Background: GSK-3β is a key pro-apoptotic kinase, and its activity is strictly regulated.
Results: GSK-3β is cleaved at both N and C termini by calpain.
Conclusion: N- or C-terminal truncation activates GSK-3β, and Ser-9/Ser-389 phosphorylation protects GSK-3β from calpain cleavage.
Significance: The GSK-3β C terminus functions as an autoinhibitory domain, and Ser-9/Ser-389 phosphorylation and calpain-mediated cleavage operate together in regulating GSK-3β activity.
Keywords: Calpain, Glycogen Synthase Kinase 3, Neurodegeneration, Neurons, Phosphorylation, Cerebellar Granule Neuron, Cleavage, Neuronal Death
Abstract
Glycogen synthase kinase-3β (GSK-3β), a key regulator of neuronal apoptosis, is inhibited by the phosphorylation of Ser-9/Ser-389 and was recently shown to be cleaved by calpain at the N terminus, leading to its subsequent activation. In this study calpain was found to cleave GSK-3β not only at the N terminus but also at the C terminus, and cleavage sites were identified at residues Thr-38–Thr-39 and Ile-384–Gln-385. Furthermore, the cleavage of GSK-3β occurred in tandem with Ser-9 dephosphorylation during cerebellar granule neuron apoptosis. Increasing Ser-9 phosphorylation of GSK-3β by inhibiting phosphatase 1/2A or pretreating with purified active Akt inhibited calpain-mediated cleavage of GSK-3β at both N and C termini, whereas non-phosphorylatable mutant GSK-3β S9A facilitated its cleavage. In contrast, Ser-389 phosphorylation selectively inhibited the cleavage of GSK-3β at the C terminus but not the N terminus. Calpain-mediated cleavage resulted in three truncated products, all of which contained an intact kinase domain: ΔN-GSK-3β (amino acids 39–420), ΔC-GSK-3β (amino acids 1–384), and ΔN/ΔC-GSK-3β (amino acids 39–384). All three truncated products showed increased kinase and pro-apoptotic activity, with ΔN/ΔC-GSK-3β being the most active form. This observation suggests that the GSK-3β C terminus acts as an autoinhibitory domain similar to the N terminus. Taken together, these findings demonstrate that calpain-mediated cleavage activates GSK-3β by removing its N- and C-terminal autoinhibitory domains and that Ser-9 phosphorylation inhibits the cleavage of GSK-3β at both termini. In contrast, Ser-389 phosphorylation inhibits only C-terminal cleavage but not N-terminal cleavage. These findings also identify a mechanism by which site-specific phosphorylation and calpain-mediated cleavage operate in concert to regulate GSK-3β activity.
Introduction
Glycogen synthase kinase-3β (GSK-3β)3 is a serine/threonine protein kinase consisting of 420 amino acids and translating into a 47-kDa protein. The core kinase domain (amino acids 55–345) of GSK-3β shares ∼33% amino acid sequence identity with other members of the serine/threonine protein kinase family, whereas the N- and C-terminal structural elements are unique (1). The N and C termini are thought to function as regulatory domains, but the underlying regulatory mechanisms remain to be fully elucidated. GSK-3β is particularly abundant in the central nervous system (2, 3). Aberrantly increased GSK-3β activity plays a key role in neuronal death (4–6) and has been implicated in the development of neurodegenerative disorders, such as Alzheimer disease (7) and Parkinson disease (8). Various GSK-3β inhibitors exert neuroprotective effects in a wide array of different neuronal death paradigms (9–11). Therefore, the tight regulation of GSK-3β is crucial for neuronal survival and is worthy of further exploration.
Inhibitory phosphorylation of Ser-9 by upstream kinases, such as Akt (12), PKA (13), and calcium/calmodulin dependent protein kinase II (14), is the most extensively studied mechanism controlling GSK-3β activity. The phosphorylated N terminus acts as a pseudosubstrate for GSK-3β, binding the pocket that normally binds prephosphorylated, or “primed,” substrates to block its own activity (15). Trophic withdrawal- (6) or activity deprivation-induced (14) loss of GSK-3β Ser-9 phosphorylation has been shown to contribute to neuronal death, indicating that Ser-9 phosphorylation plays an important role in neuronal survival. In addition, Thr-390 at the C terminus of human GSK-3β (Ser-389 in mouse and rat GSK-3β) was recently identified as a novel residue for phosphorylation by p38 MAPK (16). Phosphorylation at Thr-390/Ser-389 by p38 MAPK causes inhibition of GSK-3β comparable with that of Ser-9 phosphorylation. Thus, the GSK-3β C terminus may have an autoinhibitory property similar to that of the N terminus.
In addition to phosphorylation, enzymatic cleavage of proteins can also serve as a regulatory mechanism. Calpain, a calcium-dependent cysteine protease, has been reported to remove the inhibitory N-terminal domain of GSK-3β and thus activate it in vitro (17). This cleavage has been observed in certain neuropathological conditions, including post-mortem brain samples (18), cortical neurons exposed to glutamate (17) or ionomycin (19), and hippocampal neurons treated with 3-nitropropionic acid (20). However, the biological significance of calpain-mediated cleavage of GSK-3β remains unknown.
This study provides the first evidence that calpain truncates GSK-3β not only at the N terminus but also at the C terminus in various neuronal cell death paradigms. N- as well as C-terminal-truncated GSK-3β has enhanced kinase and pro-apoptotic activities, indicating that the GSK-3β C terminus exerts an autoinhibitory effect similar to that of the N terminus. This study also shows that Ser-9 phosphorylation inhibits the cleavage of GSK-3β at both termini, whereas Ser-389 phosphorylation inhibits only the C-terminal cleavage but not the N-terminal cleavage. Hence, the collaborative regulation of GSK-3β through Ser-9/Ser-389 phosphorylation and calpain-mediated cleavage may play an important role in neuronal death.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant calpain was from EMD Biosciences (catalog no. 208718), and N-terminally His-tagged GSK-3β (rabbit) was from Sigma (catalog no. G1663). Recombinant N-terminally His-tagged GSK-3β (rat) was expressed by the Bac-to-Bac Baculovirus Expression System (Invitrogen) and was purified by nickel-nitrilotriacetic acid-agarose (Qiagen) chromatography. Purified Akt, okadaic acid (OA), and protein G plus protein A-agarose were obtained from EMD Biosciences. Calpeptin and selective GSK-3β phosphopeptide substrate 2B-SP were purchased from Tocris Bioscience; glutamate, 1-methyl-4-phenylpyridinium (MPP+), and calpain inhibitor II (ALLM) were purchased from Sigma. Caspase inhibitor Z-VAD-FMK and endoproteinase Asp-N were acquired from Enzo Life Sciences and Roche Applied Science, respectively. Peptides, including Ser-389-tide (RIQAAASPPAN, corresponding to residues 383–393 of rat GSK-3β), Ser(P)-389-tide (RIQAAA(P)SPPAN, (P) represents a phosphate), Ser-9-tide (RPRTTSFAESC, corresponding to residues 4–14 of GSK-3β), and Ser(P)-9-tide (RPRTT(P)SFAESC) were synthesized by Genemed Synthesis, Inc.
Cell Culture and Treatments
Rat cerebellar granule neurons (CGNs) were cultured in basal modified Eagle's medium containing 10% fetal bovine serum and 25 mm KCl (25K) as previously described (21, 22). Transfections were performed on culture days 5–6 (DIV5–6), and experiments were performed after 7 days (DIV7) of culture. For potassium deprivation, DIV7 cells in basal modified Eagle's medium and 25K were switched to serum-free medium containing 25K or 5 mm KCl (5K) at various times. MPP+ (200 μm) or glutamate (75 μm) was extemporaneously prepared by solubilization into CGN culture medium. To inhibit calpain or caspases, CGNs in 5K, MPP+, or glutamate medium were treated with ALLM (25 μm), calpeptin (5 μm), or Z-VAD-FMK (10 μm) for 12 h. To inhibit phosphatase 1/2A, OA was added to CGN culture to a final concentration of 25 nm for 3 h, and the cell lysates were subsequently collected for in vitro cleavage assays. Neurons that did not receive inhibitors received DMSO as a control, and the final concentration of DMSO in the medium was no more than 0.1%. Human embryonic kidney (HEK) 293 cells were cultured in DMEM with 10% fetal bovine serum.
Immunoblotting and Antibodies
Immunoblot analysis was performed as described previously (23, 24). The primary antibodies used in this study were GSK-3β antibodies (Santa Cruz Biotechnology, no. SC9166, designated GSK-3β Mid antibody; Sigma, no. G7914, designated GSK-3β C-ter antibody); p-GSK-3β (Ser-9), HA, HA-HRP (Cell Signal Technology, no. 9336, no. 2367, no. 2999); V5 (AbD Serotec, no. 1360GA), FLAG, and tubulin (Sigma, no. F3165, no. T4026). The p-GSK-3β (Ser-389) antibody was generated by GenScript using peptide ARIQAAA(P)SPPANATA with N-terminal acetylation for immunization.
