Background: Matrix metalloproteinase-2 (MMP-2) activity is regulated by several mechanisms.
Results: We observed that Ca2+ ion is essential for MMP-2 homodimerization, which in turn results in the proteolysis of small peptide substrates and enhances thrombin-mediated activation of pro-MMP-2.
Conclusion: Pro-MMP-2 activation is modulated by MMP-2 homodimerization.
Significance: This study elucidates a novel mechanism to regulate MMP-2 activity.
Keywords: Disulfide, Enzyme Processing, Matrix Metalloproteinase (MMP), Post-translational Modification, Thrombin, MMP-2, MMP-2 Activation, Homodimer, Homodimerization, Intermolecular Disulfide Bond
Abstract
Matrix metalloproteinase-2 (MMP-2) functions in diverse biological processes through the degradation of extracellular and non-extracellular matrix molecules. Because of its potential for tissue damage, there are several ways to regulate MMP-2 activity, including gene expression, compartmentalization, zymogen activation, and enzyme inactivation by extracellular inhibitors. Enzyme regulation through zymogen activation is important for the regulation of MMP-2 activity. In our previous studies, we showed that thrombin directly cleaved the propeptide of MMP-2 at specific sites for enzyme activation. We also demonstrated that heparan sulfate was required for thrombin-mediated activation of pro-MMP-2 by binding to thrombin, presumably through conformational changes at the active site of the enzyme. This suggests a regulatory mechanism for thrombin-mediated activation of pro-MMP-2. In this study, we found that MMP-2 formed a reduction-sensitive homodimer in a controlled manner and that Ca2+ ion was essential for homodimerization of MMP-2. Homodimerization was not associated with protein kinase C-mediated phosphorylation of MMP-2. MMP-2 formed a homodimer through an intermolecular disulfide bond between Cys102 and the neighboring Cys102. Homodimerization of MMP-2 enhanced thrombin-mediated activation of pro-MMP-2. Moreover, the MMP-2 homodimer could cleave a small peptide substrate without removal of the propeptide. Taken together, our experimental data suggest a novel regulatory mechanism for pro-MMP-2 activation that is modulated through homodimerization of MMP-2.
Introduction
The matrix metalloproteinase (MMP)3 family in humans comprises 23 zinc-dependent endopeptidases, which are involved in many biological processes and diseases. These enzymes act to degrade extracellular and non-extracellular matrix molecules (1). MMP-2 is implicated in organ growth, endometrial cycling, wound healing, bone remodeling, tumor invasion, and metastasis (2). This enzyme functions through the degradation of components of the basement membrane, including type IV collagen, fibronectin, elastin, laminin, aggrecan, and fibrillin (1, 3).
Because of its potential for tissue damage, there are several ways to regulate MMP-2 activity, including gene expression, compartmentalization, zymogen activation, and enzyme inactivation by extracellular inhibitors (e.g. tissue inhibitors of metalloproteases (TIMPs)) (1). Like most MMPs, MMP-2 maintains a latent state via an interaction between a thiol group of a propeptide cysteine residue and the catalytic zinc ion in the active site (4). Disruption of this cysteine-zinc ion pairing, through conformational changes (5) or proteolytic cleavage of the propeptide (e.g. by plasmin, thrombin, factor Xa, protein C, legumain, or membrane-type MMPs) (6–14), is required for activation of the latent enzyme.
Thrombin is involved in the coagulation cascade and in multiple cellular processes, including mitogenesis of fibroblasts, lymphocytes, mesenchymal cells, and smooth muscle cells, via proteolytic activation of protease-activated receptors (15–18). It also plays a role in cell migration and invasion through MMP-2 activation (19–21).
Some controversial reports suggest the involvement of membrane-type 1 MMP (MT1-MMP) in thrombin-mediated activation of pro-MMP-2 (19, 22–24). In our previous studies, we demonstrated that thrombin directly cleaved the propeptide on the C-terminal side of Arg98 and Arg101 with a preference for Arg101; this was followed by intermolecular autoproteolytic cleavage at the Asn109–Tyr peptide bond, resulting in full enzymatic activation (7). We further showed that heparan sulfate was required for thrombin-mediated activation of pro-MMP-2 (25). Binding of heparan sulfate to thrombin at the cell surface is primarily responsible for this activation process, presumably through conformational changes at the active site of the enzyme (25). It is likely that the cleavage sites are protected from thrombin, as these sites are located immediately upstream of Cys102, which is involved in the cysteine-zinc ion pairing (4), thus making the cleavage less feasible. In this study, we elucidated the molecular mechanisms of the cleavage through the investigation of post-translational modifications in MMP-2.
EXPERIMENTAL PROCEDURES
Reagents
Human thrombin was purchased from Haematologic Technologies (Essex Junction, VT). Monoclonal antibodies specific to MMP-2 (sc-13595) and rabbit polyclonal anti-MT1-MMP antibody (sc-30074) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies recognizing phosphoserine (AB1603) and phosphothreonine (AB1607) were purchased from Millipore (Billerica, MA), and anti-phosphothreonine antibody (9381) was obtained from Cell Signaling Technology, Inc. (Danvers, MA). Anti-Myc antibody (clone 9E10) was purchased from Invitrogen. VEGF165, basic FGF, and PDGF-BB were obtained from ProSpec (Rehovot, Israel). EGF, phorbol 12-myristate 13-acetate (PMA), calphostin C, and thapsigargin were purchased from Sigma-Aldrich. IL-1β, TNF-α, and TGF-β were purchased from R&D Systems (Minneapolis, MN). Immobilized pH gradient strips (pH 3–10) and Superdex 200 HR were purchased from GE Healthcare, and calf intestinal alkaline phosphatase was from New England Biolabs (Ipswich, MA).
