Background: DynE8 is an iterative polyketide synthase (PKS) that assembles polyketide intermediates from acetate units derived from malonyl-CoA.
Results: We report the first acyltransferase (ATDYN10) crystal structure for an iterative PKS.
Conclusion: ATDYN10 protects the malonyl-enzyme, but not the acetyl-enzyme intermediate, from hydrolysis and facilitates the transfer of malonyl to the acyl carrier protein.
Significance: This differs from the dual specificity exhibited by acyltransferases of mammalian FAS and other iterative PKSs.
Keywords: Acetyl Coenzyme A, Acyl Carrier Protein, Antibiotics, Crystallography, Enzyme Structure, Polyketides
Abstract
Biosynthesis of the enediyne natural product dynemicin in Micromonospora chersina is initiated by DynE8, a highly reducing iterative type I polyketide synthase that assembles polyketide intermediates from the acetate units derived solely from malonyl-CoA. To understand the substrate specificity and the evolutionary relationship between the acyltransferase (AT) domains of DynE8, fatty acid synthase, and modular polyketide synthases, we overexpressed a 44-kDa fragment of DynE8 (hereafter named ATDYN10) encompassing its entire AT domain and the adjacent linker domain. The crystal structure at 1.4 Å resolution unveils a α/β hydrolase and a ferredoxin-like subdomain with the Ser-His catalytic dyad located in the cleft between the two subdomains. The linker domain also adopts a α/β fold abutting the AT catalytic domain. Co-crystallization with malonyl-CoA yielded a malonyl-enzyme covalent complex that most likely represents the acyl-enzyme intermediate. The structure explains the preference for malonyl-CoA with a conserved arginine orienting the carboxylate group of malonate and several nonpolar residues that preclude α-alkyl malonyl-CoA binding. Co-crystallization with acetyl-CoA revealed two noncovalently bound acetates generated by the enzymatic hydrolysis of acetyl-CoA that acts as an inhibitor for DynE8. This suggests that the AT domain can upload the acyl groups from either malonyl-CoA or acetyl-CoA onto the catalytic Ser651 residue. However, although the malonyl group can be transferred to the acyl carrier protein domain, transfer of the acetyl group to the acyl carrier protein domain is suppressed. Local structural differences may account for the different stability of the acyl-enzyme intermediates.
Introduction
As secondary metabolites produced by soil and marine microorganisms, fungi, and plants, polyketides form a structurally diverse family of natural products endowed with a variety of biological and pharmaceutical properties. Many polyketides are synthesized by type I PKSs3 that can be further classified into modular or iterative PKSs (1). Although modular type I PKSs assemble the polyketides using multiple modules, each composed of several catalytic domains, iterative type I PKSs synthesize their polyketide products iteratively using a single module (2). In contrast to the large body of knowledge accumulated for modular PKSs in the last two decades, much remains to be learned about the catalytic mechanism of iterative PKSs. Particularly, despite sharing the same repertoire of catalytic domains with modular type I PKSs, many iterative type I PKSs feature highly unusual programmed reduction, dehydration, or methylation during the chain extension processes, possibly because of some undisclosed properties of their catalytic domains.
Enediyne natural products are among the most potent antitumor and antibiotic agents ever discovered (3–5). Their remarkable antitumor properties derive from the ability of the enediyne “warhead” to induce cell apoptosis through chromosomal DNA cleavage via an oxidative radical mechanism. Recent studies revealed that enediyne biosynthetic pathways involve an iterative type I PKS that initializes the biosynthesis of the nine- or ten-membered bicyclic enediyne warhead (6–8). The PKSs responsible for the biosynthesis of several naturally occurring enediynes have been characterized by in vitro and in vivo studies (7–12). In the presence of the hot dog fold thioesterase DynE7, the enediyne PKS DynE8 produces several linear conjugated polyene products (13). Enediyne PKSs catalyze the synthesis of the linear polyenes through iterative cycles of condensation, ketoreduction, and dehydration (Fig. 1a). Based on amino acid sequence alignment and experimental evidence, the enediyne PKSs, such as CalE8, SgcE8, or DynE8, possess a domain organization that is different from known PKSs (6–8, 11). In addition to the essential ketoacyl synthase (KS), acyltransferase (AT), and acyl carrier protein (ACP) domains required for polyketide chain elongation, the processing ketoreductase and dehydratase domains were also identified. A C-terminal 4′-phosphopantetheinyl transferase domain was also documented for the addition of the phosphopantetheinyl moiety onto the ACP domain of the enediyne PKS (see Fig. 1a) (7, 8, 11, 14). The NMR structure of the ACP domain of CalE8 has revealed some interesting features of the shuttling domain (15) and crystal structures of the thioesterases DynE7 and CalE7 responsible for off loading the conjugated polyenes synthesized by CalE8 and DynE8, respectively, were also reported (16, 17). Thus, determination of the three-dimensional structures of the other catalytic domains (KS, AT, ketoreductase, and dehydratase) of the enediyne PKSs should substantially enhance our understanding of the mechanism governing enediyne biosynthesis and perhaps open up possibilities for rational alterations of the warhead.
FIGURE 1.
The enediyne polyketide biosynthesis pathway and domain composition of DynE8. a, simplified diagram of the enediyne polyketide biosynthetic pathway. KR, ketoreductase, DH, dehydratase; PPTase, phosphopantetheinyl transferase. b, construction and expression of truncated AT fragments of DynE8. Three of twelve recombinant proteins could be purified (green bars). Orange and red bars indicate low and no soluble protein expression, respectively. The boundaries of the KS-ATDYN linker domain are indicated by a gray box. The construct that was crystallized is ATDYN10.
For chain elongation, the AT domains of enediyne PKSs catalyze the transfer of the malonyl group from malonyl-CoA to the ACP domain (see Fig. 1a). Interestingly, unlike the AT domain of other iterative PKSs, the AT domains of the enediyne PKSs do not seem to use acetyl-CoA as a starter unit but solely malonyl-CoA. Here we present a high resolution crystal structure of the AT catalytic domain and of the adjacent KS-AT linker domain of DynE8 from the dynemicin biosynthetic pathway. This fragment of DynE8 is subsequently referred to as ATDYN10. In addition to the unbound structure, we also determined the structure of the malonyl-enzyme covalent complex and the structure of the enzyme in complex with acetate and glycerol. To our knowledge, this is the first crystal structure for an AT domain from an iterative PKS. The crystal structures provide insight into the evolutionary relationship between the AT domain of an iterative PKS and the AT domains of fatty acid synthase (FAS) and modular PKSs. Structural determinants of the substrate preference for malonyl-CoA are unveiled. Moreover, structural and biochemical data suggest that although ATDYN10 does not use acetyl-CoA as starter unit, ATDYN10 is able to upload the acetyl group of acetyl-CoA to form an acetyl-enzyme covalent complex that is susceptible to hydrolysis. Comparison of the structures of ATDYN10 with the acetyl- and malonyl-specific AT domain of mammalian FAS reveals structural differences in the active sites of the two AT domains that may account for the different fate of the acetyl-enzyme intermediate.
EXPERIMENTAL PROCEDURES
Reagents and Chemicals
CoA, Malonyl-CoA, acetyl-CoA, NADPH, and all other chemicals were purchased from Sigma-Aldrich and stored at −20 °C. Selenomethionine base medium was purchased from Molecular Dimensions. Crystallization screening buffers were purchased from Hampton Research.
