Background: β-Adrenergic receptors stimulate cardiac ICa via PKA-dependent phosphorylation.
Results: Deletion of the C-terminal phosphorylation sites in the β2 gene did not affect isoproterenol-stimulated ICa.
Conclusion: Phosphorylation of the C terminus of the β2 subunit in vivo does not contribute to β-adrenergic regulation of ICa.
Significance: The PKA-dependent regulation of ICa does not require the C terminus of the β2 subunit.
Keywords: Adrenergic Receptor, Calcium Channels, Heart, Phosphorylation, Protein Kinase A (PKA), Calcium-dependent Facilitation (CDF), Calcium-dependent Inhibition (CDI)
Abstract
Phosphorylation of the cardiac β subunit (Cavβ2) of the Cav1.2 L-type Ca2+ channel complex has been proposed as a mechanism for regulation of L-type Ca2+ channels by various protein kinases including PKA, CaMKII, Akt/PKB, and PKG. To test this hypothesis directly in vivo, we generated a knock-in mouse line with targeted mutation of the Cavβ2 gene by insertion of a stop codon after proline 501 in exon 14 (mouse sequence Cacnb2; βStop mouse). This mutation prevented translation of the Cavβ2 C terminus that contains the relevant phosphorylation sites for the above protein kinases. Homozygous cardiac βStop mice were born at Mendelian ratio, had a normal life expectancy, and normal basal L-type ICa. The regulation of the L-type current by stimulation of the β-adrenergic receptor was unaffected in vivo and in cardiomyocytes (CMs). βStop mice were cross-bred with mice expressing the Cav1.2 gene containing the mutation S1928A (SAβStop) or S1512A and S1570A (SFβStop) in the C terminus of the α1C subunit. The β-adrenergic regulation of the cardiac ICa was unaltered in these mouse lines. In contrast, truncation of the Cav1.2 at Asp1904 abolished β-adrenergic up-regulation of ICa in murine embryonic CMs. We conclude that phosphorylation of the C-terminal sites in Cavβ2, Ser1928, Ser1512, and Ser1570 of the Cav1.2 protein is functionally not involved in the adrenergic regulation of the murine cardiac Cav1.2 channel.
Introduction
The cardiac L-type Ca2+ current (ICa) is regulated by a number of protein kinases including PKA, CaMKII,2 PKC, Akt/PKB, and PKG (1, 2). Regulation of the cardiac Cav1.2 channel by β-adrenoceptors, cAMP, and PKA has been implicated as basic mechanism of the fight or flight reaction of an animal (3, 4). Phosphorylation of some channel subunits plays a critical role in several physiological cardiac processes, e.g. excitation-contraction coupling, the regulation of positive inotropy and chronotropy, as well as pathological processes such as heart failure (for review, see 2, 5). The molecular basis of ICa regulation by protein kinases could not be defined conclusively so far because expression studies suggested phosphorylation sites on the α1 subunit and the β2 subunit of the L-type calcium channel. Phosphorylation sites on the α1 subunit were invoked for PKA (6–8), CaMKII (9–11), PKG (12, 13), Akt/PKB (14, 15), and by PKC (16–19). In addition, phosphorylation sites in the Cavβ2 subunit were reported for PKA (20) at Ser479/480 (rabbit protein sequence (rbs)) (21), CaMKII (22) at Thr500 (rbs), PKG (12) at Ser496 (rbs), and Akt/PKB (14, 15, 23, 24) at Ser576 (rbs). The amino acids modified by protein kinases in Cavβ2 or Cav1.2 in the protein sequence from rabbit, rat, and mouse are listed in supplemental Table 1.
This very impressive work of several groups missed a clear statement, if the phosphorylation of one subunit was necessary to regulate ICa in vivo. Previously, we investigated whether phosphorylation of Ser1928 of the α1 subunit was a necessary step for the β-adrenergic regulation of the cardiac ICa in vivo. Mutation of the Ser to Ala did not affect the β-adrenergic regulation (25), raising the possibility that phosphorylation of the Cavβ2 subunit by PKA (20) might be the requested regulatory step. Therefore, we generated a mouse line that contained a stop codon in exon 14 of the mouse Cacnb2 gene after Pro501 (βStop). This stop codon resulted in a truncated β2 subunit protein that lacked the potential phosphorylation sites for PKA, PKG, Akt/PKB, and CaMKII. Basal properties of the Cav1.2 current were unaffected as expected from the report that deletion of the Cacnb2 gene in the adult heart has minimal effects on ICa (26). We crossed the βStop line with the S1928A (25) and SF (S1512A and S1570A) (27) mouse lines that contain well characterized phosphorylation sites for PKA and CaMKII, respectively. Again, the basal properties of the Cav1.2 current were unaffected, suggesting that β-adrenergic regulation of the Cav1.2 channel may be mediated by other phosphorylation sites, e.g. Ser1700 of the α1 subunit (8).
EXPERIMENTAL PROCEDURES
All substances used were of the highest purity available. Amino acid numbering is according to the Mus musculus Cacnb2 sequence (GenBank accession number Q8CC27) or to the Oryctolagus cuniculus (rabbit) Cacnb2 sequence (GenBank accession number X64297). The amino acids modified by protein kinases in Cavβ2 or Cav1.2 in the protein sequence from rabbit, rat, and mouse are listed in supplemental Table 1. Within this paper we refer to the amino acid modified in the rabbit sequence of GenBank, X64297.