Expression Plasmids and Site-directed Mutagenesis
Plasmids for expression of full-length MMP-2 or MMP-2 with C-terminal Myc and His tags were described previously (7). To make an expression plasmid encoding hemopexin-like domain-deleted pro-MMP-2, site-directed mutagenesis was used to convert Pro466 to a stop codon using a mutagenesis kit (iNtRON Biotechnology, Kyungki-Do, Korea). Site-directed mutagenesis was used to make all of the mutants used in this study. The expression plasmid for human MT1-MMP was kindly provided by Dr. Suneel Apte (Lerner Research Institute, Cleveland, OH).
Cell Culture, Transfection, and Treatment
COS-1 cells and HT-1080 human fibrosarcoma cells (ATCC CCL-121) were maintained in DMEM containing 10% FBS. Human umbilical vein endothelial cells were maintained on gelatin-coated plastic dishes in M199 culture medium containing 20% FBS, 10 units/ml heparin, and 3 ng/ml basic FGF. The endothelial cells used in the experiments were from passages 3–5. Transfection with plasmids was performed using FuGENE 6 (Promega, Madison, WI) according to the manufacturer's recommendations. For secreted MMP-2, transfected COS-1 cells were cultured and then transferred to 293 SFM II medium (Invitrogen).
Immunoblotting and Protein Purification
Cells were lysed in buffer containing 50 mm Tris-HCl (pH 7.5), 5 mm EDTA, 150 mm NaCl, 1% Triton X-100, and protease inhibitor mixture (Roche Applied Science) for 10 min at 4 °C and then centrifuged. The soluble portion of the lysate was used for Western blotting, which was performed by separation of nonreduced samples on SDS-PAGE, followed by electroblotting onto PVDF membranes and detection of bound antibody by enhanced chemiluminescence (GE Healthcare). MMP-2 was purified from the conditioned medium using gelatin-Sepharose (GE Healthcare) according to the manufacturer's recommendations. C-terminally Myc/His-tagged MMP-2 was purified using anti-c-Myc antibody-agarose (Sigma-Aldrich) according to the manufacturer's recommendations. The signal intensity of relevant bands from the Western blot was quantitated using NIH ImageJ software.
Digestion Assays Using Fluorescein-conjugated Gelatin and MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2
Substrates were incubated with purified monomeric and dimeric MMP-2 (5 μg/ml per reaction) in 50 mm Tris-HCl (pH 7.5), 5 mm CaCl2, and 10 μg/ml leupeptin at 37 °C for 1 h with fluorescein-conjugated gelatin (Molecular Probes, Eugene, OR) or for 30 min with MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2 (MP Biomedicals, Irvine, CA) according to the manufacturers' recommendations. The digested substrates were analyzed using a fluorometer (Kontron Instruments, Everett, MA) set for excitation at 495 nm and emission at 515 nm (fluorescein-conjugated gelatin) or for excitation at 320 nm and emission at 405 nm (MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2).
Statistical Analysis
Data are represented as the mean ± S.D. of n experiments. Statistical analysis was performed using Student's unpaired t test. A p value <0.05 was considered statistically significant.
RESULTS
MMP-2 Forms a Reduction-sensitive Homodimer
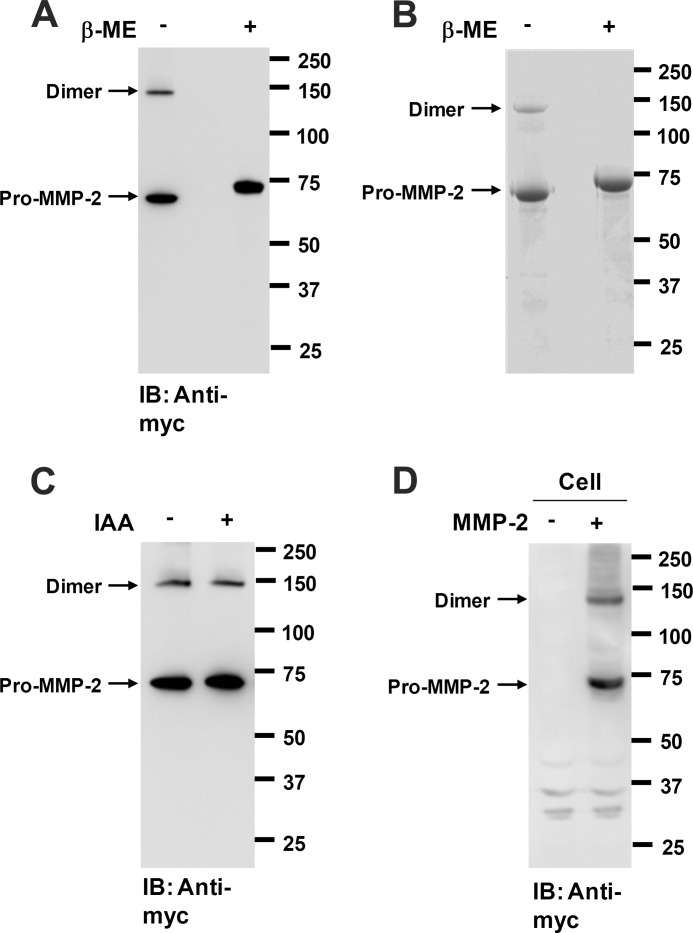
It has been shown that MMP-2 forms heterodimers with various proteins, including TIMP-2, TIMP-3, TIMP-4, and glycosaminoglycans (26–28). Herein, we also report a high molecular weight form of MMP-2 as assessed by Western blot analysis of the conditioned medium from COS-1 cells expressing pro-MMP-2-Myc/His under nonreducing conditions (Fig. 1A). The high molecular weight form disappeared under reducing conditions with a concomitant increase in the MMP-2 monomer (Fig. 1A). Similar results were obtained by SDS-PAGE analysis of purified MMP-2 (Fig. 1B). These data indicate that the high molecular weight form of MMP-2 is sensitive to reduction. These results also show that the molecular mass of the high molecular weight form is double that of the monomer, suggesting that the high molecular weight form may be a reduction-sensitive MMP-2 homodimer. We further examined this by LC-MS/MS analysis of the molecular species present in the dimer. MS analysis of the band showed that MMP-2 was exclusively present in the dimer band (data not shown), confirming that MMP-2 is a homodimer. Eleven distinct peptides from MMP-2 were identified in the band. Dimer formation may be due to oxidation of free cysteine residues after treatment of the samples with SDS. To rule out this possibility, the samples were first treated with iodoacetamide to block any free cysteine residues and then denatured with SDS in the presence of iodoacetamide, followed by Western blot analysis. The MMP-2 dimer was still observed in the iodoacetamide-treated samples (Fig. 1C). To examine whether homodimerization of MMP-2 is an intracellular process, cell lysates from the transfected cells were analyzed by Western blotting with anti-Myc antibody. Cells were pretreated with trypsin/EDTA to remove MMP-2 from the cell surface. The Western blot data revealed the presence of the MMP-2 homodimer in the cell lysates (Fig. 1D), indicating that the dimerization is an intracellular process. Taken together, these data suggest that a reduction-sensitive MMP-2 homodimer is formed intracellularly and subsequently secreted.
