Background: What is the biological function of COMP in skin ECM?
Results: COMP binds collagens XII and XIV that associate with collagen I fibrils. All three proteins localize to anchoring plaques.
Conclusion: COMP acts as an adapter in ECM of healthy skin, organizing the dermal collagen network.
Significance: COMP organizes collagen I fibrils into a suprastructure that may contribute to stabilizing cohesion between the upper dermis and the basement membrane zone.
Keywords: Collagen, Extracellular Matrix, Fibroblast, Skin, Surface Plasmon Resonance (SPR), COMP, Collagen I Fibril, FACIT Collagens, Anchoring Plaques
Abstract
The tensile and scaffolding properties of skin rely on the complex extracellular matrix (ECM) that surrounds cells, vasculature, nerves, and adnexus structures and supports the epidermis. In the skin, collagen I fibrils are the major structural component of the dermal ECM, decorated by proteoglycans and by fibril-associated collagens with interrupted triple helices such as collagens XII and XIV. Here we show that the cartilage oligomeric matrix protein (COMP), an abundant component of cartilage ECM, is expressed in healthy human skin. COMP expression is detected in the dermal compartment of skin and in cultured fibroblasts, whereas epidermis and HaCaT cells are negative. In addition to binding collagen I, COMP binds to collagens XII and XIV via their C-terminal collagenous domains. All three proteins codistribute in a characteristic narrow zone in the superficial papillary dermis of healthy human skin. Ultrastructural analysis by immunogold labeling confirmed colocalization and further revealed the presence of COMP along with collagens XII and XIV in anchoring plaques. On the basis of these observations, we postulate that COMP functions as an adapter protein in human skin, similar to its function in cartilage ECM, by organizing collagen I fibrils into a suprastructure, mainly in the vicinity of anchoring plaques that stabilize the cohesion between the upper dermis and the basement membrane zone.
Introduction
Connective tissues possess specific structural and functional properties that are largely determined by the supramolecular arrangement of the collagen network and its interactions with surrounding ECM3 proteins. To date, 28 different types of collagens are known that are categorized in eight subfamilies on the basis of their function, assembly, and domain homology (1, 2). Among these, fibrillar collagens form the major component of the ECM in connective tissues. Examples are collagen I, which is abundant in skin, bone, tendon and ligament, and collagen II, predominantly expressed in cartilage.
In skin, the arrangement of the collagen network varies considerably with anatomical location. In the reticular (deeper) dermis, well ordered thick collagen fibrils predominate, whereas in the papillary (upper) dermis underlying the epidermis and basement membrane, the collagen network is less rigid with a loose connective tissue and a distinct organization of collagen fibrils (3).
The supramolecular organization of collagen fibrils is critical for tissue integrity, biomechanical stability, and function. Collagen fibril networks are stabilized by their interaction with proteoglycans and with other collagenous and non-collagenous proteins. A large number of non-collagenous ECM components have been implicated in regulating collagen fibrillogenesis, including small leucine-rich repeat proteoglycans like decorin, fibromodulin, and lumican. Fibromodulin accelerates lateral growth of collagen I fibrils, thus enhancing fibril thickness (4). Decorin and lumican interfere with orderly lateral assembly of collagen molecules and, thus, retard fibril growth (5, 6).
Collagens themselves also regulate the formation of collagen I fibrils and fiber bundles either by directly binding the fibrillar collagens or by binding adapter molecules. Collagen III is associated with thinner collagen I fibrils in tendons (7), and collagen XI has been reported to inhibit lateral growth of collagen II fibrils in cartilage (8). Fibril-associated collagens with interrupted triple helices (FACIT), such as collagen IX, inhibit lateral growth of cartilage collagen fibrils (9). Collagens XII and XIV are also classified as FACIT collagens on the basis of their structural organization (10). Collagen XIV binds to banded collagen I fibrils, and characterization of collagen XIV-deficient mouse tendons showed a shift toward larger-diameter fibrils (11). Immunoelectron microscopy revealed that collagen XII associated with collagen I fibrils in calf, chick, and human tendons and skin and, by analogy, collagen XII has been proposed to serve similar functions as collagen XIV in connective tissues enriched in collagen I (12, 13). Structurally, both collagen XII and XIV are comprised by two collagenous domains (Col1 and Col2) separated by two short non-collagenous domains (NC1 and NC2) at the C terminus and a very large N-terminal non-collagenous NC3 domain (Fig. 1A). Collagen XII interacts with various other ECM proteins like decorin, tenascin-X, and fibromodulin via distinct or overlapping domains. For example, binding to avian tenascin-X occurs via its non-collagenous N-terminal NC3 domain (14), whereas decorin-binding is independent of the NC3 domain (15). Two collagen XII variants arise by alternative splicing and differ by N-terminal domains present exclusively in the large splice variant (Fig. 1A). This contributes a heparin binding site not present in the short splice variant. Thus, the two variants of collagen XII display differential ligand binding properties (13). In addition to structural similarities, collagen XIV also shows functional homologies to collagen XII by binding to decorin (16), tenascin-X (17), and heparin (18). However, the two proteins display different expression patterns, especially during development (19).
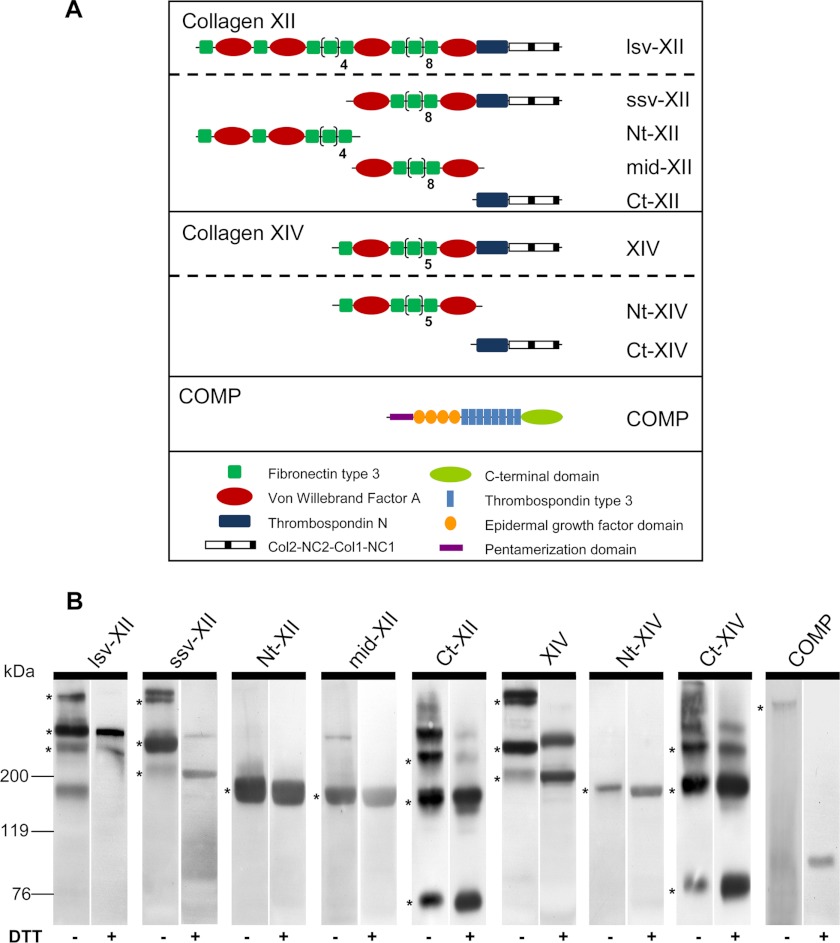
FIGURE 1.
Domain structure and Western blot analysis of recombinant proteins. A, schematic representation of the domain structure of recombinant murine collagen XII, collagen XIV, and COMP as monomers: collagen XII long splice variant (lsv-XII), short splice variant (ssv-XII), N-terminal fragment (Nt-XII), middle fragment (mid-XII), and C-terminal fragment (Ct-XII); collagen XIV full-length (XIV), N-terminal (Nt-XIV) and C-terminal fragment (Ct-XIV); and full-length COMP (COMP). B, Western blot analysis of supernatants of stably transfected HEK293-EBNA cells was carried out by SDS-PAGE separation under non-reducing (-DTT) and reducing conditions (+DTT). Recombinant proteins were detected with antibodies recognizing the StrepII tag or the His tag (Ct fragments). COMP pentamers and trimers, dimers, and monomers of lsv-XII, ssv-XII, Ct-XII, XIV, and Ct-XIV were detected under non-reducing conditions (asterisks). Stably linked dimers of collagen XII and XIV were seen even under reducing conditions. Single specific bands were detected for middle (mid-XII) and Nt (Nt-XII, Nt-XIV) fragments. Molecular weight standards (kDa) are shown on the left.
