Background: The functional impact of the Nϵ-lysine acetylation in the lumen of the ER is unknown.
Results: Analysis of the ER acetyl-lysine proteome revealed that many resident and transiting proteins are acetylated in the ER lumen.
Conclusion: The ER-based acetylation machinery regulates very diverse biological events.
Significance: The ER-based acetylation machinery is predicted to play a fundamental role in human physiology and pathology.
Keywords: Acetyl Coenzyme A, Endoplasmic Reticulum (ER), Membrane Proteins, Post-translational Modification, Proteomics, AT-1
Abstract
In addition to the nucleus, cytosol, and mitochondrial lumen, Nϵ-lysine acetylation also occurs in the lumen of the endoplasmic reticulum (ER). However, the impact of such an event on ER functions is still unknown. Here, we analyzed the “ER acetyl-lysine proteome” by nano-LC-MS/MS and discovered that a large number of ER-resident and -transiting proteins undergo Nϵ-lysine acetylation in the lumen of the organelle. The list of ER-resident proteins includes chaperones and enzymes involved with post-translational modification and folding. Grouping of all acetylated proteins into major functional categories suggests that the ER-based acetylation machinery regulates very diverse biological events. As such, it is predicted to play a fundamental role in human physiology as well as human pathology.
Introduction
The Nϵ acetylation of a lysine residue is a transient form of post-translational modification that was initially identified on histone proteins as well as a large number of cytosolic and nuclear proteins. They included proteins involved in DNA recombination and repair, transcription factors, cytoskeletal proteins, chaperones, signaling proteins, and several metabolic enzymes (1). Recognized functions of Nϵ-lysine acetylation include regulation of activity, molecular stabilization, and conformational assembly of a protein (2).
For a long time, lysine acetylation was thought to be limited to the cytosol and nucleus where both the donor of the acetyl group, acetyl-CoA, and the enzymes responsible for the biochemical reaction, acetyl-CoA:lysine acetyltransferases, were available. However, in 2006, Schwer et al. (3) reported the transient lysine acetylation of acetyl-CoA synthetase in the mitochondrial matrix, whereas in 2007, our group reported the transient lysine acetylation of the β-site APP-cleaving enzyme 1 (BACE1)3 in the lumen of endoplasmic reticulum (ER) (4). Specifically, the acetylation of nascent BACE1 requires translocation of acetyl-CoA into the ER lumen by SLC33A1/AT-1 (5) and two ER-resident acetyltransferases, ATase1 and ATase2 (6, 7).
Following up on the identification of BACE1 acetylation, two additional type I membrane proteins were shown to undergo the same process: the low density lipoprotein receptor (8) and the amyloid precursor protein (APP) (5). Finally, in 2009, a few ER-resident proteins were found to be acetylated as well (9). Despite these recent discoveries, the search for substrates of the “ER acetylation machinery” has been hampered by the transient nature of this type of modification as well as the low abundance of ER proteins in the cell. This limited information has also precluded a more comprehensive understanding of the biological impact of this machinery.
Here, we report the results of a proteomic study, which shows that a large number of ER-resident and -transiting proteins undergo Nϵ-lysine acetylation in the lumen of the organelle. Identified proteins display very diverse biochemical functions, suggesting a broad impact on cell physiology. The list of ER-resident proteins includes chaperones and enzymes involved with post-translational modification and folding.
EXPERIMENTAL PROCEDURES
Cell Cultures
Human neuroglioma (H4) cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/glutamine solution (Mediatech). Stable transfection was performed using Lipofectamine 2000 (Invitrogen), and the culture medium was supplemented with 350 μg/ml G418 sulfate (Mediatech). Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
Plasmid Constructs
Human HSPA5/BiP (NM_005347.2) cDNA was obtained from OriGene (SC108086) and subcloned with a C-terminal Myc tag into pcDNA3.1/Myc-His C vector (Invitrogen). The cDNA of human calreticulin (NM_004343.2) with a C-terminal fusion of Myc-DDK tag in the expression vector pCMV6-Entry was obtained from OriGene (RC203222). The presence of the full-length gene sequences was confirmed by DNA sequencing (performed at the DNA Sequencing Facility of the University of Wisconsin Biotechnology Center).