Generation of Mice Lacking the C Terminus of Cavβ2
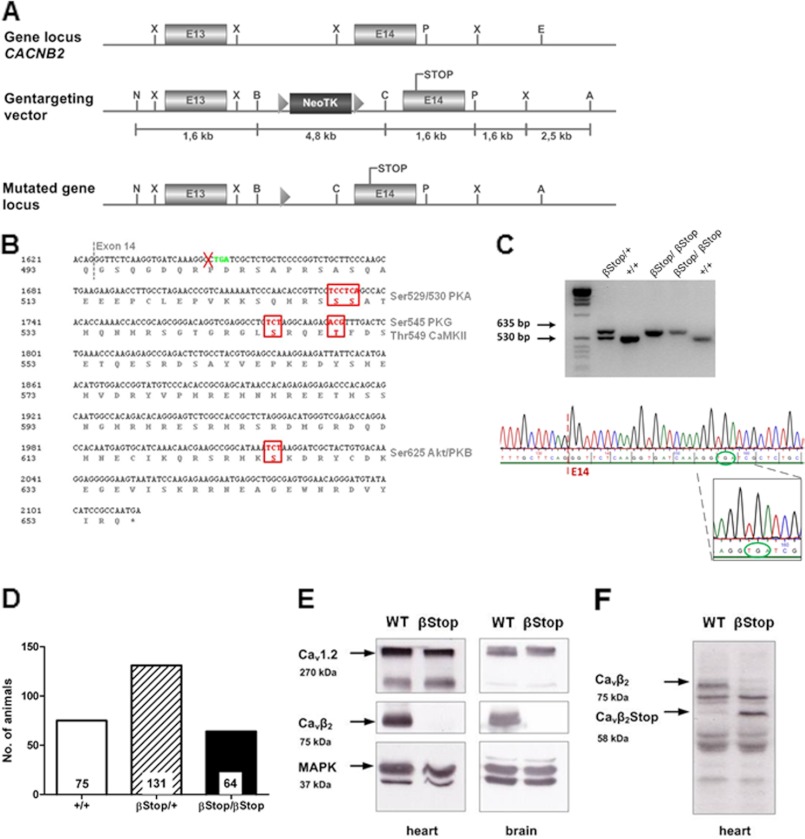
To construct the targeting vector, a 7.3-kb fragment containing exons 13–14 of CACNB2 was isolated from 129/Sv mouse genomic DNA. The targeting vector included a 1.6-kb short arm and 5.7-kb long arm with PGK-neo and the thymidine kinase gene (tk) flanked by two loxP sites. The 3′-side long arm contained exon 14 with the stop codon TGA in-frame after Pro501 and the phosphorylation sites Ser529/530 (corresponding to Ser479/480 rbs), Ser545 (corresponding to Ser496 rbs), Thr549 (corresponding to Thr498 rbs), and Ser625 (corresponding to Ser574 rbs) behind the stop codon (see supplemental Table 1). All mutation procedures were carried out by QuikChange II site-directed mutagenesis (Stratagene). The targeting construct was electroporated into R1 ES cells (129/Sv×129/Sv-CP F1). Positive clones were identified by PCR and confirmed by Southern blotting using a probe on the neo gene. One positive clone was detected and injected in C57BL/6 blastocysts. Chimeras were crossed to C57BL/6 mice. By crossing with a Cre-recombinase expressing transgenic B6.C-Tg (CMV-cre)1Cgn/J mouse strain, the neo tk marker genes were excised. Heterozygous mice were bred to produce homozygotes. The intercross of heterozygotes resulted in production of wild-type, heterozygous, and homozygous offspring at almost the expected Mendelian ratio (75:131:64). For all analyses, filial generation 2 (F2) mice with 129/Sv and C57BL/6 hybrid genetic background were used. All procedures relating to animal care and treatment were authorized by the “Regierung von Oberbayern” and conformed to the institutional, governmental, Directive 2010/63/EU of the European Parliament guidelines and to the Care and Use of Laboratory Animals published by the US National Institutes of Health. Anesthetized mice (1.5% isoflurane) were euthanized by cervical dislocation.
Antibodies
The anti-Cav1.2 and anti-Cavβ2v2 antibodies have been described previously (28). The anti-Cavβ2-N4/1195 antibody was a kind gift from Prof. Flockerzi (Universität des Saarlandes). The antibody against MAPK (p44/42) was obtained from Cell Signaling.
Membrane Preparation and Immunoblotting
Frozen heart and brain tissue were pestled to a fine powder and homogenized in membrane preparation buffer (20 mm EDTA, 20 mm EGTA, 10 mm Tris, 300 mm NaCl, pH 7.4, inhibitors per ml buffer: 8 μg of calpain inhibtor I (Roche Applied Science), 8 μg of calpain inhibitor II (Roche Applied Science), 1 μl of phenylmethylsulfonyl fluoride (PMSF; Fluka), and 2 μl of protease inhibitor mixture (Sigma)). Cell organelles were separated by centrifugation, the supernatant containing the membrane proteins was centrifuged at 100,000 × g for 30 min, and the pellet was solubilized in deoxycholate buffer (20 mm EDTA, 10 mm EGTA, 10 mm Tris-HCl, pH 7.4), 1% deoxycholate) for 20 min. Membrane proteins were separated by centrifugation at 100,000 × g for 30 min. The supernatant was aliquoted and stored at −80 °C, and protein concentration was measured according to the BCA method (Pierce). 50 μg of protein were separated per lane on 10% SDS-polyacrylamide gels, blotted, and probed with antibodies by using a chemiluminescence detection system.
Cell Isolation
Ventricular myocytes were isolated as described (AfCS Procedure Protocol PP00000125), maintained at 37 °C, and aerated with 98% O2, 2% CO2. Embryonic ventricular myocytes were isolated as described in Ref. 29.
Electrophysiological Recordings
Whole cell ICa was measured at room temperature from rod-shaped, striated, calcium-tolerant myocytes within 1–24 h after isolation. Stimulation and data acquisition were performed as described in Refs. 27, 30, 31. Facilitation of ICa was measured during a triple pulse protocol with a 200-ms control pulse to 0 mV (V1) followed by a 200-ms prepulse (Vpre) to +80 mV followed by 200-ms test pulse to 0 mV (V2) (27). The extent of voltage-dependent facilitation was calculated as the ratio of the peak current during V2 and V1. Time constants of ICa inactivation were obtained by a fit from peak current to the current value at the end of the voltage pulse by a two-exponential function using pClamp 9. Facilitation of ICa was measured as described in Ref. 30. The stimulatory effect of isoproterenol (100 nmol/liter containing an equal concentration of ascorbic acid) on ICa was examined after establishing a solid base line. Stimulation of ICa by isoproterenol was measured at a membrane potential of ±0 mV and is given as percentage of control (= 100%) determined before superfusion with isoproterenol. All fits showed a correlation coefficient >0.98.