Construction, Expression, and Purification of the ATDYN10 Fragment
Following gene annotations by Zazopoulos et al. (6), a construct encompassing residues 473–893 of DynE8 (hereafter named ATDYN10; see Fig. 1b for its subdomain composition) from Micromonospora chersina was cloned into to pNIC28-Bsa4 (GenBankTM accession number EF198106), resulting in a construct with an N-terminal hexahistidine tag and a TEV protease recognition site. The positive recombinant clone was retransformed and expressed in T1 phage-resistant BL21-DE3 Rosetta strain. For expression, the cells were grown in a LEX system (18) using 0.75 liter of Terrific Broth medium supplemented with 8 g/liter glycerol, 50 μg/ml kanamycin, and 34 μg/ml chloramphenicol. The cells were incubated at 37 °C and at A600 ∼2, the temperature was reduced to 18 °C. After 30–60 min, the expression of the target protein was induced by the addition of 0.5 mm isopropyl β-d-thiogalactopyranoside for 17–20 h. The cells were harvested by centrifugation and resuspended in lysis buffer (100 mm HEPES, 500 mm NaCl, 10 mm imidazole, 10% (v/v) glycerol, 0.5 mm TCEP, pH 8.0), supplemented with the Protease Inhibitor Mixture Set III, EDTA free (Calbiochem), and 2000 units of benzonase (Merck), and stored at −80 °C. Cell disruption was performed by sonication on ice (using Sonics Vibra cell at 70% amplitude, 3 s on/off for 3 min). The lysate was clarified by centrifugation at 47,000 × g for 25 min at 4 °C, and the supernatant was filtered through a 1.2-μm syringe filter. Filtered lysates were loaded onto 1 ml of nickel-nitrilotriacetic acid Superflow (Qiagen) in IMAC wash buffer 1 (20 mm HEPES, 500 mm NaCl, 10 mm imidazole, 10% (v/v) glycerol, 0.5 mm TCEP, pH 7.5) and subsequently washed with IMAC wash buffer 2 (20 mm HEPES, 500 mm NaCl, 25 mm imidazole, 10% (v/v) glycerol, 0.5 mm TCEP, pH 7.5). Bound proteins were eluted with 500 mm imidazole and loaded onto a HiLoad 16/60 Superdex-200 column (GE Healthcare) pre-equilibrated with 20 mm HEPES, 300 mm NaCl, 10% (v/v) glycerol, 0.5 mm TCEP, pH 7.5. Fractions containing ATDYN10 were pooled. TCEP was added to a final concentration of 2 mm, and the sample was concentrated in Vivaspin 20 filter concentrators (15-kDa molecular mass cutoff) at 15 °C. The final protein concentration was 30 mg/ml in a volume of 0.7 ml. The protein batch was then aliquoted, frozen in liquid nitrogen, and stored at −80 °C.
Preparation of Selenomethyonyl ATDYN10
The pNIC28-Bsa4-ATDYN10 plasmid was transformed into Escherichia coli B834 (DE3) (Novagen). Upon reaching an A600 of 0.4, the cells grown at 310 K in Luria Bertani medium supplemented with 50 μg/ml kanamycin were harvested by centrifugation and resuspended in selenomethionine base medium (Molecular Dimensions). The washing step was repeated, and the cells were inoculated into 2 liters of preaerated, prewarmed selenomethionine expression medium supplemented with 40 mg/liter of l-selenomethionine and 50 μg/ml kanamycin. At an A600 of 0.6, the culture was cooled to 18 °C, and protein expression was induced with 0.1 mm isopropyl β-d-thiogalactopyranoside. Protein purification was carried out as described above for the free enzyme.
Crystallization and Data Collection of Native, Substrate-Enzyme Complexes, and Selenomethyonyl Crystals
Crystals of free ATDYN10 were obtained after screening 672 crystallization conditions (Hampton Research), using the CyBio®-Crystal Creator robot (Jena Biosciences). Native ATDYN10 crystals were grown in sitting drops comprising equal volumes of protein solution (10 mg/ml) and precipitant solution (0.1 m Tris-HCl and 30% PEG 6000, pH 8.5). Crystals of malonate- and acetate-bound complexes were grown in an analogous manner with prior addition of 5 mm malonyl-CoA and acetyl-CoA, respectively, to the protein solution. The crystals of the substrate-enzyme complex were obtained at a temperature of 12 °C. After 48 h of incubation of the co-crystallization mixture, these crystals were flash frozen in liquid nitrogen following a short transfer to the precipitant solution supplemented with 25% (v/v) glycerol. The crystals of the selenomethyonyl protein were obtained in the same conditions as the wild-type enzyme. Before data collection, the crystals were transferred to a cryoprotecting solution containing the precipitating solution supplemented with 25% (v/v) glycerol and cooled to 100 K in liquid N2. Diffraction intensities were collected at the Swiss Light Source Beamlines PXI and PXIII, using a Dectris Pilatus 6M detector, to a resolution of 1.40 Å for the free enzyme, to 1.65 Å for the covalent malonate-enzyme complex, to 1.90 Å for the enzyme bound to acetate, to 1.5 Å for a complex with glycerol, and to 2.5 Å for the selenium data set. Integration, scaling, and merging of the intensities were carried out using programs MOSFLM and SCALA from the CCP4 suite (19). The data collection statistics are summarized in Table 1.
TABLE 1.
Data collection, phasing, and refinement statistics
| ATDYN10 | Selenomethionine derivative | ATDYN10-glycerol complex | ATDYN10-acetate complex | ATDYN10-malonate complex | |
|---|---|---|---|---|---|
| Data collection | |||||
| X-ray source | PXI, SLS | PXIII, SLS | PXIII, SLS | PXI, SLS | PXI, SLS |
| Wavelength (Å) | 1.000 | 0.9791 | 1.072 | 1.072 | 1.072 |
| Crystallographic parameters | |||||
| Space group | P212121 | P212121 | P212121 | P212121 | P212121 |
| Unit cell dimensions (Å) | 65.5, 66.1, 85.4 | 65.9, 67.3, 85.2 | 65.8, 66.4, 85.5 | 66.5, 68.4, 85.7 | 65.7, 66.2, 86.1 |
| a, b, c (Å); α, β, γ (°) | 90°, 90°, 90° | 90°, 90°, 90° | 90°, 90°, 90° | 90°, 90°, 90° | 90°, 90°, 90° |
| Phasing | |||||
| Rcullis (acentric)a | 0.960 | ||||
| Phasing power (acentric)b | 0.436 | ||||
| Acentric/centric | 0.115/0.034 | ||||
| Number of selenium | 5 | ||||
| Data collection statisticsc | |||||
| Resolution limit (Å) | 23.27–1.40 (1.48–1.40) | 48.14–2.48 (2.57–2.48) | 18.78–1.50 (1.58–1.50) | 29.66–1.90 (2.00–1.90) | 26.33–1.65 (1.74–1.65) |
| No. of observed reflections | 311,060 | 40,354 | 239,417 | 308,772 | 214,889 |
| No. of unique reflections | 72,439 | 8,016 | 54,690 | 30,505 | 45,596 |
| Completeness (%) | 98.6 (95.5) | 99.9 (100) | 91.0 (96.5) | 100 (100) | 99.4 (99.5) |
| Redundancy | 4.3 (3.3) | 5.0 (5.3) | 4.4 (4.4) | 10.2 (10.2) | 4.7 (4.8) |
| Average I/σ(I) | 9.7 (2.5) | 11.6 (4.9) | 11.0 (3.5) | 20.5 (5.1) | 11.1 (2.8) |
| Rmerge (%) | 0.081 (0.442) | 0.105 (0.361) | 0.069 (0.352) | 0.083 (0.430) | 0.071 (0.521) |
| Refinement | |||||
| R factor (Rwork/Rfree) (%) | 17.4/20.0 | 20.1/24.0 | 17.7/22.0 | 17.4/21.0 | |
| Model contents/average B (Å2) | |||||
| Protein atoms | 2767/20.1 | 2760/16.6 | 2677/27.5 | 2711/23.5 | |
| Water molecules | 331/31.5 | 361/30.0 | 190/36.2 | 302/36.7 | |
| Ligand atoms | 1/18.3 (chloride) | 6/21.8 (glycerol 1) | 4/47.1 (acetate 1) | 6/38.0 (malonate) | |
| 6/40.1 (glycerol 2) | 4/60.0 (acetate 2) | 6/33.2 (glycerol) | |||
| 1/14.9 (chloride) | 1/29.0 (chloride) | ||||
| Root mean square deviation | |||||
| Distances (Å) | 0.027 | 0.024 | 0.023 | 0.028 | |
| Bond angles (°) | 2.2 | 2.1 | 1.9 | 2.3 | |
| Estimated coordinate error (Å) | 0.015 | 0.016 | 0.017 | 0.021 | |
| Ramachandran plotd | |||||
| Favored | 97.9 | 98.2 | 97.3 | 98.6 | |
| Allowed | 2.1 | 1.8 | 2.7 | 1.4 | |
| Outlier | 0 | 0 | 0 | 0 | |
a Rcullis is calculated as (Σ|E|Σ||FPH| − |FP||), where FPH is the amplitude of the protein plus the heavy atom, and FP is the amplitude of the protein.
b Phasing power is calculated as the root mean square (|Fh|/E), where |Fh| is the heavy atom structure-factor amplitude, and E is the residual lack of closure error.
c Statistics for the highest resolution shell are shown in parentheses.
d As defined by PROCHECK.