In cartilage ECM, one of the pivotal proteins involved in bridging the collagen II fibril network and other cartilage components is COMP, the fifth member of the thrombospondin family (20, 21). COMP monomers consist of an N-terminal oligomerization domain followed by four EGF-like repeats, eight calcium-binding thrombospondin-like type 3 domains, and a globular C-terminal domain (21) (Fig. 1A). Five identical monomers assemble into a bouquet-like complex (20, 22) in which the N termini form a pentameric coiled-coil structure that is further stabilized by disulfide bridges (21, 23). Mutations in COMP affecting calcium binding and protein folding cause skeletal dysplasias (24, 25). These diseases are either due to intracellular retention of COMP (26) and other ECM molecules in the endoplasmic reticulum (27), leading to chondrocyte death (28, 29), or to secretion of mutant proteins that cause a disruption of extracellular matrix structures observed in chondrocyte cultures (30, 31) and in vivo (32, 33). Expression of abnormal COMP is thus deleterious to cartilage homeostasis, whereas ablation of COMP in mice did not result in obvious skeletal abnormalities (34).
In vitro studies revealed that COMP binds with high affinity to collagens I and II (35), promoting early association of collagen molecules and enhancing collagen fibril formation and organization (36). Moreover, COMP acts as a molecular bridge in maintaining the interstitial collagen II network in cartilage by binding to the FACIT collagen IX (37, 38), which decorates the surface of collagen II fibrils (39), and to other ECM proteins (40, 41). Binding to collagens is accomplished via the C-terminal globular domains of the COMP pentamer (35, 37, 38). Mutations in the C-terminal globular domain do not strongly affect binding to collagens but disrupt collagen fibrillogenesis in vitro (42, 43).
On the basis of the analogy to collagen II in cartilage, in this study we investigated whether COMP may function as an organizer of the dermal collagen I network in the skin and, if so, whether binding to the FACIT collagens XII and XIV is involved, representing molecules that decorate the major collagen I fibrils present in skin ECM. We report in vitro binding between COMP and collagens XII and XIV and colocalization of these proteins in vivo. Interestingly, the proteins also codistribute at the ultrastructural level in healthy human skin, mainly in a characteristic band-like pattern in the topmost papillary dermis adjacent to anchoring plaques in close vicinity to keratinocytes and fibroblasts.
EXPERIMENTAL PROCEDURES
Recombinant Proteins
For cloning of collagen XII (mCol12a1, accession no. NM_007730.2), first the Ct-XII fragment (Fig. 1A) was amplified from embryonic day 15.5 mouse embryo cDNA using the primer pairs P3/P8 (see list of PCR primers in supplemental Table S1). The PCR product was ligated (rapid DNA ligation kit, Roche) into a pBK II vector for sequencing, followed by cloning into a modified pCEP-Pu vector carrying a 3′ His8 tag (44). The collagen ssv-XII was generated by cloning two additional fragments using primer pairs M854/M893 (N-terminal) and P143/P144 (middle). Both fragments were ligated into a pBK II vector for sequencing. The Ct-XII as well as the N-terminal ssv-XII fragments were digested with herculase (Stratagene) to generate blunt ends. The ssv-XII was created by digestion of the N-terminal fragment with BspEI, the middle fragment with NdeI and BspEI, and the blunt end Ct-XII with NdeI, which were ligated in that order. The resulting ssv-XII was cloned via the restriction sites NheI and Psp XI into a modified pCEP-Pu vector containing a 5′ 2× StrepII tag (45). For cloning the collagen lsv-XII, an N-terminal fragment was amplified using the primers P145/P146 and cloned into a pBK II vector for sequencing. The previously cloned ssv-XII was digested with NheI and Nsi I to remove its N-terminal part and replace it with the de novo N-terminal fragment for lsv-XII via the Nsi I site. The resulting lsv-XII was cloned via NheI and Psp XI into a modified pCEP-Pu vector containing a 5′ 2× StrepII tag. The Nt-XII and mid-XII fragments were amplified with the primer pairs P145/T374 and T375/T376 using the lsv-XII cDNA as a template and cloned into the same pCEP-Pu vector as the full-length collagens.
Cloning of full-length collagen XIV (mCol14a1, accession no. NM_181277.3) was carried out by amplifying the Ct-XIV and an N-terminal fragment with the primer pairs P18/P19 and M850/P148 and ligation of the amplified product into a pBK II vector. Both fragments were fused via the internal restriction site Sbf I and cloned into a modified pCEP-Pu vector containing a 5′ 2× StrepII-tag. For the Nt-XIV fragment the primers M850/T377 were used, and the fragment was cloned via the pBK II vector for sequencing into the pCEP-Pu vector with a 5′ 2× StrepII-tag. The Ct-XIV fragment was cloned into a modified pCEP-Pu vector harboring a 5′ His8 tag. COMP (mCOMP, accession no. NM_016685.2) was amplified with the primer pair P987/P988 and cloned into a modified pCEP-Pu vector containing a 5′ 2× StrepII tag.
HEK293-EBNA cells (Invitrogen) were stably transfected with all full-length constructs and their fragments as described (46). Immunoblotting of all proteins was carried out using supernatants of transfected HEK293-EBNA cells by separating the proteins by SDS-PAGE using 4–12% gradient gels under non-reducing and reducing conditions and transfer onto nitrocellulose. Membranes were blocked in 3% BSA/TBS/0.05% Tween 20 (TBST), incubated with antibodies recognizing the 2× StrepII tag (IBA) or the His8 tag (Qiagen), followed by incubation with horseradish peroxidase-conjugated anti-mouse secondary antibodies. Proteins were visualized with Immobilon Western chemiluminescent HRP substrate (Millipore). Collected supernatants were supplemented with 1 mm phenylmethylsulfonyl fluoride (Sigma). Strep-tagged proteins (lsv-XII, ssv-XII, Nt-XII, mid-XII, XIV, Nt-XIV, and COMP) were passed over a streptactin-Sepharose column (IBA) after filtration, and the recombinant proteins were eluted with buffer (100 mm Tris, 150 mm NaCl (pH 7.4)) containing 2.5 mm d-desthiobiotin (Sigma) (45). Supernatants containing His-tagged proteins (Ct-XII and Ct-XIV) were additionally supplemented with 10% 0.5 m Na2HPO4 and, after filtration, passed over a Ni-Sepharose 6 Fast Flow (GE Healthcare) and eluted stepwise with 10–250 mm imidazole in 20 mm Tris, 150 mm NaCl (pH 8.0) (46). All proteins were dialyzed against TBS (pH 7.4) to remove imidazole as well as d-desthiobiotin.
Antibodies and Antibody Production
To produce polyclonal antibodies recognizing human collagen XII or XIV, the fibronectin type III repeats 14 to 18 of collagen XII and 5 to 8 of collagen XIV were selected for immunizing guinea pigs (11, 14). The fragments were amplified using the primers 5′-cac GCT AGC aga gga ctg gca aga aat gtc c-3′ and 5′-ttg TGA TCA tta ggt atg ttc ttt aac aga gac t-3′ for collagen XII and 5′-aaa GCT AGC gaa gtt cca gcc cag caa tac-3′ and 5′-aaa GCT AGC gaa gtt cca gcc cag caa tac-3′ for collagen XIV and cloned into a pPET vector (EMD Biosciences) carrying a 5′ His6 tag. Bacterial expression, purification, and immunization were carried out as described (14). Polyclonal antibodies recognizing mouse COMP were produced by immunizing rabbits with the purified full-length recombinant mouse protein described above. The antisera were purified by affinity chromatography on a CNBr-activated Sepharose (GE Healthcare) column coupled with the corresponding recombinant antigens and tested as described (14). The molecular specificity of the antibodies was characterized by preabsorbing antibodies with recombinant immunogens followed by immunostaining of tissue sections and Western blot (supplemental Fig. S1). The antibody against human COMP was raised in rabbit using COMP purified from human cartilage as antigen for immunization according to standard protocols. The antibody reacts with human recombinant COMP as well as with COMP from several other species and shows the expected staining in Western blot analyses of cartilage extracts.
Immunohistochemistry and Immunofluorescence
Skin biopsies from healthy donors were embedded in optimal cutting temperature compound (Sakura) for cryosectioning. To separate epidermis from dermis, whole skin was incubated with 1 m NaCl for 72 h at 4 °C, washed thoroughly, and dried briefly before being embedded in optimal cutting temperature compound. For single antibody immunohistochemistry, 6-μm sections were cut, fixed with ice-cold acetone, endogenous peroxidase-blocked (Dako) for 10 min, and unspecific binding sites-blocked with 10% fetal calf serum for 1 h. Sections were then incubated with primary antibodies for 1 h at room temperature and washed with PBS, followed by incubation with secondary antibodies conjugated with HRP (EnVision-AEC kit, Dako). Signals were visualized with 3-amino-9-ethyl-carbazole and counterstained with hematoxylin.