Telemetric Electrocardiogram (ECG) Recordings
Radiotelemetric ECG transmitters (ETA-F20; DSI, St. Paul, MN) were implanted into the peritoneal cavity under general anesthesia with isoflurane/O2. The ECG leads were sutured subcutaneously onto the upper right chest muscle and the upper left abdominal wall muscle. The animals were allowed to recover for 2 weeks before the experiments. Isoproterenol (0.1 mg/kg mouse; Sigma) or phenylephrine (3 mg/kg mouse; Sigma) was dissolved in 0.9% NaCl. After 15 min of base-line recording, the mice were injected intraperitoneally with the drugs. The ECGs were recorded for 45 min thereafter. The animals were allowed to recover for at least 48 h between experiments. Data were acquired using the DSI acquisition system.
Echocardiography
Images were obtained using a Vevo 770 Visual Sonics scanner equipped with a 30-MHz probe (Visual Sonics Inc., Toronto, ON, Canada). The mice were lightly anesthetized (1.5% isoflurane) and anchored to a warming platform in dorsal position, and ECG limb electrodes were placed. The chests were shaved and cleaned to minimize ultrasound attenuation. Fractional shortening (FS, the diameter at the end of systole minus the diameter at the end of diastole divided by the diameter at the end-diastole) was assessed from the M mode of the parasternal short axis view. Control and mice carrying the various mutations were studied before and after administration of isoproterenol (0.1 mg/kg mouse intraperitoneally).
Statistics
Data are presented as mean ± S.E. Statistical significance was tested by using a (two-tailed) unpaired Student's t test. The null hypothesis was rejected if p < 0.05.
RESULTS
We report the generation of a mouse line in which the β2 subunit of the Cav1.2 channel complex (Cavβ2) was truncated at Pro501 (βStop mice). For this purpose, we used a gene-targeting strategy that utilized a replacement vector containing a stop codon after proline 501 in exon 14 and a neo tk gene cassette flanked by loxP sites (Fig. 1A). The Cavβ2 C terminus was truncated to prevent the expression of several putative phosphorylation sites (PKA Ser479/480 rbs, PKG Ser496 rbs, CaMKII Thr498 rbs, and Akt/PKB Ser574 rbs; see supplemental Table 1) and to test the physiological relevance of these sites (Fig. 1B). All homozygous βStop mutants analyzed were chimeric F2 mice (mixed sv129 and C57BL/6 background). βStop mice were compared with litter-matched control mice (Ctr βStop). Nomenclature and genotype of mouse lines are outlined in supplemental Table 2. The correct genomic localization (supplemental Fig. 1) and mutation (Fig. 1C) in βStop mice was confirmed by Southern blotting and genomic sequencing. βStop mice were viable, fertile, and reproduced in a 1:2:1 Mendelian ratio (WT 27.7%, heterozygous Ctr βStop 48.5%, βStop 23.7%) (Fig. 1D). Western blot analysis of heart and brain membrane fraction showed no alterations in Cav1.2 expression. The expression of the C-terminal truncated Cavβ2 protein was confirmed by the C-terminal binding Cavβ2v2 antibody and the N-terminal binding antibody Cavβ2-N4/1195. The C-terminal binding Cavβ2v2 antibody detected the 75-kDa WT Cavβ2 protein, but not the truncated Cavβ2 protein (Fig. 1E), whereas the N-terminal binding Cavβ2-N4/1195 antibody detected both the truncated Cavβ2 protein (58 kDa) and the WT protein (75 kDa) (Fig. 1F). These results show that the βStop mouse expressed the truncated Cavβ2 protein that missed the reported phosphorylation sites.
FIGURE 1.
Generation and biochemical analysis of βStop mice. A, gene targeting strategy of the βStop mouse. Top, genomic DNA structure of CACNB2 with the relevant restriction enzyme sites; boxes represent exons 13 and 14 encoding the C terminus of Cavβ2. Middle, targeting vector. Neo, neomycin-resistance gene; TK, thymidine kinase gene with loxP sequence (triangles) at both sides. The insertion of the stop codon after proline 501 is shown. Bottom, knock-in locus after homologous recombination and Cre-mediated deletion of resistance markers. N, NotI; B, BamHI; C, ClaI; X, XhoI; P, PstI; E, EcoRI; A, Acc65I. B, location of PKA, PKG, CaMKII, and Akt/PKB phosphorylation sites in exon 14 of the murine Cavβ2 protein. The amino acid sequence is according to M. musculus CACNB2 sequence (GenBank accession number Q8CC27), and the nucleotide sequence is according to M. musculus CACNB2 sequence (GenBank accession number NM_023116.4). The phosphorylation sites (right) are those of the mouse amino acid sequence. C, genotyping of a Cavβ2Stop litter (635-bp βStop band, 530-bp WT band) and sequence analysis of the βStop knock-in mice. The sequence shows exon 14 of a homozygous βStop offspring. The stop mutation is in-frame within exon 14. PCR primers bind 5′ and 3′ of the loxP site remaining after Cre recombination. The primers amplify a 635-bp fragment in CavβStop DNA (including one loxP site after Cre recombination) or a 530-bp fragment in WT DNA (without loxP site). D, Mendelian ratio at birth of the Cavβ2Stop/WT strain. E and F, Western blot analysis of heart and brain membrane fractions on 10% SDS PAGE; 50 μg of protein was applied per lane. E, Cav1.2 and Cavβ2 protein expression. The truncated Cavβ2 protein is not detected by the common Cavβ2v2 antibody which binds C-terminal of the Stop mutation; loading control, MAPK. F, detection of the Cavβ2Stop protein with the N-terminal binding Cavβ2v2-N4/1195 antibody.