Two-dimensional Electrophoresis
Two-dimensional electrophoresis was performed as described previously (25) with slight modifications. Briefly, 25K- or 5K-treated CGNs were washed 3 times in ice-cold Tris-buffered sucrose solution, scraped, and lysed in ice-cold lysis buffer (30 mm Tris-HCl, pH 8.5, 7 m urea, 2 m thiourea, and 4% w/v CHAPS). Next, the two-dimensional Clean-up kit (GE Healthcare) and two-dimensional Quant kit (GE Healthcare) were used to sequentially remove non-protein material from the extract and determine the final protein concentrations. The amount of protein to be electrophoresed on one gel was set at 60 μg. The protein sample was dissolved in rehydration buffer (7 m urea, 2 m thiourea, 4% w/v CHAPS, 40 mm DTT, 1% immobilized pH gradient (IPG) buffer pH 6–11, and 0.002% w/v bromphenol blue). Rehydration and isoelectric focusing were performed according to the manufacturer's instructions using pH 6–11 IPG strips (13 cm) and an Ettan IPGphor II apparatus (GE Healthcare). After two-step equilibration, the proteins were resolved in the second dimension on a 12.5% SDS-PAGE gel (18 × 16 cm) using the Hoefer SE 600 Ruby instrument (GE Healthcare).
Calpain Cleavage Assay
CGNs with or without OA treatment and HEK293 cells transfected with GSK-3β wild type (WT), a serine 9 to alanine mutant (S9A), or a serine 389 to alanine mutant (S389A) were homogenized in lysis buffer (50 mm Tris-HCl, pH 7.4, 0.5% Nonidet P-40, 50 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 1 mm β-glycerophosphate, and 1 mm sodium pyrophosphate). After centrifugation, the supernatants were collected and incubated with 5 mm CaCl2 and 3 units/ml calpain at 30 ºC for different incubation periods. Recombinant His-tagged GSK-3β (0.5 μg) was incubated in cleavage buffer (50 mm Tris-HCl pH 7.4, 50 mm NaCl, 1 mm EDTA, 1 mm EGTA, and 1 mm DTT) containing 0.3 unit/ml calpain and 5 mm CaCl2. Samples were then incubated at 30 ºC for various times in the presence or absence of 25 μm ALLM. For the Akt phosphorylation studies, 800 ng of recombinant His-tagged GSK-3β was incubated for 30 min at 30 ºC with 1 μl of purified Akt in 30 μl of kinase reaction buffer (25 mm Tris-Cl pH 7.5, 1 mm DTT, 10 mm MgCl2, and 1 mm β-glycerophosphate) in the presence or absence of 100 mm ATP. After phosphorylation, the reaction mix was diluted with cleavage buffer to a final volume of 300 μl followed by further incubations for different times at 30 ºC with or without 0.3 unit/ml calpain and 5 mm CaCl2. For immunoblot analysis, the reactions described above were terminated by the addition of 3× Laemmli buffer followed by boiling for 5 min. For the GSK-3β kinase activity assay, in vitro cleavage of recombinant His-tagged GSK-3β was terminated with the addition of 25 μm ALLM.
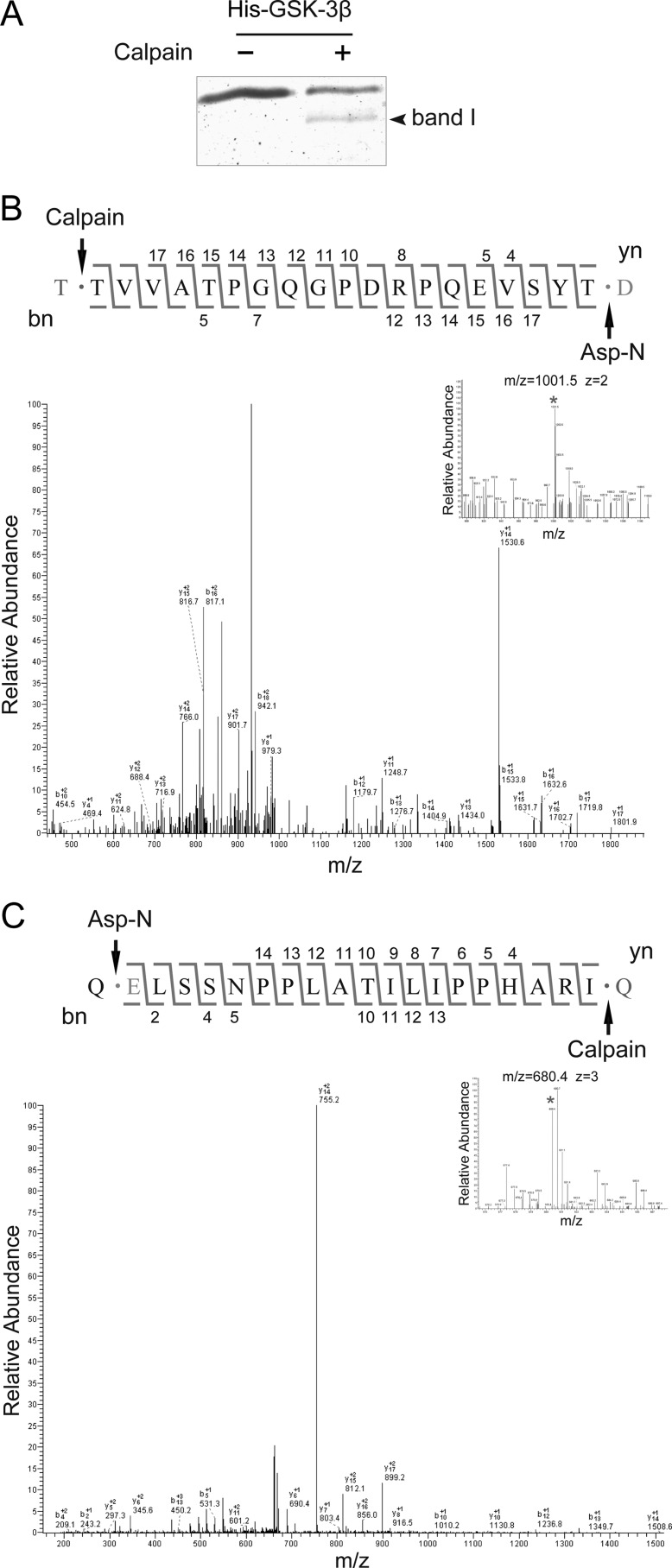
Identification of Cleavage Sites
Samples of the calpain-mediated in vitro cleavage products derived from the recombinant His-tagged GSK-3β were separated by one-dimensional SDS-PAGE. Gels were stained with Coomassie Brilliant Blue, and band I was excised and subjected to in-gel digestion with the endoproteinase Asp-N. Identification of the extracted peptides was performed using nano-HPLC MS/MS on an Agilent 1100 instrument (Agilent, Santa Clara, CA) coupled to an LTQ-Orbitrap fitted with a 10 cm long, 100 μm inner diameter fused silica column packed with 5 μm particle size reversed phase (C18) beads (Michrom Bioresources) and using a water:acetonitrile:formic acid formula as the mobile phase for gradient elution. Mass spectrometry data were analyzed by comparison with the GSK-3β sequence (UniProtKB P49841) using Mascot v.2.1.
Constructs and Transfection
The rat GSK-3β WT and its deletion mutants ΔN (amino acids 39–420), ΔC (amino acids 1–384), and ΔN/ΔC (amino acids 39–384) were subcloned into the pcDNA3.1–3×FLAG vector. The GSK-3β S9A mutant plasmid has been described previously (14). The point mutation at Ser-389 (Ala) was introduced by overlap extension PCR. The N-terminally FLAG-tagged GSK-3β WT, S9A, or S389A sequence was subsequently subcloned into the pcDNA3.1/V5-His vector (Invitrogen). The rat m-calpain coding sequence was generously provided by Dr. Hiroyuki Sorimachi (Tokyo Metropolitan Institute of Medical Science, Japan) and subcloned into the pCMV-HA vector (Clontech). MKK6 and p38 plasmids were kindly provided by Dr. Jiahuai Han (Xiamen University, China). The primer sequences used for cloning are available upon request. All constructs were confirmed by DNA sequencing. For CGN transfection, cells at DIV5–6 were transfected using the calcium phosphate transfection method as described previously (13, 23). The overall transfection efficiency using the calcium phosphate method in CGN culture was ∼1%. HEK293 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Immunoprecipitation
Immunoprecipitation assays were performed as described previously (24). HEK293 cells were transfected with 3 μg of pcDNA3.1–3×FLAG-GSK-3β WT, ΔN, ΔC, or ΔN/ΔC for 24 h. The cell lysates were centrifuged at 10,000 rpm for 10 min at 4 ºC. Before immunoprecipitation, 2 μg FLAG antibody was incubated with protein G plus protein A-agarose for 4 h at 4 ºC. Next, antibody and agarose complex were added to the cleared lysates and incubated overnight at 4 ºC. The immunoprecipitates were subsequently collected by short centrifugations and washed three times with lysis buffer and twice with GSK-3β kinase assay buffer (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, and 1 mm DTT). The precipitates were subsequently used for the GSK-3β activity assay. For co-immunoprecipitation of calpain and GSK-3β, HEK293 cells were co-transfected with 2.5 μg of FLAG-tagged GSK-3β WT, S9A, or control plasmid and a HA-tagged calpain plasmid. Twenty-four hours after transfection, the cells were lysed and immunoprecipitated with 2 μg of FLAG antibody. The precipitated immunocomplexes were subsequently subjected to immunoblotting analysis.