Western Blot Analysis and Immunoprecipitation
The following antibodies were used: anti-HSPA5/BiP (1:1000; Cell Signaling), anti-calreticulin (1:1000; Abcam), anti-PDIA3/ERp57 (1:2000; Abcam), anti-PPIB/cyclophilin B (1:1000; Abcam), anti-acetylated lysine residue (1:1000; Abcam), and anti-Myc (1:1000; Santa Cruz Biotechnology). Protein extracts were prepared in GTIP buffer (100 mm Tris, pH 7.6, 20 mm EDTA, 1.5 m NaCl) supplemented with 1% Triton X-100 (Roche Applied Science), 0.25% Nonidet P-40 (Roche Applied Science), Complete protein inhibitor mixture (Roche Applied Science), and phosphatase inhibitors (mixture set I and set II; Calbiochem). Protein concentration was measured by the bicinchoninic acid method (Pierce).
Immunoprecipitation was performed from highly purified ER fractions prepared as described previously (4–6, 8). Proteins were extracted as described above, and lysates were precleared with BioMag protein A beads (Polysciences, Inc.). Samples were then incubated overnight with specific antibodies (listed above) and recovered with BioMag protein A beads. The immunoprecipitate-bead complexes were washed three times in PBS and boiled for 5 min in reducing NuPAGE® LDS sample buffer (Invitrogen). Overexpressed HSPA5/BiP-Myc and calreticulin-Myc fusion proteins were purified using the ProFound c-Myc tag IP/Co-IP kit (Pierce) as described previously (6).
Protein samples prepared in reducing NuPAGE® LDS sample buffer (Invitrogen) were subjected to electrophoresis using precast NuPAGE® Novex 4–12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes (Invitrogen). Membranes were blocked for 1 h in Tris-buffered saline (TBS) containing 5% bovine serum albumin (BSA; Sigma) followed by an overnight incubation with the primary antibody diluted in 5% BSA in TBS-0.1% Tween®20 (TBST). After washing with TBST, membranes were incubated with goat anti-rabbit Alexa Fluor 680- or goat anti-mouse Alexa Fluor 800-conjugated secondary antibodies (LI-COR Biosciences). Membranes were imaged and quantified using the LI-COR Odyssey infrared imaging system (LI-COR Biosciences).
LC-MS/MS Analysis
Highly purified ER fractions were prepared as before (4–6, 8). Proteins were then extracted as described above and digested with trypsin prior to high-resolution high-accuracy MS. MS analysis was performed by the Mass Spectrometry facility at the University of Wisconsin-Madison as described previously (4). Peptides and proteins were identified with the Mascot search engine (Matrix Science, London, UK) via automated database searching of all tandem mass spectra. The output of the MS analysis was further processed to remove “contaminant” peptides. For the most part, these included acetylated peptides that are normally found on the cytoplasmic site of the ER membrane or that belong to structural proteins of the nuclear envelope, which is connected to the ER membrane.
RESULTS
The in Vivo ER Lysine Acetylome
To better understand the biological as well as clinical impact of the ER-based acetylation machinery, we examined the extent of the “ER acetyl-lysine proteome” by performing nano-HPLC/MS/MS analysis on highly purified ER preparations. To enrich for acetylated peptides, the ER was purified from H4 (human neuroglioma) cells overexpressing the ER membrane acetyl-CoA transporter, AT-1. In fact, these cells have increased influx of acetyl-CoA into the ER and, as a result, experience a quantitative increase in the acetylation profile of the organelle (5). Importantly, the overexpression of AT-1 per se does not cause acetylation of proteins that are normally nonacetylated (5).