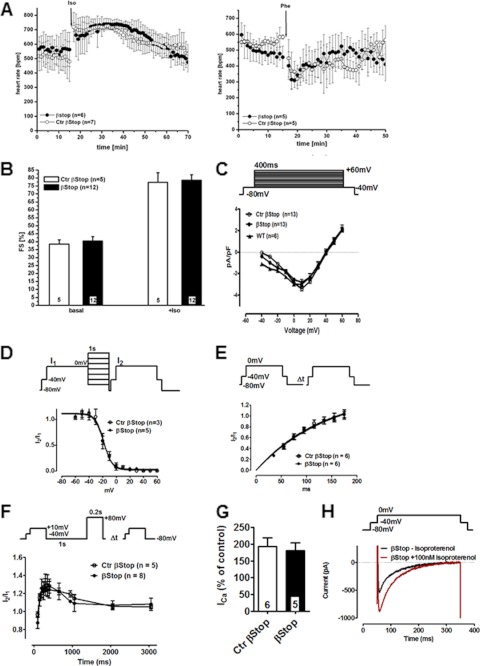
Telemetric ECG measurement of heart rate (HR) and activity revealed no differences in WT and βStop mice (supplemental Fig. 2). Both genotypes showed a typical cardiac response to isoproterenol and phenylephrine administration with an increase and a drop in HR, respectively (Fig. 2A). FS was identical in Ctr βstop and βStop mice (Fig. 2B). Isoproterenol doubled FS in both genotypes. These data indicate that the putative PKA phosphorylation sites Ser479/480 rbs of the Cavβ2 subunit are not necessary to observe the positive inotropic, β-adrenergic regulation of the heart muscle.
FIGURE 2.
βStop mutation does not prevent positive inotropic heart regulation. A, normal regulation of beating frequency by isoproterenol (0.1 mg/kg of body weight intraperitoneally) and phenylephrine (0.3 mg/kg of body weight intraperitoneally). B, FS unchanged in βStop mice before and after isoproterenol administration (0.1 mg/kg of body weight intraperitoneally). C, I/V relation of WT, Ctr βStop, and βStop CMs. D, steady-state inactivation of Ctr βStop and βStop CMs. E, recovery from inactivation of Ctr βStop and βStop CMs. F, unchanged CDF in Ctr βStop and βStop CMs. G, statistics of isoproterenol (0.1 μm) stimulation of ICa in Ctr βStop and βStop CMs. H, ICa trace ± isoproterenol (0.1 μm) in a βStop CM. Number of animal/cells is given in columns or within figures. The voltage protocol is depicted above the corresponding figure.
To further support the insignificant effect of the Cavβ2 truncation for cardiac β-adrenergic regulation, patch clamp experiments were carried out on isolated cardiomyocytes (CMs). Isolated CMs of either genotype had normal size (WT, 161.2 ± 22 pF n = 6; Ctr βStop, 170 ± 14 pF, n = 13; βStop, 149 ± 8 pF, n = 13), normal ICa density at +10 mV (WT, 2.99 ± 0.25pA/pF, n = 6; Ctr βStop, 3.39 ± 0.2 pA/pF, n = 13; βStop, 3.01 ± 0.2 pA/pF, n = 13), half-maximal activation constants (WT, −21.7 ± 1.6 mV, n = 4; Ctr βStop −16.3 ± 1.7 mV, n = 8; βStop −14.1 ± 0.9 mV, n = 9) and a normal I-V relation (Fig. 2C).3 As expected from the results of Meissner et al. (26), further analysis showed normal half-maximal steady state inactivation (Fig. 2D) (V0.5: Ctr βStop, −18.2 ± 1.6 mV (n = 3); βStop, −18.7 ± 1.2 mV (n = 5)), and normal half-maximal recovery time from inactivation (Fig. 2E) (τ1/2 (ms): Ctr βStop, 135. 3 (n = 6); βStop, 147.6 (n = 6)).
It is widely accepted that calcium-dependent facilitation (CDF) is caused by activation of CaMKII followed by phosphorylation of a component of the Cav1.2 channel complex. Recently, we reported that CDF requires phosphorylation of Cav1.2 at Ser1512/1570 (27). In contrast, Colbran and co-workers (22) reported that phosphorylation of Cavβ2 by CaMKII at Thr500 modulated CDF. Because Thr500 was not present anymore in the βStop protein, we tested whether or not the truncation of the Cavβ2 C terminus might affect ICa facilitation. We compared prepulse facilitation of ICa in CMs of both genotypes. CDF was not affected by the truncation of the Cavβ2 protein (Fig. 2F), suggesting that phosphorylation of the Cavβ2 subunit is not a necessary prerequisite to induce CDF under basal conditions.
In agreement with the ECG results, isoproterenol stimulated ICa of Ctr βStop and βStop CMs to the same level (Fig. 2G). Representative current traces for a βStop CM are shown in Fig. 2H. Isoproterenol treatment increased ICa in Ctr βStop CMs by 193 ± 25% and in βStop CMs by 180 ± 24% (Fig. 2G). Furthermore, there was no change in the slow or fast component of inactivation either with or without isoproterenol stimulation (supplemental Table 3). The fast component of inactivation describes Ca2+-dependent inactivation (CDI), the slow component the voltage-dependent inactivation. Neither inactivation pathway is affected by the C-terminal truncation of the Cavβ2 protein.
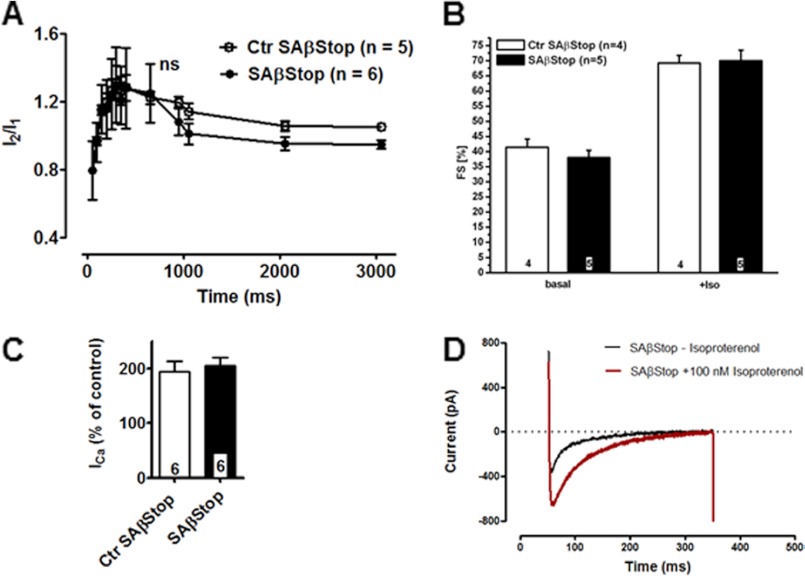
These negative experiments raised the possibility that the positive inotropic effect was mediated by phosphorylation of both the Cav1.2 and Cavβ2 subunit. We tested this hypothesis by cross-breeding the βStop line with the Cav1.2SA or the Cav1.2SF lines. The Cav1.2SA mouse line expresses a Cav1.2 channel containing the mutation S1928A (25). Mice homozygous for the double mutation Cav1.2S1928A/Cav1.2S1928A, Cavβ2P501Stop/Cavβ2P501Stop (SAβStop) had the same size and weight as the heterozygous litters. Diurnal cardiac rhythm was not altered in these mice (supplemental Fig. 3). The cell capacitance of Ctr SAβStop and double knock-in SAβStop CMs was the same (Ctr SAβStop: 168.6 ± 12 pF (n = 15); SAβStop: 163.0 ± 18 pF (n = 6)). Inactivation time constants of ICa were not affected by this double mutation (supplemental Table 3). We did not observe an effect of the double mutation on CDF (Fig. 3A). Isoproterenol stimulated FS (Fig. 3B) and HR (supplemental Fig. 4) in both mouse lines to the same extent. No statistically significant difference was noted between the curves. Phenylephrine decreased the HR to the same extent in both genotypes (supplemental Fig. 4). Stimulation of the corresponding CMs by 100 nm isoproterenol increased ICa by 194.3 ± 19.2% (n = 6) and 205.3 ± 14% (n = 6) in heterozygous Ctr SAβStop and homozygous SAβStop, respectively (Fig. 3, C and D).
