Background: Exercise and diet control are fundamental approaches to metabolic conditions caused by high fat diet (HFD).
Results: HFD-induced memory deficit and Aβ deposition were more ameliorated in the exercise- than in the diet control-induced mice.
Conclusion: Exercise was more effective than diet control in preventing HFD-induced AD development.
Significance: Exercise has the highest priority in the prevention of AD.
Keywords: Alzheimer Disease, Amyloid, Diabetes, Diet, Exercise
Abstract
Accumulating evidence suggests that some dietary patterns, specifically high fat diet (HFD), increase the risk of developing sporadic Alzheimer disease (AD). Thus, interventions targeting HFD-induced metabolic dysfunctions may be effective in preventing the development of AD. We previously demonstrated that amyloid precursor protein (APP)-overexpressing transgenic mice fed HFD showed worsening of cognitive function when compared with control APP mice on normal diet. Moreover, we reported that voluntary exercise ameliorates HFD-induced memory impairment and β-amyloid (Aβ) deposition. In the present study, we conducted diet control to ameliorate the metabolic abnormality caused by HFD on APP transgenic mice and compared the effect of diet control on cognitive function with that of voluntary exercise as well as that of combined (diet control plus exercise) treatment. Surprisingly, we found that exercise was more effective than diet control, although both exercise and diet control ameliorated HFD-induced memory deficit and Aβ deposition. The production of Aβ was not different between the exercise- and the diet control-treated mice. On the other hand, exercise specifically strengthened the activity of neprilysin, the Aβ-degrading enzyme, the level of which was significantly correlated with that of deposited Aβ in our mice. Notably, the effect of the combination treatment (exercise and diet control) on memory and amyloid pathology was not significantly different from that of exercise alone. These studies provide solid evidence that exercise is a useful intervention to rescue HFD-induced aggravation of cognitive decline in transgenic model mice of AD.
Introduction
Alzheimer disease (AD),2 the most common cause of dementia, is poised to be a significant public health crisis. The occurrence of AD is largely sporadic, typically affecting individuals aged over 65 years. Amyloid plaque is one of the pathological hallmarks of AD. Amyloid plaques are composed of β-amyloid (Aβ), which are derived from the amyloid precursor protein (APP) via proteolytic cleavages by β- and γ-secretases. A widely accepted hypothesis about AD pathogenesis is the amyloid cascade hypothesis in which Aβ plays a crucial role in neurodegeneration (1). Importantly, recent studies have implied that soluble Aβ oligomers may be the main culprit of AD pathogenesis (2–4).
High fat diet (HFD) is prevalent in modern society, and HFD-induced metabolic condition is becoming a worldwide issue because it leads to obesity, type 2 diabetes mellitus, and hypercholesterolemia. More importantly, accumulating evidence suggests that some dietary patterns, specifically HFD, increase the risk of developing sporadic AD (5). Experimental studies also support this idea. For example, application of HFD for APP transgenic mice exacerbated pathological alterations in the brain and their memory deficits (6, 7). In contrast, it is widely known that composite dietary patterns such as the Mediterranean diet, characterized by high intake of vegetables, unsaturated fatty acids, and wine, are related to lower risk for AD (8). These studies clearly indicate that there is an association between metabolic conditions and a higher risk of sporadic AD.
Exercise and diet control are fundamental approaches in the treatment of metabolic conditions. They might also become useful ways to prevent the development of AD. For example, a prospective study revealed that physical activity is protective against the development of cognitive impairment in AD and that the highest activity group showed a lowered incidence of AD (9). Recently, we also demonstrated that voluntary exercise ameliorates HFD-induced memory deficit and Aβ deposition in APP transgenic mice (7), indicating that interventions to reduce metabolic conditions can become the preventive method for AD. However, it still remains unknown what kind of intervention targeting metabolic conditions is more effective.
In the present study, we used the AD model mice with metabolic conditions through HFD (APP-HFD mice), which we had previously established (7). In addition to voluntary exercise, we preventively conducted diet control or the combination of diet control with exercise using APP-HFD mice followed by comparing the effect of these interventions on cognitive function and amyloid pathology. Here, we show that diet control improved metabolic conditions including hyperinsulinemia and hypercholesterolemia of APP-HFD mice better than exercise. However, exercise more effectively ameliorated HFD-induced memory deficit and Aβ deposition than diet control (despite higher serum insulin/cholesterol levels). Exercise specifically enhanced the activity of neprilysin, which we speculate may be responsible for the reduction of the Aβ level. These results clearly indicated that exercise, affecting the process of Aβ degradation, could be a more effective way to ameliorate the AD progression caused by metabolic dysfunctions than diet control.
EXPERIMENTAL PROCEDURES
Animals and Dietary Conditions
Human APP transgenic mice overexpressing the familial AD-linked mutations bearing both Swedish (K670N/M671L) and Indiana (V717F) mutation (APPSwe/Ind) (10), which have been imported from The Jackson Laboratory, were used in the present study. They were maintained as heterozygotes, and male and female mice were housed separately. Age- and sex-matched (1:1, male: female) mice were exposed to either a standard diet (10% fat, 70% carbohydrate, and 20% protein, Oriental Yeast Co., Ltd., Tokyo, Japan) or an established HFD (caloric composition, 60% fat, 20% carbohydrate, and 20% protein, Research Diet, Inc., New Brunswick, NJ) for 20 weeks, from 2–3 to 7–8 months of age. To examine the effect of voluntary exercise (Ex) on APP transgenic mice fed HFD (APP-HFD mice), the cage of the mice was changed to a 2.4 times larger one equipped with a running wheel as well as objects after 10 weeks of HFD (APP-HFD+Ex mice) (7). The mice spent 10 weeks in the exercise condition in the presence of HFD. To examine the effect of diet control (Dc) on APP-HFD mice, HFD was replaced with standard diet after 10 weeks of HFD and mice were fed with standard diet for another 10 weeks (APP-HFD+Dc mice). To examine the effect of the combinatory intervention of Ex and Dc, the mice spent 10 weeks in the exercise condition in the presence of standard diet after 10 weeks of HFD (APP-HFD+Ex+Dc mice). After the dietary manipulation, metabolic changes in these mice were analyzed followed by the assessment of memory function through the Morris water maze test, as described below. After the analysis of memory function, the brains were extracted and cut sagittally into left and right hemispheres. The left hemisphere was fixed in 4% paraformaldehyde for histological analysis. After removing the olfactory lobe and cerebellum, the right hemisphere was rapidly frozen in liquid nitrogen for biochemical analysis. All animal experiments were performed in compliance with the Guidelines for the Care and Use of Laboratory Animals of the Kyoto University.
Assessment of Metabolic Changes
Blood glucose content was measured by using LabAssay glucose (Wako Pure Chemical Industries, Osaka, Japan). To assess glucose intolerance in these mice, we conducted the intraperitoneal glucose tolerance test. Mice were given a single dose of intraperitoneal injection of glucose (2 g/kg of body weight) after 14 h of fasting, and blood was collected from the tail vein periodically over 2 h (fasting, 30 min, 60 min, and 120 min). Plasma insulin concentration was measured by enzyme-linked immunosorbent assay (ELISA) kit specific to insulin (Morinaga Institute of Biological Science). Plasma concentrations of total cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride were measured by using cholesterol E, HDL cholesterol E, and triglyceride E (Wako Pure Chemical Industries).