For coimmunofluorescence, 6-μm cryo sections were fixed in 2% paraformaldehyde for 10 min at room temperature, and sections were blocked with 5% BSA/0.1% Triton X-100 for 1 h. A combination of primary antibodies was applied for 1 h at room temperature, followed by guinea pig and rabbit secondary antibodies coupled to Alexa Fluor 488 (green) and Alexa Fluor 568 (red), respectively (Molecular Probes). Nuclei were counterstained with DAPI. Photo micrographs of the stained sections were taken using either a DM 4000B microscope (Leica) or a Nikon Eclipse 800E fluorescence microscope equipped with a digital camera (DXM 1200F, Nikon). Images were further processed with Adobe Photoshop.
Isolation of Primary Fibroblasts and Cell Culture
Primary fibroblasts were isolated from human skin by explant culture as described (47) and used between passages 2 and 5. Fibroblasts and HaCaT cells (kindly provided by Dr. Petra Boukamp, Heidelberg) were cultured in DMEM (Invitrogen) containing 10% FCS (PAA), 50 μg/ml Na-ascorbate (Sigma), 2 mm glutamine, and antibiotics (Seromed-Biochrom) at 37 °C in 5% CO2 on 6-well culture plastic (BD Biosciences) for 24 h.
Real-time Quantitative RT-PCR
RNA was extracted from separated dermis and epidermis or cultured primary human dermal fibroblasts and HaCaT cells using the RNeasy kit (Qiagen). The epidermis was separated from the dermis by incubating thin strips of whole human skin in thermolysin (Protease type X; Sigma) dissolved at 1 mg/ml in buffer (10 mm HEPES, 150 mm NaCl, 6 mm KCl, 1 mm CaCl2, 1 mm MgCl2), overnight at 4°C (48). Thereafter, the epidermis was carefully peeled away from the dermis, and the separated tissues were stored in RNAlater (Qiagen).
Total RNA was reverse transcribed using oligo(dT) primers and the revertAidTM first strand cDNA synthesis kit (Fermentas). Primers were as follows: COMP, 5′-tgc gac gac gac atc gac ggc-3′ (forward) and 5′-cgc tgt cac aag cat ctc cca caa-3′ (reverse); Thy-1, 5′-atg aac ctg gcc atc agc atc gc-3′ (forward) and 5′-cga ggt gtt ctg agc cag cag gc-3′ (reverse); α1(I) procollagen, 5′-cca gaa gaa ctg gta cat cag ca-3′ (forward) and 5′-cgc cat act cga act gga at-3′ (reverse); K14, 5′-cga cct gga agt gaa gat cc-3′ (forward) and 5′-gtc cac tgt ggc tgt gag aa-3′ (reverse); S26, 5′-ccg tgc ctc caa gat gac aa-3′ (forward) and 5′-aga act cag ctc ctt aca-3′ (reverse) (Metabion). Amplification reactions were set up in triplicate, including PowerSYBR Green PCR Master Mix, using a 7300 real-time PCR system (Applied Biosystems). The comparative method of relative quantification (2−ΔΔCt) was used to calculate expression levels of target genes after normalizing to S26.
Solid Phase Binding Assays
10 μg/ml recombinant collagen XII, collagen XIV, or fragments thereof were immobilized on 96-well plates (Nunc Maxisorb) overnight at 4 °C. After washing with TBS containing 0.05% Tween 20 and blocking with 5% skim milk for 1 h at room temperature, recombinant COMP was added in indicated concentrations and incubated for 2 h at room temperature. Excess ligand was removed by washing thrice with TBS containing 0.05% Tween 20. Bound COMP was detected by an affinity-purified rabbit antibody against mouse COMP (1:3000) and swine anti-rabbit horseradish peroxidase-coupled IgG (1:3000) (Dako). HRP activity was detected by incubation with 50 μl of 0.25 mm tetramethylbenzidine and 0.005% (v/v) H2O2 in 0.1 m sodium acetate (pH 6.0) for 10 min. The reaction was stopped with 50 μl/well of 0.25 m H2SO4, and the absorbance was measured at 450 nm using a multilabel counter (Victor3, PerkinElmer Life Sciences). All measurements were done in triplicate, and identically treated uncoated wells or wells without ligand were used as blanks. All buffers contained 1 mm ZnCl2, considered to be essential for the interaction of COMP with collagen I and II (35).
Surface Plasmon Resonance Spectroscopy
Surface plasmon resonance spectroscopy was performed using a BIAcore 2000 (BIAcore AB) system. The recombinant pentameric mouse COMP (360 response units) was coupled to the surface of a CM5 chip in 25 mm sodium acetate (pH 4) as described (14). To measure protein interactions, the recombinant full-length collagen lsv-XII and XIV were passed over the chip as soluble analytes in serial dilutions (3–300 nm) in running buffer (20 mm HEPES, 150 mm NaCl, 500 nm ZnCl2 0.005% P20 (pH 7.4)). The experiments were performed at a constant flow rate of 30 μl/min with an association time of 300 s and a dissociation time of 500 s. Fitting of the data and calculation of the apparent KD value was performed with the BIAevaluation 4.1 software using the 1:1 Langmuir binding model.
Immunoelectron Microscopy of Skin Samples
Skin samples from a human neonate, a 1-year-old, and a 2.5-year-old were rinsed in Dulbecco's serum-free medium (SFM), immunolabeled en bloc by immersing in primary antibody diluted 1:5 in SFM overnight at 4 °C, rinsed extensively in SFM, immersed in appropriate 1 nm of gold conjugate (Amersham Biosciences), diluted 1:3 in SFM, and rinsed extensively in SFM. The 1-nm gold particles were enhanced using the Nanoprobe gold-enhanced EM (GEEM) kit. Briefly, tissue in buffer was chilled on ice, incubated on ice for 15 min in complete enhance solution, warmed quickly to 25 °C, and incubated for 5 min. Thereafter, tissue was rinsed in ice-cold SFM and fixed. For simultaneous localization of COMP with either collagen XII or XIV, tissues were immersed in a combination of the antibodies diluted 1:5 with SFM overnight at 4 °C and then in the appropriate combination of colloidal gold conjugated secondary antibodies (6 or 10 nm as specified in the figure legends) diluted 1:3 in SFM overnight at 4 °C. The specific reactivity of the gold-conjugated secondary antibodies with primary antibodies raised in rabbit and guinea pig was confirmed experimentally (supplemental Fig. S2). Tissues were rinsed in SFM and fixed in 1.5% glutaraldehyde/1.5% paraformaldehyde containing 0.05% tannic acid in SFM followed by 1% OsO4, then rinsed, dehydrated, and embedded in Spurrs epoxy. Ultrathin sections were cut at 60–80 nm, mounted onto formvar-coated 1 × 2 mm slot grids, stained with uranyl acetate and lead citrate, and imaged at 120 kilovolts using a FEI Tecnai G2 transmission electron microscope. Images were recorded on an AMT 2 K × 2 K side-mounted digital camera.
Rotary Shadowing Electron Microscopy
A 30 μl sample at a concentration of 100 μg/ml in ammonium bicarbonate was mixed with 70 μl glycerol and nebulized, using an airbrush, onto freshly cleaved mica. The sample was dried in vacuum and rotary-shadowed using a Pt-C electron beam gun angled at 6 degrees relative to the mica surface within a Balzers BAE 250 evaporator. The replica was backed with carbon, floated onto distilled water, and picked up onto 600 mesh grids. Photomicrographs were taken using a FEI Tecnai G2 operated at 120 KV as described above.
RESULTS
Expression and Purification of Recombinant Full-length COMP, Collagen XII, Collagen XIV and Fragments Thereof
To characterize binding properties of COMP, collagen XII, and collagen XIV, full-length recombinant proteins were generated in an eukaryotic expression system (Fig. 1A). Complete open reading frames of COMP, collagen XII (long and short splice variants denoted as lsv-XII and ssv-XII, respectively), and collagen XIV were amplified (supplemental Table S1) from cDNA, confirmed by sequencing, and ligated into a modified episomal expression vector (45). Recombinant plasmid DNA was stably transfected into HEK293-EBNA cells, and recombinant proteins were purified from cell culture supernatants. The oligomerization patterns of these high molecular weight proteins (i.e. pentamers for COMP and trimers for collagen XII and XIV) were confirmed by immunoblotting the supernatant of transfected HEK293-EBNA cells (Fig. 1B). Recombinant collagen lsv-XII (Mr 360 kDa), ssv-XII (Mr 200 kDa), and XIV (Mr 200 kDa) were detected as monomers as well as non-reducible dimers under reducing conditions and additionally as trimers under non-reducing conditions. Recombinant COMP monomers were detected at an expected Mr of ∼110 kDa under reducing conditions and as pentamers under non-reducing conditions.
To delineate the putative binding sites for COMP in collagens XII and XIV, smaller fragments of these proteins were generated (Fig. 1A). Expression and purification of these fragments was carried out as described under “Experimental Procedures” and protein oligomerization of these fragments was analyzed by immunoblotting supernatants of transfected HEK293-EBNA cells under non-reducing and reducing conditions (Fig. 1B). As the collagenous domains of C-terminal fragments oligomerize, multiple bands representing trimers, dimers, and monomers were detected, whereas specific single bands were detected for the middle and N-terminal fragments. The identity and molecular shape of the full-length proteins and the fragments were confirmed by mass spectrometry peptide mass fingerprinting (data not shown).