FIGURE 3.
Unchanged β-adrenergic regulation of ICa in SAβStop mice. A, unchanged CDF in Ctr SAβStop and SAβStop CMs. B, FS unchanged in βStop mice before and after isoproterenol administration (0.1 mg/kg of body weight intraperitoneally). C, statistics of isoproterenol (0.1 μm) stimulation of ICa in Ctr SAβStop and SAβStop CMs. D, ICa trace ± isoproterenol (0.1 μm) in a SAβStop CMs. Number of animal/cells is given in columns or within figures.
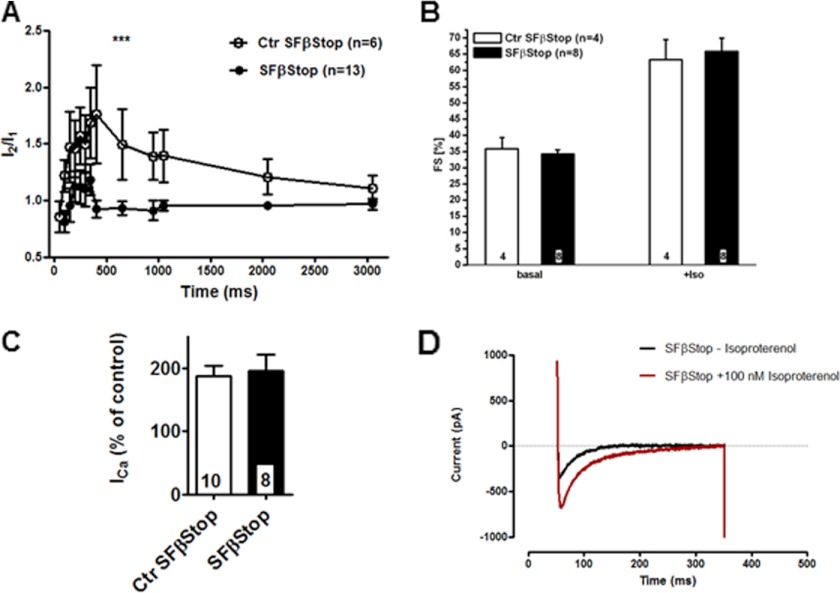
In the next series of experiments we tested the double mutation Cav1.2S1512/1570A/Cav1.2S1512/1570A, Cavβ2P501Stop/Cavβ2P501Stop (SFβStop). Mice homozygous for the double mutation SFβStop had the same size and weight as the heterozygous litters. Diurnal cardiac rhythm was not altered in these mice (supplemental Fig. 3). The cell capacitance of Ctr SFβStop and double knock-in SFβStop CMs was the same (Ctr SFβStop: 213 ± 13 pF (n = 9); SFβStop: 195 ± 17 pF (n = 8)). Inactivation time constants of ICa were not affected by this double mutation (supplemental Table 3). As shown for the SF mice (27), CDF was also significantly decreased in the SFβStop mice (Fig. 4A).
FIGURE 4.
Unchanged β-adrenergic regulation of ICa in SFβStop mice. A, CDF in SFβStop CMs decreased compared with Ctr SFβStop. B, FS unchanged in βStop mice before and after isoproterenol administration (0.1 mg/kg of body weight intraperitoneally). C, statistics of isoproterenol (0.1 μm) stimulation of ICa in Ctr SFβStop and SFβStop CMs. D, ICa trace ± isoproterenol (0.1 μm) in a SFβStop CM. Number of animal/cells is given in columns or within figures.
Isoproterenol stimulated FS (Fig. 4B) and HR (supplemental Fig. 5) in both mouse lines to the same extent. Phenylephrine decreased the HR to the same extent in both genotypes (supplemental Fig. 5). Stimulation of these CMs by 100 nm isoproterenol increased ICa by 187 ± 17% (n = 10) and 196 ± 26% (n = 8) in the heterozygous Ctr SFβStop and homozygous SFβStop line, respectively (Fig. 4, C and D). We concluded from this analysis that neither double mutation affected the β-adrenergic stimulation of FS in the intact mouse nor that of ICa in the CMs.