Morris Water Maze Test
To assess spatial navigation learning and memory retention, the Morris water maze test was conducted. Initially, animals received a habituation trial during which the animals were allowed to explore the pool of water (diameter 120 cm, height 25 cm, temperature 21 ± 1 °C) without the platform present.
Visual Cue Phase
Following habituation, visible platform training was performed to measure motivation and swimming speed of mice to find a platform. Briefly, distal cues were removed from around the pool, and the platform was labeled with a flag and placed 1 cm above the surface of the water in the center of a quadrant. Mice were placed in the maze and allowed to explore the maze for 60 s, and if they reached the visible platform, they were allowed to remain there for 20 s before being returned to their cages. If they did not find the platform within 60 s, the experimenter led them to the platform and let them remain there for 20 s. Animals were trained in groups of five, and training was completed once each animal received six trials. This training was performed for 1 day.
Acquisition Phase
We measured the ability of mice to understand the spatial relationship between a safe but invisible platform of 10 cm in diameter (submerged 1 cm below the water level) and visual cues surrounding the maze. The platform was located in the center of one of the four quadrants, and several extramaze cues were distributed across the walls surrounding the pool. During the acquisition phase of training, each mouse received four daily hidden platform training trials with 10–12-min intervals for 5 consecutive days. Animals were allowed 60 s to locate the platform and 20 s to rest on it. Mice that failed to find the platform were led there by the experimenter and allowed to rest there for 20 s.
Probe Trial Phase
24 h following the last acquisition trial, a single 60-s probe trial was administered to assess spatial memory retention. For the probe trial, animals were returned to the pool without the platform present, and parameters were recorded to assess the ability of the mouse to remember the previous location of the platform.
Performance was recorded with an automated tracking system (TARGET series/2, Japan) during all phases of training. During the visual cue phase of training, speed and latency to the platform were used to compare the activity of the performance between each group. During the acquisition phase, acquisition time (latency to reach the platform) was used to analyze and compare the performance between different treatment groups. The time to the platform quadrant and the number of entries into the target quadrant were recorded and analyzed during the probe trials.
Immunoblotting and Filter Trap Assay
For immunoblotting analysis, the brain was taken and rapidly frozen using liquid nitrogen. The brain samples from the cerebrum of the male mice were extracted in radioimmunoprecipitation assay buffer (50 mm Tris-HCl, 150 mm NaCl, 1% Triton X-100, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, pH 8.0) with protease inhibitor mixture (Roche Applied Science) and sufficiently homogenized on ice. Then, the samples were incubated for one night at 4 °C and centrifuged at 14,000 × g for 20 min. The supernatants were directly used for Western blot analysis. The detailed protocol has been described previously (11). 5–20% polyacrylamide gradient gels (Atto) were used to detect full-length APP, β-actin, neprilysin, and insulin-degrading enzyme. Both 5–20% polyacrylamide gradient gels (Atto) and 4–12% NuPAGE Bis-Tris gel (Invitrogen) were used to detect APP CTFs. Mouse monoclonal anti-β-actin and rabbit polyclonal anti-APP C-terminal antibodies were from Sigma. Rabbit polyclonal anti-neprilysin and mouse monoclonal anti-insulin degrading enzyme antibodies were from Abcam.
The filter trap assay was conducted as described previously (7, 12). Briefly, the protein concentration of the cerebrum samples in Tris-buffered saline (TBS)-extracted fraction was measured, and an equal amount of protein was subjected to vacuum filtration through a 96-well dot blot apparatus (Bio-Rad Laboratories) containing 200-nm pore size nitrocellulose membrane. The resultant membrane was then incubated with primary antibody at 4 °C overnight. The membrane was then blocked by TBS containing 4% skim milk and incubated with HRP-linked secondary antibody (GE Healthcare; diluted 1:2,000) for 1 h. The membrane was developed using the ECL Western blotting analysis system (GE Healthcare). Anti-Aβ oligomer antibody (A11, Invitrogen) was used for the detection of Aβ oligomer in TBS soluble fraction.
Immunohistochemistry
The paraformaldehyde-fixed and paraffin-embedded tissue sections of male mice were incubated with primary antibodies. The sections were then incubated with biotinylated anti-second IgG antibody (1:2,000; Vector Laboratories) followed by the incubation with avidin peroxidase (ABC Elite kit; 1:4,000; Vector Laboratories). Subsequently, the labeling was visualized by incubation with 50 mm Tris-HCl buffer (pH 7.6) containing 0.02% 3,3-diaminobenzidine and 0.0045% hydrogen peroxide. All images were visually analyzed using an ECLIPSE 80i microscope (Nikon Corp.). For each animal, the sections of the hippocampus were captured then were imported into ImageJ, and an intensity threshold level was set that allowed for discrimination between the antigen and background labeling. Anti-Aβ (6E10) antibody (1:1,000; Sigma) was used for the detection of Aβ plaque.
Neprilysin Activity Assay
The proteolytic activity of neprilysin was measured as described previously with minor modifications (13). Briefly, the brain samples from the cerebrum of the male mice were extracted in radioimmunoprecipitation assay buffer, and protein concentrations were analyzed. 100 μg of brain lysates were incubated with 50 μm the substrate3-dansyl-d-Ala-Gly-p-(nitro)-Phe-Gly (Sigma) and 1 μm captopril, angiotensin-converting enzyme inhibitor, in 200 μl of 50 mm Tris-HCl buffer (pH 7.6) for 1 h at 37 °C. Reactions were stopped by heating samples to 100 °C for 5 min followed by 5,000 g × 5 min centrifugation. The 180 μl of supernatant were diluted into 400 μl of 50 mm Tris-HCl buffer (pH 7.6), and fluorescence was determined using Infinite 200 PRO (Tecan Japan Co., Ltd.) (excitation 342 nm, emission 562 nm).
Statistical Analysis
All values are given in means ± S.E. Comparisons were performed using an unpaired Student's t test. For comparison of multiparametric analysis, one-way factorial analysis of variance followed by the post hoc analysis by Fisher's protected least significant difference was used. Statistical significance of differences between mean scores during acquisition phase of training in the Morris water maze test was assessed with two-way repeated measures analysis of variance (general linear model) and Fisher's post hoc analysis for multiple comparisons. p < 0.05 was considered to indicate a significant difference.
