO3 treatment. C, summary of BM labeling. Results were analyzed the same as described in Fig. 3. Data represent mean of 3–6 experiments ± S.E. (error bars).
Intracellular Border of Integrin β3-TM in Fully Active State
The flow cytometry assay showed that none of the cysteine substitutions except K716C in the β3 TM/MP region activated αIIbβ3. Therefore, the first labeled cysteine-substituted residue, F727C, marks the intracellular border of β3 TM in αIIbβ3 inactive state in living cells. To determine the intracellular border of β3 TM in activated αIIbβ3, we co-expressed β3 cysteine-substituted constructs with αIIb F992A/F993A, a construct that fully activates the receptor (16). Fig. 3, B and C, shows that activation of αIIbβ3 did not expose any β3 endogenous cysteines to aqueous. Interestingly, F727C again remained the first residue labeled with BM, indicating its aqueous accessibility. We were somewhat surprised that no differences were observed in the β3 TM BM labeling between the active and the inactive state of αIIbβ3, and therefore we performed additional BM labeling assays on the co-expressed β3 cysteine-substituted constructs with a truncated form of αIIb at Gly991, which generates the constitutively active state of αIIbβ3 (6). Indeed, the same results were obtained (data not shown). These results suggested that β3 TM/MP regions do not repartition between lipid bilayer and aqueous medium upon the receptor activation as seen in αIIb subunit.
Aqueous Accessibility of the Predicted Membrane-anchored Integrin β3 Intracellular Tail
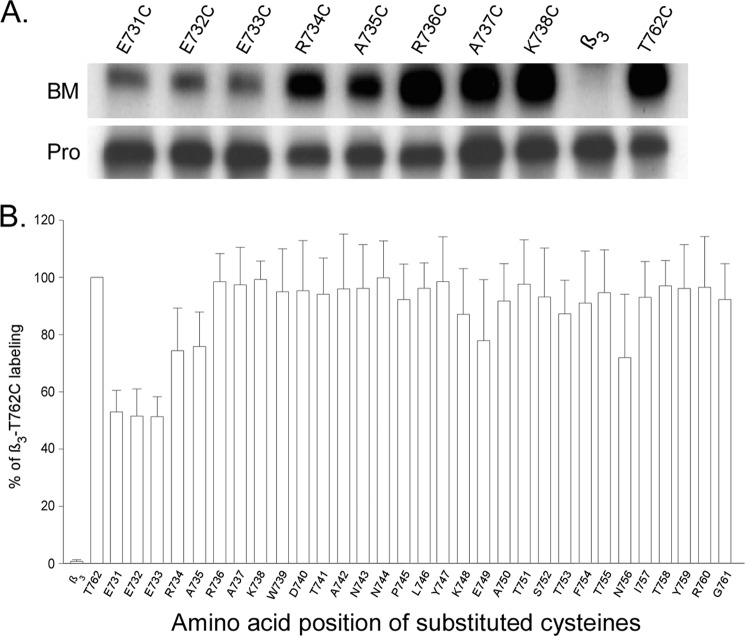
A recent NMR analysis on the complex of fragmented αIIb and β3 intracellular tails has suggested that regions of Phe727-Trp739 and Tyr747-Tyr759 in the β3 tail form membrane-anchored α-helices in loose contact with the lipid bilayer (31). To determine whether these predicted membrane-anchored helices are accessible to BM labeling, we individually substituted amino acids from Glu731 to Thr762 (in wild-type β3) with cysteines that cover the whole intracellular tail of β3. The cysteine-substituted constructs were co-expressed with wild-type αIIb in HEK 293 cells and subjected to whole cell BM labeling. Fig. 7 shows that all of the substituted cysteines are increasingly strongly labeled. Therefore, these predicted membrane-anchored helices are accessible to aqueous that differs from Leu712-Glu726 region that is embedded in the lipid bilayer.
FIGURE 7.
BM labeling of cysteine-substituted integrin β3 intracellular tail. Amino acids between Glu731 and Thr762 in the β3 subunit were individually substituted with cysteines and labeled with BM. A, representative results of BM labeling on substituted cysteines in integrin β3 intracellular tail co-expressed with wild-type αIIb. B, summary of BM labeling of cysteine-substituted β3 intracellular tail. Results were analyzed the same as described in Fig. 3. Data represent mean of 3 experiments ± S.E. (error bars).