Interaction of COMP with Collagens XII and XIV
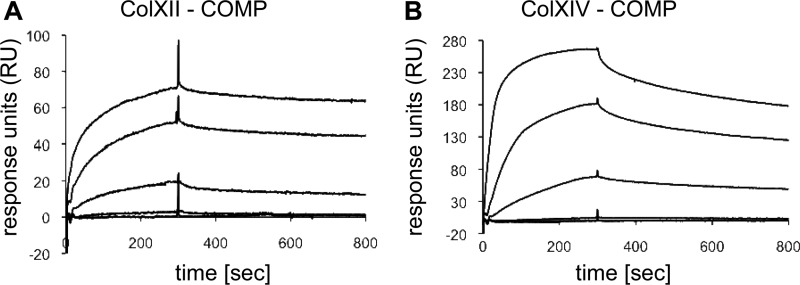
The interaction of COMP with collagen XII and collagen XIV was analyzed by surface plasmon resonance spectroscopy. Pentameric COMP was immobilized to a CM5 sensor chip, and the long splice variant of collagen XII was injected as soluble analyte at increasing concentrations (3–300 nm). The association and the dissociation curves were analyzed in a Langmuir 1:1 binding model (Fig. 2A). The long splice variant of collagen XII bound to COMP with an apparent KD of 2.32 nm. As collagen XII shares high structural and functional homology with collagen XIV, we determined whether COMP interacts with collagen XIV. In surface plasmon resonance spectroscopy, soluble full-length collagen XIV bound to immobilized COMP with an apparent KD of 11.8 nm (Fig. 2B). These binding analyses confirmed that COMP binds to the two FACIT collagens XII and XIV with affinities of the same order of magnitude.
FIGURE 2.
COMP binds to FACIT collagens XII and XIV. Interaction of soluble lsv-XII (A) and XIV (B) with immobilized pentameric COMP was evaluated by surface plasmon resonance spectroscopy. The curves are drawn in ascending order, reflecting the concentrations of 3, 10, 30, 100, and 300 nm of the soluble analyte. The amounts of interacting analytes were monitored by measuring the variation in plasmon resonance angle over time expressed in response units (RU).
COMP Binds to the Collagenous Domains of Collagen XII but Not to the Large NC3 Domain
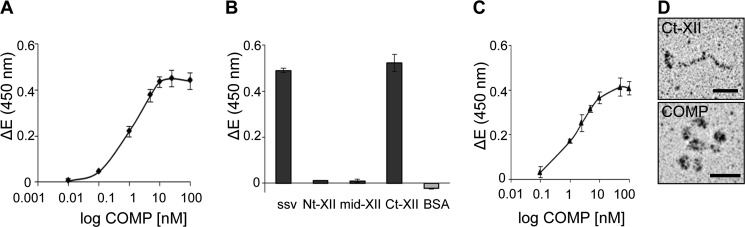
Two splice variants of collagen XII are known that differ in their expression depending on the tissue and the developmental stage (13). We explored whether COMP binds to the short splice variant of collagen XII with similar affinity as to the long splice variant. Instead of SPR spectroscopy, ELISA-style binding assays were used with immobilized collagen XII and increasing concentrations of COMP as soluble ligand because of the limitation of the purified proteins. For these experiments, the molar concentration of COMP was calculated assuming the molecular mass of pentameric COMP to be 524 kDa (20). The titrations gave saturable binding with half maximal binding at a concentration of 1.05 nm (Fig. 3A), indicating that COMP binds both collagen XII splice variants with comparable affinity.
FIGURE 3.
COMP binds to the C-terminal collagenous domain of collagen XII but not to the NC3 domain. A, binding of soluble COMP to the immobilized short splice variant of collagen XII (ssv-XII) was determined by solid phase ELISA-style assay using our antibody against mouse COMP to detect the bound ligand. The resulting saturation curve was used to calculate the apparent KD value of about 1 nm. B, recombinant fragments of collagen XII (ssv, Nt, mid, Ct) and BSA were immobilized at a concentration of 10 μg/ml, and binding of COMP (100 nm) was determined by an ELISA-style assay using the antibody against mouse COMP. BSA served as a negative control. The measurements clearly demonstrated that the binding site for COMP is located in the collagenous region of collagen XII and not in the NC3 domain. C, Ct-XII was immobilized at 10 μg/ml, and binding of pentameric COMP in concentrations ranging from 0.1 to 100 nm was analyzed. Saturable binding curves confirmed the interaction of COMP with the C-terminal collagenous domain of collagen XII. For all measurements shown in this figure, ΔE represents the measured extinction minus the blank value. Each value depicts mean ± S.D. (n = 3). D, rotary shadowing of the interacting Ct-XII fragments and pentameric COMP indicates that the recombinant proteins maintained the appropriate conformation. Scale bars = 25 nm.
To narrow down the exact binding site in collagen XII responsible for the interaction, we analyzed the binding of COMP to selected collagen XII fragments. Single concentration ELISA-style binding experiments using COMP (100 nm) as soluble ligand and Nt, mid, and Ct (10 μg/ml) fragments of collagen XII as solid analyte revealed that COMP binds to the Ct-XII fragment, which contains the collagenous domains Col1 and Col2 along with the short non-collagenous NC1 and NC2 domains and a thrombospondin N domain (Fig. 3B). No binding was observed with the middle and Nt fragments, which collectively form the large non-collagenous NC3 domain of collagen XII. The short splice variant of collagen XII and bovine serum albumin were used as positive and negative controls. Binding of COMP to Ct-XII was saturable with half-maximal binding at 1.53 nm, a value in good agreement with the apparent KD calculated for the binding of COMP to the long and short splice variants of full-length collagen XII (Fig. 3C). The intact structure of the interacting proteins used in binding assays was demonstrated by rotary shadowing (Fig. 3D). Taken together, COMP binds the collagenous region of collagen XII with an apparent KD of ∼1 nm, as demonstrated by two independent methods.
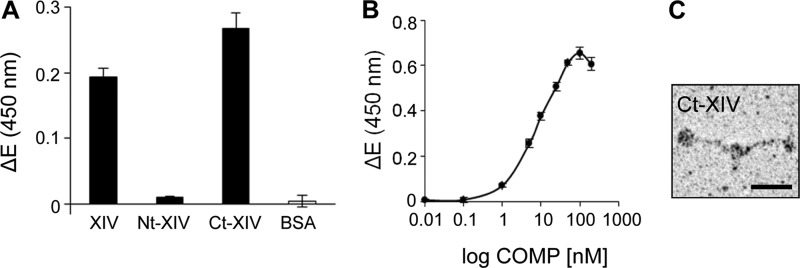
Collagen XIV Binds to COMP via Its Collagenous Domains
To narrow down the binding site for COMP in collagen XIV, binding of COMP to the N- and C-terminal fragments of collagen XIV was studied in an ELISA-style binding assay. COMP as a soluble ligand (100 nm) binds the immobilized (10 μg/ml) Ct fragment of collagen XIV (Fig. 1A), whereas nearly no binding was observed with the Nt fragment (large non-collagenous NC3 domain) (Fig. 4A). Full-length collagen XIV and bovine serum albumin were used as positive and negative controls. Binding of soluble COMP to immobilized collagen XIV Ct fragment was with a saturable half maximal binding at 6.99 nm (Fig. 4B). Rotary shadowing of the Ct-XIV fragment confirmed its domain structure and shape (Fig. 4C).
FIGURE 4.
COMP binds to the C-terminal collagenous domain of collagen XIV. A, recombinant full-length collagen XIV (XIV), collagen XIV fragments (Nt-XIV, Ct-XIV), and BSA were coated at 10 μg/ml on microtiter plates overnight at 4 °C, and binding of COMP (100 nm) was determined by ELISA-style assay using the antibody against mouse COMP. The measurements pointed to a binding site for COMP localized in the collagenous region of collagen XIV, whereas no binding was observed with the non-collagenous Nt fragment. B, saturable binding was observed for COMP to immobilized C-terminal collagenous region of collagen XIV (Ct-XIV) as determined by ELISA-style assay, confirming the presence of the binding site for COMP in the collagenous domain of collagen XIV. For all measurements shown in this figure, ΔE represents the measured extinction minus the blank value. Each value depicts the mean ± S.D. (n = 3). C, rotary shadowing of the Ct fragment of collagen XIV demonstrated that the fragment maintained an appropriate conformation. Scale bar = 25 nm.