RESULTS
Effects of Diet Control on Metabolic Conditions of APP-HFD mice
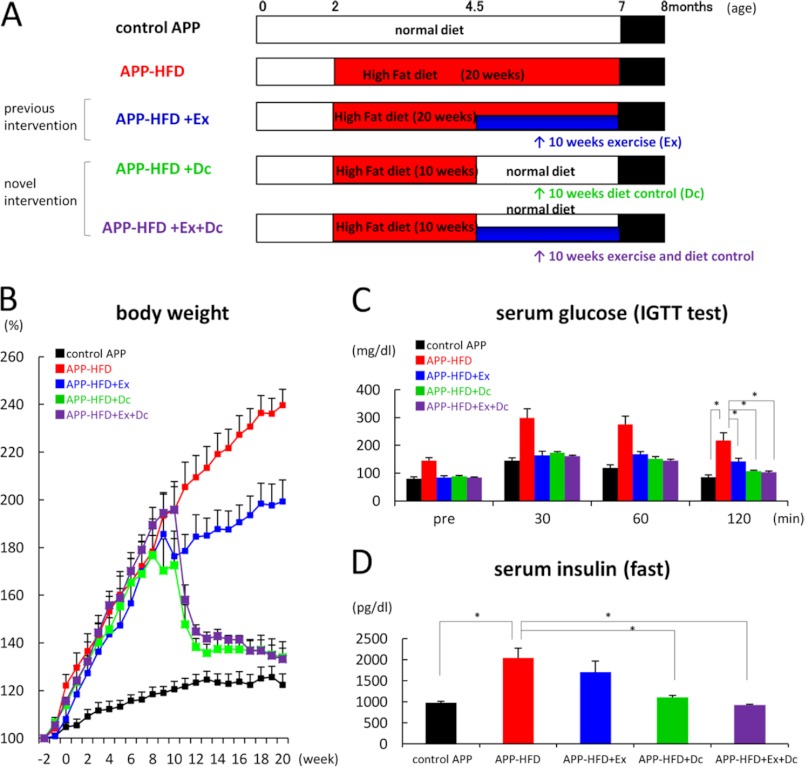
In our recent study, voluntary exercise is shown to be a useful tool for preventing the progress of cognitive dysfunction and amyloid pathology in APP-HFD mice (7). Notably, voluntary exercise does not ameliorate HFD-induced hyperinsulinemia or hypercholesterolemia but improves glucose intolerance as well as cognitive impairment. Diet therapy is another way to control metabolic dysfunctions. Thus, in the present study, we aimed to elucidate which environmental factor contributes more to metabolic and cognitive functions in APP-HFD mice by controlling the diet (Dc; diet control, APP-HFD+Dc) or combined diet control with exercise (Ex; exercise, APP-HFD+Ex+Dc) after mice were fed HFD for 10 weeks. Then, we compared the effects of these interventions on cognitive function and Aβ pathology (Fig. 1A).
FIGURE 1.
Diet control ameliorated HFD-induced obesity and diabetic conditions when compared with exercise. A, schematic presentation of the interventions targeting metabolic conditions. As described previously (7), APPSwe/Ind mice were maintained with a standard diet in standard laboratory cages until they were 2–3 months old. Then, age- and sex-matched mice were separated into five groups. In the control group, the mice were fed with a standard diet in standard laboratory cages for 20 weeks (control APP mice) (top row, n = 9). In the HFD-induced group, mice were fed HFD in standard laboratory cages for 20 weeks (APP-HFD mice) (second row, n = 10). In the HFD with exercise-induced group, mice spent 10 weeks in standard laboratory cages and then spent 10 weeks in enrichment cages with HFD (APP-HFD+Ex mice) (third row, n = 8). As a novel intervention, in the diet-control-induced group, after 10 weeks of HFD, we used a standard diet for another 10 weeks (APP-HFD+Dc mice) (fourth row, n = 7). In the combination group with exercise plus diet control, mice spent 10 weeks in standard laboratory cages with HFD and then spent 10 weeks in enrichment cages with a standard diet (APP-HFD+Ex+Dc mice) (bottom row, n = 7). After 20 weeks, metabolic conditions of these mice were analyzed followed by ethological, histochemical, and biochemical analyses targeting AD pathophysiology. B, relative body weight changes over 20 weeks. The body weight 2 weeks before each diet was regarded as the baseline (100%). Diet control and its combination with exercise significantly inhibited the HFD-induced increase of body weight. C, blood glucose levels during glucose tolerance test after an intraperitoneal injection of glucose (2 g/kg of body weight). The fasting glucose level (pre) and glucose tolerance in APP-HFD+Dc mice (F(4,159) = 26.49, *, p < 0. 001) and APP-HFD+Ex+Dc mice (*, p < 0.001) were clearly improved. D, serum insulin levels during fasting. The serum insulin levels in APP-HFD+Dc mice (F(4, 36) = 9.3, *, p = 0.003) and APP-HFD+Ex+Dc mice (*, p < 0.001) were significantly decreased when compared with that in APP-HFD mice.
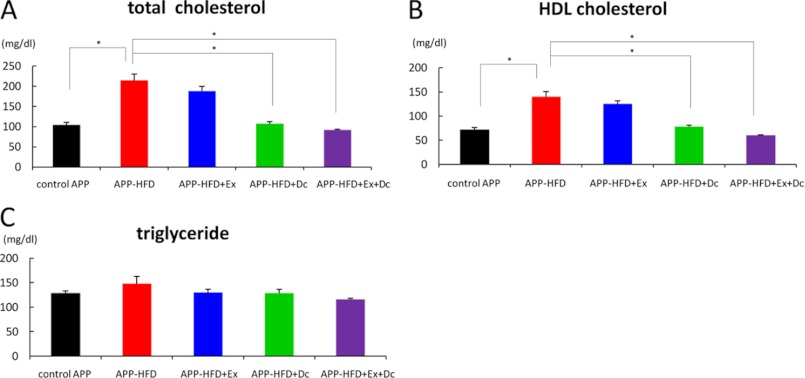
As reported previously, APP-HFD mice gained significantly more body weight than the control APP mice, and after the introduction of voluntary exercise, APP-HFD+Ex mice gained less body weight than APP-HFD mice (7). On the other hand, the body weight decrease of APP-HFD+Dc and APP-HFD+Ex+Dc mice was much more drastic than that of APP-HFD+Ex mice (Fig. 1B), showing that diet control was more effective in body weight reduction than voluntary exercise. Weekly monitoring of food intake showed that the food intake of the APP-HFD+Dc and APP-HFD+Ex+Dc mice did not change or mildly increased (supplemental Fig. 1), indicating that the diet control-mediated attenuation of body weight was not caused by the reduction of food intake. Fasting serum glucose level was not different among APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice, being significantly lower in the three groups than in APP-HFD mice (Fig. 1C, pre columns). In addition, the result of the intraperitoneal glucose tolerance test indicated that the impaired glucose tolerance was improved in the APP-HFD+Ex as well as the APP-HFD+Dc and APP-HFD+Ex+Dc mice (Fig. 1C). To examine whether diet control reverses or prevents the development of glucose intolerance, we conducted the intraperitoneal glucose tolerance test 10 weeks after HFD introduction (at the time point when exercise or diet control was started). The fasting glucose level and glucose tolerance of APP-HFD+Dc and APP-HFD+Ex+Dc mice were better than those of APP mice 10 weeks after HFD introduction (supplemental Fig. 2). Therefore, diet control reversed glucose intolerance as well as exercise did (7). The plasma insulin level of APP-HFD+Dc and APP-HFD+Ex+Dc mice was significantly decreased when compared with that of APP-HFD mice (Fig. 1D). The plasma lipid analyses indicated that both total and HDL-cholesterol were significantly decreased in APP-HFD+Dc and APP-HFD+Ex+Dc mice (Fig. 2, A and B), suggesting that diet control ameliorated HFD-induced hypercholesterolemia. The level of plasma triglycerides was not different among them (Fig. 2C).
FIGURE 2.
Diet control ameliorated HFD-induced lipid dysfunction when compared with exercise. A, plasma total cholesterol levels. The total cholesterol levels in APP-HFD+Dc mice (F(4, 36) = 30.29, *, p < 0.001) and APP-HFD+Ex+Dc mice (*, p < 0.001) were significantly decreased when compared with the level in APP-HFD mice. B, plasma HDL cholesterol levels. The HDL cholesterol levels in APP-HFD+Dc mice (F(4, 36) = 30.96, *, p < 0.001) and APP-HFD+Ex+Dc mice (*, p < 0.001) were significantly decreased when compared with the level in APP-HFD mice. C, plasma triglyceride levels. There was no difference among control, APP-HFD, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice (F(4, 36) = 1.65, not significant).