Structure Determination and Refinement
The structure was determined using a combination of the molecular replacement and single anomalous diffraction (SAD) techniques with data recorded at the selenium absorption edge from the selenomethionine-substituted protein. A partial model of ATDYN10 comprising 223 amino acids was obtained using program MOLREP and the SpnMCAT (20) structure from Streptococcus pneumoniae (Protein Data Bank code 3IM8) as a search probe. Phases calculated from this partial model were used to locate four selenium sites (of a total of five selenium per asymmetric unit) using an anomalous Fourier map to 5 Å resolution at the selenium edge. The position of the four peaks in the anomalous Fourier map matched the location of Met680 (Se1), Met690 (Se2), Met757 (Se3), and Met828 (Se4) of the ATDYN10 partial model and were also in agreement with the selenium positions generated by SHELXD (21), giving confidence in this partial solution from molecular replacement. The program SHARP (22) was then used to refine the four selenium positions and calculate initial SAD phases, and the residual map yielded the fifth selenium position corresponding to Met853. A new set of SAD phases was calculated with an overall figure of merit of 0.12–2.5 Å resolution. The program REFMAC (19) was then used to refine the partial model using the maximum likelihood target and the SAD phase distribution calculated using program SHARP (22), encoded as Hendrickson-Lattman coefficients. After several rounds of manual and automatic iterative model building using BUCANNEER (19) and the graphic display program COOT (23) followed by refinement with REFMAC (19), the updated partial model could be successfully fed into program Arp/Warp (24), which delivered a much more complete model with an Rfree of 26.3%, using native data to 1.4 Å resolution. The connectivity of this model was modified manually using COOT (23). For each complex, a difference Fourier synthesis map revealed residual electron density in the active site corresponding to the bound ligand. Statistics for the binary complexes are summarized in Table 1. Electron density maps in the ATDYN10 active site are displayed in supplemental Fig. S1. The quality of final models were assessed with PROCHECK (25). All of the structural graphics were generated using PyMOL (49).
Modeling of the ATDYN10-ACPDYN Complex
A model for the ACP domain of DynE8 (ACPDYN) was generated by homology using the iterative threading assembly refinement (I-TASSER) server (26, 27). Macromolecular docking calculations for ATDYN10 and ACPDYN were performed with ClusPro (28, 29). In the simulation, the orientation of ATDYN10 was kept fixed, whereas ACPDYN, as ligand, was allowed to rotate and translate. A total of 57 conformations with lower energies and larger cluster sizes were generated based on the multistage protocol that includes rigid body docking, energy-based filtering, clustering properties ranking, and energy minimization refinement. The model for the ATDYN10-ACPDYN complex was selected based on the lowest energy and largest cluster size criteria as well as comparisons with previously reported AT/ACP complexes.
Enzymatic Assay and Product Analysis by HPLC
All of the enzymatic reactions were performed in a final volume of 15 μl. Each enzymatic reaction was measured at different time points of incubation. A reaction mixture includes 2.38 μl of ATDYN10 (630 mm), 3 μl of acetyl-CoA (50 mm), and 9.62 μl of reaction buffer (100 mm HEPES, pH 8.5, and 100 mm NaCl). The reaction mixtures were incubated at 30 °C for 1, 2, 9, and 16 h. Each sample was boiled and spun down before application onto an analytical eclipse XDB C18 column (4.6 × 250 mm) using an Agilent 1200 HPLC. A 40-min linear gradient was used starting with 100% buffer A (HPLC grade water with 0.045% trifluoroacetic acid) to 40% buffer B (acetonitrile with 0.045% trifluoroacetic acid). The standards, acetyl-CoA and CoA, were diluted to 10 mm with the reaction buffer and incubated at 30 °C for 16 h prior to application onto a XDB C18 column. The diode array detector was set at 254 nm with a reference wavelength of 600 nm.
Acetyl-CoA Inhibition Assay
The co-purified DynE8 and thioesterase DynE7 prepared from a co-expression system (17) were used for the inhibition assay. The assays were carried out with 1.12 μm DynE8, 10.5 μm DynE7, 0.25 mm NADPH, 2.5 mm malonyl-CoA, and various concentrations of acetyl-CoA (1.0, 2.0, 2.5, and 5.0 mm) in a total volume of 200 μl of buffer (25 mm HEPES, pH 8.0, 300 mm NaCl, 1 mm DTT) at 30 °C. The reaction mixture without acyl-CoA was equilibrated at 30 °C for 10 min in the temperature-controlled sample chamber of Shimadzu UV-visible 1700 spectrometer. Enzymatic reaction was initiated by the addition of malonyl- or acetyl-CoA. A full wavelength spectrum scan was performed at 2-min intervals throughout the course of the experiment. Positive control with 2.5 mm malonyl-CoA as a substrate was used for the comparison.
Accession Numbers
The refined coordinates and structure factor amplitudes have been deposited with the Protein Data Bank with accession code 4AMP for the malonate-enzyme complex, 4AMO for the acetate-enzyme complex, 4AMN for the glycerol-enzyme complex, and 4AMM for the unliganded enzyme.
RESULTS
Cloning and Expression of the ATDYN10 Domain
We cloned and overexpressed a soluble fragment (ATDYN10 residues 473–893) comprising the predicted acyltransferase domain of the enediyne PKS DynE8 (1905 residues) from M. chersina (Fig. 1b). Construct boundaries were assigned based on annotations of the enediyne PKS biosynthesis genes reported by Thorson and co-workers (6, 7, 11) and sequence comparisons with other structurally characterized AT domains. The segment flanked by the KS and AT catalytic domain (named “KS-ATDYN linker domain”; Fig. 2) and the helix immediately downstream of the AT catalytic domain were retained because of their possible importance for the recognition of the ACPDYN domain of DynE8 and for acyl transfer (30–34). The KS-ATDYN linker domain appears critical for solubility because constructs (ATDYN1- ATDYN3; Fig. 1b) that lack the KS-ATDYN linker domain yielded insoluble proteins. Similar results were observed for constructs (ATDYN6- ATDYN9) that include a partially truncated KS-ATDYN linker domain. Three fragments with N termini at residues 473, 469, and 465, respectively, (named ATDYN10–ATDYN12; Fig. 1b) yielded soluble proteins that could be purified and concentrated to ∼30 mg/ml. These results demonstrate the crucial role played by residues 473–480 for the stability and solubility of the recombinant protein. The construct that spans residues 473–893 (named ATDYN10) was used for crystallization and structure determination.
FIGURE 2.