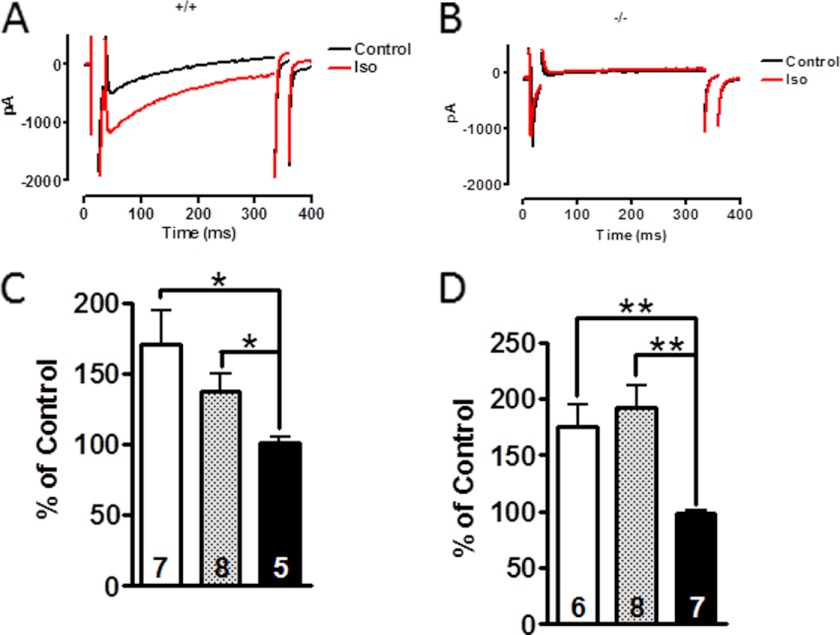
The results presented so far suggested that none of the mutated potential PKA or CaMKII phosphorylation sites was necessary for β-adrenergic stimulation of the cardiac ICa. PKA needs to bind to the L-type channel complex through a PKA-anchoring protein (AKAP) before it can phosphorylate the necessary subunit. The CMs contain several AKAPs that may be an essential part of the β-adrenergic regulation (32). Disruption of AKAP5 interfered with β-adrenergic-stimulated intracellular Ca2+ transients but not with ICa (33). AKAPs bind to the C terminus of Cav1.2 between amino acids 2026 and 2085. This sequence was not modified in the mouse lines studied, suggesting that PKA was still targeted to the β-adrenergic-regulated site. Truncation of the Cav1.2 sequence at Asp1904 (31) or at Gly1796 (34) leads to a channel that does not bind any more AKAPs. In contrast to a previous in vitro study (20) but in agreement with Fu et al. (34), ICa of embryonic Cav1.2Stop CMs is not stimulated anymore by isoproterenol (Fig. 5, A and C) or forskolin (Fig. 5, B and D), suggesting that the amino acids C-terminal to Asp1904 are essential for the adrenergic up-regulation of ICa in the heart.
FIGURE 5.
ICa is not regulated by β-adrenergic stimulation in Cav1.2Stop CMs. A and B, ICa traces of WT (A, +/+) and Cav1.2Stop (B, −/−) embryonic CM ± 0.1 μm isoproterenol. The CMs were obtained from day 18.5 embryos as described (31). C and D, statistics of isoproterenol (C) and forskolin (D)-dependent increase in peak current of WT (open columns), heterozygous (gray columns), and Cav1.2Stop (black columns) CMs. Number of cells is given in columns. *, p = < 0.05; **, p = < 0.01.
DISCUSSION
Adrenergic up-regulation of the cardiac ICa is an extensively studied physiological phenomenon that was recognized in the seventies (35) to be regulated by cAMP. Since then evidence has been published that β-adrenergic stimulation requires a PKA-mediated phosphorylation step (3, 4, 7, 20, 36–39). However, the molecular mechanism of β-adrenergic regulation of Cav1.2 channel remains unsolved. Previously, it was found that the mutation S1928A of the Cav1.2 protein did not abolish β-adrenergic regulation of the heart and ICa (25), supporting the notion that phosphorylation of S1928 by PKA was not an obligatory step to allow β-adrenergic regulation of the murine heart.
The Cavβ2 subunit has been promoted as an alternative substrate for PKA (20, 40, 41). Initially, it was suggested that PKA-dependent up-regulation of the expressed ICa requires truncation of the Cav1.2 protein at amino acid 1905 and the co-expression of the Cavβ2 subunit (20). Truncation of the murine Cav1.2 channel at Asp1904 resulted in death around birth (31) and the inability of isoproterenol to stimulate the truncated channel (Fig. 5). Similar results have been reported, when the Cav1.2 protein was truncated at Gly1796 (34). These negative results are most likely caused by the deletion of the AKAP binding sequence (32). AKAPs are components that target various proteins of the β-adrenergic signaling cascade to Cav1.2 (38). These results clearly demonstrate that truncation of the Cav1.2 protein in vivo is not required for the adrenergic regulation.
Truncation of the Cavβ2 subunit at P501 by site-directed mutagenesis removed the “classical” PKA phosphorylation sites and that for PKG, CaMKII, and PKB. Removing these reported phosphorylation sites had no effect on the basic properties of the murine cardiac ICa. The Cavβ2Stop mice showed normal β-adrenergic regulation, CDI, CDF, and basic behavior. From these results we conclude that these reported phosphorylation sites are not necessary for the basic regulation of the channel by PKA, CaMKII, PKG, and PKB.
The reported results do not rule out the possibility that PKA modified an additional site on the truncated Cavβ2 subunit that was necessary for β-adrenergic regulation of the channel (42). This consideration appears unlikely in view of the report that deletion of the Cavβ2 subunit in the adult heart does not result in an severe phenotype (26). The negative results reported here are only relevant for the relative classical tests carried out in this study. It could be that removal of these phosphorylation sites may alter more subtle cardiac functions that have not been associated so far with a phosphorylation event at the Cavβ2 subunit.
An alternative possibility is that PKA phosphorylates simultaneously sites at the Cavβ2 and the Cav1.2 subunit. This possibility was tested with two additional mouse lines. However, the combination of the Cavβ2 mutation with mutation at the C terminus of the Cav1.2 protein did not influence the cardiac response to β-adrenergic stimulation. However, the Cavβ2 mutation did not affect the reduced CDF caused by the SF mutation (27). Our results do not rule out the possibility that phosphorylation at these sites might affect parameter of the Cav1.2 channel that have not been studied under our condition. However, the physiological significance of these putative parameters appears to be restricted because deletion of the cardiac Cavβ2 gene in the adult mouse caused only negligible changes in the cardiac performance (26).