Exercise Was More Effective in Ameliorating Memory Deficit of APP-HFD Mice than Diet Control
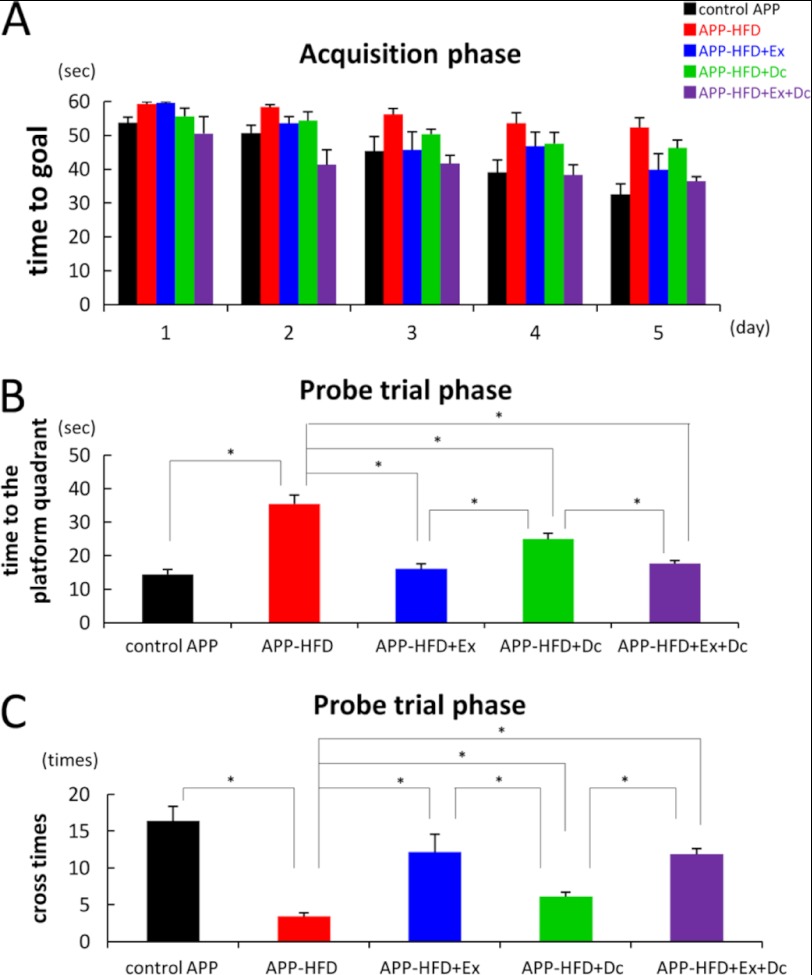
To compare the effect of diet control on HFD-induced memory deficit with that of exercise, we conducted the Morris water maze test. The locomotor activity of APP mice was not affected by HFD, HFD+Ex, HFD+Dc, or HFD+Ex+Dc, as exemplified by swimming speed (supplemental Fig. 3). During the acquisition phase, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice showed a daily improvement in their acquisition time. However, APP-HFD+Dc mice took a longer time to reach the goal than APP-HFD+Ex mice did (Fig. 3A). In the probe trial phase, the time to get to the platform quadrant in APP-HFD+Dc mice was significantly longer than that in APP-HFD+Ex mice (Fig. 3B). Moreover, the number to cross the previous location of the platform in APP-HFD+Dc mice was significantly smaller than that in APP-HFD+Ex mice (Fig. 3C). These results demonstrated that APP-HFD+Dc mice took a longer time to get to the platform quadrant and failed to cross the previous location of the platform when compared with APP-HFD+Ex mice. From these results, we concluded that exercise was the more effective way of ameliorating HFD-induced memory dysfunction than diet control in APP-HFD mice.
FIGURE 3.
Exercise ameliorated HFD-induced memory deficit when compared with diet control. A, escape latency in the acquisition phase. APP-HFD+Ex mice took a shorter time to the platform than APP-HFD+Dc mice. B, the time to the target quadrant in the probe trial phase. APP-HFD+Ex mice took a shorter time to the platform than APP-HFD+Dc mice (F(4, 36) = 23. 03, *, p = 0. 041). C, the number of entries into the target quadrant in the probe trial phase. APP-HFD+Dc mice were significantly impaired in the number of times they crossed the platform when compared with APP-HFD+Ex mice (F(4, 36) = 13.59, *, p = 0. 013).
Aβ Pathology of APP-HFD Mice Was Ameliorated Better by Exercise than by Diet Control
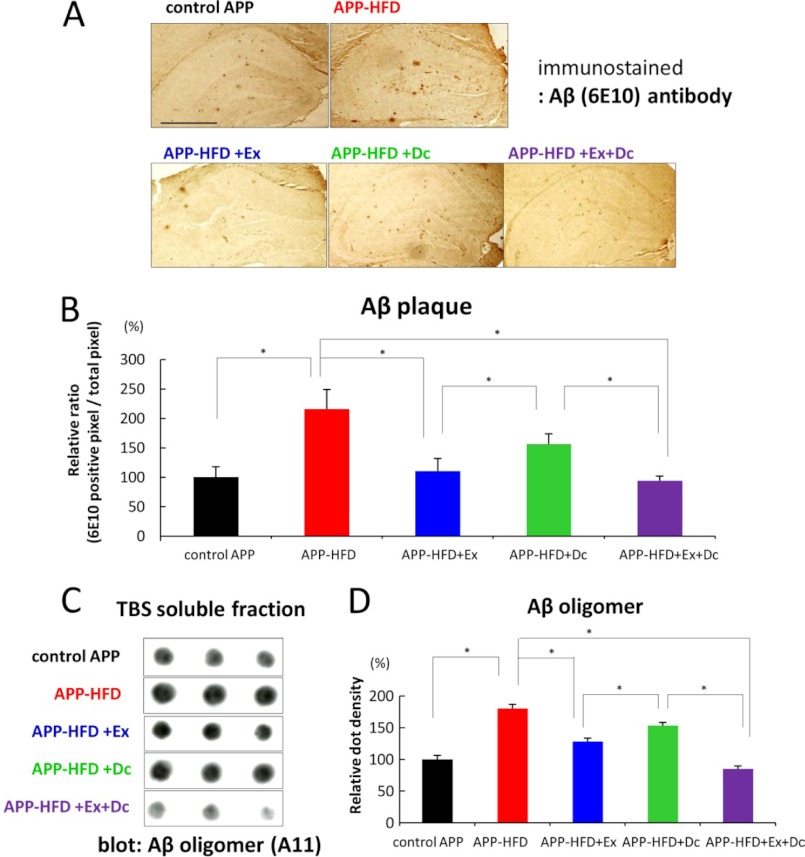
Because ample Aβ deposition is a critical hallmark of AD, we compared the effect of diet control on HFD-induced Aβ accumulation with that of exercise. For this, we conducted immunohistochemical analysis using anti-Aβ (6E10) antibody to quantitatively examine Aβ deposition. As shown in Fig. 4A, Aβ deposition was aggravated by feeding HFD (7), whereas a marked reduction of HFD-induced Aβ deposition was observed in APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice. Interestingly, the level of deposited Aβ in APP-HFD+Ex was significantly lower than that in APP-HFD+Dc mice (Fig. 4B). Therefore, exercise was more effective in reducing Aβ accumulation than diet control.