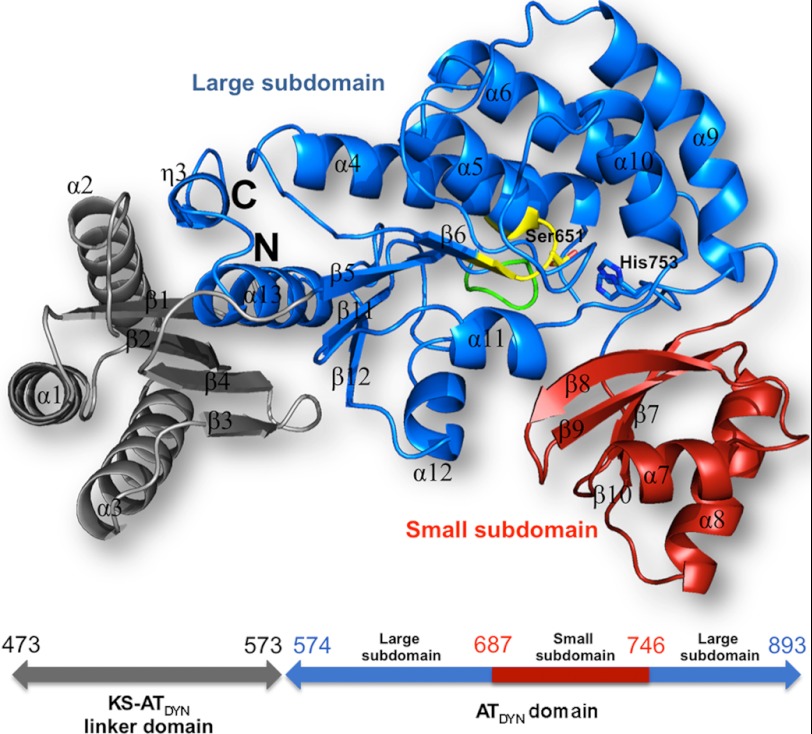
Overall structure of ATDYN10. ATDYN10 is composed of a KS-ATDYN linker domain (gray) and the catalytic acyltransferase ATDYN domain. The catalytic ATDYN domain (in ribbon representation) consists of large hydrolase subdomain (blue) and a ferrodoxin-like smaller subdomain (red). The N and C termini and the secondary structure elements of ATDYN10 are labeled. The Ser651–His753 catalytic residues are shown as yellow and blue sticks, respectively. Residues from the GHSXG and PGQGXQ motifs are colored yellow and green, respectively.
Crystal Structure Determination
The crystal structure of ATDYN10 (Fig. 2) was solved using a combination of molecular replacement and SAD at the selenium absorption edge using the seleniated protein (see “Experimental Procedures”). The structure was refined to Rwork of 17.4% and Rfree of 20.0% using data to a resolution of 1.4 Å (Table 1). Statistics for the structures of the binary complexes of the protein fragment with glycerol, acetate, and malonate are given in Table 1. The ligands are well defined, and examples of electron density maps are shown in supplemental Fig. S1.
One ATDYN10 protein is present in the asymmetric unit, and the final model comprises residues 476–594 and 612–876 of the DynE8 protein. Because of poor electron density in this region, residues 595–611 are absent from the model. This region that corresponds to a helical flap in homologous AT proteins is presumably flexible in the absence of other DynE8 domains. However, the N-terminal region of the helical flap adopts two markedly different orientations, suggesting that the positioning of the helical flap differs between the free enzyme and substrate-enzyme complexes (supplemental Fig. S2) The overall structure of ATDYN10 is consistent with the important role played by the KS-ATDYN linker domain for protein solubility: close packing at the interface between the linker domain and the larger subdomain of ATDYN10 shields nonpolar surfaces and is important to overall protein stability (see below).
Overall Structure of ATDYN10
The ATDYN10 protein fragment has approximate overall dimensions 46 Å × 61 Å × 62 Å and is composed of three globular α/β domains (Fig. 2). The AT catalytic domain (ATDYN) folds into a large α/β hydrolase subdomain (residues 574–686 and 747–876) composed of a four-stranded parallel β-sheet, eight α-helices, and the short left-handed C-terminal helix η3. The small subdomain of ATDYN (residues 687–746) adopts a ferredoxin-like fold comprising a four-stranded anti-parallel β-sheet and two α-helices (Fig. 2). The fold of ATDYN is similar to several structurally characterized AT domains or individual AT proteins with an average amino acid sequence identity of ∼30%. A structure-based sequence alignment of ATDYN with various AT catalytic domains is shown in supplemental Fig. S3. Structure comparison with AT catalytic domains from type I mammalian FAS (Protein Data Bank code 2VZ9) and discrete type II AT domains such as the malonyl CoA:ACP transferases from Streptomyces coelicolor (Protein Data Bank code 1NM2) and E. coli (Protein Data Bank code 2G2Z) suggests that ATDYN bears closer similarity with type I AT domains. Noticeable differences are found in the length of the C-terminal α-helix α13 and η3 of ATDYN that are two turns longer.
An automatic search for structurally homologous proteins using the Dali server (35) shows that ATDYN10 shares closely related tertiary structures with type I modular PKS deoxyerythronolide B synthase (DEBS) module 5 (Protein Data Bank code 2HG4, z score of 35.2), despite the fact that ATDYN10 and the corresponding region of DEBS module 3 or 5 only share ∼25% sequence identity (supplemental Fig. S4). The superimposition of ATDYN10 with the corresponding domains of DEBS module 5 is displayed in Fig. 3a. This superimposition yields a root mean square deviation of 3.7 Å for 347 equivalent residues. In the complete DynE8 protein, the C-terminal residue of ATDYN10 (position 876) makes the connection to the N-terminal residue of the ACPDYN domain (position 925) via a linker region that was not included in the present protein fragment. The corresponding linker region adopts an extended conformation in the DEBS protein (30).
FIGURE 3.
a, structure superposition of ATDYN10 and [KS5][AT5] (Protein Data Bank code 2HG4). The helical flap of the [AT5] domain, which is absent in ATDYN10, is indicated with a dotted red circle. b, superposition of the linker domains from [KS5][AT5] and ATDYN10. The secondary structure elements of the ATDYN10 linker domain are labeled. The extra strand β4 (compared with [KS5][AT5], see text for details) is labeled in red.
In addition to the AT catalytic domain, the fragment of DynE8 that was crystallized contains an extra 10-kDa domain (residues 473–573) that connects the adjacent KSDYN domain to the N-terminal end of the AT catalytic domain and that is crucial for solubility (Figs. 1b and 2). The linker domain also adopts a α/β fold that abuts on the large ATDYN subdomain with a buried surface area of 2436 Å2 at the interface. The linker domain comprises four anti-parallel β strands and three α-helices running roughly parallel to each other. A search for similar protein structures also reveals the closest homology with the corresponding [KS5][AT5] linker domain in the DEBS protein (36), and a magnified view of their superimposition is displayed in Fig. 3b. Hence, the overall structure of the KS-AT linker domain of modular PKSs is conserved in iterative PKSs. Nevertheless, compared with the [KS5][AT5] linker domain, KS-ATDYN possesses the extra β-strand β4 at its C terminus that makes the connection to the AT domain (Figs. 2 and 3b). Compared with the [KS5][AT5] linker domain of the DEBS protein, KS-ATDYN has longer helices (spanning 14–17 residues) and shorter strands (4–5 residues). Interestingly, in the case of the DEBS protein, the linker regions were proposed to confer rigidity to the protein and to serve as anchoring sites to ACP (30). Given the overall structural homology, helices α1–α3 helix of KS-ATDYN may also interact with the neighboring KS domain in DynE8.
Active Site of ATDYN and Amino Acid Sequence Motifs
In agreement with previously characterized AT structures including the malonyl CoA:ACP acyltransferases from S. coelicolor and E. coli, and the AT domains of mammalian FAS and 6-DEBS, the active site of ATDYN is located in a deep cleft between its large and small subdomains (Fig. 2). Inspection of this cleft reveals the presence of several evolutionary conserved amino acid sequence motifs required for catalysis and substrate specificity. The essential Ser651 residue from the highly conserved GHSXG motif (GHSLG in ATDYN; motif b in supplemental Fig. S3) is located in a turn between strand β6 and helix α5 (Fig. 2). Two histidine residues: His650 and His753 can be seen in the ATDYN active site (Fig. 4a). In other characterized AT domains from FAS or PKS, the residue corresponding to His753 was suggested to act as a general base/acid catalyst in the acyl transfer reaction. Here, the catalytic role played by His753 is supported by the short distance of 2.7 Å between its Nϵ2 atom and the Oγ atom of Ser651. In contrast, the Nϵ2 atom of His650 is located 5 Å away from the Oγ atom of Ser651, excluding a direct role in catalysis for His650. Spatial conservation of the catalytic Ser-His dyad suggests that ATDYN uses a catalytic mechanism similar to other FAS and PKS AT domains, where transfer of the malonyl group from malonyl-CoA onto Ser651 occurs via a ping-pong kinetic bi bi mechanism, followed by the transfer of the malonate group to the shuttling ACP domain (Fig. 1a) (37, 38). In the malonyl acetyl transferase (MAT) FabD, the backbone amide groups of Gln11 and Leu93 form the oxyanion hole stabilizing the transition state. Previous work showed that the conformation of the PGQ11GXQ motif has a direct influence on the integrity of the oxyanion hole (38–40). In ATDYN, motif PGQGXQ is replaced by PGQ581AAP (labeled motif a in supplemental Fig. S3 and Fig. 2) that contains the conserved Gln581, and Leu652, the corresponding residue Leu652 from motif b, is also conserved. Moreover, it was proposed that the ZTXX[AT][QE] motif (where Z is a hydrophilic residue and X is hydrophobic) located 30 residues upstream of the essential Ser determines the enzyme specificity for malonyl-CoA (41). In ATDYN, the corresponding motif (DTAVAQ623) is present with Gln623 hydrogen-bonded to the highly conserved Arg676 residue. Finally, a HAFH753 motif is also found in ATDYN (supplemental Fig. S3), which further supports the correlation between malonyl-CoA specificity and the presence of the [HTVY]AFH motif (41).