COMP Is Present in the ECM of Human Skin and Is Produced by Dermal Fibroblasts
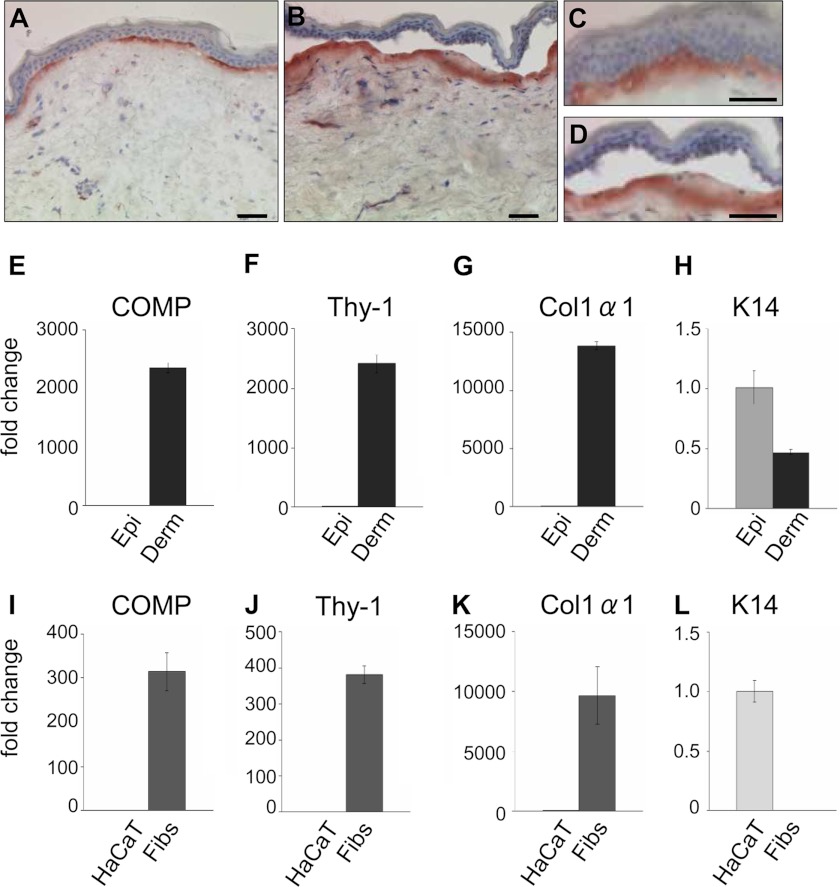
Connective tissues rich in collagen I, e.g. skin, bone, tendon, and ligaments are also abundant in collagens XII and XIV. To determine the physiological relevance of COMP binding to these FACIT collagens, we first analyzed the COMP presence in skin. Immunostaining of skin biopsies from healthy donors with antibodies raised against human COMP demonstrated the protein mainly in the superficial papillary dermis, just below the epidermal keratinocytes, in a continuous linear pattern (Fig. 5, A and C). Much lower expression was found in the reticular dermis. Absence of signals upon preincubation of the antibody with recombinant human COMP and detection of the protein in human cartilage and skin extracts upon immunoblotting confirmed the specificity of the antibody (not shown). Immunostaining of separated epidermis and dermis (salt-split human skin) revealed COMP protein almost exclusively in the dermal compartment (Fig. 5, B and D). Further, quantitative real-time RT-PCR demonstrated abundant COMP transcripts in extracts of separated dermis (Fig. 5E) and cultured primary dermal fibroblasts (I), which were also highly positive for the specific human fibroblast marker Thy-1 (49) and α1(I) procollagen (G and K). In contrast, no COMP transcripts were detected in extracts of epidermis (Fig. 5E) or cultured epidermal HaCaT cells (I), which showed a high expression of keratin 14 (H and L), a marker for proliferating keratinocytes in the basal layer of the epidermis (50, 51). Keratin 14 signals in the dermis (Fig. 5H) derive from keratinocytes of the hair follicles that are embedded in the dermis but are absent from extracts of cultured dermal fibroblasts (Fig. 5L). Thus, COMP is produced by dermal fibroblasts and is deposited in a characteristic pattern, mainly in the papillary dermis of human skin.
FIGURE 5.
COMP is produced by dermal fibroblasts and deposited in the papillary dermis of healthy human skin. A, COMP deposition and localization in human skin as detected by immunohistochemistry (n ≥ 10). Cryosections were immunostained using antibodies against human COMP showing a subepidermal localization in the superficial papillary dermis. B, the immunostaining remained restricted to the dermal part of the skin upon separation of the epidermis from the dermis by 1 m NaCl. C and D are higher-magnification images of A and B, respectively. Scale bars = 50 μm. mRNA levels were determined by real-time RT-PCR for COMP (E and I), Thy-1 (F and J), α1(I) procollagen (G and K), and keratin-14 (K14) (H and L) in epidermis (Epi) and dermis (Derm) separated after treatment with thermolysin, in cultured HaCaT cells and human primary dermal fibroblasts (Fibs). COMP expression was detected exclusively in extracts of dermis and dermal fibroblasts. Restricted expression of Thy-1 and α1(I) procollagen only in dermis and cultured fibroblasts and absence of keratin 14 (K14) expression in fibroblast cultures confirmed the dermoepidermal separation and the purity of the cell cultures. The signals obtained were normalized to S26 mRNA used as internal control. Expression levels are expressed relative to signals in epidermis and HaCaT cells. Each value depicts mean ± S.D. Results are representative of n ≥ 3 independent experiments using different donors/cell strains.
COMP Partially Codistributes with the FACIT Collagens XII and XIV in Human Skin
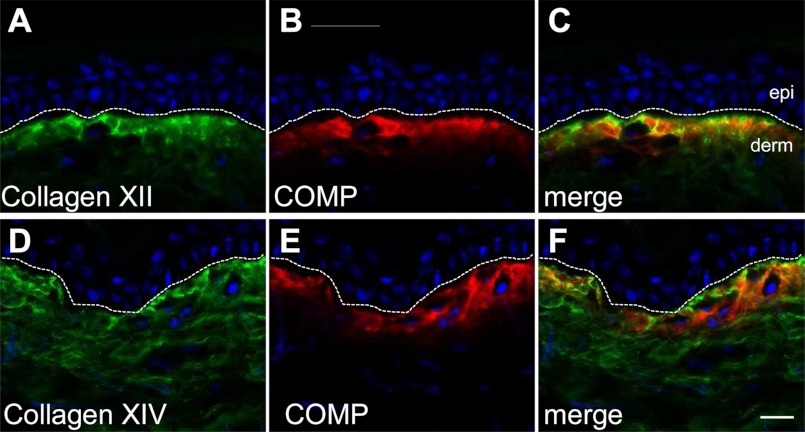
To assess whether the interaction of COMP with collagens XII and XIV observed in vitro may be functionally relevant in vivo, coimmunofluorescence was performed to reveal the distribution of all three proteins in adult human skin. Collagen XII is concentrated predominantly in the papillary dermis in a similar distribution as COMP (Fig. 6, A and B), whereas collagen XIV is located throughout the dermis, including the reticular compartment (D). Overlay of the signals demonstrates substantial colocalization of COMP and collagen XII (Fig. 6C) and also of COMP and collagen XIV in the upper papillary dermis (F). This codistribution strongly suggests that COMP may interact with FACIT collagens in human skin and thus may function as a structural bridge in maintaining collagen fibril architecture, comparable with COMP and collagen IX in cartilage (37, 38).
FIGURE 6.
COMP and FACIT collagens XII and XIV show partial codistribution in the papillary dermis of human skin. Skin biopsies from healthy human donors (n ≥ 3) were coimmunostained with antibodies recognizing human collagen XII (A, green) and human COMP (B, red) raised in guinea pig and rabbit, respectively. Nuclei were stained using DAPI (blue). Coimmunofluorescence (C, merge) demonstrated extensive codistribution of COMP and collagen XII in the papillary dermis, just below the epidermis. Consecutive skin samples were coimmunostained with antibodies recognizing human collagen XIV (D, green) and human COMP (E, red) raised in guinea pig and rabbit, respectively. Collagen XIV showed deposition throughout the dermis with strong subepidermal signals, which partially codistributed with COMP (F, merge). The broken line indicates the dermoepidermal junctional zone. epi and derm depict the epidermis and dermis, respectively. Scale bar = 10 μm.