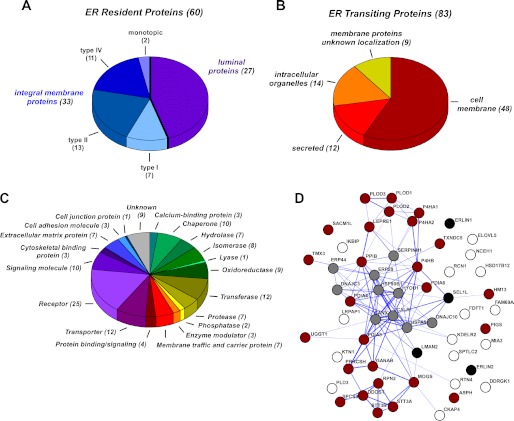
This strategy identified 549 lysine acetylation sites from 143 proteins. Only 12 of them had already been reported to be acetylated before (9). Of the above 143 acetylated proteins, 60 were classified as ER-resident, and 83 were classified as ER-transiting (Fig. 1, A and B; see also supplemental Tables S1 and S2). The latter included both membrane and secreted proteins that are synthesized in the ER but reside in a different cellular location or in the extracellular milieu (Fig. 1B and supplemental Table S2). As with previously identified membrane proteins (4–6, 8), these are likely to undergo a transient form of lysine acetylation as part of their post-translational modifications in the secretory pathway. The ER-resident proteins included both membrane-bound and luminal proteins (Fig. 1A and supplemental Table S1). Importantly, all identified acetylation sites listed in supplemental Table S1 and S2 are in peptides that face the lumen of the ER, indicating that Nϵ-lysine acetylation is an important post-translational modification in the lumen of the organelle. Grouping of all acetylated proteins into major functional categories based on their known or predicted biochemical functions suggests that Nϵ-lysine acetylation regulates very diverse biological events (Fig. 1C). A similar conclusion can also be drawn when studying the reported association of acetylated proteins with human diseases (see supplemental Tables S1 and S2).
FIGURE 1.
Overview of the ER acetyl-lysine proteome analysis. A and B, topological classification of acetylated resident (A) and transiting (B) proteins. The complete list of proteins and peptides is in supplemental Tables S1 and S2. C, functional classification of all acetylated proteins (resident and transiting). Proteins were assigned based on the Protein Analysis THrough Evolutionary Relationships (PANTHER) classification system. D, interaction network from STRING analysis of acetylated resident proteins. STRING analysis was performed using “experiments, database, and text mining evidences” as prediction methods (using STRING 9.0). Description of the current STRING database as well as analysis procedures can be found in Ref. 11. Thickness of the connecting lines indicates the confidence score of the association (thicker lines indicate stronger confidence). Resident proteins were grouped based on functions as follows: gray, chaperones; red, proteins involved with post-translational modification; black, proteins involved with quality control and ER-associated protein degradation; white, other.
The great majority of ER-resident proteins are represented by chaperones or enzymes that are involved with folding and post-translational modification in the lumen of the ER (supplemental Table S1). Direct assessment of the physical and functional associations of the resident proteins listed in supplemental Table S1 by using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database showed significantly high network connectivity among the above classes of acetylated proteins (Fig. 1D). The high degree of functional association and network connectivity suggests that the Nϵ-lysine acetylation plays a major role in regulating the activity of ER-resident proteins involved with the folding and post-translational modification of newly synthesized proteins.
Direct Targeting of ER-resident Proteins Confirms the Results of the Proteomic Study
The proteomic assessment was performed with ER fractions that were purified from AT-1-overexpressing cells. In fact, the overexpression of AT-1 increases the acetylation profile of the organelle, thus improving our ability to detect modified peptides, without affecting proteins that are normally not acetylated (5). However, to confirm that our results were not skewed by the increased availability of acetyl-CoA in the lumen of the ER, we decided to assess the acetylation status of four randomly chosen ER-resident proteins: 78-kDa glucose-regulated protein (GRP78; also known as BiP and HSPA5), calreticulin, protein disulfide-isomerase A3 (PDIA3; also known as ERp57), and peptidyl-prolyl cis-trans isomerase B (PPIB; also known as cyclophilin B). We decided to focus on ER-resident proteins because the lysine acetylation nature of ER-transiting proteins has already been fully characterized (4–6, 8).