DISCUSSION
Intracellular Borders of Integrin αIIb and β3 TMs in Living Cells
Here, we present for the first time the application of SCSAM in analyzing the conformational changes of integrin αIIbβ3, a membrane adhesion receptor in living cells. The intracellular MP region of integrin αIIbβ3 has been the target of intensive study because of its critical role in controlling the receptor inside-out activation. In this study, we have unambiguously resolved the intracellular borders of integrin αIIb and β3 TM domain in the receptor active and inactive states on the surface of living cells. Our data revealed that, unlike the results from in vitro analysis, the intracellular borders of the active state integrin αIIb and β3 TMs reside at amino acids Pro998 and Phe727, respectively. This located the proposed MP regions of both subunits in the lipid bilayer in the active state. The conclusion is supported by two sets of BM labeling experiments by using highly active mutation (K716P and G708I) or truncation (at Lys716) in the β3 subunit, and highly active mutation (F992A/F993A) or truncation (at Gly991) in the αIIb subunit that generates constitutive active states of integrin αIIbβ3.
A puzzling pattern of BM labeling was observed on the determination of αIIb TM border in the receptor inactive state. When co-expressed with wild-type β3 subunit, weak labeling was detected on αIIb K994C; strong labeling on N996C, R997C, and P998C; however, no labeling on F992C, F993C, and R995C. This result is readily explained by the functional data from the current and the previous studies (16), which showed cysteine substitution of Arg995 activated the receptor close to the maximum level in the HEK 293 cells, whereas Phe992, Phe993, Lys994, Asn996, Arg997, and Pro998 were ∼0–40% activation, indicating that >60% of the population of the mutant receptors on the cell surface were not active. Additionally, the recent Rosetta model on the αIIb MP region showed that the side chain of Lys994 points away from the dimer interface in the receptor inactive state, suggesting its accessibility to the aqueous medium (16). Therefore, labeled K994C, N996C, R997C, and P998C represent the major population of inactive receptors. Consistent with this, no BM labeling was detected on αIIb R995C co-expressed either with wild-type or activating β3 subunit because both conditions activated the receptor at a high level. Based on these observations, we concluded that the labeled K994C defines the intracellular border of αIIb TM in the inactive state. One possibility is that in the active state, intracellular proteins may bind to the unclasped αIIb tail that shields the substituted cysteines to BM labeling. We ruled out this possibility by stripping the isolated membranes with 0.1 m or 3 m Na2CO3 that removes potential bound peripheral proteins prior to BM labeling and showed no detectable differences compared with the samples without treatment.
In contrast to αIIb, determination of the β3TM intracellular border was clear and compelling because none of the cysteine substitutions in the proposed MP region except Lys716 activated the receptor. Our data showed that none of the cysteine substitutions prior to Phe727, including the highly charged 722HDRKE726 stretch, was accessible to BM labeling, suggesting their lipid embedment. Residues starting at Phe727 became strongly labeled, indicating their aqueous exposure. Therefore, we assigned the intracellular border of β3 TM in the inactive state to Phe727. Indeed, the strong labeling of the cysteine-substituted β3 tail membrane-anchored α-helices further supports that Phe727 marks the intracellular lipid/aqueous interface of β3 TM in the living cells.
Comparison with the in Vitro Determined TM Borders
Our findings differ significantly from the results obtained in the in vitro studies, including glycosylation mapping in the cell-free system (32, 33) and the recent NMR structures of αIIb (34) and β3 TM (35) peptides in the lipid bicelles, which both placed the intracellular borders of αIIb TM at Lys994 and β3 TM at Asp723. Because integrin TMs are fully separated in the active state (36), these in vitro findings may represent the TM borders of the integrin active state. Because the isolated TM fragments do not contain the intracellular clasp in the MP region and more importantly do not carry the extracellular domains, the physiologic significance is questionable. It is known that the extracellular domain of integrin αIIb and β3 forms a dimer independent of the TM domains (13), and further, dimerization of the extracellular domains proceed the dimerization of the TM domains during biosynthesis. It is conceivable that the αIIbβ3 TM domain dimerization and lipid embedding are influenced or guided by the dimerized extracellular domain. In support of this, mutations that separated the dimerized αIIbβ3 TM fragment in the lipid bicelles failed to activate the intact receptor in the cells (20), confirming that the extracellular domain is involved in stabilizing the αIIbβ3 TM dimer and that the previous in vitro observations may not be physiological.