Ultrastructural Localization of COMP and FACIT Collagens XII and XIV in Human Skin
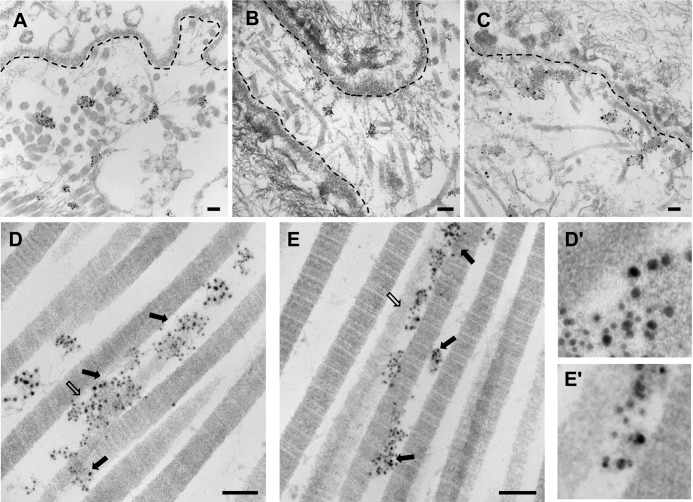
Having established the ability of COMP to bind to collagen XII and XIV and their presence in close proximity in the upper papillary dermis, we sought further evidence for a potential interaction of these proteins in skin. Therefore, freshly obtained human skin was processed for ultrastructural localization of COMP, collagen XII, and collagen XIV and immunostained by the en bloc method (see “Experimental Procedures”) with specific primary and gold-conjugated secondary antibodies. Electron microscopy confirmed that COMP localizes subepidermally and revealed that it is distributed in clusters overlapping with, but not limited to, anchoring plaques (Fig. 7A). A similar localization pattern was observed for collagen XII (Fig. 7B) and XIV (C) in the papillary dermis adjacent to the basement membrane (depicted as a broken line in Fig. 7). All three proteins were present in clusters that did not show an obvious orientation relative to collagen fibrils. Coimmunolabeling of COMP and collagen XII with secondary antibodies conjugated with differently sized gold particles showed that COMP (10 nm) and collagen XII (6 nm) indeed colocalize in some areas (Fig. 7, D and D′) but that the two proteins are also present in discrete areas. Coimmunogold labeling for COMP (10 nm) and collagen XIV (6 nm) similarly demonstrated that there are regions of colocalization but also regions of independent deposition (Fig. 7, E and E′). Taken together, these results indicate the presence of complexes between COMP and FACIT collagens that are concentrated in anchoring plaques and decorate collagen I fibrils.
FIGURE 7.
Ultrastructural colocalization of COMP and FACIT collagens XII and XIV in the dermis of human skin. Human skin was incubated en bloc with a rabbit antibody against mouse COMP (A), with a guinea pig antibody against human collagen XII (B), and with a guinea pig antibody against human collagen XIV (C), followed by incubation with secondary antibodies conjugated to 1 nm colloidal gold and by gold enhancement. The localization of COMP (A), collagen XII (B), and collagen XIV (C) was associated with anchoring plaques in the papillary dermis. The broken line indicates the basement membrane. Colocalization of COMP with collagen XII (D) and collagen XIV (E) at the ultrastructural level was determined by double labeling of human skin with the respective primary antibodies followed by secondary antibodies conjugated to differently sized colloidal gold particles. For colocalization of COMP with collagen XII (D) and collagen XIV (E), gold particles of 10-nm diameter were used to detect COMP, and gold particles of 6-nm diameter were used to detect either collagen XII or XIV. Colabeling of COMP/collagen XII (D) and COMP/collagen XIV (E) is indicated by arrows. D′ and E′ show magnified images of areas marked by open arrows in D and E, respectively. Scale bars = 100 nm.
DISCUSSION
COMP has been implicated in various human skeletal diseases. Mutations in COMP cause chondrodysplasia, such as multiple epiphyseal dysplasia (MED) and pseudoachondroplasia (PSACH) (24, 25). The presence of COMP fragments in serum and synovial fluid serves as a marker for cartilage degradation in osteoarthritis and rheumatoid arthritis (52). Still, the biological function of COMP has remained obscure, especially because the constitutive ablation of COMP in mice failed to show major skeletal defects (34). The current view is that COMP functions as an adapter molecule in cartilage ECM by interacting with fibrillar collagen II (35), FACIT collagen IX (37, 38), matrilins (40), aggrecan (41), and fibronectin (53).
Because COMP has the capacity to bind collagen IX, which shares structural and molecular similarities with collagens XII and XIV, we investigated the expression and function of COMP in skin ECM with high concentrations of collagen XII and XIV and put forward the hypothesis that COMP may act as a matrix modifier by interacting with collagen I and associated FACIT collagens. The antibodies used for these investigations have been characterized in detail, and cross-reactivity has been ruled out as demonstrated in supplemental Figs. 1 and 2. COMP expression in skin has been reported for certain pathological conditions, e.g. fibrosis, yet no COMP deposition was noted in healthy human skin (54–56). This is the first report showing that COMP is a matrix component of healthy skin with a restricted localization mainly in the papillary dermis, where it interacts with collagens XII and XIV found on the surface of collagen I fibrils. COMP is exclusively produced by fibroblasts, although keratinocytes may play a role in modulating expression. This conclusion is on the basis of detection of specific COMP transcripts in dermal extracts after separation of epidermis from dermis and in lysates of cultured fibroblasts but not epidermal cells, as well as on the immunolocalization of COMP in the dermis and not epidermis of salt-split skin.
By surface plasmon resonance spectroscopy, we observed significant binding of COMP to FACIT collagens XII and XIV with apparent KD values of the same order of magnitude. Binding was confirmed in the reverse direction by ELISA-style assays, yielding a similar apparent KD. Two splice variants of collagen XII occur with distinct expression patterns (13). The large splice variant (Fig. 1A, lsv) is mostly restricted to embryonic tissues, whereas the short variant (Fig. 1A, ssv) and only small amounts of lsv are detected postnatally, as shown for avian skin (13). The NC3 region present only in the long splice variant accounts for some differential binding properties. For example, a heparin binding site is located in the seventh fibronectin type III repeat of the NC3 domain in lsv and is absent in the short variant (13). Here we show that COMP binds to both splice variants of collagen XII with similar affinity, demonstrating that COMP binding is independent of the NC3 domain. This domain is thought to extend into the pericellular space and to be available for interaction with other ECM proteins, e.g. tenascin-X (14). Our binding studies using defined molecular fragments clearly show that binding of COMP to both collagen XII and XIV is conferred by the collagenous C-terminal region and abrogated by collagenase treatment (not shown). The collagenous regions of collagens XII and XIV also bind decorin (15), and they are the regions binding to the major collagen I fibrils (10, 57). All these interactions are thought to be important for regulating fibril spacing and diameter (13, 58). On the basis of the codistribution of COMP with collagen XII and XIV and the propensity for mutual interactions, COMP may contribute to such control mechanisms in skin ECM. Halasz et al. (36) showed that collagen fibrils formed in vitro in the presence of COMP are uniform and distinct. The COMP-null mouse does not show obvious skeletal alterations (34), and no study of the skin has been reported. Interestingly, patients with PSACH or MED and mice expressing mutant COMP exhibit thicker collagen fibrils in tendon and ligaments (59). Further, high levels of COMP in equine tendon correlated with thinner collagen fibrils (60). This agrees well with the distribution of COMP in human skin, where it is mainly localized in the papillary dermis, which is characterized by collagen fibrils of smaller diameter.
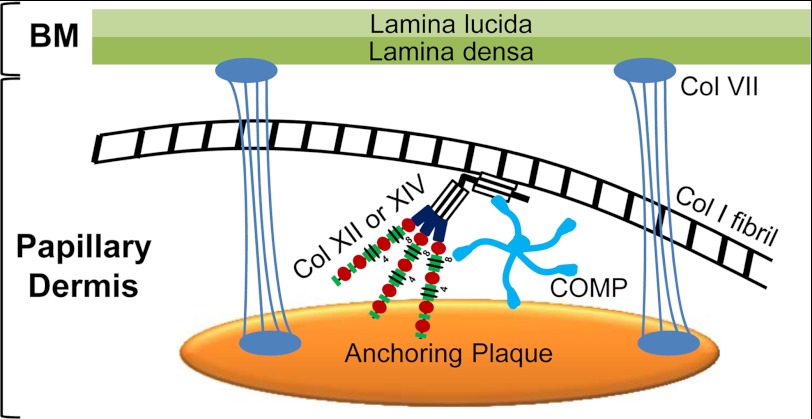
We demonstrated, by immunoelectron microscopy, codistribution of COMP with collagens XII and XIV aligning with major banded collagen I fibrils. All three components were found at much higher abundance in papillary dermis (adjacent to the basement membrane zone), and all three proteins codistributed in anchoring plaques. The latter are amorphous structures in the skin basement membrane zone containing collagen IV, laminin 332, and collagen VII that are interspersed into the banded fibril network of the papillary dermis and thought to contribute to the stability of the dermoepidermal junctional zone (61, 62). Although these data are largely descriptive at this point, they have several functional implications. They indicate that anchoring plaques are complex structures which, on the one hand, connect to the basement membrane via anchoring fibrils (61, 62, 63) and, on the other hand, are interconnected to the interstitial collagen network in the papillary dermis by COMP and the FACIT collagens XII and XIV (Fig. 8).
FIGURE 8.
Proposed model for the function of COMP in skin. COMP binds to collagen XII and XIV. All these proteins cluster in and around anchoring plaques. We postulate that anchoring plaques are not only amorphous patches where anchoring fibrils end but serve as docking sites for important multimeric proteins like COMP and collagens XII and XIV. Thus, anchoring plaques may connect to interstitial collagen I fibrils via COMP and FACIT collagens XII and XIV in the papillary dermis. BM, basement membrane; col VII, collagen VII that forms anchoring fibrils.