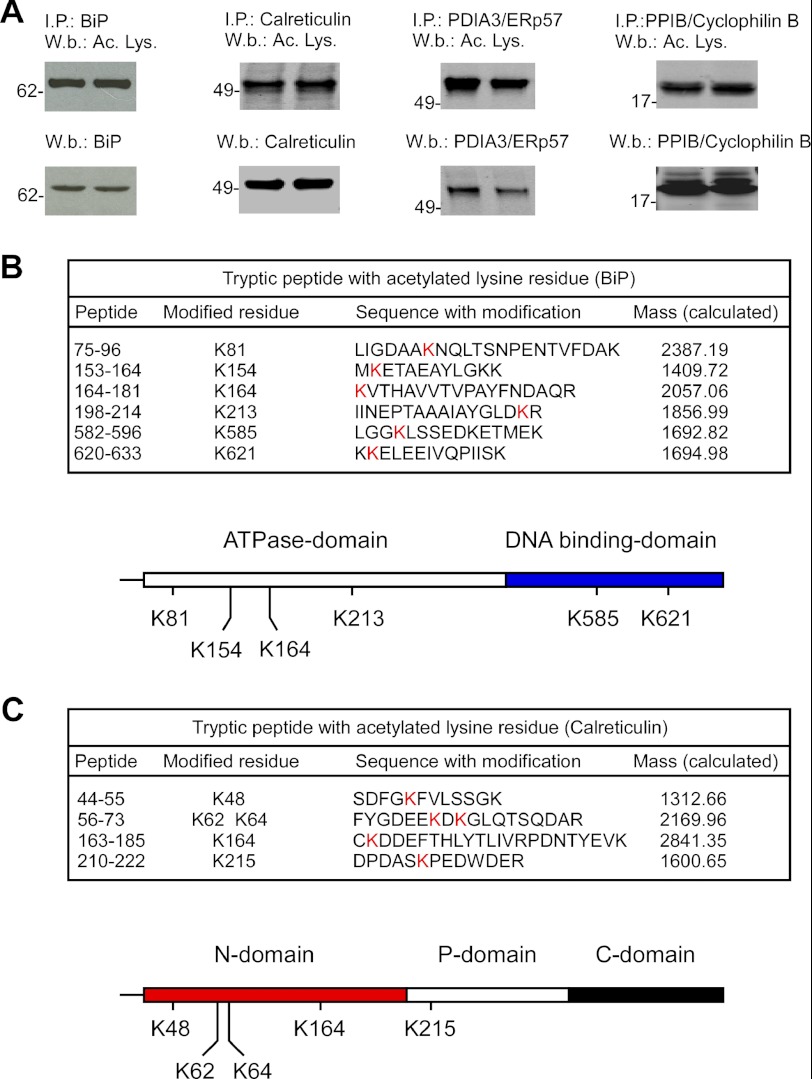
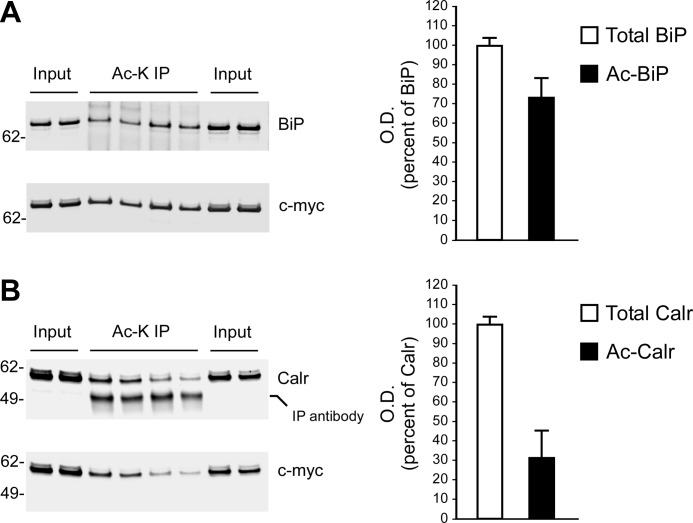
The above proteins were immunoprecipitated from control (nontransfected) H4 cells and then analyzed by immunoblotting with an antibody raised against acetylated lysine residues. Importantly, the assessment was done with the endogenous proteins. Fig. 2A shows that we could successfully determine the acetylation status of all four proteins. To further confirm our results, we generated Myc-tagged versions of BiP and calreticulin and then overexpressed them in control H4 cells. The C-terminal Myc tag was used for affinity purification on immobilized anti-Myc columns. The purified proteins were then analyzed by LC-MS/MS. The results are reported in Fig. 2, B and C. We were able to confirm the acetylation nature in both cases. Interestingly, direct assessment of the transgenic proteins allowed us to detect additional sites that were missed with large-scale proteomics. In fact, the proteomic approach identified Lys-81, Lys-213, and Lys-621 on BiP and Lys-48 on calreticulin, whereas direct assessment of the transgenic proteins identified three additional sites (Lys-154, Lys-164, and Lys-585) on BiP and four (Lys-62, Lys-64, Lys-164, and Lys-215) on calreticulin. These results were in part expected because of the intrinsic transient nature of the modification. However, it is important to point out that the modified residues identified with the proteomic approach in AT-1-overexpressing cells were also identified by direct assessment of the proteins in control cells, excluding possible artifacts induced by AT-1 overexpression. Analysis of the domain organization of BiP and calreticulin revealed preferential distribution of the modified residues. In the case of BiP, four acetylated lysine residues were located on the ATPase domain, and two were located on the DNA-binding domain (Fig. 2B; lower panel). In the case of calreticulin, four acetylated residues were found on the N-domain, and one was found on the P-domain, whereas no modified lysine residues were found on the C-domain (Fig. 2C; lower panel). The biological impact of this distribution remains to be addressed. Finally, quantitative assessment revealed that under the experimental conditions used here, about 70% of BiP and 30% of calreticulin was found to be acetylated (Fig. 3). These results indicate that the levels of acetylation might vary dramatically among different proteins. Obviously, the acetylation status of a protein could also be affected by the cell type and/or physiological conditions of the cell.
FIGURE 2.
Validation of the acetylation status of ER-resident proteins. A, the indicated ER-resident proteins were immunoprecipitated (I. P.) prior to Western blot (W. b.) analysis with the indicated antibodies. Results are in duplicate. Ac. Lys., acetylated lysine. B and C, transgenic Myc-tagged BiP (B) or calreticulin (C) were purified with an immobilized anti-Myc column and then analyzed by LC-MS/MS to detect acetylated lysine residues. The domain organization of BiP (B; lower panel) and calreticulin (C; lower panel) with modified lysine residues is also shown. The domain organization of BiP is based on Refs. 12–14, whereas the domain organization of calreticulin is based on Refs. 15 and 16.