FIGURE 4.
Exercise ameliorated HFD-induced Aβ accumulation when compared with diet control. A, immunohistochemical analysis using anti-Aβ (6E10) antibody. Representative images of Aβ-immunostained hippocampus sections from control APP, APP-HFD, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc induced mice, respectively, are shown. Scale bar, 2 mm. B, cerebral Aβ loads determined by immunohistochemical and morphometric analyses. The cerebral Aβ deposition was significantly decreased in APP-HFD+Ex mice when compared with that in APP-HFD+Dc mice (F(4, 15) = 18.35, *, p = 0. 039). C, the amount of Aβ oligomers in the TBS-soluble fractions of control-APP, APP-HFD, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice analyzed by filter trap assay using anti-Aβ oligomer (A11) antibody. As described in Ref. 12, the Aβ monomer and low weight oligomers passed through the membrane pore (200-nm pore size), and high weight oligomers were detected in this assay. D, statistical analysis of dot density. The average band density of the control APP samples was regarded as 100%, and that of other groups was relatively indicated. The relative density of APP-HFD+Ex mice was significantly decreased when compared with that of APP-HFD+Dc mice (F(4, 10) = 47.42, *, p = 0.011).
An increasing number of studies show that the level of TBS-soluble Aβ oligomers correlates with memory deficits in AD model mice (2–4). We showed that HFD increases the level of soluble Aβ oligomers in APP mice, which is reduced by voluntary exercise (7). We further extended this result by comparing the amount of Aβ oligomers in the above three conditions. The filter trap analysis indicated that the levels of Aβ oligomers in the APP-HFD+Ex and APP-HFD+Ex+Dc mice were significantly decreased when compared with the level in the APP-HFD mice (Fig. 4, C and D). Remarkably, the level of Aβ oligomers in the APP-HFD+Ex mice was statistically lower than that in APP-HFD+Dc mice. Thus, exercise plays a much more significant role than diet control in APP-HFD mice in modulating not only Aβ deposition but also the level of Aβ oligomers. This is in line with the result of behavioral experiments.
HFD-enhanced APP Processing Was Equally Inhibited by Exercise and by Diet Control
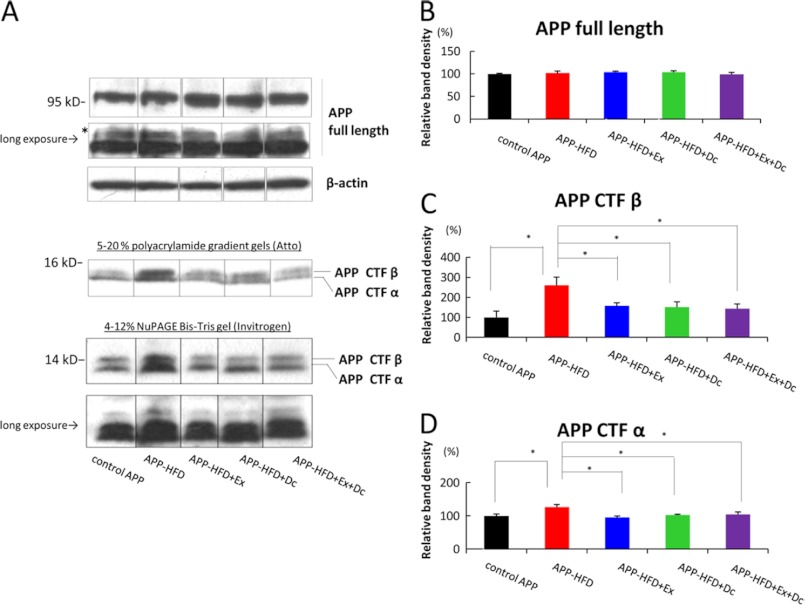
The above results led us to wonder whether the level of Aβ in our mice was regulated by APP processing or by Aβ degradation in exercise/diet control conditions. To examine how these environmental conditions affected the HFD-induced Aβ pathology, we first investigated the effect on APP processing in both conditions and compared the result of exercise with that of diet control. For this, we analyzed the level of APP C terminus fragments through immunoblotting assay using anti-APP C-terminal antibody. APP is cleaved by α- and β-secretases at the extramembrane domain, which produce APP-CTFα and APP-CTFβ, respectively. APP-CTFα and APP-CTFβ were further cleaved by γ-secretase at the intramembrane domain, producing p3 and Aβ, respectively. In the present study, the level of full-length APP was not different among control-APP, APP-HFD, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice (Fig. 5, A and B). Moreover, the change of fully glycosylated form was not observed among them. On the other hand, the level of APP-CTFβ in the APP-HFD mice was higher than that in the control APP, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice. Notably, the level of APP-CTFβ in APP-HFD+Ex was the same as that in APP-HFD+Dc mice (Fig. 5, A and C), indicating that both exercise and diet control inhibited β-secretase-mediated APP cleavage. This tendency was also observed in the level of APP-CTFα (Fig. 5, A and D).
FIGURE 5.
Both diet control and exercise reduced APP-CTFβ accumulation. A, immunoblotting analysis of full-length APP and APP-CTFα and APP-CTFβ. Full-length APP and APP CTFs (CTFα and CTFβ) were detected by anti-APP C terminus antibody. β-Actin was detected as loading control. Long exposure indicated that the same film was exposed for a longer time. The asterisk indicates glycosylated full-length APP. To analyze APP CTFs in detail, two kinds of gels (5–20% polyacrylamide gradient gels and 4–12% NuPAGE Bis-Tris gels) were used. Unfortunately, we could not clarify the mobility of CTFs bands caused by phosphorylation presumably because of the gel conditions of our experiment. B, statistical analysis of full-length APP. The band of full-length APP was normalized by that of β-actin. The band density of the control was regarded as 100%, and that of other groups was relatively indicated. There was no statistically significant difference among control APP, APP-HFD, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice (F(4, 10) = 0.47, not significant). C, statistical analysis of APP-CTFβ. The band of APP-CTFβ was normalized by that of full-length APP. The band density of APP-CTFβ in APP-HFD mice was increased when compared with that in control APP mice. However, the band densities of APP-CTFβ in APP-HFD+Ex (F(4, 10) = 4.27, *, p = 0.003), APP-HFD+Dc (*, p = 0.021), and APP-HFD+Ex+Dc (*, p = 0.023) mice were significantly decreased when compared with that in APP-HFD mice. There was no difference between APP-CTFβ in APP-HFD+Ex mice and that in APP-HFD+Dc mice. D, statistical analysis of APP-CTFα. The band of APP-CTFα was normalized by that of full-length APP. The band density of APP-CTFα in APP-HFD mice was increased when compared with that in control APP mice. The band densities of APP-CTFα in APP-HFD+Ex (F(4, 10) = 4.36, *, p = 0.034), APP-HFD+Dc (*, p = 0.014), and APP-HFD+Ex+Dc (*, p = 0.024) mice were significantly decreased when compared with that in APP-HFD mice. There was no difference between APP-CTFα in APP-HFD+Ex mice and that in APP-HFD+Dc mice.