The precise biological function of COMP in the papillary dermis is not understood. We propose that the interaction of COMP with FACIT collagens associated with major collagen fibrils in the dermoepidermal junction zone may play an important role in regulating the suprastructure of collagen I fibrils in skin and, thus, may represent an additional molecular mechanism providing tissue integrity and homeostasis in the uppermost dermis. Although the involvement of COMP in skin disease has not yet been explored comprehensively, it is interesting that pronounced COMP expression was detected in scleroderma (55, 56), where skin alterations with irregularly packed thin collagen fibrils in the papillary and reticular layers of the dermis have been reported (64, 65).
Future studies are required to precisely delineate the role of COMP in scleroderma and other fibrotic conditions. Very recently, COMP was shown to enhance the cellular response to TGFβ (66), which is considered a pivotal player in the pathophysiology of these disease states (67, 68). Thus, COMP could be directly involved in the persistent activation of fibroblasts, leading to the excessive deposition of altered collagen fibrils.
Supplementary Material
Acknowledgments
We thank Gabriele Scherr, Semra Özcelik, Dr. Gundula Grimberg, Dr. Markus Schmitz, Dr. Andreas Klatt (all from Cologne) and Dr. Kristofer Andreasson (from Lund) for help and critical discussion. We also thank Sara Tufa (Portland) for contribution to electron microscopy studies.
This work was funded by Deutsche Forschungsgemeinschaft through Grants KR558/14-1 (to T. K. and B. E.), ZA561/2-1 (to F. Z.), and SFB 829 (to M. P. and M. K.).

This article contains supplemental Figs. S1 and S2 and Table S1.
- ECM
- extracellular matrix
- FACIT
- fibril-associated collagens with interrupted triple helices
- COMP
- cartilage oligomeric matrix protein
- SFM
- serum-free medium.
REFERENCES
- 1. Kadler K. E., Baldock C., Bella J., Boot-Handford R. P. (2007) Collagens at a glance. J. Cell Sci. 120, 1955–1958 [DOI] [PubMed] [Google Scholar]
- 2. Myllyharju J., Kivirikko K. I. (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20, 33–43 [DOI] [PubMed] [Google Scholar]
- 3. Sorrell J. M., Caplan A. I. (2004) Fibroblast heterogeneity. More than skin deep. J. Cell Sci. 117, 667–675 [DOI] [PubMed] [Google Scholar]
- 4. Ezura Y., Chakravarti S., Oldberg A., Chervoneva I., Birk D. E. (2000) Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 151, 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danielson K. G., Baribault H., Holmes D. F., Graham H., Kadler K. E., Iozzo R. V. (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 136, 729–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rada J. A., Cornuet P. K., Hassell J. R. (1993) Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp. Eye Res. 56, 635–648 [DOI] [PubMed] [Google Scholar]
- 7. Birk D. E., Mayne R. (1997) Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 72, 352–361 [PubMed] [Google Scholar]
- 8. Blaschke U. K., Eikenberry E. F., Hulmes D. J., Galla H. J., Bruckner P. (2000) Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J. Biol. Chem. 275, 10370–10378 [DOI] [PubMed] [Google Scholar]
- 9. Wotton S. F., Duance V. C., Fryer P. R. (1988) Type IX collagen. A possible function in articular cartilage. FEBS Lett. 234, 79–82 [DOI] [PubMed] [Google Scholar]
- 10. Shaw L. M., Olsen B. R. (1991) FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem. Sci. 16, 191–194 [DOI] [PubMed] [Google Scholar]
- 11. Ansorge H. L., Meng X., Zhang G., Veit G., Sun M., Klement J. F., Beason D. P., Soslowsky L. J., Koch M., Birk D. E. (2009) Type XIV collagen regulates fibrillogenesis: Premature collagen fibril growth and tissue dysfunction in null mice. J. Biol. Chem. 284, 8427–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keene D. R., Lunstrum G. P., Morris N. P., Stoddard D. W., Burgeson R. E. (1991) Two type XII-like collagens localize to the surface of banded collagen fibrils. J. Cell Biol. 113, 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koch M., Bohrmann B., Matthison M., Hagios C., Trueb B., Chiquet M. (1995) Large and small splice variants of collagen XII. Differential expression and ligand binding. J. Cell Biol. 130, 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Veit G., Hansen U., Keene D. R., Bruckner P., Chiquet-Ehrismann R., Chiquet M., Koch M. (2006) Collagen XII interacts with avian tenascin-X through its NC3 domain. J. Biol. Chem. 281, 27461–27470 [DOI] [PubMed] [Google Scholar]
- 15. Font B., Eichenberger D., Rosenberg L. M., van der Rest M. (1996) Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 15, 341–348 [DOI] [PubMed] [Google Scholar]
- 16. Font B., Aubert-Foucher E., Goldschmidt D., Eichenberger D., van der Rest M. (1993) Binding of collagen XIV with the dermatan sulfate side chain of decorin. J. Biol. Chem. 268, 25015–25018 [PubMed] [Google Scholar]
- 17. Lethias C., Carisey A., Comte J., Cluzel C., Exposito J. Y. (2006) A model of tenascin-X integration within the collagenous network. FEBS Lett. 580, 6281–6285 [DOI] [PubMed] [Google Scholar]
- 18. Giry-Lozinguez C., Aubert-Foucher E., Penin F., Deleage G., Dublet B., van der Rest M. (1998) Identification and characterization of a heparin binding site within the NC1 domain of chicken collagen XIV. Matrix Biol. 17, 145–149 [DOI] [PubMed] [Google Scholar]
- 19. Wälchli C., Koch M., Chiquet M., Odermatt B. F., Trueb B. (1994) Tissue-specific expression of the fibril-associated collagens XII and XIV. J. Cell Sci. 107, 669–681 [DOI] [PubMed] [Google Scholar]
- 20. Hedbom E., Antonsson P., Hjerpe A., Aeschlimann D., Paulsson M., Rosa-Pimentel E., Sommarin Y., Wendel M., Oldberg A., Heinegård D. (1992) Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 267, 6132–6136 [PubMed] [Google Scholar]
- 21. Oldberg A., Antonsson P., Lindblom K., Heinegård D. (1992) COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J. Biol. Chem. 267, 22346–22350 [PubMed] [Google Scholar]
- 22. Mörgelin M., Heinegård D., Engel J., Paulsson M. (1992) Electron microscopy of native cartilage oligomeric matrix protein purified from the Swarm rat chondrosarcoma reveals a five-armed structure. J. Biol. Chem. 267, 6137–6141 [PubMed] [Google Scholar]
- 23. Efimov V. P., Lustig A., Engel J. (1994) The thrombospondin-like chains of cartilage oligomeric matrix protein are assembled by a five-stranded α-helical bundle between residues 20 and 83. FEBS Lett. 341, 54–58 [DOI] [PubMed] [Google Scholar]
- 24. Briggs M. D., Hoffman S. M., King L. M., Olsen A. S., Mohrenweiser H., Leroy J. G., Mortier G. R., Rimoin D. L., Lachman R. S., Gaines E. S. (1995) Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat. Genet. 10, 330–336 [DOI] [PubMed] [Google Scholar]
- 25. Hecht J. T., Nelson L. D., Crowder E., Wang Y., Elder F. F., Harrison W. R., Francomano C. A., Prange C. K., Lennon G. G., Deere M. (1995) Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat. Genet. 10, 325–329 [DOI] [PubMed] [Google Scholar]
- 26. Maddox B. K., Keene D. R., Sakai L. Y., Charbonneau N. L., Morris N. P., Ridgway C. C., Boswell B. A., Sussman M. D., Horton W. A., Bächinger H. P., Hecht J. T. (1997) The fate of cartilage oligomeric matrix protein is determined by the cell type in the case of a novel mutation in pseudoachondroplasia. J. Biol. Chem. 272, 30993–30997 [DOI] [PubMed] [Google Scholar]
- 27. Merritt T. M., Bick R., Poindexter B. J., Alcorn J. L., Hecht J. T. (2007) Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes. Am. J. Pathol. 170, 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashimoto Y., Tomiyama T., Yamano Y., Mori H. (2003) Mutation (D472Y) in the type 3 repeat domain of cartilage oligomeric matrix protein affects its early vesicle trafficking in endoplasmic reticulum and induces apoptosis. Am. J. Pathol. 163, 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hecht J. T., Hayes E., Haynes R., Cole W. G. (2005) COMP mutations, chondrocyte function and cartilage matrix. Matrix Biol. 23, 525–533 [DOI] [PubMed] [Google Scholar]
- 30. Dinser R., Zaucke F., Kreppel F., Hultenby K., Kochanek S., Paulsson M., Maurer P. (2002) Pseudoachondroplasia is caused through both intra- and extracellular pathogenic pathways. J. Clin. Invest. 110, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitz M., Becker A., Schmitz A., Weirich C., Paulsson M., Zaucke F., Dinser R. (2006) Disruption of extracellular matrix structure may cause pseudoachondroplasia phenotypes in the absence of impaired cartilage oligomeric matrix protein secretion. J. Biol. Chem. 281, 32587–32595 [DOI] [PubMed] [Google Scholar]