An alternative target for PKA and β-adrenergic regulation of the heart has been proposed recently (8). Expression studies implicated PKA-dependent phosphorylation of Ser1700 and Thr1704 in the C terminus of Cav1.2 to be important for the β-adrenergic regulation (8). The phosphorylation needs to be combined with proteolytic cleavage of the mature Cav1.2 protein close to amino acid 1800. The cleaved distal C terminus has to stay with the truncated Cav1.2 channel to allow β-adrenergic regulation. The distal part inhibits ICa of the truncated Cav1.2 channel. The inhibition is then removed by β-adrenergic stimulation (8).
For β-adrenergic regulation of the expressed ICa, an additional function of the distal part, its AKAP binding site, is required. The AKAP binding site allows the close positioning of PKA to the Cav1.2 channel. Partial verification of the AKAP concept has been given by Nichols et al. (33), Fuller et al. (8) and by the results of this report. The ICa of embryonic CMs expressing a truncated Cav1.2 channel (see Fig. 7 in Ref. 34) was not stimulated by isoproterenol or forskolin. To support further the above concept, in vivo mutation of Ser1700/Thr1704 is necessary to show that β-adrenergic regulation of ICa requires the phosphorylation of Ser1700/Thr1704.
The reported results suggest that the C-terminal phosphorylation sites of the Cavβ2 subunit are not used to regulate basic properties of the murine cardiac ICa. In contrast, CaMKII-dependent phosphorylation of the C terminus of Cav1.2 is necessary for CDF. The results support again the previous notion (31, 34) that the distal C terminus of the Cav1.2 channel is necessary for β-adrenergic regulation of murine ICa.
Supplementary Material
Acknowledgment
We thank Teodora Kennel for expert technical help.
This work was supported by grants from Deutsche Forschungsgemeinschaft and Fond der Chemischen Industrie.

This article contains supplemental Tables 1–3, Figs. 1–5, and additional references.
These results indicated to us that there is no gene dose effect through the deletion of the C terminus of one β2 gene. Almost identical results have been reported by Meissner et al. (26), which reported the inactivation of both β2 alleles. Comparison of WT and heterozygous animals should allow the detection of a gene dose effect more easily. However, if no different phenotype has been found between the WT and heterozygous animals, it is generally requested that the heterozygous litter-matched animals are the correct controls to the knockout mice because they carry one WT chromosome and one chromosome carrying the mutated gene. Based on these generally accepted βStop considerations, we tested against the heterozygous, litter-matched CTR animals. Furthermore, heart-specific inactivation of the β2 subunit gene in adult mice yielded minimal or no effect on ICa kinetics (see Ref. 26, Figs. 4 and 5).
- CaMKII
- calmodulin kinase II
- AKAP
- PKA-anchoring protein
- CDF
- calcium-dependent facilitation
- CDI
- calcium-dependent inactivation
- CM
- cardiac myocyte
- Ctr
- control
- FS
- fractional shortening
- HR
- heart rate
- rbs
- rabbit protein sequence.
REFERENCES
- 1. Catterall W. A., Hulme J. T., Jiang X., Few W. P. (2006) Regulation of sodium and calcium channels by signaling complexes. J. Recept. Signal Transduct. Res. 26, 577–598 [DOI] [PubMed] [Google Scholar]
- 2. Dai S., Hall D. D., Hell J. W. (2009) Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 89, 411–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. (1982) Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature 298, 576–578 [DOI] [PubMed] [Google Scholar]
- 4. Hartzell H. C., Méry P. F., Fischmeister R., Szabo G. (1991) Sympathetic regulation of cardiac calcium current is due exclusively to cAMP-dependent phosphorylation. Nature 351, 573–576 [DOI] [PubMed] [Google Scholar]
- 5. Bers D. M. (2002) Cardiac excitation-contraction coupling. Nature 415, 198–205 [DOI] [PubMed] [Google Scholar]
- 6. Kamp T. J., Hell J. W. (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 87, 1095–1102 [DOI] [PubMed] [Google Scholar]
- 7. Hulme J. T., Westenbroek R. E., Scheuer T., Catterall W. A. (2006) Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac Cav1.2 channels during β1-adrenergic regulation. Proc. Natl. Acad. Sci. U.S.A. 103, 16574–16579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuller M. D., Emrick M. A., Sadilek M., Scheuer T., Catterall W. A. (2010) Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci. Signal. 3, ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee T. S., Karl R., Moosmang S., Lenhardt P., Klugbauer N., Hofmann F., Kleppisch T., Welling A. (2006) Calmodulin kinase II is involved in voltage-dependent facilitation of the L-type Cav1.2 calcium channel: identification of the phosphorylation sites. J. Biol. Chem. 281, 25560–25567 [DOI] [PubMed] [Google Scholar]
- 10. Hudmon A., Schulman H., Kim J., Maltez J. M., Tsien R. W., Pitt G. S. (2005) CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J. Cell Biol. 171, 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dzhura I., Wu Y., Colbran R. J., Balser J. R., Anderson M. E. (2000) Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat. Cell Biol. 2, 173–177 [DOI] [PubMed] [Google Scholar]
- 12. Yang L., Liu G., Zakharov S. I., Bellinger A. M., Mongillo M., Marx S. O. (2007) Protein kinase G phosphorylates Cav1.2 α1c and β2 subunits. Circ. Res. 101, 465–474 [DOI] [PubMed] [Google Scholar]
- 13. Jiang L. H., Gawler D. J., Hodson N., Milligan C. J., Pearson H. A., Porter V., Wray D. (2000) Regulation of cloned cardiac L-type calcium channels by cGMP-dependent protein kinase. J. Biol. Chem. 275, 6135–6143 [DOI] [PubMed] [Google Scholar]
- 14. Sun H., Kerfant B. G., Zhao D., Trivieri M. G., Oudit G. Y., Penninger J. M., Backx P. H. (2006) Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kα/PKB signaling. Circ. Res. 98, 1390–1397 [DOI] [PubMed] [Google Scholar]
- 15. Lawlor M. A., Alessi D. R. (2001) PKB/Akt: a key mediator of cell proliferation, survival, and insulin responses? J. Cell Sci. 114, 2903–2910 [DOI] [PubMed] [Google Scholar]
- 16. Singer-Lahat D., Gershon E., Lotan I., Hullin R., Biel M., Flockerzi V., Hofmann F., Dascal N. (1992) Modulation of cardiac Ca2+ channels in Xenopus oocytes by protein kinase C. FEBS Lett. 306, 113–118 [DOI] [PubMed] [Google Scholar]