FIGURE 4.
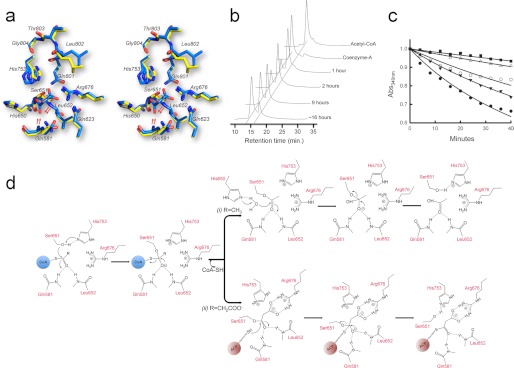
The catalytic pocket of the unbound ATDYN domain and binary complexes with several ligands. a, the active site of the free enzyme. b–d, covalent complex with malonate (MLA, b), with acetate (ACY, c), and with glycerol (GLY, d). The left panels show cross-sections through the electrostatic potential surface of the catalytic pocket (positive potential, blue; negative potential, red at the 10 kT e−1 level). The right panels show omit Fo − Fc difference electron density maps contoured at a level of 3.0 σ, where the ligand has been omitted from phase calculation. In each case, the hydrogen bond network stabilizing the ligand is indicated as dotted lines. Water molecules (red spheres) are labeled W. The views are in the same orientation. The stereo views were generated using program PyMOL (49).
Enzyme-Malonate Covalent Complex
ATDYN catalyzes the transfer of the malonate group from malonyl-CoA onto ACPDYN. According to studies on the AT domains of FAS and modular PKSs, catalysis is likely to proceed through a covalent intermediate with the malonate group attached to Ser651. By co-crystallizing ATDYN10 with malonyl-CoA, the malonyl group was transferred onto the AT domain yielding the structure of the enzyme-malonate covalent complex (Fig. 4b). The crystal structure of this complex was refined to Rwork and Rfree values of 17.4 and 21.0%, respectively (Table 1). After refinement, the distance between the Cβ atom of the Ser651 side chain and the oxygen of the malonate moiety is 1.4 Å, suggesting formation of a covalent complex that represents the acyl-enzyme intermediate of the transacylation reaction. A bidendate salt bridge is formed between the carboxylate of the malonate and the guanidinium group of Arg676 (Fig. 4b). The oxygen atoms of the malonate moiety are anchored through a water-mediated hydrogen bond with the Nδ1 atoms of the surrounding His650 and His753 and with the amide nitrogen of Gln581 (Fig. 4b). The structure of the enzyme-malonate covalent complex also reveals the presence of a Phe residue in the active site that is likely to play a key role in discriminating against methylmalonyl-CoA as a substrate. The bulky side chain of Phe752 is in direct contact with the methylene carbon of the covalently tethered malonate (Fig. 5). The close distance excludes the possibility to accommodate methylmalonate in a similar position in the active site.
FIGURE 5.
a, cross-sections of the AT mFAS and ATDYN catalytic pocket. The electrostatic potential surface represents positive potential in blue, and negative potential in red at the 10 kT e−1 level. The catalytic pocket of the AT domain of mFAS (left panel) is indicated by a red asterisk, whereas the catalytic pocket of ATDYN is occupied by malonate, covalently linked to Ser651 (right panel). b, overlay of ATDYN10-malonate adduct with AT mammalian FAS. Only residues of ATDYN are labeled. The malonate moiety is covalently attached to residue Ser651 of ATDYN. Residues Met680 and Phe752 of ATDYN would hinder binding of a bulkier substrate (e.g. methylmalonyl-CoA) with substituents projecting from the α-carbon. The active site of mammalian FAS is more hydrophobic compared with ATDYN and other AT because of the substitution of Gln581 and Gln623 by Met499 and Phe553, respectively. The stereo view was generated using PyMOL (49).
Comparison of the free enzyme and malonate-enzyme structures (Figs. 4, a and b, and 6a) reveals several subtle conformational changes in the active site upon formation of the Ser652-malonate linkage. Following malonate attachment, the rotamer conformation of Ser652 changes (Fig. 6a). In the free enzyme structure, the Nδ1 atom of His753 and the Oγ atom of Ser651 are hydrogen-bonded. This is in agreement with the role of His753 as a general base catalyst to activate Ser651 for nucleophilic attack of the thioester carbonyl of malonyl-CoA. In the enzyme-malonate complex structure, the imidazole ring of His753 is reoriented, and the surrounding hydrogen bond network is altered. The active site pocket also expands slightly following the reorientation of the His753 and Leu802. The transfer of the malonate moiety from malonyl-CoA to the enzyme and its subsequent transfer from the enzyme to the ACP domain is believed to proceed through tetrahedral transition states and intermediates. An oxyanion hole formed by two main chain amide groups was proposed to play an essential role in stabilizing these transition states and intermediates (38). The hydrogen bond network formed between malonate and nearby residues and structural comparison with FabD (Protein Data Bank: 1MLA) points to the amide groups of Leu652 and Gln581 to constitute the oxyanion hole in ATDYN. In the malonyl-enzyme structure, the Gln581 shifts toward the Leu652 amide, and the distance between the two amides is decreased from a value of 5.5 Å (unliganded structure) to 5.2 Å (malonyl complex) (Fig. 6a).
FIGURE 6.
The active site of ATDYN and the catalytic mechanism for acyltransfer. a, stereo view of a superposition of the active site for the free enzyme (yellow sticks) and malonate-bound ATDYN (blue sticks). Residues His753 and Ser651 form the catalytic dyad. Compared with their positions in the unliganded structure, the backbone amides of residues Gln581 and Leu652 are shifted by about 3 Å to shape the oxyanion hole (the movement is indicated by red arrows). Note that the malonate-bound Ser651 rotates downwards by about 30° (also indicated by red arrows). b, HPLC analysis of ATDYN-catalyzed hydrolysis of acetyl-CoA. The HPLC chromatograms show the depletion of the acetyl-CoA substrate along with the increment of the CoA by-product. c, substrate competition inhibition assay. The absorption spectra of NADPH is monitored using UV-visible spectrophotometry at an absorbance of 340 nm. The positive control (black circles), with only 2.5 mm malonyl-CoA as substrate, shows depletion of NADPH, whereas the reaction with the addition of 1.0 mm (black triangles), 2.0 mm (white circles), 2.5 mm (white triangles), and 5.0 mm (black square) acetyl-CoA shows slower depletion of NADPH with increasing concentrations of acetyl-CoA. d, proposed events following post-catalytic mechanism for acetyl- and malonyl-enzyme intermediate. i, acetyl-ATDYN intermediate: His650 deprotonate the adjacent water molecule and subsequently hydrolyzes the covalent bond of acetyl-Ser651. ii, malonate-ATDYN intermediate. A phosphopantetheined-ACP domain extracts malonate by breaking the malonyl-Ser651 covalent bond.