- 32. Piróg-Garcia K. A., Meadows R. S., Knowles L., Heinegård D., Thornton D. J., Kadler K. E., Boot-Handford R. P., Briggs M. D. (2007) Reduced cell proliferation and increased apoptosis are significant pathological mechanisms in a murine model of mild pseudoachondroplasia resulting from a mutation in the C-terminal domain of COMP. Hum. Mol. Genet. 16, 2072–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suleman F., Gualeni B., Gregson H. J., Leighton M. P., Piróg K. A., Edwards S., Holden P., Boot-Handford R. P., Briggs M. D. (2012) A novel form of chondrocyte stress is triggered by a COMP mutation causing pseudoachondroplasia. Hum. Mutat. 33, 218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Svensson L., Aszódi A., Heinegård D., Hunziker E. B., Reinholt F. P., Fässler R., Oldberg A. (2002) Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol. Cell Biol. 22, 4366–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg K., Olsson H., Mörgelin M., Heinegård D. (1998) Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 273, 20397–20403 [DOI] [PubMed] [Google Scholar]
- 36. Halász K., Kassner A., Mörgelin M., Heinegård D. (2007) COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 282, 31166–31173 [DOI] [PubMed] [Google Scholar]
- 37. Holden P., Meadows R. S., Chapman K. L., Grant M. E., Kadler K. E., Briggs M. D. (2001) Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J. Biol. Chem. 276, 6046–6055 [DOI] [PubMed] [Google Scholar]
- 38. Thur J., Rosenberg K., Nitsche D. P., Pihlajamaa T., Ala-Kokko L., Heinegård D., Paulsson M., Maurer P. (2001) Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J. Biol. Chem. 276, 6083–6092 [DOI] [PubMed] [Google Scholar]
- 39. Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. (1988) D-periodic distribution of collagen type IX along cartilage fibrils. J. Cell Biol. 106, 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mann H. H., Ozbek S., Engel J., Paulsson M., Wagener R. (2004) Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J. Biol. Chem. 279, 25294–25298 [DOI] [PubMed] [Google Scholar]
- 41. Chen F. H., Herndon M. E., Patel N., Hecht J. T., Tuan R. S., Lawler J. (2007) Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J. Biol. Chem. 282, 24591–24598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hansen U., Platz N., Becker A., Bruckner P., Paulsson M., Zaucke F. (2011) A secreted variant of cartilage oligomeric matrix protein carrying a chondrodysplasia-causing mutation (p.H587R) disrupts collagen fibrillogenesis. Arthritis Rheum. 63, 159–167 [DOI] [PubMed] [Google Scholar]
- 43. Spitznagel L., Nitsche D. P., Paulsson M., Maurer P., Zaucke F. (2004) Characterization of a pseudoachondroplasia-associated mutation (His-587 → Arg) in the C-terminal, collagen-binding domain of cartilage oligomeric matrix protein (COMP). Biochem. J. 377, 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koch M., Schulze J., Hansen U., Ashwodt T., Keene D. R., Brunken W. J., Burgeson R. E., Bruckner P., Bruckner-Tuderman L. (2004) A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 279, 22514–22521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gara S. K., Grumati P., Urciuolo A., Bonaldo P., Kobbe B., Koch M., Paulsson M., Wagener R. (2008) Three novel collagen VI chains with high homology to the α3 chain. J. Biol. Chem. 283, 10658–10670 [DOI] [PubMed] [Google Scholar]
- 46. Koch M., Veit G., Stricker S., Bhatt P., Kutsch S., Zhou P., Reinders E., Hahn R. A., Song R., Burgeson R. E., Gerecke D. R., Mundlos S., Gordon M. K. (2006) Expression of type XXIII collagen mRNA and protein. J. Biol. Chem. 281, 21546–21557 [DOI] [PubMed] [Google Scholar]
- 47. Kessler D., Dethlefsen S., Haase I., Plomann M., Hirche F., Krieg T., Eckes B. (2001) Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J. Biol. Chem. 276, 36575–36585 [DOI] [PubMed] [Google Scholar]
- 48. Walzer C., Benathan M., Frenk E. (1989) Thermolysin treatment. A new method for dermo-epidermal separation. J. Invest. Dermatol. 92, 78–81 [DOI] [PubMed] [Google Scholar]
- 49. Saalbach A., Aust G., Haustein U. F., Herrmann K., Anderegg U. (1997) The fibroblast-specific MAb AS02: a novel tool for detection and elimination of human fibroblasts. Cell Tissue Res. 290, 593–599 [DOI] [PubMed] [Google Scholar]
- 50. Fuchs E., Green H. (1980) Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19, 1033–1042 [DOI] [PubMed] [Google Scholar]
- 51. Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. (1982) The catalog of human cytokeratins. Patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 [DOI] [PubMed] [Google Scholar]
- 52. Heinegård D., Saxne T. (2011) The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 7, 50–56 [DOI] [PubMed] [Google Scholar]
- 53. Di Cesare P. E., Chen F. S., Mörgelin M., Carlson C. S., Leslie M. P., Perris R., Fang C. (2002) Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 21, 461–470 [DOI] [PubMed] [Google Scholar]
- 54. Farina G., Lemaire R., Korn J. H., Widom R. L. (2006) Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 25, 213–222 [DOI] [PubMed] [Google Scholar]
- 55. Farina G., Lemaire R., Pancari P., Bayle J., Widom R. L., Lafyatis R. (2009) Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann. Rheum. Dis. 68, 435–441 [DOI] [PubMed] [Google Scholar]
- 56. Hesselstrand R., Kassner A., Heinegård D., Saxne T. (2008) COMP. A candidate molecule in the pathogenesis of systemic sclerosis with a potential as a disease marker. Ann. Rheum. Dis. 67, 1242–1248 [DOI] [PubMed] [Google Scholar]
- 57. Gordon M. K., Olsen B. R. (1990) The contribution of collagenous proteins to tissue-specific matrix assemblies. Curr. Opin. Cell Biol. 2, 833–838 [DOI] [PubMed] [Google Scholar]
- 58. Young B. B., Gordon M. K., Birk D. E. (2000) Expression of type XIV collagen in developing chicken tendons. Association with assembly and growth of collagen fibrils. Dev. Dyn. 217, 430–439 [DOI] [PubMed] [Google Scholar]
- 59. Piróg K. A., Jaka O., Katakura Y., Meadows R. S., Kadler K. E., Boot-Handford R. P., Briggs M. D. (2010) A mouse model offers novel insights into the myopathy and tendinopathy often associated with pseudoachondroplasia and multiple epiphyseal dysplasia. Hum. Mol. Genet. 19, 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Södersten F., Ekman S., Eloranta M. L., Heinegård D., Dudhia J., Hultenby K. (2005) Ultrastructural immunolocalization of cartilage oligomeric matrix protein (COMP) in relation to collagen fibrils in the equine tendon. Matrix Biol. 24, 376–385 [DOI] [PubMed] [Google Scholar]
- 61. Keene D. R., Sakai L. Y., Lunstrum G. P., Morris N. P., Burgeson R. E. (1987) Type VII collagen forms an extended network of anchoring fibrils. J. Cell Biol. 104, 611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Villone D., Fritsch A., Koch M., Bruckner-Tuderman L., Hansen U., Bruckner P. (2008) Supramolecular interactions in the dermo-epidermal junction zone. Anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J. Biol. Chem. 283, 24506–24513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sakai L. Y., Keene D. R., Morris N. P., Burgeson R. E. (1986) Type VII collagen is a major structural component of anchoring fibrils. J. Cell Biol. 103, 1577–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krieg T., Perlish J. S., Mauch C., Fleischmajer R. (1985) Collagen synthesis by scleroderma fibroblasts. Ann. N.Y. Acad. Sci. 460, 375–386 [DOI] [PubMed] [Google Scholar]
- 65. Perlish J. S., Lemlich G., Fleischmajer R. (1988) Identification of collagen fibrils in scleroderma skin. J. Invest. Dermatol. 90, 48–54 [DOI] [PubMed] [Google Scholar]
- 66. Haudenschild D. R., Hong E., Yik J. H., Chromy B., Mörgelin M., Snow K. D., Acharya C., Takada Y., Di Cesare P. E. (2011) Enhanced activity of transforming growth factor β1 (TGF-β1) bound to cartilage oligomeric matrix protein. J. Biol. Chem. 286, 43250–43258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gabrielli A., Avvedimento E. V., Krieg T. (2009) Scleroderma. N. Engl. J. Med. 360, 1989–2003 [DOI] [PubMed] [Google Scholar]
- 68. Varga J., Abraham D. (2007) Systemic sclerosis. A prototypic multisystem fibrotic disorder. J. Clin. Invest. 117, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.