- 17. Shistik E., Ivanina T., Blumenstein Y., Dascal N. (1998) Crucial role of N terminus in function of cardiac L-type Ca2+ channel and its modulation by protein kinase C. J. Biol. Chem. 273, 17901–17909 [DOI] [PubMed] [Google Scholar]
- 18. Yang L., Doshi D., Morrow J., Katchman A., Chen X., Marx S. O. (2009) Protein kinase C isoforms differentially phosphorylate Cav1.2α1c. Biochemistry 48, 6674–6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McHugh D., Sharp E. M., Scheuer T., Catterall W. A. (2000) Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc. Natl. Acad. Sci. U.S.A. 97, 12334–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bünemann M., Gerhardstein B. L., Gao T., Hosey M. M. (1999) Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the β2 subunit. J. Biol. Chem. 274, 33851–33854 [DOI] [PubMed] [Google Scholar]
- 21. Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. (1992) Calcium channel βsubunit heterogeneity: functional expression of cloned cDNA from heart, aorta, and brain. EMBO J. 11, 885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grueter C. E., Abiria S. A., Dzhura I., Wu Y., Ham A. J., Mohler P. J., Anderson M. E., Colbran R. J. (2006) L-type Ca2+ channel facilitation mediated by phosphorylation of the β subunit by CaMKII. Mol. Cell 23, 641–650 [DOI] [PubMed] [Google Scholar]
- 23. Catalucci D., Zhang D. H., DeSantiago J., Aimond F., Barbara G., Chemin J., Bonci D., Picht E., Rusconi F., Dalton N. D., Peterson K. L., Richard S., Bers D. M., Brown J. H., Condorelli G. (2009) Akt regulates L-type Ca2+ channel activity by modulating Cavα1 protein stability. J. Cell Biol. 184, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viard P., Butcher A. J., Halet G., Davies A., Nürnberg B., Heblich F., Dolphin A. C. (2004) PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat. Neurosci. 7, 939–946 [DOI] [PubMed] [Google Scholar]
- 25. Lemke T., Welling A., Christel C. J., Blaich A., Bernhard D., Lenhardt P., Hofmann F., Moosmang S. (2008) Unchanged β-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J. Biol. Chem. 283, 34738–34744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meissner M., Weissgerber P., Londoño J. E., Prenen J., Link S., Ruppenthal S., Molkentin J. D., Lipp P., Nilius B., Freichel M., Flockerzi V. (2011) Moderate calcium channel dysfunction in adult mice with inducible cardiomyocyte-specific excision of the Cacnb2 gene. J. Biol. Chem. 286, 15875–15882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blaich A., Welling A., Fischer S., Wegener J. W., Köstner K., Hofmann F., Moosmang S. (2010) Facilitation of murine cardiac L-type Cav1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of Ser-1512 and Ser-1570. Proc. Natl. Acad. Sci. U.S.A. 107, 10285–10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ludwig A., Flockerzi V., Hofmann F. (1997) Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J. Neurosci. 17, 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seisenberger C., Specht V., Welling A., Platzer J., Pfeifer A., Kühbandner S., Striessnig J., Klugbauer N., Feil R., Hofmann F. (2000) Functional embryonic cardiomyocytes after disruption of the L-type α1C (Cav1.2) calcium channel gene in the mouse. J. Biol. Chem. 275, 39193–39199 [DOI] [PubMed] [Google Scholar]
- 30. Poomvanicha M., Wegener J. W., Blaich A., Fischer S., Domes K., Moosmang S., Hofmann F. (2011) Facilitation and Ca2+-dependent inactivation are modified by mutation of the Cav1.2 channel IQ motif. J. Biol. Chem. 286, 26702–26707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Domes K., Ding J., Lemke T., Blaich A., Wegener J. W., Brandmayr J., Moosmang S., Hofmann F. (2011) Truncation of murine Cav1.2 at Asp-1904 results in heart failure after birth. J. Biol. Chem. 286, 33863–33871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McConnachie G., Langeberg L. K., Scott J. D. (2006) AKAP signaling complexes: getting to the heart of the matter. Trends Mol. Med. 12, 317–323 [DOI] [PubMed] [Google Scholar]
- 33. Nichols C. B., Rossow C. F., Navedo M. F., Westenbroek R. E., Catterall W. A., Santana L. F., McKnight G. S. (2010) Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ. Res. 107, 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fu Y., Westenbroek R. E., Yu F. H., Clark J. P., 3rd, Marshall M. R., Scheuer T., Catterall W. A. (2011) Deletion of the distal C terminus of Cav1.2 channels leads to loss of β-adrenergic regulation and heart failure in vivo. J. Biol. Chem. 286, 12617–12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reuter H. (1974) Localization of β-adrenergic receptors and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents, and tension in mammalian cardiac muscle. J. Physiol. 242, 429–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Jongh K. S., Murphy B. J., Colvin A. A., Hell J. W., Takahashi M., Catterall W. A. (1996) Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry 35, 10392–10402 [DOI] [PubMed] [Google Scholar]
- 37. Sculptoreanu A., Rotman E., Takahashi M., Scheuer T., Catterall W. A. (1993) Voltage-dependent potentiation of the activity of cardiac L-type calcium channel α1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 90, 10135–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao T., Yatani A., Dell'Acqua M. L., Sako H., Green S. A., Dascal N., Scott J. D., Hosey M. M. (1997) cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron 19, 185–196 [DOI] [PubMed] [Google Scholar]
- 39. Ganesan A. N., Maack C., Johns D. C., Sidor A., O'Rourke B. (2006) β-Adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of α1C but not serine 1928. Circ. Res. 98, e11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perez-Reyes E., Castellano A., Kim H. S., Bertrand P., Baggstrom E., Lacerda A. E., Wei X. Y., Birnbaumer L. (1992) Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J. Biol. Chem. 267, 1792–1797 [PubMed] [Google Scholar]
- 41. Buraei Z., Yang J. (2010) The β subunit of voltage-gated Ca2+ channels. Physiol. Rev. 90, 1461–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gerhardstein B. L., Puri T. S., Chien A. J., Hosey M. M. (1999) Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the β2 subunit of L-type voltage-dependent calcium channels. Biochemistry 38, 10361–10370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.