Enzyme-Acetate Complex
We previously showed that DynE8 and other enediyne PKSs do not use acetyl-CoA to generate the acetate starter unit (13, 42). Instead, the starter acetate unit of the enediyne PKS products is derived from malonyl-CoA through an intrinsic decarboxylation mechanism. This is in contrast to the AT domain of the mammalian FAS that exhibits dual specificity for malonyl- and acetyl-CoA. It was hypothesized that the inability of enediyne PKSs to utilize acetyl-CoA derives from their AT domains that bind acetyl-CoA in an unproductive configuration that prohibits acyl transfer. To understand the molecular basis for substrate discrimination, we co-crystallized ATDYN10 with acetyl-CoA (Fig. 4c). Intriguingly, co-crystallization of ATDYN10 with acetyl-CoA yielded a noncovalent enzyme-acetate complex (1.9 Å resolution) (Fig. 4c). The electron density observed near Ser651 rules out the formation of an acetyl-enzyme complex. Instead, two noncovalently bound acetates ACY1 and ACY2 are observed with average temperature factors of 47 and 60 Å2, respectively, although the average temperature factor for the protein is 28 Å2 (supplemental Fig. S1b). ACY1 is bound in the active site, with its carboxylic end anchored by two hydrogen bonds with Arg676 and the Nϵ2 atom of the side chain of Gln581 (Fig. 4c). A weak hydrogen bond is also formed with the backbone amide group of Leu652. This mode of interaction, where the acetate moiety mimics interactions formed by the carboxylic end of the malonyl group, is similar to the ScMAT-acetate complex (38, 43). However, the orientation of ACY1 in ATDYN differs slightly from the one adopted in ScMAT because ACY1 is positioned parallel to Ser651 and almost perpendicular to the Arg676 guanidino plane. Upon ACY1 binding, an alternate conformation of Ser651 side chain is observed with a change in chi angle of 104°. Based on the electron density map, however, we cannot rule out an alternative binding mode for ACY1 with its carboxylate group hydrogen-bonded to the Oγ atom of Ser651 and backbone amide group of Gln581. The second acetate ACY2 is located in the immediate vicinity of the AT active site in a pocket formed by His650, Asn715, Gly580, Gln581, and Ala582 (supplemental Fig. S1b). ACY2 is stacked against the imidazole ring of His650 and is stabilized through a weak hydrogen bond with the carbonyl oxygen of Gln581 and a water-mediated hydrogen bond with Asn715. ACY2 is found in a position equivalent to the second acetate bound to ScMAT, demonstrating the similarity between the two active sites.
Enzyme-Glycerol Complex
A native ATDYN10 crystal soaked with 25% glycerol as cryoprotectant clearly showed two bound glycerol molecules. One glycerol molecule, with a temperature factor of 21.8 Å2, is located in the active site (Fig. 4d). Its oxygen atoms make hydrogen bonds with the side chains of Ser651, Arg676 and with His650 via a bridging water molecule. Comparison with the malonyl-enzyme covalent complex reveals that one hydroxyl group overlaps with the oxygen atom of the thioester carbonyl group. The hydroxyl group also forms hydrogen bonds with the main chain amide groups of Leu652 and Gln581 that form the oxyanion hole. A second glycerol molecule, with a temperature factor of 40.0 Å2, is found at the surface of the large ATDYN subdomain next to loop α4 -α6 (supplemental Fig. S5).
ATDYN10 Is Able to Hydrolyze the Thioester Bond of Acetyl-CoA
The surprising observation of acetate ligands in the ATDYN10 crystal structure raised the question of how they were generated from acetyl-CoA during the crystallization process. To distinguish between an enzyme or a buffer catalyzed hydrolysis mechanism, we performed enzymatic assays with acetyl-CoA. This experiment showed that ATDYN is able to hydrolyze the thioester bond of acetyl-CoA to produce CoA and acetate (Fig. 6b), whereas under the same conditions, acetyl-CoA remains intact in the absence of enzyme. This shows that the acetate moieties seen in the crystal structure are generated by enzyme-catalyzed hydrolysis of acetyl-CoA. Further support for the idea that ATDYN can accept acetyl-CoA as substrate comes from competitive inhibition experiments using the full-length DynE8 protein. We found that the addition of acetyl-CoA to the reaction mixture can significantly slow down the PKS reaction in a concentration-dependent fashion (Fig. 6c), indicating that acetyl-CoA is competing with malonyl-CoA for the substrate-binding pocket. The possible mechanisms for the trans-acylation and hydrolysis reactions are depicted in Fig. 6d. In contrast to the previous belief, this mechanism indicates that ATDYN can also use acetyl-CoA as a substrate by transferring the acetyl moiety onto Ser651. However, unlike the malonyl moiety that stays attached to Ser651 and is later transferred to the ACP domain, the acetyl moiety is likely to be removed from the protein by hydrolysis.
ACP Docking Site of ATDYN10
In fatty acid and polyketide synthesis, specific protein interaction between the ACP and the AT domains are critical for the efficient transfer of acyl groups (38, 44). Considerable efforts have been devoted to identify the structural factors influencing the interaction between ACP and AT in FAS and modular PKSs (38, 44, 45), whereas little information is available for iterative PKSs. We found that the weak and transient interaction between ATDYN10 and ACPDYN precluded crystallization of the complex. As an alternative strategy, we performed protein-protein docking simulations using the crystal structure of ATDYN10 and a model for ACPDYN generated using the highly similar meACP (15) domain structure (Protein Data Bank code 2L9F) as template and adding the phosphopantetheine group to Ser965. The resulting model resembles the [KS3][AT3]-ACP3 complex from DEBS (30). The ACP-tethered phosphopantetheine extends into the AT active site (Fig. 7a). The distance between the active site Ser651 of the ATDYN domain and the phosphopantetheine attachment site on ACPDYN is ∼18 Å, consistent with a fully extended phosphopantetheine arm (Fig. 7a). The ACPDYN protein makes contact with the KS-ATDYN linker domain and the larger ATDYN subdomain. Interestingly, this mode of interaction differs from the predicted HpMCAT-HpACP (44) and FabH-ACP (46) complexes, where the AT-ACP interactions mainly involve the larger and smaller AT subdomains. The predicted interface includes an area (area A; see Fig. 7b) from the larger subdomain located at the entrance of the substrate tunnel leading to the active site serine. Electrostatic interactions between the KS-AT linker domain (area B) and loops I and II of ACPDYN are probably also important for the interaction. Residues Asp477, Arg534, and Arg562 (area B) of KS-ATDYN are predicted to form salt bridges with Arg956, Asp960, and Glu945 on the surface of ACPDYN (Fig. 7b and supplemental Fig. S6 and supplemental Table S1). Finally, interactions between the helical flap of the larger subdomain of ATDYN (area C) with ACPDYN could also play a role for orienting the ACP domain.
FIGURE 7.
Docking model of ATDYN10 and ACPDYN. a, the ACPDYN homology model (colored in yellow), generated by I-TASSER (26, 27), was used as a ligand protein to dock with the unliganded ATDYN10 structure. The phosphopantetheine arm and malonate were modeled into the catalytic pocket of ATDYN. Distance between the Ser651 of the ATDYN and the Ser965 phosphopantetheine attachment site on ACPDYN is ∼18 Å. b, electrostatic surface maps of ACPDYN and ATDYN10 docking interfaces are generated with PyMOL (49). The colors range from blue (positive) to white to red (negative). Three main electrostatic surface contact areas with the interacting residues are labeled. A blue asterisk indicates the entrance of the catalytic pocket. Docked ACPDYN is rotated 180° from ATDYN10-ACPDYN interface such that annotations of A and B on ATDYN10 and ACPDYN interfaces should match up.
DISCUSSION
AT domains or discrete AT proteins are responsible for the loading of starter or extender units for FASs and modular and iterative PKSs. Extensive structural and biochemical studies have been conducted to understand the catalytic mechanism and substrate specificity of the AT domains of FASs and modular PKSs. The crystal structure of ATDYN10, which represents the first AT domain structure for an iterative PKSs, provides valuable information on the relationship between AT domains from the iterative PKS, FASs, and modular PKSs. Given the conserved KS-AT linker domain, the overall sequence homology, and similarities in the active site, ATDYN is most closely related with the malonyl-CoA-specific AT domains of modular PKSs.