GSK-3β Kinase Assay
GSK-3β activity assay was described in our previous reports (13, 14). Briefly, samples from in vitro cleavage or immunoprecipitation were incubated in a total volume of 40 μl of GSK-3β kinase assay buffer containing 100 μm 2B-SP and 100 μm [γ-32P]ATP (2 μCi) (PerkinElmer Life Sciences). After incubation for 30 min at 30 ºC, the reaction was terminated by spotting onto P81 phosphocellulose paper (GE Healthcare). Filters were washed in 3 changes of 0.75% phosphoric acid for a total of 15 min, rinsed in acetone, and dried. The filter radioactivity was determined by Cerenkov counting.
Immunofluorescence and Quantification of Neuronal Apoptosis
Immunofluorescence and imaging were performed as described previously (14). Briefly, CGNs were transfected with GFP (for marking the transfected cells) and either FLAG-GSK-3β or one of its deletion mutants. Twenty-four hours after transfection, cells were fixed and incubated with FLAG antibody and later with an Alexa Fluor®555 secondary antibody (Invitrogen). Anti-FLAG fluorescence intensity was determined in GFP-positive cells using ImageJ software. The expression level of FLAG-tagged GSK-3β was determined by the relative fluorescence intensity of the transfected cells. The relative fluorescence intensity of GSK-3β WT-transfected cells was set to 1.0. For the apoptosis quantification assay, 36 h after transfection, the neurons were switched to 25K medium for 12 h and stained with Hoechst 33258 to visualize nuclear morphology. Apoptotic rates were quantified by scoring the percentage of GFP-positive cells with condensed or fragmented nuclei as described previously (14, 21). Cells were counted in an unbiased manner, with at least 500 transfected cells being counted for each group.
Statistical Analysis
All experiments were independently repeated at least three times, and all measurements were performed blindly. The significance of the difference between the means was analyzed by analysis of variance followed by the Bonferroni post hoc test for multiple comparisons between groups. Student's t test was used for single comparison between treatment and control groups. All values represent the mean ± S.E. For this study, p < 0.05 was considered to be statistically significant.
RESULTS
Calpain Cleaves GSK-3β at Both N and C Termini during CGN Death
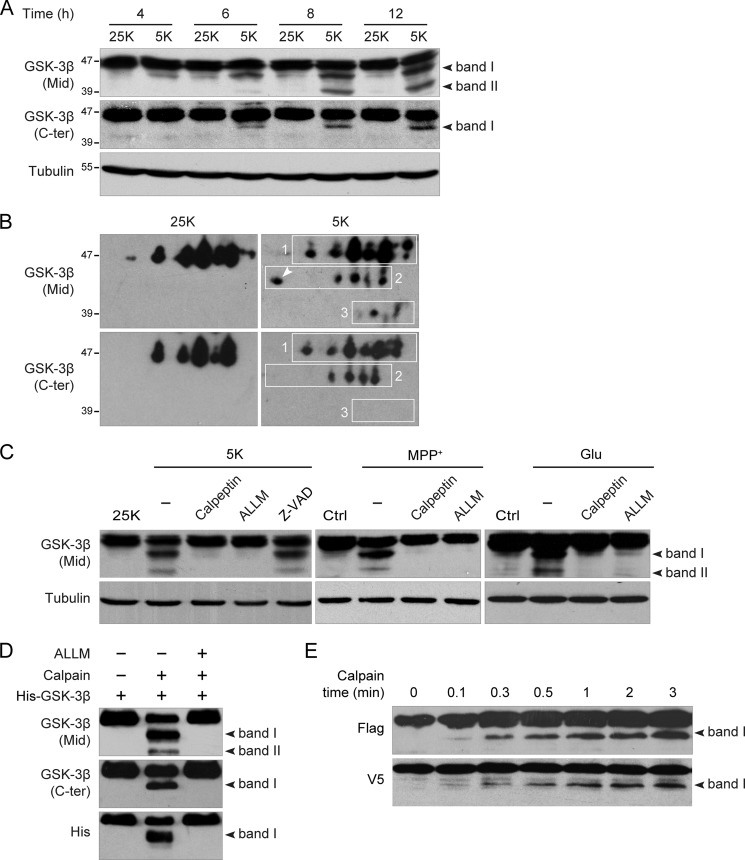
Cultured CGNs are healthy in serum-free medium containing 25K but undergo apoptosis after switching to 5K medium, as has been described by our group (21, 23, 24) and others (22, 26). A time-course analysis of GSK-3β using a polyclonal antibody against amino acids 345–420 of GSK-3β (designated the Mid antibody) showed a time-dependent decrease in the 47-kDa full-length GSK-3β in 5K compared with 25K. Concomitantly, a ∼43-kDa fragment (band I) appeared at 4 h, and its intensity gradually increased over time as the 5K treatment progressed (Fig. 1A, upper panel). Another fragment, which exhibited a molecular mass of ∼39-kDa (band II), emerged at 6 h, becoming more apparent at 8 h and later (Fig. 1A, upper panel). Cleavage of GSK-3β into two fragments by calpain has been described by others (17), but the cleavage sites have not been identified. The cleavage sites were assumed to be located at the N terminus based on the observation that a His-tag antibody was unable to recognize the truncated fragments of recombinant N-terminally His-tagged GSK-3β in vitro.
FIGURE 1.
Calpain cleaves GSK-3β at both N and C termini during CGN death. A, activity deprivation-induced GSK-3β cleavage is shown. DIV7 CGNs were incubated in 25K (control) or 5K medium for the indicated times. Cell lysates were subjected to immunoblotting with the indicated antibodies and with a tubulin as the loading control. The two black arrowheads indicate band I and band II, which are the truncated fragments of GSK-3β. B, CGNs incubated in 25K or 5K for 8 h were lysed, subjected to two-dimensional gel electrophoresis, and immunoblotted with either GSK-3β Mid or C-ter antibody. The spots in rectangle 1 represent full-length GSK-3β; the white arrow denotes ΔC-GSK-3β. The remaining spots in rectangle 2 represent ΔN-GSK-3β; the spots in rectangle 3 represent ΔN/ΔC-GSK-3β. C, DIV7 CGNs were treated with 5K, MPP+, or glutamate (Glu) in the presence or absence of 25 μm ALLM, 5 μm calpeptin, or 10 μm Z-VAD-FMK (Z-VAD) for 12 h. D, in vitro cleavage of GSK-3β by calpain is shown. Recombinant His-GSK-3β was incubated with or without 0.3 unit/ml calpain plus 5 mm calcium in the presence or absence of 25 μm ALLM for 2 min. E, time course of GSK-3β cleavage is shown. FLAG-GSK-3β-V5 was transfected into HEK293 cells for 24 h, and cell lysates were added with 3 units/ml calpain plus 5 mm calcium for the various time intervals.
If GSK-3β is truncated only at the N terminus, the cleavage patterns exhibited by the Mid and GSK-3β C-terminal antibodies should be identical. Surprisingly, when the immunoblots were probed with a C-terminal antibody against the last 17 C-terminal residues of GSK-3β (designated GSK-3β C-ter antibody), band I, but not band II, was observed (Fig. 1A, middle panel) even when the blot was overexposed (data not shown). This finding implies that GSK-3β was truncated at the C terminus as well as the N terminus. Thus, band I, which was clearly detectable by both Mid and C-ter antibodies, was definitely N-terminal-truncated GSK-3β (ΔN-GSK-3β), whereas band II, which was positive to the Mid antibody but negative to the C-ter antibody, may be C-terminal-truncated GSK-3β (ΔC-GSK-3β) or both N- and C-terminal-truncated GSK-3β (ΔN/ΔC-GSK-3β). Closer observation revealed that the ratio of band I to full-length GSK-3β recognized by the C-ter antibody was much less than that recognized by the Mid antibody, implying that band I did not consist entirely of ΔN-GSK-3β but may have overlapped with ΔC-GSK-3β. Thus, band II was deduced to be ΔN/ΔC-GSK-3β from the apparent molecular masses.
An N-terminal antibody would directly recognize ΔC-GSK-3β and verify this hypothesis; however, no commercially available GSK-3β N-terminal antibody is appropriate for this purpose. As an alternative, two-dimensional electrophoresis was performed to separate the cleavage products based on their molecular masses as well as their net charges. Full-length GSK-3β was separated into a series of spots (Fig. 1B, rectangle 1) corresponding to the different phosphorylation states of GSK-3β. Accordingly, two groups of truncated products, the spots in rectangle 2 (equivalent to band I) and the spots in rectangle 3 (equivalent to band II), were detected by the Mid antibody in 5K but not in 25K (Fig. 1B), which was consistent with the immunoblotting results (Fig. 1A). Interestingly, the C-ter antibody missed one spot in rectangle 2 (Fig. 1B, denoted by the white arrow), which represented ΔC-GSK-3β. The remaining spots in rectangle 2 clearly represented ΔN-GSK-3β. Therefore, the spots in rectangle 3 were supposed to be ΔN/ΔC-GSK-3β according to molecular weight. Thus, as speculated above, band I represents an overlap of ΔN-GSK-3β and ΔC-GSK-3β.
The calpain inhibitors, calpeptin and ALLM, but not the caspase inhibitor, Z-VAD-FMK, inhibited the generation of the truncated products (Fig. 1C, left panel), confirming that the cleavage of GSK-3β was dependent on calpain activity. Similar results were also obtained in other calpain-dependent neuronal death models, such as MPP+- (27) or glutamate-treated (28) CGNs, and the resulting cleavage patterns were identical to those observed after potassium deprivation (Fig. 1C). These observations suggest that cleavage of GSK-3β by calpain is a general phenomenon during neuronal death.