FIGURE 1.
MMP-2 forms a reduction-sensitive homodimer. A, Western blot analysis using anti-Myc antibody on conditioned medium from COS-1 cells expressing pro-MMP-2-Myc/His under nonreducing and reducing conditions. β-ME, β-mercaptoethanol. Arrows indicate the monomeric and dimeric forms of pro-MMP-2 (n = 3). B, SDS-PAGE analysis of purified pro-MMP-2-Myc/His under nonreducing and reducing conditions. Pro-MMP-2-Myc/His was purified from the conditioned medium of the transfected cells using anti-c-Myc antibody-agarose. C, effect of SDS on dimer formation of MMP-2 in vitro. Purified pro-MMP-2-Myc/His (10 μg/ml) was treated with 10 mm iodoacetamide (IAA) at 37 °C for 1 h and then denatured with SDS-PAGE sample buffer in the presence of 10 mm iodoacetamide, followed by Western blot analysis using anti-Myc antibody (n = 2). IB, immunoblot. D, Western blot analysis using anti-Myc antibody of cell lysates from COS-1 cells expressing pro-MMP-2-Myc/His under nonreducing conditions. Cells were pretreated with 0.05% trypsin and 0.53 mm EDTA for 30 min on ice (n = 2).
Homodimerization of MMP-2 Is Increased by PMA Treatment
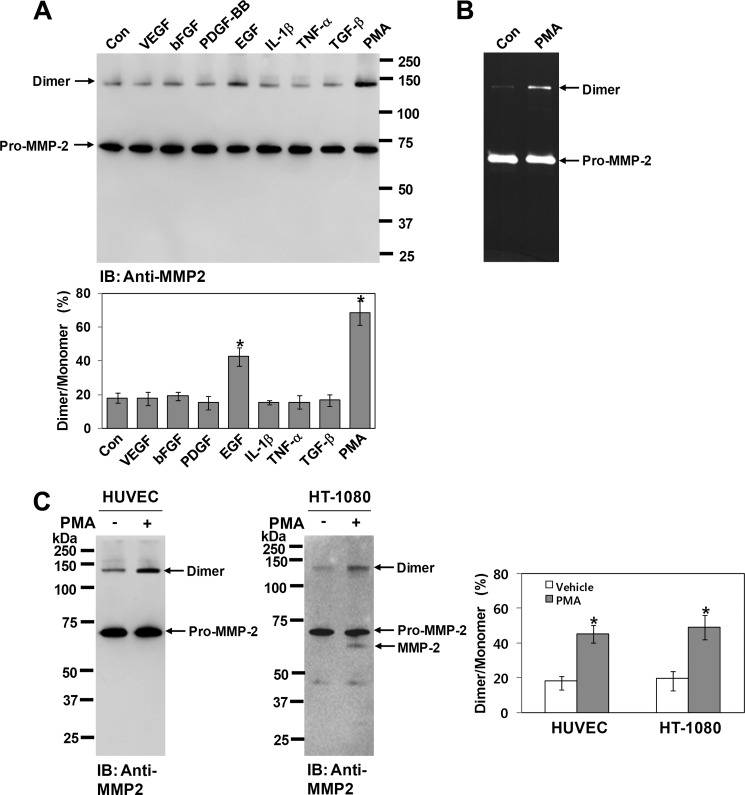
Several studies have reported the spontaneous and controlled homodimerization of proteins. We explored whether MMP-2 forms a homodimer spontaneously or in a controlled manner. When COS-1 cells expressing pro-MMP-2 were treated with various agonists and the conditioned medium was analyzed by Western blotting with anti-MMP-2 antibody, the results revealed that treatment with EGF or PMA led to an increase in homodimerization of MMP-2, with PMA treatment showing the most robust effect (Fig. 2A). Zymographic analysis also showed an increase in homodimerization of MMP-2 by PMA treatment (Fig. 2B). Because endogenous MMP-2 expression in human umbilical vein endothelial cells and HT-1080 cells was sufficient for Western blot analysis, parallel experiments using PMA were performed on these cells. Western blot analysis of the conditioned medium using anti-MMP-2 antibody showed that PMA treatment also resulted in an increase in homodimerization of MMP-2 (Fig. 2C). Taken together, these data suggest that MMP-2 forms a homodimer in a controlled manner and not spontaneously.