Neprilysin Activity Was Up-regulated by Exercise
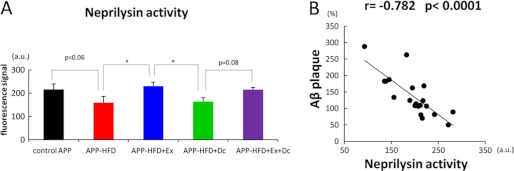
Next, we examined the effect of environmental intervention on Aβ degradation process in APP-HFD mice. Aβ-degrading proteases, including neprilysin, were reported to degrade Aβ both in vitro and in vivo (14, 15). To compare the effect of exercise on the degradation of Aβ with that of diet control, we analyzed the enzymatic neprilysin activity. As shown in Fig. 6A, the activity of neprilysin had a tendency to be suppressed by HFD, although it did not reach statistical significance. More importantly, the enzymatic activity of neprilysin in APP-HFD+Ex mice was significantly higher than that in APP-HFD+Dc mice. On the other hand, the neprilysin activity in APP-HFD+Dc mice was the same as that in APP-HFD mice. These results indicated that exercise could up-regulate the enzymatic activity of neprilysin. To clarify whether the up-regulation of neprilysin depended on its expression level, we conducted an immunoblotting assay. As shown in supplemental Fig. 4, the levels of neprilysin were not different among APP-HFD, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice, although those levels were slightly larger than that in control APP mice. In addition, we examined the expression levels of insulin-degrading enzyme, another Aβ-degrading protease, and determined that its levels were also not different among APP-HFD, APP-HFD+Ex, APP-HFD+Dc, and APP-HFD+Ex+Dc mice. To confirm the effect of neprilysin activity on the Aβ deposition in our mice, we conducted correlation analysis between neprilysin activity and the level of Aβ plaque. The activity of neprilysin was significantly correlated with the level of Aβ deposition (Fig. 6B).
FIGURE 6.
Exercise specifically rescued HFD-induced deterioration of neprilysin activity. A, In vitro enzyme activity assay of neprilysin using the fluorescence substrate. The activity of neprilysin in APP-HFD mice tends to be decreased when compared with that in control APP mice (F(4, 15) = 5.58, p = 0.061). On the other hand, neprilysin activity in APP-HFD+Ex mice was significantly higher than that in APP-HFD mice (*, p = 0.023) or that in APP-HFD+Dc mice (*, p = 0.032). B, significant correlation was established by comparing the activity of neprilysin and the level of cerebral Aβ deposition, using Pearson's correlation coefficients. The activity of neprilysin was negatively correlated with the level of accumulated Aβ (r = −0.782, *, p = 0.00003).
DISCUSSION
HFD is prevalent in modern society, and HFD-induced metabolic conditions are becoming a worldwide issue. Notably, an increasing number of studies have suggested that diet and nutrition are important epigenetic factors for the development of sporadic AD (16–18). Several epidemiological studies have reported that people with a higher body mass index during midlife are at greater risk for developing AD (19–21) and that obesity is associated with lower brain volumes in patients with mild cognitive impairment or AD (22). Obesity promotes a cascade of pathological conditions including type 2 diabetes mellitus and dyslipidemia. Remarkably, several drugs targeting type 2 diabetes mellitus and hypercholesterolemia have been shown to improve cognitive performance in mouse models of AD as well as in patients with early AD (23–26). In our experiment, serum glucose, serum insulin, and serum cholesterol levels were positively correlated with the result of the Morris test in APP-HFD mice, confirming that both type 2 diabetes mellitus and hypercholesterolemia could deteriorate memory deficit in APP mice (supplemental Fig. 5). However, serum glucose level was correlated with memory function in APP-HFD mice better than serum cholesterol and serum insulin levels. In addition, we previously demonstrated that exercise in our experimental condition does not improve HFD-induced hyperinsulinemia and hypercholesterolemia but ameliorates glucose intolerance (7). Therefore, intervention in patients with hyperglycemia and glucose intolerance might be important for the prevention of AD.
Magkos et al. (27) reviewed that first-line intervention of metabolic dysfunctions involves lifestyle modifications including diet control and physical activity and that metabolic dysfunctions may be reversible if addressed early on. They proposed that long-term engagement in lifestyle changes may result in the resolution of such dysfunctions (27). However, there has been no solid evidence on whether diet control or exercise is more effective in the prevention of AD. Therefore, in the present study, we aimed to compare the effect of diet control on metabolic dysfunctions as well as AD pathology with that of exercise using APP-HFD mice. Our present study clearly revealed that exercise ameliorated HFD-induced memory impairment better than diet control (Fig. 3). Exercise is reported to enhance neurogenesis and results in increased numbers of synapses per neuron (28). In addition, exercise increases the expression of the brain-derived neurotrophic factor (BDNF), which regulates neuronal development as well as plasticity (29). In this sense, exercise might specifically induce “cognitive reserve,” which increases cognitive function and enhances complex mental activity as protective factors against dementia (reviewed in Ref. 30). On the other hand, we showed that exercise decreased the level of soluble Aβ oligomers as well as deposited Aβ more than diet control (Fig. 4). Thus, the exercise-induced inhibition of Aβ oligomers might be involved in better cognitive performance in APP-HFD mice because the level of soluble Aβ oligomers is known to correlate with memory deficits due to their synaptotoxicity (2–4). In line with our present result, Hu et al. (28) previously showed that exercise reduces oligomeric Aβ levels in the cortex and hippocampus of AD model mice. However, there are a couple of differences between our experimental conditions and theirs. Our mice were fed HFD, but their mice were given a standard diet. Moreover, we analyzed oligomeric Aβ levels after onset of Aβ deposition, but they examined them before Aβ accumulation. Therefore, our results clearly supported that exercise reduced oligomeric Aβ levels after onset of Aβ deposition even if the mice were fed with HFD.
The level of Aβ within the brain is determined by the balance between its production and its degradation. Because Aβ is generated by the proteolytic processing of APP, we first examined whether exercise or diet control was acting directly on Aβ production. Our detailed immunoblotting analysis showed that the level of APP-CTFβ in APP-HFD+Ex mice was almost the same as that in APP-HFD+Dc mice (Fig. 5), indicating that the production of Aβ might not be different between APP-HFD mice treated with exercise and those treated with diet control. On the contrary, the enzymatic activity of neprilysin, the Aβ-degrading enzyme, was up-regulated in APP-HFD+Ex mice more than in APP-HFD+Dc mice (Fig. 6). Lazarov et al. (31) have reported that voluntary exercise elevates neprilysin activity in the brain of APP transgenic mice, contributing to the lowering of Aβ levels, which is in line with our findings. However, their result is different from ours in that their APP mice, which were allowed voluntary exercise, were fed a standard diet. Intriguingly, we found that feeding HFD itself reduced the activity of neprilysin. We further observed that exercise strengthened neprilysin activity even if the mice were fed with HFD. In the present study, the activity of neprilysin was negatively correlated with the level of deposited Aβ (Fig. 6). Therefore, we assumed that exercise-mediated up-regulation of neprilysin may critically reduce HFD-induced Aβ deposition. Importantly, the expression level of neprilysin did not change between the exercise- and the diet control-treated APP-HFD mice (supplemental Fig. 4). Because the activity of neprilysin was clearly up-regulated by exercise only, we concluded that exercise could modulate its activity in an expression level-independent manner. We speculate that exercise may specifically modulate the upstream molecules of neprilysin or may regulate posttranslational modification of neprilysin. The mechanism of neprilysin up-regulation by exercise should be clarified in future studies.