To determine whether calpain directly cleaves GSK-3β and to further confirm that GSK-3β is truncated at both its N and C termini, we performed an in vitro cleavage assay using recombinant N-terminally His-tagged GSK-3β as the substrate of calpain as previously reported (17). Our results showed that the His-tag antibody reliably recognized ΔC-GSK-3β, which overlapped with ΔN-GSK-3β in band I. Therefore, band II, which was positive by Mid antibody staining but not by the His-tag or C-ter antibody staining, represented ΔN/ΔC-GSK-3β (Fig. 1D).
To examine which terminus of GSK-3β was more sensitive to calpain truncation, we generated the N-terminally FLAG-tagged and C-terminally V5-tagged GSK-3β construct, FLAG-GSK-3β-V5. Total protein lysates from HEK293 cells transfected with this construct were incubated with calpain plus calcium for various time intervals, and the resulting fragments were detected by immunoblot analysis using either the FLAG or the V5 antibody. Consistent with the above mentioned data, both FLAG and V5 antibodies could detect only band I, suggesting that the FLAG fragment and the V5 fragment represent ΔC-GSK-3β and ΔN-GSK-3β, respectively (Fig. 1E). The two fragments both appeared in as short a time period as 0.1 min and gradually increased over time after calpain treatment (Fig. 1E), indicating that the N and C termini of GSK-3β have equal susceptibility to calpain.
Taken together, these results demonstrate that calpain truncates GSK-3β not only at the N terminus but also at the C terminus in various neuronal death models. Moreover, we show that calpain truncates GSK-3β in a limited fashion, generating three truncated products: ΔN-GSK-3β, ΔC-GSK-3β, and ΔN/ΔC-GSK-3β.
Ser-9 Phosphorylation Protects GSK-3β from Calpain-mediated Cleavage at Both N and C Termini
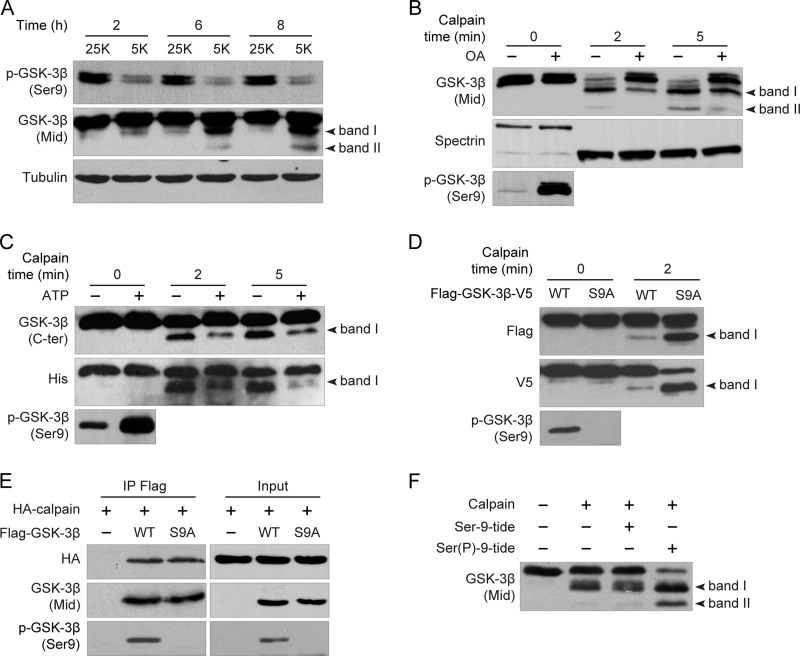
As has been previously reported by our group (14), Ser-9 phosphorylation of GSK-3β is significantly decreased within 2 h and thereafter remains stable (Fig. 2A, upper panel) during potassium deprivation-induced CGN apoptosis. In a parallel time-course experiment, we found that GSK-3β was cleaved by calpain soon after Ser-9 dephosphorylation (Fig. 2A, middle panel). This interesting observation raises the possibility that Ser-9 phosphorylation of GSK-3β may reduce its vulnerability to calpain cleavage.
FIGURE 2.
Ser-9 phosphorylation protects GSK-3β from calpain-mediated cleavage at both N and C termini. A, Ser-9 dephosphorylation precedes calpain cleavage of GSK-3β. DIV7 CGNs were incubated in 25K or 5K for the indicated times. Cell lysates were subjected to immunoblotting with the indicated antibodies. B, effect of OA-induced Ser-9 phosphorylation on calpain-mediated cleavage of GSK-3β is shown. DIV7 CGNs cultured in 5K were treated with or without 25 nm OA for 3 h, and the cell homogenates were added with 3 units/ml calpain plus 5 mm calcium for the indicated times. C, shown is the effect of Akt-mediated Ser-9 phosphorylation on calpain-mediated cleavage of GSK-3β. Recombinant His-GSK-3β was first incubated with purified Akt in the presence or absence of ATP for 30 min, and 0.3 unit/ml calpain plus 5 mm calcium were subsequently added for the subsequent in vitro cleavage. D, shown is the effect of S9A on calpain-mediated cleavage of GSK-3β. The cell homogenates from HEK293 cells transfected with either FLAG-GSK-3β(WT)-V5 or FLAG-GSK-3β(S9A)-V5 were incubated with 3 units/ml calpain plus 5 mm calcium for 2 min. E, shown is the effect of S9A on the interaction between GSK-3β and calpain. HA-calpain was transfected into HEK293 cells together with either FLAG-GSK-3β WT or FLAG-GSK-3β S9A. After 24 h cell lysates were immunoprecipitated (IP) with a FLAG antibody, and immunoblot analysis was performed using GSK-3β Mid or HA-HRP antibody (left panel). F, shown is the effect of Ser(P)-9-tide on calpain-mediated cleavage of GSK-3β. Recombinant His-tagged GSK-3β was incubated with 0.3 unit/ml calpain and 5 mm calcium in the presence or absence of Ser-9-tide or Ser(P)-9-tide (100 μm) for 2 min.
To test this possibility, CGNs in 5K medium were treated with the phosphatase 1/2A inhibitor OA to increase the Ser-9 phosphorylation of GSK-3β, as previously reported (29). After 3 h of incubation, the cell lysates were collected and added with the purified calpain plus calcium for different incubation times. The OA, which dramatically increased Ser-9 phosphorylation levels (Fig. 2B, lower panel), substantially attenuated the cleavage of GSK-3β (Fig. 2B, upper panel). By contrast, OA had no discernible effect on the cleavage of spectrin, an acknowledged calpain substrate (Fig. 2B, middle panel). These results indicate that the Ser-9 phosphorylation state of GSK-3β affects the cleavage of GSK-3β.
To confirm further that Ser-9 phosphorylation reduced the susceptibility of GSK-3β to calpain, purified GSK-3β was first phosphorylated by Akt and subsequently incubated with calpain and calcium. In the presence of Akt and the absence of ATP, GSK-3β Ser-9 was rarely phosphorylated and was cleaved by calpain, as monitored by the His-tag and C-ter antibodies (Fig. 2C). By contrast, in the presence of ATP, GSK-3β was largely phosphorylated at Ser-9 by Akt and subsequently became resistant to calpain-mediated cleavage at both N and C termini (Fig. 2C).
Next, we examined whether blocking Ser-9 phosphorylation would facilitate calpain-mediated cleavage with a non-phosphorylatable mutant of GSK-3β in which Ser-9 was mutated to alanine (S9A). With a similar quantity of GSK-3β wild type (WT), S9A promoted the production of both ΔN-GSK-3β and ΔC-GSK-3β upon calpain treatment, indicating that GSK-3β S9A is more susceptible to calpain (Fig. 2D). Thus, these results suggest that Ser-9 phosphorylation protects GSK-3β from calpain-mediated cleavage at both N and C termini.
To investigate how Ser-9 phosphorylation inhibits the calpain-mediated cleavage of GSK-3β, we performed a co-immunoprecipitation experiment to test whether Ser-9 phosphorylation negatively regulates the interaction between GSK-3β and calpain. As shown in Fig. 2E, calpain equivalently interacted with GSK-3β WT and S9A, suggesting that Ser-9 phosphorylation did not affect the interaction between calpain and GSK-3β.
The conformation of GSK-3β is known to be affected by Ser-9 phosphorylation (15). As previously noted by our group and by others (14, 15), the Ser-9-phosphorylated peptide encompassing residues 4–14 (Ser(P)-9-tide) can compete for the endogenous priming phosphate binding pocket of GSK-3β and thus may suppress the three-dimensional structural transition resulting from Ser-9 phosphorylation. By using the Ser(P)-9-tide with a non-phosphorylated peptide (Ser-9-tide) as a control, we found that Ser(P)-9-tide promoted the cleavage of GSK-3β, whereas Ser-9-tide had no effect (Fig. 2F). This result implies that Ser-9 phosphorylation may lead to a conformational change that may hinder the access of calpain to its recognition sites and thus protect GSK-3β from calpain-mediated cleavage at both its N and C termini.