FIGURE 2.
MMP-2 homodimer is increased by PMA treatment. A, effect of various agonists on homodimerization of MMP-2 in COS-1 cells. Cells expressing pro-MMP-2 were treated with VEGF (10 μg/ml), basic FGF (bFGF; 10 μg/ml), PDGF-BB (20 μg/ml), EGF (20 μg/ml), IL-1β (10 μg/ml), TNF-α (10 μg/ml), TGF-β (10 μg/ml), or PMA (10 ng/ml). After a 16-h incubation, conditioned media were analyzed by Western blotting using anti-MMP-2 antibody. The bar graph shows the ratio of dimeric to monomeric MMP-2. Arrows indicate the monomeric and dimeric forms of pro-MMP-2. Data represent the mean ± S.D. of three experiments. *, p < 0.05 versus the untreated control (Con). IB, immunoblot. B, zymogram of conditioned medium from cells expressing pro-MMP-2 treated with or without PMA (10 ng/ml) (n = 3). C, Western blot analysis of conditioned media from human umbilical vein endothelial cells (HUVEC) and HT-1080 cells using anti-MMP-2 antibody. Cells were treated with PMA (10 ng/ml) in serum-free medium for 16 h. The conditioned medium (500 μl) from human umbilical vein endothelial cells was concentrated using gelatin-Sepharose, and the bound sample was used for Western blot analysis. The conditioned medium from HT-1080 cells was analyzed without concentration. Note that activated MMP-2 was detected in the conditioned medium from PMA-treated HT-1080 cells. The bar graph shows the ratio of dimeric to monomeric MMP-2. Data represent the mean ± S.D. of three experiments. *, p < 0.05 versus the untreated control.
Homodimerization of MMP-2 Is Not Associated with PKC-mediated Phosphorylation
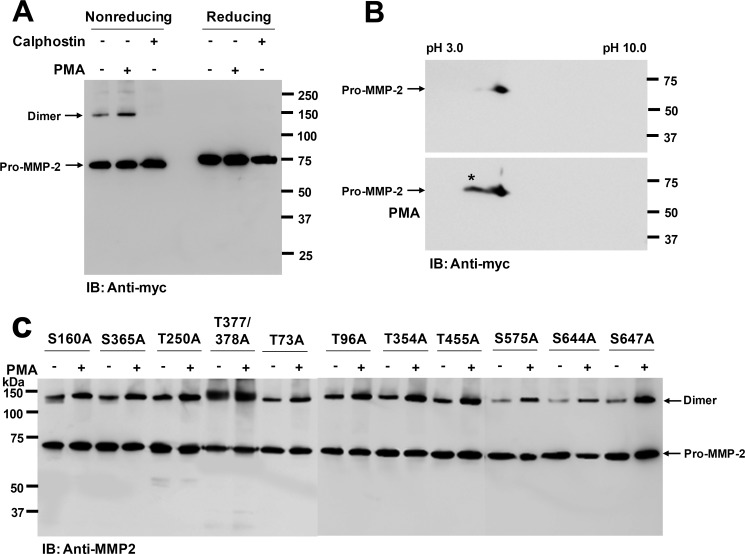
PMA is an established activator of PKC in many kinds of cells (29). Therefore, we assumed that PKC may play a role in this process. We treated COS-1 cells expressing pro-MMP-2-Myc/His with calphostin C, a potent inhibitor of PKC (30), and the conditioned medium was analyzed by Western blotting with anti-Myc antibody. The Western blot data showed that homodimerization of MMP-2 was completely inhibited by treatment with the PKC inhibitor, whereas PMA treatment increased homodimerization (Fig. 3A, left). As expected, the dimer was not detected under reducing conditions (Fig. 3A, right). Human MMP-2 contains 29 potential phosphorylation sites, and some of them are phosphorylated by PKC (31). Thus, we examined the phosphorylation status of MMP-2 in the conditioned medium of transfected COS-1 cells treated with or without PMA by Western blotting with antibodies against phosphothreonine and phosphoserine. These experiments were unsuccessful because the antibodies could not specifically recognize phosphorylated proteins (data not shown). Alternatively, we analyzed pro-MMP-2-Myc/His in the conditioned medium of the transfected cells by two-dimensional electrophoresis, followed by Western blotting with anti-Myc antibody. The Western blot data showed that MMP-2 from untreated cells resolved as a single spot and that MMP-2 from PMA-treated cells resolved as two spots, one with a more acidic pI (as indicated by the asterisk in Fig. 3B). This suggested phosphorylation of MMP-2 by PKC (Fig. 3B). These results clearly revealed that phosphorylated MMP-2 was only slightly detectable in the conditioned medium of untreated cells. In contrast, MMP-2 formed appreciable amounts of homodimer in untreated cells, although homodimerization and phosphorylation of MMP-2 were both increased by PMA treatment (Fig. 3, A and B). Because phosphorylation of several serine and threonine residues in MMP-2 has been reported (31) and probability scores for phosphorylation of serine and threonine in MMP-2 can be estimated using NetPhos 2.0, pro-MMP-2 mutants incapable of phosphorylation by PKC were generated (S160A, S365A, T250A, T377A/T378A, T73A, T96A, T354A, T455A, S575A, S644A, and S647A). In agreement with the data shown in Fig. 3 (A and B), transient transfection of these mutant plasmids into COS-1 cells did not affect homodimerization of MMP-2, as the homodimers were still increased in the conditioned medium from PMA-treated cells (Fig. 3C). Some of the pro-MMP-2 mutants exhibited greater levels of dimerization than others (e.g. T377A/T378A and T354A) (Fig. 3C). This might occur due to conformational changes in MMP-2 by the mutation. Taken together, these data suggest that homodimerization of MMP-2 is not linked to PKC-mediated phosphorylation of MMP-2.