Our study has considerable advantages over the in vitro studies because the analyses were performed on intact integrin αIIbβ3 protein in living cells. Our data showed, in contrast to the in vitro findings, that Lys994 marks the intracellular border of inactive state αIIb TM. When the receptor is active, the border shifts to Pro998, indicating a significant peptide repartitioning from aqueous medium into the lipid bilayer. The extracellular domain of integrin was shown to switch from a bent to an extended conformation in the active state (36). We suggest that the peptide repartitioning is caused by the pulling of the extended extracellular domain rather by a spontaneous repartition of the peptide itself. Indeed, upon close examination of the lipid-embedded extracellular end of αIIb TM, two amphiphilic residues (Trp997, Trp998) immediately follow the first lipid embedded residue Ile996, making it possible for them to repartition into the aqueous medium as a result of the stretching forces induced by the extracellular domain extension. In support of this, when the extracellular domain was missing, the 994KRNRP998 stretch of αIIb TM was not embedded in the lipid bilayer, indicating that spontaneous lipid repartitioning of the peptide is not favorable (32–34). This phenomenon can only be observed in intact integrin in a cellular environment.
Compared with the reported in vitro determined β3 TM intracellular border, our data are more complex: exposure to the aqueous starts at amino acid Phe727, leaving the stretch of charged residues 722HDRKE726 in the membrane. In the in vitro system, to place such a highly charged peptide in the lipid environment is energetically unfavorable. The β3 TM NMR structure showed that the TM helix terminates at Asp723 (20, 35), whereas cysteine cross-linking analyses in the intact protein suggested that the helical structure extended to Phe730 (16). Following the latter observation, our findings suggest that there is an additional helix turn at the C terminus of β3 TM in the lipid bilayer, ∼5.4 Å distant from the aqueous medium. Residue Asp723 has been shown to face the dimer interface (16, 20); therefore Arg724, Lys725, and Glu726 would be 100 degrees apart, which potentially interacts with the negatively charged phosphate groups and the positively charged choline head of membrane phospholipids, which forms ionic interactions to stabilize the end of TM in the lipid bilayer. In agreement with this, we observed that activation of the receptor had no effect on membrane embedding of this region, suggesting that it is unlikely that the C terminus of β3 TM could move out of the lipid bilayer upon activation. Lipid embedding of heavily charged peptides has been shown in the crystallized voltage sensor of the voltage-gated potassium channel (37), and potentially, these charged residues are hydrated (38).
A recent analysis of the integrin β3 TM intracellular border using a β3 TM fragment suggested that β3 Lys716 helps determine the integrin β3 TM topography (39). Removal of the positive charge at this position (active state) induced aqueous exposure of the residues in the proposed β3 MP region. Because our experiments analyzed the intact receptor in a cellular environment, the difference between our findings and this observation is potentially due to differences in the experimental approaches used.