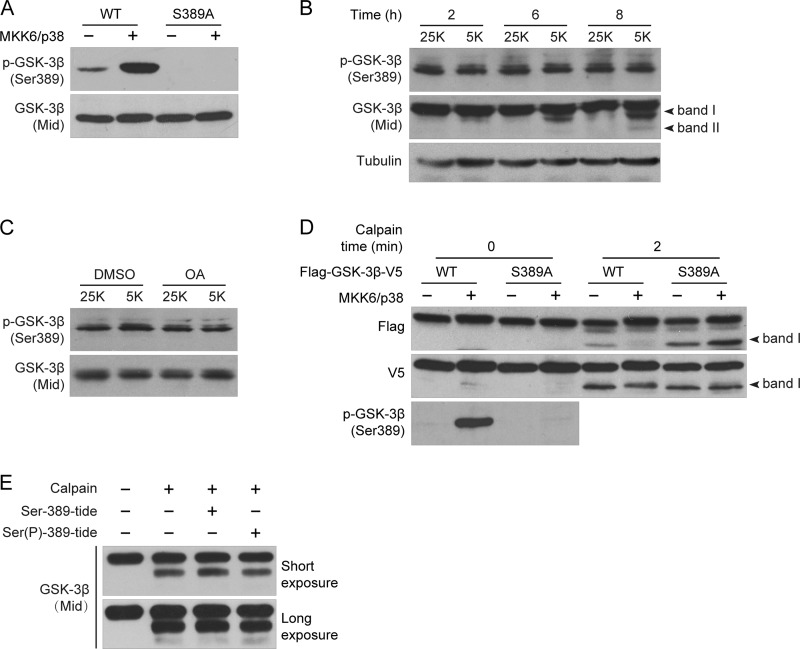
Ser-389 Phosphorylation Protects GSK-3β from Its C-terminal but Not N-terminal Cleavage
MAPK-dependent phosphorylation of Ser-389/Thr-390 (rat/human) has been demonstrated recently as an alternative pathway for GSK-3β inactivation (16). To investigate whether Ser-389 phosphorylation affects calpain-mediated cleavage in CGNs as well, we generated a phospho-Ser-389-specific antibody against a synthetic phosphopeptide that corresponds to the residues surrounding Ser-389 of rat GSK-3β. A specific band corresponding to GSK-3β was detected by this antibody in HEK293 cells transfected with a WT GSK-3β but not with the GSK-3β S389A mutant (Fig. 3A). The intensity of the band increased in magnitude when GSK-3β WT was co-transfected with MKK6/p38 (Fig. 3A) as previously reported (16), suggesting that this antibody is both efficient and specific for p-GSK-3β (Ser-389). Utilizing the same time-course experiment used for examining Ser-9 phosphorylation status (Fig. 2A) with this antibody, we found that there was no change in the level of Ser-389 phosphorylation between the 25K and 5K cultures (Fig. 3B). Furthermore, the phosphatase inhibitor OA, which was able to increase Ser-9 phosphorylation (Fig. 2B), had no effect on Ser-389 phosphorylation (Fig. 3C). Thus, Ser-389 phosphorylation is not regulated by an OA-sensitive phosphatase in CGNs.
FIGURE 3.
Ser-389 phosphorylation protects GSK-3β from its C-terminal but not N-terminal cleavage. A, validation of the p-GSK-3β (Ser-389) antibody is shown. Either FLAG-GSK-3β(WT)-V5 or FLAG-GSK-3β(S389A)-V5 was transfected into HEK293 cells with or without MKK6/p38. The cell lysates were analyzed by immunoblotting with the indicated antibodies. B, Ser-389 phosphorylation between 25K and 5K treatment is shown. DIV7 CGNs were incubated in 25K or 5K for the indicated times. Cell lysates were subjected to immunoblotting with the indicated antibodies. C, shown is the effect of OA on Ser-389 phosphorylation. DIV7 CGNs incubated in 25K or 5K were treated with 25 nm OA for 3 h, and cell lysates were subjected to immunoblotting with the indicated antibodies. D, shown is the effect of Ser-389 phosphorylation on calpain-mediated cleavage of GSK-3β. Either FLAG-GSK-3β(WT)-V5 or FLAG-GSK-3β(S389A)-V5 was transfected into HEK293 cells with or without MKK6/p38. The cell lysates were incubated with or without 3 units/ml calpain plus 5 mm calcium followed by immunoblot analysis with either FLAG or V5 antibody. E, shown is the effect of Ser(P)-389-tide on calpain-mediated cleavage of GSK-3β. Recombinant His-tagged rat GSK-3β was incubated with 0.3 unit/ml calpain and 5 mm calcium in the presence or absence of Ser(P)-389-tide or Ser-389-tide (100 μm) for 2 min.
To examine whether Ser-389 phosphorylation also inhibits the calpain-mediated cleavage of GSK-3β, FLAG-GSK-3β-V5 WT was transfected into HEK293 cells with or without MKK6/p38 to induce Ser-389 phosphorylation. The increase in Ser-389 phosphorylation by MKK6/p38 inhibited the production of the FLAG fragment but not the V5 fragment (Fig. 3D), suggesting that Ser-389 phosphorylation inhibits GSK-3β cleavage at its C terminus rather than its N terminus. Consistently, the non-phosphorylatable mutant S389A promotes the production of the FLAG but not the V5 fragment compared with WT (Fig. 3D). Together, these results demonstrate that Ser-389 phosphorylation protects GSK-3β from C-terminal but not N-terminal cleavage.
In addition, we found that the Ser-389-phosphorylated peptide (Ser(P)-389-tide) derived from residues 383–393 of GSK-3β could inhibit GSK-3β activity (see Fig. 5C), just as Ser(P)-9-tide did (14), but that it had no effect on the cleavage of GSK-3β (Fig. 3E) even at higher concentrations (data not shown). This result suggests that the underlying mechanisms by which Ser-389 phosphorylation regulates calpain-mediated cleavage of GSK-3β are different from those of Ser-9 phosphorylation (for a more detailed explanation, please see “Discussion”).
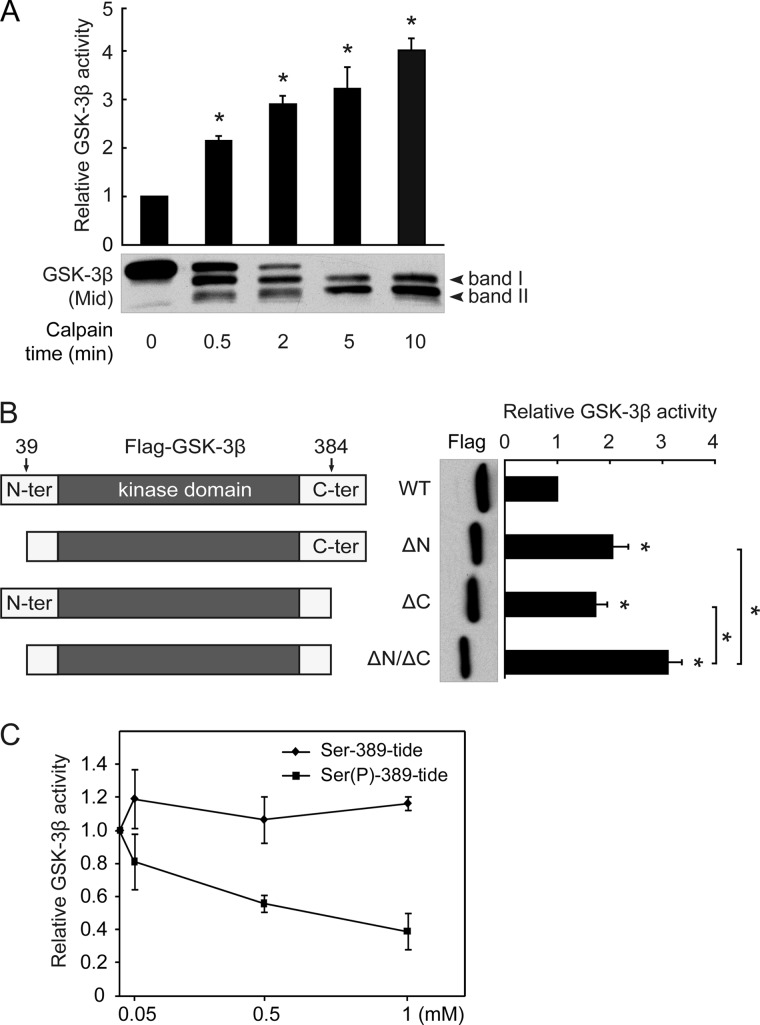
FIGURE 5.
Removal of either N- or C-terminal autoinhibitory domain by calpain activates GSK-3β. A, shown is the effect of calpain-mediated cleavage on the activity of GSK-3β. Recombinant His-tagged GSK-3β was first cleaved by calpain, after which GSK-3β activity was assayed. B, schematic diagrams of the FLAG-GSK-3β WT, ΔN, ΔC, and ΔN/ΔC constructs (left panel) are shown. These constructs were transfected into HEK293 cells, and the resulting cell lysates were immunoprecipitated with the FLAG antibody. The precipitates were subsequently subjected to a GSK-3β kinase assay. C, recombinant GSK-3β was preincubated with the indicated concentrations of Ser-389-tide or Ser(P)-389-tide for 5 min, and a GSK-3β activity assay was performed with 2B-SP as the substrate. All data in this figure represent the mean ± S.E. of at least three independent experiments. *, p < 0.05.