FIGURE 3.
Homodimerization of MMP-2 is not linked to PKC-mediated phosphorylation of MMP-2. A, effect of calphostin C on homodimerization of MMP-2 in COS-1 cells. After cells were treated with 50 nm calphostin C for 16 h, the conditioned medium was analyzed by Western blotting using anti-Myc antibody under nonreducing and reducing conditions. PMA was used as a positive control to increase MMP-2 homodimer formation. Arrows indicate the monomeric and dimeric forms of pro-MMP-2 (n = 3). B, two-dimensional electrophoresis of pro-MMP-2 using immobilized pH gradient strips (pH 3–10) for the first dimension and SDS-polyacrylamide gels for the second dimension. After electroblotting onto PVDF, Western blotting was done using anti-Myc antibody (n = 2). C, Western blotting using anti-MMP-2 antibody of conditioned media from cells expressing pro-MMP-2 mutants S160A, S365A, T250A, T377A/T378A, T73A, T96A, T354A, T455A, S575A, S644A, and S647A treated with or without PMA (10 ng/ml) for 16 h. IB, immunoblot.
Ca2+ Ions Are Responsible for Homodimerization of MMP-2
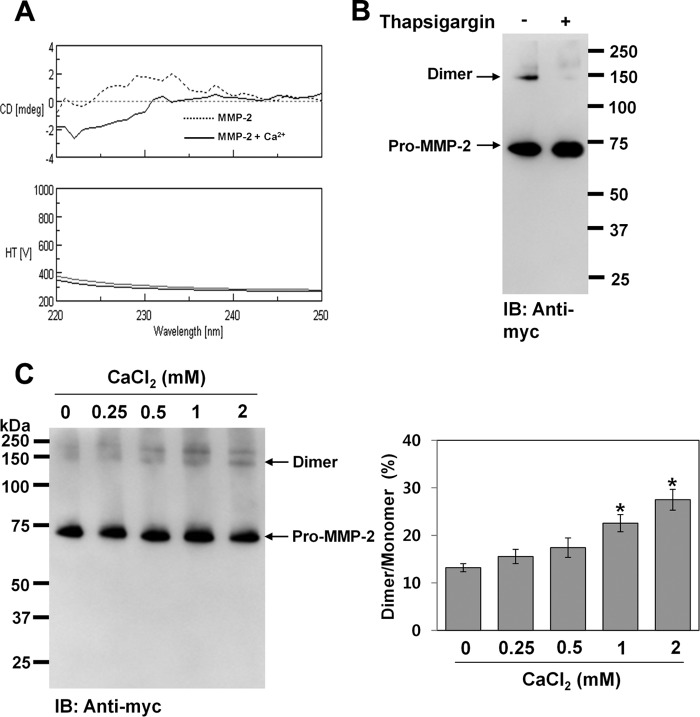
In addition to the role of PMA in activating PKC by binding to its diacylglycerol-binding domain (29), it also stimulates calcium influx in various cell types through activation of Ca2+-ATPase (32). Calphostin C, a known inhibitor of PKC, induces Ca2+ release from intracellular Ca2+ stores (33). We also showed Ca2+ ion-induced conformational changes in MMP-2 using circular dichroism spectroscopy (Fig. 4A). Thus, we postulated that Ca2+ ion might play a role in homodimerization of MMP-2 during protein folding in the endoplasmic reticulum. To test this, we examined homodimerization of MMP-2 in COS-1 cells expressing pro-MMP-2-Myc/His after depleting intracellular Ca2+ stores by treatment with thapsigargin, an inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (34). Western blot analysis of the conditioned medium showed that the MMP-2 homodimer disappeared after treatment with thapsigargin (Fig. 4B). To further explore the effect of Ca2+ ions on homodimerization of MMP-2, in vitro refolding experiments were performed using purified pro-MMP-2-Myc/His. When unfolded pro-MMP-2-Myc/His was dialyzed against refolding buffer (50 mm Tris-HCl (pH 8.0)) containing various concentrations of Ca2+ ions and the dialyzed samples were analyzed by Western blotting with anti-Myc antibody, the results showed that homodimerization of MMP-2 was increased in the presence of Ca2+ ions (Fig. 4C). These data suggest that Ca2+ ions in the endoplasmic reticulum are required for homodimerization of MMP-2.
FIGURE 4.
Ca2+ ions are required for homodimerization of MMP-2. A, circular dichroism spectra of pro-MMP-2 in the presence or absence of Ca2+ ions. Purified pro-MMP-2 was further dialyzed against 50 mm Tris-HCl (pH 7.5) for 18 h. Pro-MMP-2 (0.2 mg/ml) was treated with or without 2 mm CaCl2 at 27 °C for 20 min, and circular dichroism spectra were obtained at 27 °C in the wavelength range of 220–260 nm. HT represents high tension. Six scans were averaged for each sample with a scan speed of 10 nm/min. mdeg, millidegrees. B, effect of thapsigargin on homodimerization of MMP-2 in COS-1 cells. Cells were treated with 0.5 μm thapsigargin for 16 h, and the conditioned medium was analyzed by Western blotting using anti-Myc antibody. Arrows indicate the monomeric and dimeric forms of pro-MMP-2 (n = 2). IB, immunoblot. C, effect of Ca2+ ions on homodimerization of MMP-2 during in vitro refolding. Purified pro-MMP-2-Myc/His was unfolded using 8 m urea in 50 mm Tris-HCl (pH 8.0). Unfolded pro-MMP-2-Myc/His was dialyzed against refolding buffer containing various concentrations of Ca2+ ions at 4 °C for 2 days, and the dialyzed samples were analyzed by Western blotting with anti-Myc antibody. The bar graph shows the ratio of dimeric to monomeric MMP-2. Data represent the mean ± S.D. of three experiments. *, p < 0.01 versus the untreated control.