Implications for Integrin Inside-out Activation
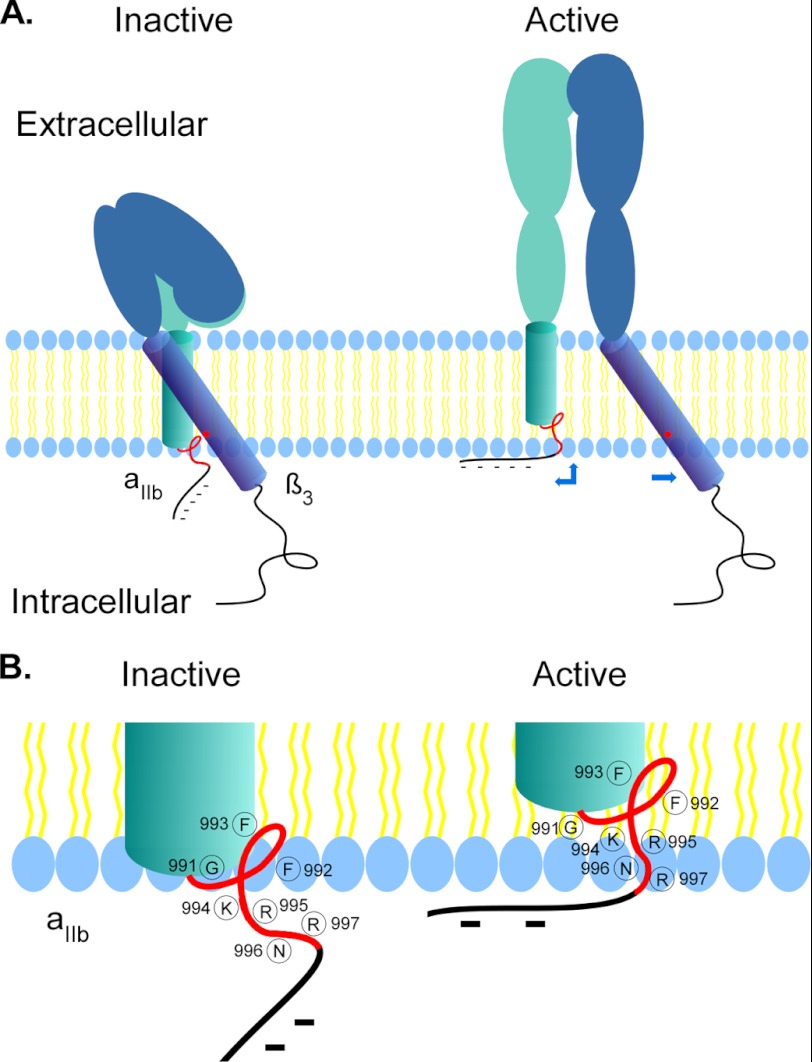
The TMs of integrin have been proposed to undergo “separation” (9), “piston” (40), or “scissors” (40, 41) movement upon inside-out activation. We found that, upon activation of integrin αIIbβ3 in the living cells, the intracellular border of αIIb TM undergoes an upward shift that repartitions into the lipid bilayer, whereas the position of β3 TM border remains unchanged. It has been shown that the αIIb TM is perpendicular in the lipid bilayer whereas β3 TM tilts at a significant angle when dimerized (16, 20). Our finding that the membrane embedded region of β3 TM extends to Glu726 suggests that the TM tilts at more extended angles. Taking our in vivo findings together with the reported in vitro TM structures (16, 20, 23), we propose a new mechanism for integrin inside-out activation (Fig. 8). In the resting state, the MP region of αIIb 991GFFKR995 interacts with β3 Lys716 forming a clasp that keeps the receptor inactive. Upon inside-out activation, the breakage of the intracellular clasp leads to extensive conformational changes in the extracellular domain from a bent to an extended conformation. Because the two TMs are dissociated, the extended αIIb extracellular domain pulls its TM upward causing four consecutive residues in the MP region repartition into the lipid bilayer. The NMR structure shows the region after αIIb Val990 has a left reverse turn rather than a helical structure; this would minimize the energy required for the peptide repartition into the lipid bilayer. Additionally, the up shift of αIIb TM would draw the negatively charged 1001EEDDEEGE1008 stretch close to the surface of lipid bilayer, which may form ionic interactions with the positively charged choline head of membrane phospholipids to serve as a “break” for the prevention of “whiplash” of αIIb extracellular domain, to keep integrin in the correct active state conformation on the surface of cells. Indeed, the unique reverse folding structure prior to the acidic stretch introduced by two prolines (998 and 999) in the αIIb tail makes the break hypothesis plausible (42).
FIGURE 8.
Proposed mechanism of integrin inside-out activation. A, schemes showing that in the inactive state, the αIIb TM (aqua) associates with β3 TM (blue) in the lipid bilayer with a clasp formed by GFFKRNR (red line) and Lys716 (red dot) at the intracellular lipid/aqueous interface. Upon inside-out activation, extension of the αIIb extracellular domain applies stretching forces on the αIIb TM, causing its upward shift that results repartition of four amino acids in the MP region (red line) into the lipid bilayer and drawing the negatively charged tail (dashed black lines) close to the inner leaflet of the plasma membrane that may form ionic interactions with the positive head groups of membrane phospholipids. B, detailed positions of the residues in the αIIb MP region involving lipid bilayer repartitioning upon inside-out activation. The structure of the αIIb MP region was adapted from Refs. 16 and 34.
Acknowledgment
We thank Dr. Mark Ginsberg for helpful discussions.
This work was supported by the Factor Foundation.
- TM
- transmembrane domain
- BM
- biotin maleimide (3-(N-maleimidylpropionyl) biocytin)
- MP
- membrane-proximal
- SCSAM
- substituted cysteine scanning accessibility method.