Our previous studies have shown that the iterative PKSEs (CalE8, SgcE8, and DynE8) assemble their polyketide intermediates using the acetate units of malonyl-CoA alone (13, 16, 42). The crystal structure of ATDYN10 reveals the molecular basis for substrate preference, with several conserved key residues/motifs identified for malonate binding. Similar to the malonyl-specific AT domains from FAS and modular PKSs, the specificity toward malonyl-CoA is determined by several key residues as well as the properties of the active site. In addition to the catalytic residues Ser651 and His753, the other key residues contributing to substrate specificity include Arg676, Phe752, Gln581, Leu652, Met680, and Gln623. The key determinant Arg676 not only defines the depth of the binding pocket but also forms a salt bridge with the carboxylate of the malonyl group. The specificity for malonyl-CoA, but not α-substituted malonyl-CoA (e.g. methylmalonyl CoA), appears primarily achieved by the bulky residues Phe752 (from the HAFH motif) and Met680, as the latter would restrict the ability of Phe752 to move out of the way to accommodate the additional methyl group (Fig. 5b).
Both DynE8 and mammalian FAS assemble their products using a single set of catalytic domains iteratively, and they both contain an acetate starter unit in their final products. However, ATmFAS is known to be an acetyl- and malonyl-dual specific domain (47), whereas the ATDYN domain does not use acetyl-CoA as a substrate. The terminal acetate unit of the DynE8 product is derived from malonyl-CoA through an intrinsic decarboxylation mechanism. Previously, it was not known whether the decarboxylation is catalyzed by the KS domain or the AT domain. Observation of the malonyl-enzyme covalent complex confirms that the AT domain does not convert the malonyl group to acetyl group and that the decarboxylation is thus most likely catalyzed by the KS domain. Our structural and biochemical data also suggest that ATDYN can transfer the acetyl moiety of acetyl-CoA onto the enzyme (Fig. 6d). In contrast to ATmFAS, which subsequently transfers the acetyl moiety to the ACP domain, the acetyl group attached to ATDYN is likely to be off-loaded from the protein by a hydrolytic process. Hence, one of the key differences between these two AT domains is that although ATmFAS is able to protect both the acetyl- and malonyl-enzyme intermediate from hydrolysis, ATDYN only protects the malonyl-enzyme intermediate but not the acetyl-enzyme intermediate. Comparison of the two AT domains points to some subtle structural differences that may account for this different mode in acetyl transfer. Compared with ATmFAS, the active site of ATDYN is more spacious and hydrophilic with Gln581 and Gln623 replacing residues Met499 and Phe553 in ATmFAS (Fig. 5). As a result, the active site of ATDYN is more likely to contain water molecules to hydrolyze the acetyl-enzyme intermediate. In support of this model, a few water molecules are seen in the substrate-binding pocket of the free enzyme occupying the position of the malonate carboxylate in the malonyl-enzyme complex (Fig. 4a). In summary, the crystal structures reported here yield insight into the close evolutionary relationship with the malonyl-specific AT domains of modular PKSs and the acetyl/malonyl-specific AT domain of mammalian FAS. Observation of a malonyl-enzyme covalent complex settles some uncertainties in the enediyne biosynthetic mechanism by suggesting that the terminal acetate unit of the PKS products is likely to be generated by a KS domain-catalyzed decarboxylation. In contrast to the previous belief that the malonyl-specific AT domains of enediyne PKSs do not recognize acetyl-CoA, the current structural and biochemical data reveal the surprising uploading of both acetyl and malonyl groups to the malonyl-specific ATDYN domain. However, ATDYN only transfers malonyl group, but not the acetyl group, to the ACP domain by protecting the malonyl-enzyme intermediate from hydrolysis and facilitating the second malonyl transfer, presumably by activating the plastic oxyanion hole.
Supplementary Material
This work is supported by the Ministry of Education of Singapore through Academic Research Council grants (to Z.-X. L.), Biomedical Research Council grant 08/1/22/19/589, and an Action Thematique et Incitative sur Programme from the CNRS (to J. L.).

This article contains supplemental Tables S1 and Figs. S1–S6.
The atomic coordinates and structure factors (codes 4AMP and 4AMM) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- PKS
- polyketide synthase
- AT
- acyltransferase
- FAS
- fatty acid synthase
- ACP
- acyl carrier protein
- KS
- ketoacyl synthase
- SAD
- single anomalous diffraction
- DEBS
- deoxyerythronolide B synthase
- MAT
- malonyl acetyl transferase
- DynE8
- Dynemicin Polyketide Synthase
- CoA
- coenzyme A
- TCEP
- tris(2-carboxyethyl) phosphine
- IMAC
- immobilized metal ion affinity chromatography.
REFERENCES
- 1. Walsh C. T. (2004) Polyketide and nonribosomal peptide antibiotics. Modularity and versatility. Science 303, 1805–1810 [DOI] [PubMed] [Google Scholar]
- 2. Cox R. J. (2007) Polyketides, proteins and genes in fungi: programmed nano-machines begin to reveal their secrets. Org. Biomol. Chem. 5, 2010–2026 [DOI] [PubMed] [Google Scholar]
- 3. Liang Z. X. (2010) Complexity and simplicity in the biosynthesis of enediyne natural products. Nat. Product Reports 27, 499–528 [DOI] [PubMed] [Google Scholar]
- 4. Smith A. L., Nicolaou K. C. (1996) The enediyne antibiotics. J. Med. Chem. 39, 2103–2117 [DOI] [PubMed] [Google Scholar]
- 5. Shen B., Liu W., Nonaka K. (2005) Enediyne natural products: biosynthesis and prospect towards engineering novel antitumor agents. Front. Med. Chem. 2, 357–369 [DOI] [PubMed] [Google Scholar]
- 6. Zazopoulos E., Huang K., Staffa A., Liu W., Bachmann B. O., Nonaka K., Ahlert J., Thorson J. S., Shen B., Farnet C. M. (2003) A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat. Biotechnol. 21, 187–190 [DOI] [PubMed] [Google Scholar]
- 7. Ahlert J., Shepard E., Lomovskaya N., Zazopoulos E., Staffa A., Bachmann B. O., Huang K., Fonstein L., Czisny A., Whitwam R. E., Farnet C. M., Thorson J. S. (2002) The calicheamicin gene cluster and its iterative type I enediyne PKS. Science 297, 1173–1176 [DOI] [PubMed] [Google Scholar]
- 8. Liu W., Christenson S. D., Standage S., Shen B. (2002) Biosynthesis of the enediyne antitumor antibiotic C-1027. Science 297, 1170–1173 [DOI] [PubMed] [Google Scholar]
- 9. Liu W., Shen B. (2000) Genes for production of the enediyne antitumor antibiotic C-1027 in Streptomyces globisporus are clustered with the cagA gene that encodes the C-1027 apoprotein. Antimicrob. Agents Chemother. 44, 382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu W., Nonaka K., Nie L., Zhang J., Christenson S. D., Bae J., Van Lanen S. G., Zazopoulos E., Farnet C. M., Yang C. F., Shen B. (2005) The neocarzinostatin biosynthetic gene cluster from Streptomyces carzinostaticus ATCC 15944 involving two iterative type I polyketide synthases. Chem. Biol. 12, 293–302 [DOI] [PubMed] [Google Scholar]
- 11. Gao Q., Thorson J. S. (2008) The biosynthetic genes encoding for the production of the dynemicin enediyne core in Micromonospora chersina ATCC53710. FEMS Microbiol. Lett. 282, 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Lanen S. G., Oh T. J., Liu W., Wendt-Pienkowski E., Shen B. (2007) Characterization of the maduropeptin biosynthetic gene cluster from Actinomadura madurae ATCC 39144 supporting a unifying paradigm for enediyne biosynthesis. J. Am. Chem. Soc. 129, 13082–13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun H., Kong R., Zhu D., Lu M., Ji Q., Liew C. W., Lescar J., Zhong G., Liang Z. X. (2009) Products of the iterative polyketide synthases in 9- and 10-membered enediyne biosynthesis. Chem. Commun. (Camb.) 47, 7399–7401 [DOI] [PubMed] [Google Scholar]