MMP-2 Forms a Homodimer through an Intermolecular Disulfide Bond
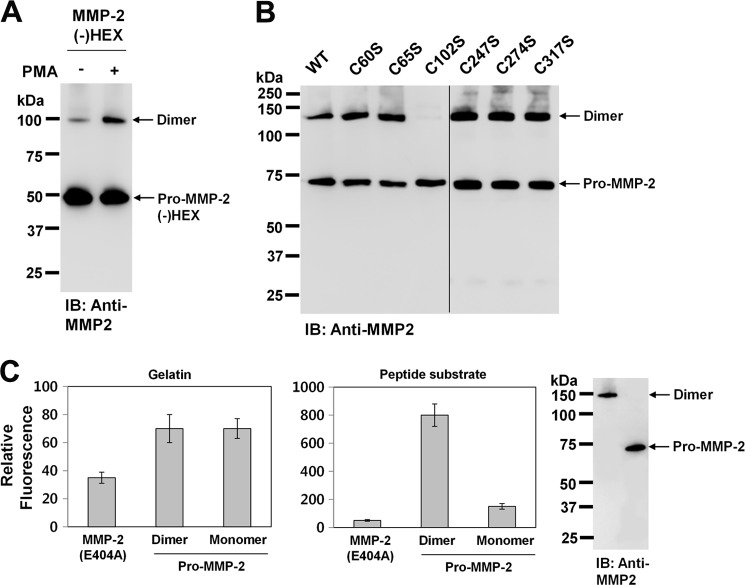
It has been reported that MMP-9 forms a reduction-sensitive homodimer through hydrophobic interactions and a salt bridge, which are mediated by hemopexin-like domains within the protease (35, 36). Thus, we examined whether hemopexin-like domains of MMP-2 also play a role in homodimerization of MMP-2. When COS-1 cells expressing hemopexin-like domain-deleted pro-MMP-2 were treated with PMA and the conditioned medium was analyzed by Western blotting with anti-MMP-2 antibody, the data showed that the MMP-2 mutant still formed a homodimer, and PMA treatment resulted in a significant increase in homodimerization of MMP-2 (Fig. 5A), ruling out the involvement of the hemopexin-like domain in homodimerization of MMP-2. The homodimer was reduction-sensitive (Fig. 1) and did not dissociate into monomers upon incubation in 8 m urea at room temperature for 24 h (data not shown). Therefore, we assumed that MMP-2 formed the homodimer through intermolecular disulfide bonds. To further determine which cysteine residue is involved in this process, cysteine residues in pro-MMP-2, except those in the hemopexin-like domain, were individually mutated to serine residues (C60S, C65S, C102S, C233S, C247S, C259S, C274S, C291S, C305S, C317S, C332S, C349S, C363S, C375S, and C395S), and COS-1 cells were transfected with these mutant plasmids. When the transfected cells were treated with PMA and the conditioned medium was analyzed by Western blotting with anti-MMP-2 antibody, the results showed that the pro-MMP-2 C102S mutant did not form the homodimer (Fig. 5B). This indicates that MMP-2 forms the homodimer through an intermolecular disulfide bond between Cys102 and the neighboring Cys102. In these experiments, MMP-2 was not detected in the conditioned media of COS-1 cells transfected with pro-MMP-2 mutants C233S, C259S, C291, C305, C332S, C349S, C363S, C375S, and C395S, possibly due to their proteasomal degradation (data not shown). Cys102 plays a role in inhibition of catalytic activity through a cysteine-zinc ion pairing (4). Therefore, we postulated that this pairing might be disrupted by the intermolecular disulfide bond in the MMP-2 homodimer, resulting in enzyme activation. To test this, we performed proteolysis experiments on fluorescein-conjugated gelatin with the MMP-2 dimer purified from the conditioned medium of COS-1 cells expressing pro-MMP-2 treated with PMA. As a negative control, the proteolytically inactive pro-MMP-2 E404A mutant was used. Our data showed that the enzymatic activity of the MMP-2 monomer was barely detectable, and the activity was not altered by homodimerization of MMP-2 (Fig. 5C, left panel). Although homodimerization of MMP-2 can open the active site, it is not likely to be big enough to cleave large substrates, including gelatin, as shown in Fig. 5C. However, the opened active site may be sufficient to allow small peptides to diffuse into the active site. Thus, we examined whether the MMP-2 homodimer could cleave low molecular weight peptides using MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2. The results showed that the MMP-2 homodimer catalyzed the proteolysis of the small peptide (Fig. 5C, right panel). Taken together, these data suggest that disruption of the cysteine-zinc ion pairing by homodimerization of MMP-2 opens the active site, but it may accommodate only small peptide substrates. Thus, MMP-2 propeptide processing is a prerequisite to gain its proteolytic activity against large substrates, including gelatin.
FIGURE 5.