GSK-3β Is Cleaved at Thr-38–Thr-39 and Ile-384–Gln-385
To generate constructs mimicking the truncated forms of GSK-3β to investigate their individual functions, we identified the cleavage sites using electrospray ionization-MS/MS. The ∼43-kDa band I comprising ΔN-GSK-3β and ΔC-GSK-3β was visualized by Coomassie Blue staining, whereas band II was not, due to its low intensity (Fig. 4A). Therefore, band I was subjected to in-gel digestion with endoproteinase Asp-N, which specifically cleaves at the N-terminal side of aspartic acid or glutamic acid residues. Analysis of the MS1 spectra (Fig. 4, B and C, top right corner) revealed that two interesting peaks at m/z 1001.5 and 680.4 (denoted by asterisks) did not match the predicted molecular weight of the peptides derived from Asp-N digestion, suggesting that these peptides resulted from calpain cleavage. MS2 analysis identified the m/z 1001.5 peak as the peptide T↓39TVVATPGQGPDRPQEVSYT57↓D (Fig. 4B) and the m/z 680.4 peak as the peptide Q↓366ELSSNPPLATILIPPHARI384↓Q (Fig. 4C). Therefore, the residues Thr-38–Thr-39 and Ile-384–Gln-385, which are not Asp-N recognition sites, clearly are calpain cleavage sites.
FIGURE 4.
GSK-3β is cleaved at Thr-38–Thr-39 and Ile-384–Gln-385. A, calpain cleavage of GSK-3β was performed in vitro as described above (Fig. 1D). Samples were resolved by SDS-PAGE and visualized by Coomassie Brilliant Blue staining. Band I was excised for in-gel digestion with endoproteinase Asp-N and subsequent identification. B, MS1 spectra (top right corner) identifying a peak at m/z 1002.0 (asterisk) did not match the predicted molecular weight of peptides derived from Asp-N digestion. The peptide was then sequenced by a series of b- and y-ions in the MS/MS spectrum and corresponded to TVVATPGQGPDRPQEVSYT at the N terminus of GSK-3β. C, as described in B, MS/MS identified another non-Asp-N derived peptide, ELSSNPPLATILIPPHARI, at the C terminus of GSK-3β.
Based on these cleavage sites, the resulting calculated molecular masses for both ΔN-GSK-3β and ΔC-GSK-3β were ∼43-kDa, supporting the previous observation that they overlapped in band I in the SDS-PAGE gels. These data demonstrate that calpain-mediated cleavage of GSK-3β occurs at both Thr-38–Thr-39 and Ile-384–Gln-385, resulting in the removal of the N- and C-terminal domains of GSK-3β while leaving the kinase domain intact.
Calpain Truncation Activates GSK-3β by Removing Its N/C-terminal Autoinhibitory Domains
Consistent with a previous report (17), GSK-3β activity increased over time after calpain treatment in vitro (Fig. 5A). We further found that the abundance of ΔN/ΔC-GSK-3β gradually increased along with an increase in GSK-3β activity, suggesting that ΔN/ΔC-GSK-3β may be the main product contributing to the enhanced activity of GSK-3β. In other words, the GSK-3β C terminus acts additively with the N terminus in GSK-3β activity inhibition.
We next examined whether the truncated GSK-3βs, particularly ΔC-GSK-3β, showed increased activity when compared with the full-length GSK-3β. Plasmids expressing N-terminally FLAG-tagged GSK-3β WT, ΔN-GSK-3β, ΔC-GSK-3β, or ΔN/ΔC-GSK-3β were constructed (Fig. 5B, left panel) and overexpressed in HEK293 cells. At the same protein levels, each of the three truncated forms of GSK-3β (ΔN-GSK-3β, ΔC-GSK-3β, and ΔN/ΔC-GSK-3β) exhibited significantly increased kinase activity compared with the WT (Fig. 5B, right panel). Moreover, the activity of ΔN/ΔC-GSK-3β demonstrated a significant (p < 0.05) increase over either ΔN-GSK-3β or ΔC-GSK-3β, suggesting that N- and C-terminal cleavage have an additive effect on the activation of GSK-3β.
The N-terminal domain of GSK-3β has been established as autoinhibitory; however, the features of the GSK-3β C-terminal domain are not yet fully understood. To confirm that the C terminus of GSK-3β has an autoinhibitory effect, we synthesized a series of peptides covering the GSK-3β C-terminal residues from 383 to 420 and found that none of these peptides inhibited GSK-3β activity (data not shown). Thornton et al. (16) reported an inhibitory phosphorylation site, Thr-390, located at the GSK-3β C-terminal domain; the corresponding site in the rat is Ser-389. Based on this report (16), we synthesized two other peptides encompassing residues 383–393, with Ser-389 phosphorylated or not (Ser(P)-389-tide, Ser-389-tide). The results showed that Ser(P)-389-tide but not Ser-389-tide inhibited GSK-3β activity (Fig. 5C), indicating that the C terminus of GSK-3β acts as an autoinhibitory domain in a Ser-389 phosphorylation-dependent manner. Taken together, these results confirm that the GSK-3β C terminus serves as an autoinhibitory domain and demonstrate that calpain-mediated truncation activates GSK-3β through the removal of its N- and C-terminal autoinhibitory domains.
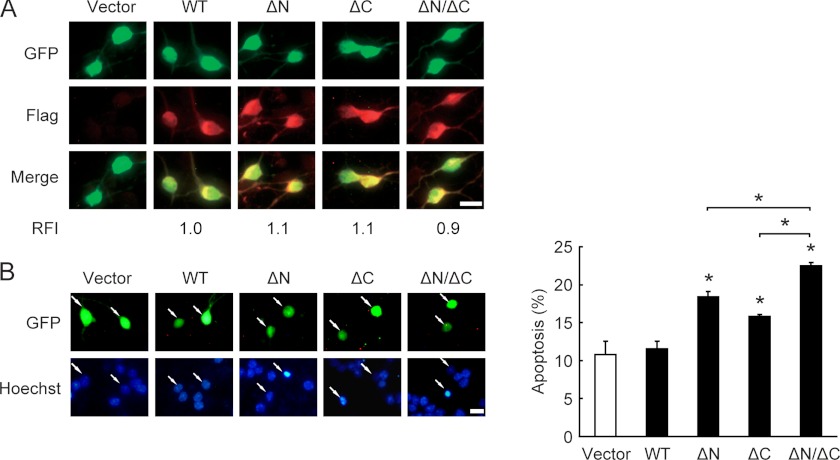
Truncated Forms of GSK-3β Promote Neuronal Death
GSK-3β plays a critical role in neuronal death (5, 6). This study shows that calpain-truncated forms of GSK-3β have higher activity than full-length GSK-3β. Next, we asked whether the truncated GSK-3β also possesses stronger pro-apoptotic property. FLAG-tagged WT GSK-3β, ΔN-GSK-3β, ΔC-GSK-3β, or ΔN/ΔC-GSK-3β was transiently transfected into CGNs. After 36 h of transfection, CGNs were switched to serum-free medium containing 25K for 24 h followed by immunofluorescence and apoptosis assays. Because of the low transfection efficiency in cultured primary neurons (∼1% for differentiated neurons), the protein expression levels in the transfected neurons were impossible to detect by immunoblotting. Therefore, as previously described (21, 24), GFP was used to mark the transfected cells, and the relative fluorescence intensity of FLAG immunostaining was employed to indicate FLAG-tagged GSK-3β protein levels in the transfected CGNs. At a comparable expression level (Fig. 6A), the GSK-3β WT-transfected cells were morphologically indistinguishable from the cells transfected with the vector (Fig. 6B). In contrast, transfection of ΔC-GSK-3β or ΔN-GSK-3β resulted in a significant (p < 0.05) increase in neuronal apoptosis (Fig. 6B). Moreover, ΔN/ΔC-GSK-3β was the most toxic isoform of the three truncated products. Consistent with the notion that aberrant activation of GSK-3β promotes neuronal death, these data suggest that both the N- and C-terminal calpain-mediated truncation of GSK-3β act collaboratively to contribute to GSK-3β activation and subsequent neuronal death.
FIGURE 6.
Truncated forms of GSK-3β promote neuronal death. A, DIV5 CGNs were transfected with FLAG-tagged GSK-3β WT, ΔN, ΔC, or ΔN/ΔC along with GFP for 36 h, and the fluorescence intensity was quantified by immunostaining with the FLAG antibody. The relative fluorescence intensity (RFI) of GSK-3β WT-transfected cells was set to 1.0. Scale bar, 10 μm. B, apoptosis was determined according to the percentage of GFP-positive cells showing pyknotic nuclei (stained by Hoechst 33258). Representative images are shown (left panel). Data represent the mean ± S.E. of three experiments. *, p < 0.05. Scale bar, 10 μm.
DISCUSSION
To the best of our knowledge this study is the first to demonstrate the following: 1) GSK-3β is cleaved by calpain not only at the N terminus but also at the C terminus. 2) Ser-9 phosphorylation inhibits calpain-mediated cleavage of GSK-3β at both termini, whereas Ser-389 phosphorylation protects GSK-3β from only C-terminal but not N-terminal cleavage. 3) Truncated GSK-3β shows increased kinase and pro-apoptotic activity, further supporting the notion that, similar to the N terminus, the GSK-3β C terminus is an autoinhibitory domain.