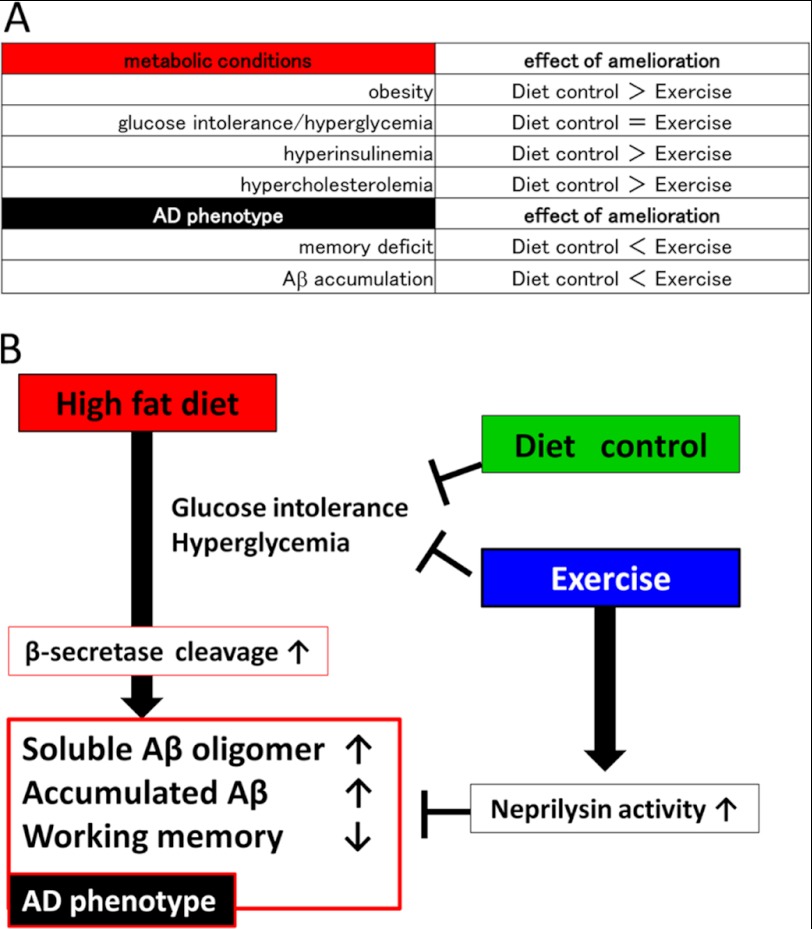
As classified in Fig. 7A, our result, for the first time, clarified the differential effects of diet control and exercise on metabolic and cognitive dysfunctions. According to our data, diet control significantly improved HFD-induced metabolic conditions, including obesity, hyperinsulinemia, and hypercholesterolemia (Figs. 1 and 2), when compared with exercise. However, exercise decreased Aβ oligomers as well as deposited Aβ (Fig. 4) and ameliorated memory impairment (Fig. 3) when compared with diet control. From these results, we conclude that exercise was more effective than diet control in the prevention of HFD-induced amyloid pathology. In Fig. 7B, we present our hypothesis on how diet control and exercise differently ameliorate HFD-induced Aβ deposition and memory deficit in APP transgenic mice. As described in our previous study, HFD leads to glucose intolerance and hyperglycemia, which may lead to the up-regulation of β-secretase activity. This up-regulation increases soluble Aβ oligomers as well as deposited Aβ levels followed by memory deficit (7). The up-regulation of β-secretase was also reported in HFD-feeding mice from another laboratory (32) and consistently reported in AD cases by several previous studies (33–35). Thus, this phenomenon might represent the actual pathology of sporadic AD. On the other hand, both diet control and exercise ameliorate HFD-induced glucose intolerance and hyperglycemia, thereby decreasing Aβ load by inhibiting Aβ production. However, exercise specifically strengthens the enzymatic activity of neprilysin, which decreases the level of Aβ in the brain. Surprisingly, the effect of the combination treatment (exercise and diet control) on cognitive function and amyloid pathology was not significantly different from that of exercise only, indicating that exercise is an effective behavioral intervention sufficient to inhibit Aβ pathology. We suppose that this is because exercise affects both the production and the degradation of Aβ, but diet control modifies only the production of Aβ. Therefore, for the introduction of intervening metabolic functions targeting the prevention of AD, we provide the first evidence-based comparison of effective interventions, concluding that exercise has the highest priority. Although the beneficial effect of exercise was obtained even under HFD, the magnitude and the nature (i.e. voluntary versus forced, aerobic versus anaerobic) of the exercise required to prevent HFD-induced AD progression should be elucidated in future studies. Because metabolic dysfunctions are epidemiologically considered to be risk factors of sporadic AD, evidence-based interventions for metabolic dysfunctions should be carried out to prevent AD.
FIGURE 7.
Schematic presentation of our study. A, the classification of the results in the present study. The items we analyzed in this study are included in the left column, whereas the effect of amelioration is showed in the right column. As shown in this table, Diet control > Exercise indicated that diet control ameliorated better than exercise. Diet control significantly improved HFD-induced metabolic conditions, including obesity, hyperinsulinemia, and hypercholesterolemia, better than exercise. However, exercise decreased soluble Aβ oligomers as well as deposited Aβ and ameliorated memory impairment better than diet control. B, schematic presentation of our hypothesis: how diet control or exercise ameliorated HFD-induced memory deficits and Aβ accumulation. HFD leads to glucose intolerance and hyperglycemia, which may lead to the up-regulation of β-secretase activity. This up-regulation increases soluble Aβ oligomers as well as deposited Aβ levels followed by memory deficit (7). On the other hand, both diet control and exercise ameliorate HFD-induced glucose intolerance and hyperglycemia, thereby decreasing soluble Aβ oligomer and fibrillar Aβ levels by inhibiting Aβ production. However, exercise specifically strengthens the enzymatic activity of neprilysin, which degrades Aβ in the brain.
Supplementary Material
Acknowledgments
We appreciate Dr. S. Oka (Kyoto University, Kyoto, Japan), Mr. J. Morise (Kyoto University), Dr. Y. Kitamura (Kyoto Pharmaceutical University, Kyoto, Japan), and Dr. K. Takata (Kyoto Pharmaceutical University) for technical assistance in the brain sample preparation. We thank Dr. K. Saito (Kyoto University) for kind support of the Morris water maze test. We greatly appreciate the advice for metabolic analysis from Dr. M. Okuda (PHARMAEIGHT, Kyoto, Japan). We thank Dr. Y. Tashiro (Kyoto University) for kind discussion.
The work was financially supported by a Core Backup Stage Grant from Kyoto University (to A. Kinoshita), Grant-in-Aid 23591243 from the Ministry of Education, Culture, Sports, Science and Technology (to K. U.) and a research grant from the Takeda Science Foundation (to K. U.).
This article was selected as a Paper of the Week.

This article contains supplemental Figs. 1–5.