REFERENCES
- 1. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2. Anthis N. J., Campbell I. D. (2011) The tail of integrin activation. Trends Biochem. Sci. 36, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shattil S. J., Kim C., Ginsberg M. H. (2010) The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell. Biol. 11, 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wegener K. L., Campbell I. D. (2008) Transmembrane and cytoplasmic domains in integrin activation and protein-protein interactions. Mol. Membr. Biol. 25, 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Toole T. E., Katagiri Y., Faull R. J., Peter K., Tamura R., Quaranta V., Loftus J. C., Shattil S. J., Ginsberg M. H. (1994) Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 124, 1047–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Toole T. E., Mandelman D., Forsyth J., Shattil S. J., Plow E. F., Ginsberg M. H. (1991) Modulation of the affinity of integrin αIIbβ3 (GPIIb-IIIa) by the cytoplasmic domain of αIIb. Science 254, 845–847 [DOI] [PubMed] [Google Scholar]
- 7. Takagi J., Petre B. M., Walz T., Springer T. A. (2002) Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611 [DOI] [PubMed] [Google Scholar]
- 8. Adair B. D., Yeager M. (2002) Three-dimensional model of the human platelet integrin αIIbβ3 based on electron cryomicroscopy and x-ray crystallography. Proc. Natl. Acad. Sci. U.S.A. 99, 14059–14064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim M., Carman C. V., Springer T. A. (2003) Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720–1725 [DOI] [PubMed] [Google Scholar]
- 10. Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. (2008) Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325–330 [DOI] [PubMed] [Google Scholar]
- 11. Moser M., Legate K. R., Zent R., Fässler R. (2009) The tail of integrins, talin, and kindlins. Science 324, 895–899 [DOI] [PubMed] [Google Scholar]
- 12. Ma Y. Q., Qin J., Wu C., Plow E. F. (2008) Kindlin-2 (Mig-2): a co-activator of β3 integrins. J. Cell Biol. 181, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu J., Luo B. H., Xiao T., Zhang C., Nishida N., Springer T. A. (2008) Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell 32, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes P. E., O'Toole T. E., Ylänne J., Shattil S. J., Ginsberg M. H. (1995) The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J. Biol. Chem. 270, 12411–12417 [DOI] [PubMed] [Google Scholar]
- 15. Hughes P. E., Diaz-Gonzalez F., Leong L., Wu C., McDonald J. A., Shattil S. J., Ginsberg M. H. (1996) Breaking the integrin hinge: a defined structural constraint regulates integrin signaling. J. Biol. Chem. 271, 6571–6574 [DOI] [PubMed] [Google Scholar]
- 16. Zhu J., Luo B. H., Barth P., Schonbrun J., Baker D., Springer T. A. (2009) The structure of a receptor with two associating transmembrane domains on the cell surface: integrin αIIbβ3. Mol. Cell 34, 234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J., Ma Y. Q., Page R. C., Misra S., Plow E. F., Qin J. (2009) Structure of an integrin αIIbβ3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proc. Natl. Acad. Sci. U.S.A. 106, 17729–17734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vinogradova O., Velyvis A., Velyviene A., Hu B., Haas T., Plow E., Qin J. (2002) A structural mechanism of integrin αIIbβ3 “inside-out” activation as regulated by its cytoplasmic face. Cell 110, 587–597 [DOI] [PubMed] [Google Scholar]
- 19. Vinogradova O., Vaynberg J., Kong X., Haas T. A., Plow E. F., Qin J. (2004) Membrane-mediated structural transitions at the cytoplasmic face during integrin activation. Proc. Natl. Acad. Sci. U.S.A. 101, 4094–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lau T. L., Kim C., Ginsberg M. H., Ulmer T. S. (2009) The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 28, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim C., Lau T. L., Ulmer T. S., Ginsberg M. H. (2009) Interactions of platelet integrin αIIb and β3 transmembrane domains in mammalian cell membranes and their role in integrin activation. Blood 113, 4747–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gottschalk K. E. (2005) A coiled-coil structure of the αIIbβ3 integrin transmembrane and cytoplasmic domains in its resting state. Structure 13, 703–712 [DOI] [PubMed] [Google Scholar]