In contrast to the report of Goñi-Oliver et al. (17) showing that calpain cleaves GSK-3β at the N terminus in vitro, this study found that calpain cleaved GSK-3β at both the N terminus and the C terminus in vitro and in vivo. One possible explanation for this discrepancy is that when determining which terminus of GSK-3β is cleaved by calpain, Goñi-Oliver et al. (17) showed that the His-tag antibody failed to recognize the truncated products. This finding may have resulted from the excessive cleavage of GSK-3β, as excess calpain, or overly long calpain incubation times generate more ΔN/ΔC-GSK-3β (band II in Fig. 5A) than primary truncated product (band I in Fig. 5A); therefore, the remaining small amounts of ΔC-GSK-3β are beyond the detection limit of the His-tag antibody. In this study we provide several lines of evidence, including the same in vitro cleavage experiment previously performed, to confirm our conclusion. First, the fragment patterns of GSK-3β were not identical when examined with the GSK-3β C-ter and Mid antibodies, suggesting that GSK-3β was cleaved at the both the N terminus and the C terminus. Second, three truncated products, ΔN-GSK-3β, ΔC-GSK-3β, and ΔN/ΔC-GSK-3β, were separated by two-dimensional gel electrophoresis and visualized by probing with either C-ter or Mid antibody. Third, the His-tag antibody clearly recognized ΔC-GSK-3β, resulting from in vitro cleavage of recombinant N-terminally His-tagged GSK-3β by calpain. Finally, the identified cleavage sites were located at the N-terminal Thr-38–Thr-39 and C-terminal Ile-384–Gln-385 residues of GSK-3β. Thus, calpain-mediated cleavage removed both the N and C termini of GSK-3β, leaving the kinase domain intact.
The GSK-3β N terminus is known to be an autoinhibitory domain (12, 15, 30); therefore, it is reasonable to believe that ΔN-GSK-3β has increased activity. Unexpectedly, we found that ΔC-GSK-3β also showed enhanced kinase activity similar to that of ΔN-GSK-3β, and the activity of ΔN/ΔC-GSK-3β exceeded that of ΔN-GSK-3β and ΔC-GSK-3β. These data indicate that, similar to the N terminus, the C terminus of GSK-3β is an autoinhibitory domain and that both termini control GSK-3β activity additively. To further confirm that the C terminus of GSK-3β has autoinhibitory effects, we synthesized a series of peptides derived from the GSK-3β C-terminal region and found that only the Ser(P)-389-tide had an autoinhibitory effect, which is consistent with a previous report showing that phosphorylation at the C-terminal Thr-390 site (the corresponding residue in rat is Ser-389) inhibits GSK-3β activity (16). Moreover, a study in patients with Parkinson disease revealed a disease-related SNP site that results in an alternative GSK-3β splice isoform (Δexon9 + 11) that lacks residues 366–398 (31). Interestingly, this isoform also lost the phosphorylation site Thr-390 and showed increased kinase activity compared with full-length GSK-3β. The present data in combination with those of the reports mentioned above establish the GSK-3β C terminus as an important functional inhibitory domain. Further exploration of how the GSK-3β C-terminal domain exerts its autoinhibitory effect may redefine and deepen our understanding of its functions.
Although the crystal structure of GSK-3β has been solved by many groups (1, 15), clear electron density is evident only for the 351 residues from Lys-35 to Ser-386; the regions preceding residue 35 and following residue 386 lack organized structure. Referring to the literature (32), we realized that these regions may be intrinsically disordered. Prediction of GSK-3β disordered regions by the DISOPRED server (33) indicates that the regions from residues 1 to 36 at the N terminus and from residues 381 to 420 at the C terminus are disordered (data not shown). Disordered protein sequences, which are characterized by a lack of stable tertiary structure, a low proportion of bulky hydrophobic amino acids, and a high proportion of polar and charged amino acids, have been recognized for many years (32, 34). The flexibility of the disordered regions is proposed to correlate with sites of post-translational modification, such as phosphorylation (35) and protease degradation (36), both of which occur at the N- and C-terminal domains of GSK-3β. Another classic feature of disordered regions is their ability to acquire different conformational arrangements for binding target proteins or interacting with different molecules (32, 37). The N terminus of GSK-3β was recently reported to be necessary for 14–3-3ζ, p53, and PKB interactions (38); however, the interaction partners of the GSK-3β C terminus have not yet been thoroughly investigated. We are currently studying this topic.
It has been proposed that phosphorylation could influence the neighboring cleavage sites to decrease or increase the accessibility of the recognition sites to calpain (39, 40). However, several of our experimental approaches support the notion that phosphorylation of GSK-3β at Ser-9, distant from the cleavage sites Thr-38–Thr-39 and Ile-384–Gln-385, inhibits calpain-mediated cleavage at both sites by altering the conformation of GSK-3β. First, increasing Ser-9 phosphorylation of GSK-3β via inhibition of phosphatase 1/2A or via pretreatment with purified active Akt protects GSK-3β from calpain-mediated cleavage at both N and C termini. Second, the non-phosphorylatable mutant S9A increases calpain-mediated cleavage of GSK-3β at the N terminus as well as the C terminus. Third, the three-dimensional conformation of protein substrates has been speculated to determine calpain access to the cleavage site (41), and the conformation of GSK-3β has been proposed to change upon Ser-9 phosphorylation (15). Furthermore, the peptide Ser(P)-9-tide, which could mimic the Ser-9-phosphorylated N terminus of GSK-3β and block the Ser-9 phosphorylation-dependent conformational change, promotes the cleavage of GSK-3β at both sites. Taken together, these data indicate that Ser-9 phosphorylation can induce tertiary structure transformation, which in turn reduces the accessibility of calpain to its cleavage sites and contributes to the Ser-9 phospho-GSK-3β cleavage resistance.
Ser-389 is an alternative inhibitory phosphorylation site of GSK-3β, although the mechanism underlying its autoinhibitory effect remains unclear. In this study we found that MKK6/p38-mediated Ser-389 phosphorylation inhibits, whereas a non-phosphorylatable mutant S389A promotes the cleavage of GSK-3β at the C terminus but not the N terminus. This result demonstrates that unlike Ser-9 phosphorylation, Ser-389 phosphorylation protects GSK-3β from only C-terminal rather than N-terminal cleavage. Furthermore, Ser(P)-389-tide, which inhibits GSK-3β activity just as Ser(P)-9-tide does, has no effect on the cleavage of GSK-3β. These results suggest that the phosphorylation of Ser-389 affects only its vicinal calpain cleavage site Ile-384–Gln-385 instead of altering its tertiary structure, as Ser-9 phosphorylation does.
In potassium deprivation-induced CGN apoptosis, Ser-9 dephosphorylation precedes and facilitates calpain-mediated cleavage. The latter subsequently serves in an irreversible regulatory manner to activate GSK-3β, providing the opportunity to amplify cell death signals through the production of “strategic” cleavage products under cell death stimuli. Coordinated regulation of GSK-3β by Ser-9 phosphorylation and calpain cleavage may allow for the fine-tuning of the cell death threshold. Both calpain and GSK-3β play critical roles in many neurological diseases characterized by deregulated neuronal death, such as Alzheimer disease, Parkinson disease, and brain ischemia (9, 10, 42). In this study we found that calpain cleaves GSK-3β after treatment with various neuronal death stimuli, including potassium deprivation, MPP+, and glutamate. Thus, it will be interesting to examine whether calpain-mediated cleavage of GSK-3β promotes neuronal death in animal models of these neurological diseases, which is a possibility that may have important implications for their pathogenesis.
Acknowledgments
We thank Dr. Hiroyuki Sorimachi for generously providing us with the rat calpain coding sequence.
This work was supported by National Basic Research Program of China 973 Program Grant 2011CB504105, National Natural Science Foundation of China Grant 81030024, Natural Science Foundation of Guangdong Province China Grant 9351008901000003, and Key Program for Scientific and Technological Innovations of Higher Education Institutes in Guangdong Province China Grant cxzd1002.
- GSK-3
- glycogen synthase kinase-3
- CGN
- cerebellar granule neuron
- 25K
- 25 mM KCl
- 5K
- 5 mM KCl
- DIV
- days in vitro
- ΔN
- N-terminal-truncated
- ΔC
- C-terminal-truncated
- ΔN/ΔC
- both N- and C-terminal-truncated
- OA
- okadaic acid
- ALLM
- calpain inhibitor II
- Z-VAD-FMK
- benzyloxycarbonyl-VAD-fluoromethyl ketone.