- AD
- Alzheimer disease
- HFD
- high fat diet
- APP
- amyloid precursor protein
- Aβ
- β-amyloid
- Ex
- exercise
- Dc
- diet control
- CTF
- C terminus fragments
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Finder V. H. (2010) Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. J. Alzheimers Dis. 22, Suppl. 3, 5–1920858960 [Google Scholar]
- 2. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 3. Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 4. Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008) Amyloid-β protein dimers isolated directly from Alzheimer brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luchsinger J. A., Tang M. X., Shea S., Mayeux R. (2002) Caloric intake and the risk of Alzheimer disease. Arch. Neurol. 59, 1258–1263 [DOI] [PubMed] [Google Scholar]
- 6. Ho L., Qin W., Pompl P. N., Xiang Z., Wang J., Zhao Z., Peng Y., Cambareri G., Rocher A., Mobbs C. V., Hof P. R., Pasinetti G. M. (2004) Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer disease. FASEB J. 18, 902–904 [DOI] [PubMed] [Google Scholar]
- 7. Maesako M., Uemura K., Kubota M., Kuzuya A., Sasaki K., Asada M., Watanabe K., Hayashida N., Ihara M., Ito H., Shimohama S., Kihara T., Kinoshita A. (2012) Environmental enrichment ameliorated high fat diet-induced Aβ deposition and memory deficit in APP transgenic mice. Neurobiol. Aging 33, 1011.e11–23 [DOI] [PubMed] [Google Scholar]
- 8. Scarmeas N., Stern Y., Tang M. X., Mayeux R., Luchsinger J. A. (2006) Mediterranean diet and risk for Alzheimer disease. Ann. Neurol. 59, 912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laurin D., Verreault R., Lindsay J., MacPherson K., Rockwood K. (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 58, 498–504 [DOI] [PubMed] [Google Scholar]
- 10. Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., McConlogue L. (2000) High level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maesako M., Uemura K., Kuzuya A., Sasaki K., Asada M., Watanabe K., Ando K., Kubota M., Kihara T., Kinoshita A. (2011) Presenilin regulates insulin signaling via a γ-secretase-independent mechanism. J. Biol. Chem. 286, 25309–25316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitaguchi H., Tomimoto H., Ihara M., Shibata M., Uemura K., Kalaria R. N., Kihara T., Asada-Utsugi M., Kinoshita A., Takahashi R. (2009) Chronic cerebral hypoperfusion accelerates amyloid-β deposition in APPSwInd transgenic mice. Brain res. 1294, 202–210 [DOI] [PubMed] [Google Scholar]
- 13. Rose J. B., Crews L., Rockenstein E., Adame A., Mante M., Hersh L. B., Gage F. H., Spencer B., Potkar R., Marr R. A., Masliah E. (2009) Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer disease. J. Neurosci. 29, 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., Kawashima-Morishima M., Lee H. J., Hama E., Sekine-Aizawa Y., Saido T. C. (2000) Identification of the major Aβ1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat. Med 6, 143–150 [DOI] [PubMed] [Google Scholar]
- 15. Shirotani K., Tsubuki S., Iwata N., Takaki Y., Harigaya W., Maruyama K., Kiryu-Seo S., Kiyama H., Iwata H., Tomita T., Iwatsubo T., Saido T. C. (2001) Neprilysin degrades both amyloid-β peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J. Biol. Chem. 276, 21895–21901 [DOI] [PubMed] [Google Scholar]
- 16. Solfrizzi V., Panza F., Capurso A. (2003) The role of diet in cognitive decline. J. Neural. Transm. 110, 95–110 [DOI] [PubMed] [Google Scholar]
- 17. Panza F., Capurso C., D'Introno A., Colacicco A. M., Del Parigi A., Seripa D., Pilotto A., Capurso A., Solfrizzi V. (2006) Diet, cholesterol metabolism, and Alzheimer disease: apolipoprotein E as a possible link? J. Am Geriatr Soc. 54, 1963–1965 [DOI] [PubMed] [Google Scholar]
- 18. Scarmeas N., Luchsinger J. A., Mayeux R., Stern Y. (2007) Mediterranean diet and Alzheimer disease mortality. Neurology 69, 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kivipelto M., Ngandu T., Fratiglioni L., Viitanen M., Kåreholt I., Winblad B., Helkala E. L., Tuomilehto J., Soininen H., Nissinen A. (2005) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 62, 1556–1560 [DOI] [PubMed] [Google Scholar]
- 20. Whitmer R. A., Gunderson E. P., Quesenberry C. P., Jr., Zhou J., Yaffe K. (2007) Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr. Alzheimer Res. 4, 103–109 [DOI] [PubMed] [Google Scholar]
- 21. Fitzpatrick A. L., Kuller L. H., Lopez O. L., Diehr P., O'Meara E. S., Longstreth W. T., Jr., Luchsinger J. A. (2009) Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 66, 336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho A. J., Raji C. A., Becker J. T., Lopez O. L., Kuller L. H., Hua X., Lee S., Hibar D., Dinov I. D., Stein J. L., Jack C. R., Jr., Weiner M. W., Toga A. W., Thompson P. M. (2010) Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging 31, 1326–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watson G. S., Cholerton B. A., Reger M. A., Baker L. D., Plymate S. R., Asthana S., Fishel M. A., Kulstad J. J., Green P. S., Cook D. G., Kahn S. E., Keeling M. L., Craft S. (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr Psychiatry 13, 950–958 [DOI] [PubMed] [Google Scholar]
- 24. Pedersen W. A., McMillan P. J., Kulstad J. J., Leverenz J. B., Craft S., Haynatzki G. R. (2006) Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 199, 265–273 [DOI] [PubMed] [Google Scholar]
- 25. Reger M. A., Watson G. S., Green P. S., Wilkinson C. W., Baker L. D., Cholerton B., Fishel M. A., Plymate S. R., Breitner J. C., DeGroodt W., Mehta P., Craft S. (2008) Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology 70, 440–448 [DOI] [PubMed] [Google Scholar]
- 26. Shepardson N. E., Shankar G. M., Selkoe D. J. (2011) Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 68, 1239–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magkos F., Yannakoulia M., Chan J. L., Mantzoros C. S. (2009) Management of the metabolic syndrome and type 2 diabetes through lifestyle modification. Annu Rev. Nutr 29, 223–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu Y. S., Xu P., Pigino G., Brady S. T., Larson J., Lazarov O. (2010) Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer disease-linked APPswe/PS1ΔE9 mice. FASEB J. 24, 1667–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neeper S. A., Gómez-Pinilla F., Choi J., Cotman C. (1995) Exercise and brain neurotrophins. Nature 373, 109. [DOI] [PubMed] [Google Scholar]
- 30. Tucker A. M., Stern Y. (2011) Cognitive reserve in aging. Curr Alzheimer Res. 8, 354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lazarov O., Robinson J., Tang Y. P., Hairston I. S., Korade-Mirnics Z., Lee V. M., Hersh L. B., Sapolsky R. M., Mirnics K., Sisodia S. S. (2005) Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell 120, 701–713 [DOI] [PubMed] [Google Scholar]
- 32. Thirumangalakudi L., Prakasam A., Zhang R., Bimonte-Nelson H., Sambamurti K., Kindy M. S., Bhat N. R. (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 106, 475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukumoto H., Cheung B. S., Hyman B. T., Irizarry M. C. (2002) β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 59, 1381–1389 [DOI] [PubMed] [Google Scholar]
- 34. Yang L. B., Lindholm K., Yan R., Citron M., Xia W., Yang X. L., Beach T., Sue L., Wong P., Price D., Li R., Shen Y. (2003) Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 9, 3–4 [DOI] [PubMed] [Google Scholar]
- 35. Li R., Lindholm K., Yang L. B., Yue X., Citron M., Yan R., Beach T., Sue L., Sabbagh M., Cai H., Wong P., Price D., Shen Y. (2004) Amyloid-β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer disease patients. Proc. Natl. Acad. Sci. U.S.A. 101, 3632–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.