MMP-2 forms a homodimer through an intermolecular disulfide bond. A, Western blot analysis using anti-MMP-2 antibody of the conditioned medium from cells expressing hemopexin-like domain-deleted pro-MMP-2 (MMP-2(−)HEX). Cells were treated with PMA (10 ng/ml) for 16 h. Arrows indicate the monomeric and dimeric forms of hemopexin-like domain-deleted pro-MMP-2 (n = 2). IB, immunoblot. B, Western blotting using anti-MMP-2 antibody of the conditioned media from cells expressing wild-type pro-MMP-2 (WT) and mutants C60S, C65S, C102S, C247S, C274S, and C317S (n = 3). C, digestion assays using fluorescein-conjugated gelatin and MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2. MMP-2 was first purified from the conditioned medium of cells expressing pro-MMP-2 treated with PMA (10 ng/ml) using gelatin-Sepharose and subsequently separated into monomeric and dimeric MMP-2 on Superdex 200 HR. The digested substrates were analyzed as described under “Experimental Procedures.” Data represent the mean ± S.D. of five experiments. The results from Western blot analysis of purified monomeric and dimeric MMP-2 using anti-MMP-2 antibody are shown on the right.
Thrombin-mediated Activation of Pro-MMP-2 Is Enhanced by Homodimerization of MMP-2
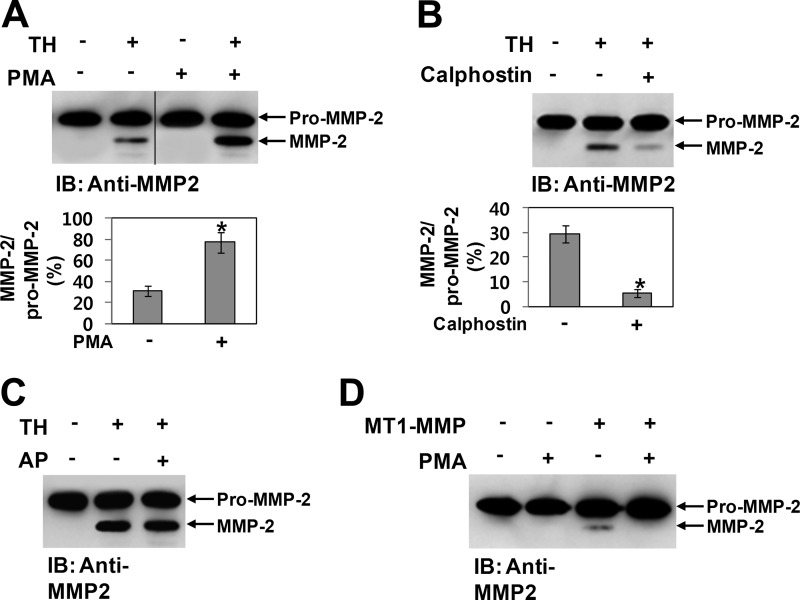
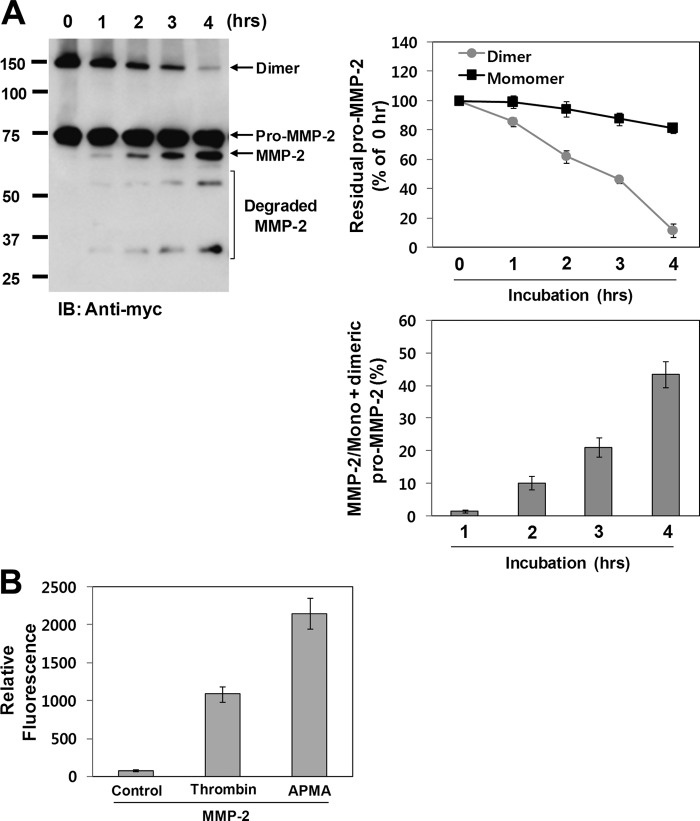
Because Cys102 is located immediately downstream of the thrombin cleavage sites (7), the cysteine-zinc ion pairing may hinder thrombin-mediated activation of pro-MMP-2. Moreover, these cleavage sites are likely to be easily accessible to thrombin following formation of the intermolecular disulfide bond due to disruption of the cysteine-zinc ion pairing. Thus, to examine whether thrombin-mediated activation of pro-MMP-2 could be increased by homodimerization of MMP-2, we compared thrombin-mediated MMP-2 activation using pro-MMP-2 from transfected COS-1 cells treated with or without PMA. Western blot data showed that thrombin-mediated MMP-2 activation was greatly increased by PMA treatment (Fig. 6A). When similar experiments were performed using calphostin C, the activation was significantly decreased by treatment with the inhibitor (Fig. 6B). Because it has been reported that MMP-2 and its phosphorylated form possess different biochemical properties (31), we tested whether the effects of PMA and calphostin C on thrombin-mediated MMP-2 activation are exerted through phosphorylation of MMP-2. When pro-MMP-2 was dephosphorylated using alkaline phosphatase and the dephosphorylated MMP-2 was incubated with thrombin under cell-associated conditions, Western blot analysis of the reaction mixture showed that thrombin-mediated MMP-2 activation was not affected by dephosphorylation of MMP-2 (Fig. 6C), confirming that MMP-2 phosphorylation does not influence thrombin-mediated activation of pro-MMP-2. Dephosphorylation of MMP-2 was confirmed by Western blotting with anti-MMP-2 antibody following two-dimensional electrophoresis (data not shown). We also examined the role of MMP-2 homodimerization in MT1-MMP-mediated activation of pro-MMP-2. When COS-1 cells coexpressing pro-MMP-2 and MT1-MMP were treated with or without PMA, Western blot analysis of the conditioned medium showed that pro-MMP-2 was activated in cells expressing MT1-MMP, but activated MMP-2 was not detected in PMA-treated cells (Fig. 6D), indicating that MT1-MMP-mediated activation of pro-MMP-2 is not increased by homodimerization of MMP-2. In this experiment, one possible explanation for the absence of activated MMP-2 in PMA-treated cells is that phosphorylation of MMP-2 may prevent its activation by MT1-MMP. Next, experiments were performed using C-terminally Myc/His-tagged pro-MMP-2, followed by immunoblotting with anti-Myc antibody. When pro-MMP-2-Myc/His was incubated with thrombin under cell-associated conditions, the MMP-2 homodimer disappeared rapidly with a concomitant increase in activated MMP-2, whereas the monomer was slightly decreased (Fig. 7A). Moreover, MMP-2 from