REFERENCES
- 1. Bax B., Carter P. S., Lewis C., Guy A. R., Bridges A., Tanner R., Pettman G., Mannix C., Culbert A. A., Brown M. J., Smith D. G., Reith A. D. (2001) The structure of phosphorylated GSK-3β complexed with a peptide, FRATtide, that inhibits β-catenin phosphorylation. Structure 9, 1143–1152 [DOI] [PubMed] [Google Scholar]
- 2. Woodgett J. R. (1990) Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 9, 2431–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leroy K., Brion J. P. (1999) Developmental expression and localization of glycogen synthase kinase-3β in rat brain. J. Chem. Neuroanat. 16, 279–293 [DOI] [PubMed] [Google Scholar]
- 4. Beurel E., Jope R. S. (2006) The paradoxical pro- and anti-apoptotic actions of GSK-3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 79, 173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pap M., Cooper G. M. (1998) Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273, 19929–19932 [DOI] [PubMed] [Google Scholar]
- 6. Hetman M., Cavanaugh J. E., Kimelman D., Xia Z. (2000) Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 20, 2567–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takashima A. (2006) GSK-3 is essential in the pathogenesis of Alzheimer disease. J. Alzheimers Dis. 9, 309–317 [DOI] [PubMed] [Google Scholar]
- 8. Chen G., Bower K. A., Ma C., Fang S., Thiele C. J., Luo J. (2004) Glycogen synthase kinase 3β (GSK-3β) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 18, 1162–1164 [DOI] [PubMed] [Google Scholar]
- 9. Hernández F., de Barreda E. G., Fuster-Matanzo A., Goñi-Oliver P., Lucas J. J., Avila J. (2009) The role of GSK-3 in Alzheimer disease. Brain Res. Bull. 80, 248–250 [DOI] [PubMed] [Google Scholar]
- 10. Wang W., Yang Y., Ying C., Li W., Ruan H., Zhu X., You Y., Han Y., Chen R., Wang Y., Li M. (2007) Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 52, 1678–1684 [DOI] [PubMed] [Google Scholar]
- 11. Chin P. C., Majdzadeh N., D'Mello S. R. (2005) Inhibition of GSK-3β is a common event in neuroprotection by different survival factors. Mol. Brain Res. 137, 193–201 [DOI] [PubMed] [Google Scholar]
- 12. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 13. Li M., Wang X., Meintzer M. K., Laessig T., Birnbaum M. J., Heidenreich K. A. (2000) Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3β. Mol. Cell. Biol. 20, 9356–9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song B., Lai B., Zheng Z., Zhang Y., Luo J., Wang C., Chen Y., Woodgett J. R., Li M. (2010) Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival. J. Biol. Chem. 285, 41122–41134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dajani R., Fraser E., Roe S. M., Young N., Good V., Dale T. C., Pearl L. H. (2001) Crystal structure of glycogen synthase kinase 3β. Structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105, 721–732 [DOI] [PubMed] [Google Scholar]
- 16. Thornton T. M., Pedraza-Alva G., Deng B., Wood C. D., Aronshtam A., Clements J. L., Sabio G., Davis R. J., Matthews D. E., Doble B., Rincon M. (2008) Phosphorylation by p38 MAPK as an alternative pathway for GSK-3β inactivation. Science 320, 667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goñi-Oliver P., Lucas J. J., Avila J., Hernández F. (2007) N-terminal cleavage of GSK-3 by calpain. A new form of GSK-3 regulation. J. Biol. Chem. 282, 22406–22413 [DOI] [PubMed] [Google Scholar]
- 18. Goñi-Oliver P., Avila J., Hernández F. (2009) Calpain-mediated truncation of GSK-3 in post-mortem brain samples. J. Neurosci. Res. 87, 1156–1161 [DOI] [PubMed] [Google Scholar]
- 19. Goñi-Oliver P., Avila J., Hernández F. (2009) Memantine inhibits calpain-mediated truncation of GSK-3 induced by NMDA. Implications in Alzheimer disease. J. Alzheimers Dis. 18, 843–848 [DOI] [PubMed] [Google Scholar]
- 20. Crespo-Biel N., Camins A., Gutiérrez-Cuesta J., Melchiorri D., Nicoletti F., Pallàs M., Canudas A. M. (2010) Regulation of GSK-3β by calpain in the 3-nitropropionic acid model. Hippocampus 20, 962–970 [DOI] [PubMed] [Google Scholar]
- 21. Xie B., Wang C., Zheng Z., Song B., Ma C., Thiel G., Li M. (2011) Egr-1 transactivates Bim gene expression to promote neuronal apoptosis. J. Neurosci. 31, 5032–5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Mello S. R., Galli C., Ciotti T., Calissano P. (1993) Induction of apoptosis in cerebellar granule neurons by low potassium. Inhibition of death by insulin-like growth factor I and cAMP. Proc. Natl. Acad. Sci. U.S.A. 90, 10989–10993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma C., Ying C., Yuan Z., Song B., Li D., Liu Y., Lai B., Li W., Chen R., Ching Y. P., Li M. (2007) dp5/HRK is a c-Jun target gene and required for apoptosis induced by potassium deprivation in cerebellar granule neurons. J. Biol. Chem. 282, 30901–30909 [DOI] [PubMed] [Google Scholar]
- 24. Yuan Z., Gong S., Luo J., Zheng Z., Song B., Ma S., Guo J., Hu C., Thiel G., Vinson C., Hu C. D., Wang Y., Li M. (2009) Opposing roles for ATF2 and c-Fos in c-Jun-mediated neuronal apoptosis. Mol. Cell. Biol. 29, 2431–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu W., Zhou X. W., Liu S., Hu K., Wang C., He Q., Li M. (2009) Calpain-truncated CRMP-3 and -4 contribute to potassium deprivation-induced apoptosis of cerebellar granule neurons. Proteomics 9, 3712–3728 [DOI] [PubMed] [Google Scholar]
- 26. Miller T. M., Johnson E. M., Jr. (1996) Metabolic and genetic analyses of apoptosis in potassium/serum-deprived rat cerebellar granule cells. J. Neurosci. 16, 7487–7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvira D., Tajes M., Verdaguer E., de Arriba S. G., Allgaier C., Matute C., Trullas R., Jiménez A., Pallàs M., Camins A. (2007) Inhibition of cyclin-dependent kinases is neuroprotective in 1-methyl-4-phenylpyridinium-induced apoptosis in neurons. Neuroscience 146, 350–365 [DOI] [PubMed] [Google Scholar]
- 28. Bano D., Young K. W., Guerin C. J., Lefeuvre R., Rothwell N. J., Naldini L., Rizzuto R., Carafoli E., Nicotera P. (2005) Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120, 275–285 [DOI] [PubMed] [Google Scholar]
- 29. Hernández F., Langa E., Cuadros R., Avila J., Villanueva N. (2010) Regulation of GSK-3 isoforms by phosphatases PP1 and PP2A. Mol. Cell. Biochem. 344, 211–215 [DOI] [PubMed] [Google Scholar]
- 30. Salas T. R., Reddy S. A., Clifford J. L., Davis R. J., Kikuchi A., Lippman S. M., Menter D. G. (2003) Alleviating the suppression of glycogen synthase kinase-3β by Akt leads to the phosphorylation of cAMP-response element-binding protein and its transactivation in intact cell nuclei. J. Biol. Chem. 278, 41338–41346 [DOI] [PubMed] [Google Scholar]
- 31. Kwok J. B., Hallupp M., Loy C. T., Chan D. K., Woo J., Mellick G. D., Buchanan D. D., Silburn P. A., Halliday G. M., Schofield P. R. (2005) GSK-3B polymorphisms alter transcription and splicing in Parkinson's disease. Ann. Neurol. 58, 829–839 [DOI] [PubMed] [Google Scholar]
- 32. Dyson H. J., Wright P. E. (2005) Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6, 197–208 [DOI] [PubMed] [Google Scholar]
- 33. Ward J. J., McGuffin L. J., Bryson K., Buxton B. F., Jones D. T. (2004) The DISOPRED server for the prediction of protein disorder. Bioinformatics 20, 2138–2139 [DOI] [PubMed] [Google Scholar]
- 34. Dunker A. K., Garner E., Guilliot S., Romero P., Albrecht K., Hart J., Obradovic Z., Kissinger C., Villafranca J. E. (1998) Protein disorder and the evolution of molecular recognition: theory, predictions and observations. Pac. Symp. Biocomput., 473–484 [PubMed] [Google Scholar]
- 35. Iakoucheva L. M., Radivojac P., Brown C. J., O'Connor T. R., Sikes J. G., Obradovic Z., Dunker A. K. (2004) The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 32, 1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prakash S., Tian L., Ratliff K. S., Lehotzky R. E., Matouschek A. (2004) An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat. Struct. Mol. Biol. 11, 830–837 [DOI] [PubMed] [Google Scholar]
- 37. Fink A. L. (2005) Natively unfolded proteins. Curr. Opin. Struct. Biol. 15, 35–41 [DOI] [PubMed] [Google Scholar]
- 38. Goñi-Oliver P., Avila J., Hernández F. (2011) Calpain regulates N-terminal interaction of GSK-3β with 14–3-3ζ, p53, and PKB but not with axin. Neurochem. Int. 59, 97–100 [DOI] [PubMed] [Google Scholar]
- 39. Yuen E. Y., Liu W., Yan Z. (2007) The phosphorylation state of GluR1 subunits determines the susceptibility of AMPA receptors to calpain cleavage. J. Biol. Chem. 282, 16434–16440 [DOI] [PubMed] [Google Scholar]
- 40. Kamei H., Saito T., Ozawa M., Fujita Y., Asada A., Bibb J. A., Saido T. C., Sorimachi H., Hisanaga S. (2007) Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J. Biol. Chem. 282, 1687–1694 [DOI] [PubMed] [Google Scholar]
- 41. Sakai K., Akanuma H., Imahori K., Kawashima S. (1987) A unique specificity of a calcium-activated neutral protease indicated in histone hydrolysis. J. Biochem. 101, 911–918 [DOI] [PubMed] [Google Scholar]
- 42. Vosler P. S., Brennan C. S., Chen J. (2008) Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 38, 78–100 [DOI] [PMC free article] [PubMed] [Google Scholar]