FIGURE 3.
Quantitative assessment of the acetylation status of BiP and calreticulin. Transgenic (Myc-tagged) BiP and calreticulin (Calr) were purified with an immobilized anti-Myc column. The affinity-purified protein (Input) was immunoprecipitated with an anti-acetylated lysine antibody (Ac-K IP) prior to Western blot assessment with the indicated antibodies. Quantitative analysis of the acetylation status of both BiP (A) and calreticulin (B) is shown. Representative Western blots are shown in the left panels, whereas the average (n = 4) ± S.D. is shown in the right panels. O. D., optical density.
DISCUSSION
Collectively, our studies indicate that Nϵ-lysine acetylation of resident and transiting proteins in the lumen of the ER constitutes a fundamental function of the organelle. The acetylation of the ϵ-amino group of lysine residues was initially described on nuclear and cytosolic proteins, where it regulates activity, molecular stabilization, and conformational assembly of the modified proteins (1, 2). However, it is now known that the same event also occurs in the lumen of the mitochondria (3, 9, 10) as well as ER (4–6, 8).
Here, we expand the list of Nϵ-lysine acetylated proteins and show that this modification is common among both ER-transiting and ER-resident proteins. Interestingly, none of the previously identified transiting proteins undergoing transient lysine acetylation (BACE1, APP, and the low-density lipoprotein receptor) emerged from the screen reported here, suggesting that the proteomic analysis underestimates the actual number of proteins that undergo this modification while trafficking in the secretory pathway. Obviously, the transient nature of the modification as well as the expression levels of the individual proteins represent a great limitation to studies assessing global modifications. Perhaps this is particularly important for transiting proteins, which are rapidly moved out of the ER after biosynthesis. However, a similar limitation was also observed when mapping the modified residues of two ER-resident proteins (BiP and calreticulin). Despite these limitations, the proteomic study reported here has unexpectedly revealed that a large number of ER-resident proteins involved with post-translational modification and folding of newly synthesized proteins are acetylated. Functional grouping of all acetylated proteins into major biochemical categories suggests that the ER-based acetylation machinery regulates very diverse biological events. As such, it is predicted to play a fundamental role in human physiology as well as human pathology.