- 23. Wegener K. L., Partridge A. W., Han J., Pickford A. R., Liddington R. C., Ginsberg M. H., Campbell I. D. (2007) Structural basis of integrin activation by talin. Cell 128, 171–182 [DOI] [PubMed] [Google Scholar]
- 24. Zhu Q., Lee D. W., Casey J. R. (2003) Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J. Biol. Chem. 278, 3112–3120 [DOI] [PubMed] [Google Scholar]
- 25. Zhu Q., Kao L., Azimov R., Newman D., Liu W., Pushkin A., Abuladze N., Kurtz I. (2010) Topological location and structural importance of the NBCe1-A residues mutated in proximal renal tubular acidosis. J. Biol. Chem. 285, 13416–13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karlin A., Akabas M. H. (1998) Substituted-cysteine accessibility method. Methods Enzymol. 293, 123–145 [DOI] [PubMed] [Google Scholar]
- 27. Zhu Q., Casey J. R. (2007) Topology of transmembrane proteins by scanning cysteine accessibility mutagenesis methodology. Methods 41, 439–450 [DOI] [PubMed] [Google Scholar]
- 28. Zhu Q., Kao L., Azimov R., Abuladze N., Newman D., Pushkin A., Liu W., Chang C., Kurtz I. (2010) Structural and functional characterization of the C-terminal transmembrane region of NBCe1-A. J. Biol. Chem. 285, 37178–37187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan B., Smith J. W. (2000) A redox site involved in integrin activation. J. Biol. Chem. 275, 39964–39972 [DOI] [PubMed] [Google Scholar]
- 30. Barry W. T., Boudignon-Proudhon C., Shock D. D., McFadden A., Weiss J. M., Sondek J., Parise L. V. (2002) Molecular basis of CIB binding to the integrin αIIb cytoplasmic domain. J. Biol. Chem. 277, 28877–28883 [DOI] [PubMed] [Google Scholar]
- 31. Metcalf D. G., Moore D. T., Wu Y., Kielec J. M., Molnar K., Valentine K. G., Wand A. J., Bennett J. S., DeGrado W. F. (2010) NMR analysis of the αIIbβ3 cytoplasmic interaction suggests a mechanism for integrin regulation. Proc. Natl. Acad. Sci. U.S.A. 107, 22481–22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armulik A., Nilsson I., von Heijne G., Johansson S. (1999) Determination of the border between the transmembrane and cytoplasmic domains of human integrin subunits. J. Biol. Chem. 274, 37030–37034 [DOI] [PubMed] [Google Scholar]
- 33. Stefansson A., Armulik A., Nilsson I., von Heijne G., Johansson S. (2004) Determination of N- and C-terminal borders of the transmembrane domain of integrin subunits. J. Biol. Chem. 279, 21200–21205 [DOI] [PubMed] [Google Scholar]
- 34. Lau T. L., Dua V., Ulmer T. S. (2008) Structure of the integrin αIIb transmembrane segment. J. Biol. Chem. 283, 16162–16168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lau T. L., Partridge A. W., Ginsberg M. H., Ulmer T. S. (2008) Structure of the integrin β3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry 47, 4008–4016 [DOI] [PubMed] [Google Scholar]
- 36. Luo B. H., Carman C. V., Springer T. A. (2007) Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Long S. B., Tao X., Campbell E. B., MacKinnon R. (2007) Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 [DOI] [PubMed] [Google Scholar]
- 38. Krepkiy D., Mihailescu M., Freites J. A., Schow E. V., Worcester D. L., Gawrisch K., Tobias D. J., White S. H., Swartz K. J. (2009) Structure and hydration of membranes embedded with voltage-sensing domains. Nature 462, 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim C., Schmidt T., Cho E. G., Ye F., Ulmer T. S., Ginsberg M. H. (2012) Basic amino acid side chains regulate transmembrane integrin signalling. Nature 481, 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams M. J., Hughes P. E., O'Toole T. E., Ginsberg M. H. (1994) The inner world of cell adhesion: integrin cytoplasmic domains. Trends Cell Biol. 4, 109–112 [DOI] [PubMed] [Google Scholar]
- 41. Kalli A. C., Campbell I. D., Sansom M. S. (2011) Multiscale simulations suggest a mechanism for integrin inside-out activation. Proc. Natl. Acad. Sci. U.S.A. 108, 11890–11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vinogradova O., Haas T., Plow E. F., Qin J. (2000) A structural basis for integrin activation by the cytoplasmic tail of the αIIb subunit. Proc. Natl. Acad. Sci. U.S.A. 97, 1450–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]