- 14. Murugan E., Liang Z. X. (2008) Evidence for a novel phosphopantetheinyl transferase domain in the polyketide synthase for enediyne biosynthesis. FEBS Lett. 582, 1097–1103 [DOI] [PubMed] [Google Scholar]
- 15. Lim J., Kong R., Murugan E., Ho C. L., Liang Z. X., Yang D. (2011) Solution structures of the acyl carrier protein domain from the highly reducing type I iterative polyketide synthase CalE8. PLoS ONE 6, e20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kotaka M., Kong R., Qureshi I., Ho Q. S., Sun H., Liew C. W., Goh L. P., Cheung P., Mu Y., Lescar J., Liang Z. X. (2009) Structure and catalytic mechanism of the thioesterase CalE7 in enediyne biosynthesis. J. Biol. Chem. 284, 15739–15749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liew C. W., Sharff A., Kotaka M., Kong R., Sun H., Qureshi I., Bricogne G., Liang Z. X., Lescar J. (2010) Induced-fit upon ligand binding revealed by crystal structures of the hot-dog fold thioesterase in dynemicin biosynthesis. J. Mol. Biol. 404, 291–306 [DOI] [PubMed] [Google Scholar]
- 18. Vedadi M., Lew J., Artz J., Amani M., Zhao Y., Dong A., Wasney G. A., Gao M., Hills T., Brokx S., Qiu W., Sharma S., Diassiti A., Alam Z., Melone M., Mulichak A., Wernimont A., Bray J., Loppnau P., Plotnikova O., Newberry K., Sundararajan E., Houston S., Walker J., Tempel W., Bochkarev A., Kozieradzki I., Edwards A., Arrowsmith C., Roos D., Kain K., Hui R. (2007) Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol. Biochem. Parasitol. 151, 100–110 [DOI] [PubMed] [Google Scholar]
- 19. Collaborative (1994) The CCP4 suite. Programs for protein crystallography. Acta Crystallogr. D 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 20. Hong S. K., Kim K. H., Park J. K., Jeong K. W., Kim Y., Kim E. E. (2010) New design platform for malonyl-CoA-acyl carrier protein transacylase. FEBS Lett. 584, 1240–1244 [DOI] [PubMed] [Google Scholar]
- 21. Sheldrick G. (2008) A short history of SHELX. Acta Crystallogr. A 64, 112–122 [DOI] [PubMed] [Google Scholar]
- 22. Bricogne G. (1993) Direct phase determination by entropy maximization and likelihood ranking. Status report and perspectives. Acta Crystallogr. D 49, 37–60 [DOI] [PubMed] [Google Scholar]
- 23. Emsley P., Cowtan K. (2004) Coot. Model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 24. Langer G., Cohen S. X., Lamzin V. S., Perrakis A. (2008) Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nature Prot. 3, 1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 26. Roy A., Kucukural A., Zhang Y. (2010) I-TASSER. A unified platform for automated protein structure and function prediction. Nat. Prot. 5, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y. (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Comeau S. R., Gatchell D. W., Vajda S., Camacho C. J. (2004) ClusPro. An automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 20, 45–50 [DOI] [PubMed] [Google Scholar]
- 29. Comeau S. R., Gatchell D. W., Vajda S., Camacho C. J. (2004) ClusPro. A fully automated algorithm for protein-protein docking. Nucleic Acids Res. 32, W96–W99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong F. T., Chen A. Y., Cane D. E., Khosla C. (2010) Protein-protein recognition between acyltransferases and acyl carrier proteins in multimodular polyketide synthases. Biochemistry 49, 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen A. Y., Cane D. E., Khosla C. (2007) Structure-based dissociation of a type I polyketide synthase module. Chem. Biol. 14, 784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen A. Y., Schnarr N. A., Kim C. Y., Cane D. E., Khosla C. (2006) Extender unit and acyl carrier protein specificity of ketosynthase domains of the 6-deoxyerythronolide B synthase. J. Am. Chem. Soc. 128, 3067–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim C. Y., Alekseyev V. Y., Chen A. Y., Tang Y., Cane D. E., Khosla C. (2004) Reconstituting modular activity from separated domains of 6-deoxyerythronolide B synthase. Biochemistry 43, 13892–13898 [DOI] [PubMed] [Google Scholar]
- 34. Alekseyev V. Y., Liu C. W., Cane D. E., Puglisi J. D., Khosla C. (2007) Solution structure and proposed domain domain recognition interface of an acyl carrier protein domain from a modular polyketide synthase. Protein Sci. 16, 2093–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holm L., Rosenström P. (2010) Dali server. Conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang Y., Kim C. Y., Mathews I. I., Cane D. E., Khosla C. (2006) The 2.7-Angstrom crystal structure of a 194-kDa homodimeric fragment of the 6-deoxyerythronolide B synthase. Proc. Natl. Acad. Sci. U.S.A. 103, 11124–11129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joshi V. C., Wakil S. J. (1971) Studies on the mechanism of fatty acid synthesis. XXVI. Purification and properties of malonyl-coenzyme A. Acyl carrier protein transacylase of Escherichia coli. Arch. Biochem. Biophys. 143, 493–505 [DOI] [PubMed] [Google Scholar]
- 38. Keatinge-Clay A. T., Shelat A. A., Savage D. F., Tsai S. C., Miercke L. J., O'Connell J. D., 3rd, Khosla C., Stroud R. M. (2003) Catalysis, specificity, and ACP docking site of Streptomyces coelicolor malonyl-CoA:ACP transacylase. Structure 11, 147–154 [DOI] [PubMed] [Google Scholar]
- 39. Liu W., Han C., Hu L., Chen K., Shen X., Jiang H. (2006) Characterization and inhibitor discovery of one novel malonyl-CoA. Acyl carrier protein transacylase (MCAT) from Helicobacter pylori. FEBS Lett. 580, 697–702 [DOI] [PubMed] [Google Scholar]
- 40. Serre L., Swenson L., Green R., Wei Y., Verwoert I. I., Verbree E. C., Stuitje A. R., Derewenda Z. S. (1994) Crystallization of the malonyl coenzyme A-acyl carrier protein transacylase from Escherichia coli. J. Mol. Biol. 242, 99–102 [DOI] [PubMed] [Google Scholar]
- 41. Yadav G., Gokhale R. S., Mohanty D. (2003) Computational approach for prediction of domain organization and substrate specificity of modular polyketide synthases. J. Mol. Biol. 328, 335–363 [DOI] [PubMed] [Google Scholar]
- 42. Kong R., Goh L. P., Liew C. W., Ho Q. S., Murugan E., Li B., Tang K., Liang Z. X. (2008) Characterization of a carbonyl-conjugated polyene precursor in 10-membered enediyne biosynthesis. J. Am. Chem. Soc. 130, 8142–8143 [DOI] [PubMed] [Google Scholar]
- 43. Dreier J., Li Q., Khosla C. (2001) Malonyl-CoA:ACP transacylase from Streptomyces coelicolor has two alternative catalytically active nucleophiles. Biochemistry 40, 12407–12411 [DOI] [PubMed] [Google Scholar]
- 44. Zhang L., Liu W., Xiao J., Hu T., Chen J., Chen K., Jiang H., Shen X. (2007) Malonyl-CoA. Acyl carrier protein transacylase from Helicobacter pylori. Crystal structure and its interaction with acyl carrier protein. Protein Sci. 16, 1184–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wong F. T., Jin X., Mathews I. I., Cane D. E., Khosla C. (2011) Structure and mechanism of the trans-acting acyltransferase from the disorazole synthase. Biochemistry 50, 6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Y. M., Rao M. S., Heath R. J., Price A. C., Olson A. J., Rock C. O., White S. W. (2001) Identification and analysis of the acyl carrier protein (ACP) docking site on β-ketoacyl-ACP synthase III. J. Biol. Chem. 276, 8231–8238 [DOI] [PubMed] [Google Scholar]
- 47. Maier T., Leibundgut M., Ban N. (2008) The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 [DOI] [PubMed] [Google Scholar]
- 48.Deleted in proof
- 49. Delano W. L. (2002) PyMOL Molecular Graphics System, Delano Scientific, San Carlos, CA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.