Supplementary Material
Acknowledgments
We are grateful to Grzegorz Sabat at the University of Wisconsin Mass Spectrometry Facility for help. We are also grateful to Antonio Puglielli and Abilash Seshadri for helping with the manual analysis of the MS output data. We are grateful for use of the resources and facilities of the William S. Middleton Memorial Veterans Hospital, Madison, WI.
This work was supported, in whole or in part, by National Institutes of Health Grants AG028569 and AG033514 (to L.P.) through the NIA.

This article contains supplemental Tables S1 and S2.
- BACE1
- β-site APP-cleaving enzyme 1
- APP
- amyloid precursor protein
- ER
- endoplasmic reticulum
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Yang X. J., Seto E. (2007) HATs and HDACs: from structure, function, and regulation to novel strategies for therapy and prevention. Oncogene 26, 5310–5318 [DOI] [PubMed] [Google Scholar]
- 2. Kouzarides T. (2000) Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19, 1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwer B., Bunkenborg J., Verdin R. O., Andersen J. S., Verdin E. (2006) Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. U.S.A. 103, 10224–10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costantini C., Ko M. H., Jonas M. C., Puglielli L. (2007) A reversible form of lysine acetylation in the ER and Golgi lumen controls the molecular stabilization of BACE1. Biochem. J. 407, 383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jonas M. C., Pehar M., Puglielli L. (2010) AT-1 is the ER membrane acetyl-CoA transporter and is essential for cell viability. J. Cell Sci. 123, 3378–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ko M. H., Puglielli L. (2009) Two endoplasmic reticulum (ER)/ER Golgi intermediate compartment-based lysine acetyltransferases post-translationally regulate BACE1 levels. J. Biol. Chem. 284, 2482–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding Y., Ko M. H., Pehar M., Kotch F., Peters N. R., Luo Y., Salamat S. M., Puglielli L. (2012) Biochemical inhibition of the acetyltransferases ATase1 and ATase2 reduces β-secretase (BACE1) levels and Aβ generation. J. Biol. Chem. 287, 8424–8433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jonas M. C., Costantini C., Puglielli L. (2008) PCSK9 is required for the disposal of non-acetylated intermediates of the nascent membrane protein BACE1. EMBO Rep. 9, 916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 10. Kim S. C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., Grishin N. V., White M., Yang X. J., Zhao Y. (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618 [DOI] [PubMed] [Google Scholar]
- 11. Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., Minguez P., Doerks T., Stark M., Muller J., Bork P., Jensen L. J., von Mering C. (2011) The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561–D568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaut J. R., Hendershot L. M. (1993) Mutations within the nucleotide binding site of immunoglobulin-binding protein inhibit ATPase activity and interfere with release of immunoglobulin heavy chain. J. Biol. Chem. 268, 7248–7255 [PubMed] [Google Scholar]
- 13. Hendershot L. M., Wei J. Y., Gaut J. R., Lawson B., Freiden P. J., Murti K. G. (1995) In vivo expression of mammalian BiP ATPase mutants causes disruption of the endoplasmic reticulum. Mol. Biol. Cell 6, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wisniewska M., Karlberg T., Lehtiö L., Johansson I., Kotenyova T., Moche M., Schüler H. (2010) Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70–2, HSPA6/Hsp70B′, and HSPA5/BiP/GRP78. PLoS One 5, e8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellgaard L., Riek R., Herrmann T., Güntert P., Braun D., Helenius A., Wüthrich K. (2001) NMR structure of the calreticulin P-domain. Proc. Natl. Acad. Sci. U.S.A. 98, 3133–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chouquet A., Païdassi H., Ling W. L., Frachet P., Houen G., Arlaud G. J., Gaboriaud C. (2011) X-ray structure of the human calreticulin globular domain reveals a peptide-binding area and suggests a multimolecular mechanism. PLoS One 6, e17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.