calphostin C-treated cells that secreted exclusively the MMP-2 monomer was very slowly activated by thrombin (data not shown). Next, we tested whether thrombin does not simply cleave the MMP-2 dimer by comparing the enzymatic activity of pro-MMP-2 treated with or without thrombin. Our data showed that thrombin-processed MMP-2 from the dimer possessed the enzymatic activity, although the level of the activity was lower than that of p-aminophenylmercuric acetate-treated pro-MMP-2 (Fig. 7B). These results indicate that the MMP-2 homodimer may be more readily activated by thrombin but that the thrombin cleavage site in the monomer may not be easily accessible to thrombin, leading to resistance to thrombin cleavage. Taken together, our experimental data suggest that the MMP-2 monomer is not readily activated by thrombin and that its homodimerization enhances thrombin-mediated activation of pro-MMP-2.
FIGURE 6.
Thrombin-mediated activation of pro-MMP-2 is enhanced by homodimerization of MMP-2. A and B, Western blot analyses of 10 μg/ml pro-MMP-2 incubated with 50 nm thrombin (TH) in COS-1 cells for 4 h using anti-MMP-2 antibody. Prior to these experiments, pro-MMP-2 was purified from the conditioned medium of cells expressing pro-MMP-2 treated with or without PMA (10 ng/ml) or calphostin C (50 nm). The bar graph shows the ratio of activated MMP-2 to pro-MMP-2. Data represent the mean ± S.D. of three experiments. *, p < 0.05 versus the untreated control. IB, immunoblot. C, Western blot analysis of 10 μg/ml pro-MMP-2 incubated with 50 nm thrombin in COS-1 cells for 4 h using anti-MMP-2 antibody. Purified pro-MMP-2 was pretreated with or without calf intestinal alkaline phosphatase (AP; 50 units/ml) at 37 °C for 1 h (n = 2). D, Western blotting of the conditioned medium from cells coexpressing pro-MMP-2 and MT1-MMP treated with or without PMA (10 ng/ml) using anti-MMP-2 antibody (n = 2).
FIGURE 7.
A, Western blot analysis of 10 μg/ml pro-MMP-2-Myc/His incubated with 50 nm thrombin in COS-1 cells for the indicated times using anti-Myc antibody. Pro-MMP-2 was purified from the conditioned medium of PMA (10 ng/ml)-treated cells expressing pro-MMP-2-Myc/His. The line graph shows the ratio of pro-MMP-2 (monomeric or dimeric) to pro-MMP-2 (monomeric or dimeric) at 0 h. Data represent the mean ± S.D. of three experiments. The bar graph shows the ratio of activated MMP-2 to monomeric plus dimeric MMP-2. Data represent the mean ± S.D. of three experiments. IB, immunoblot. B, digestion assay of fluorescein-conjugated gelatin. MMP-2 was purified from the conditioned medium of cells expressing pro-MMP-2 treated with or without thrombin (50 nm) or p-aminophenylmercuric acetate (APMA; 0.5 mm) for 6 h. The digested substrates were analyzed as described under “Experimental Procedures.” Data represent the mean ± S.D. of five experiments.
DISCUSSION
The data provided herein include several novel observations. First, MMP-2 forms a reduction-sensitive homodimer in a controlled manner. Second, homodimerization of MMP-2 is not linked to PKC-mediated phosphorylation of MMP-2. Third, Ca2+ ions are required for homodimerization of MMP-2. Fourth, MMP-2 forms the homodimer through an intermolecular disulfide bond between Cys102 and the neighboring Cys102. Finally, homodimerization of MMP-2 enhances thrombin-mediated activation of pro-MMP-2.
Protein dimerization or oligomerization plays a key role in the regulation of proteins such as enzymes, ion channels, receptors, and transcription factors. Protein dimerization results in the formation of hetero- and homodimers. Heterodimers are formed when two different molecules are linked, and proteins exist as homodimers when the same molecules are linked together. Proteins are covalently linked to each other either through disulfide bonds or through hydrophobic interactions and/or salt bridges for dimerization to occur. Some snake venom metalloproteases form homodimers through disulfide bonds (37, 38). In addition, dimeric forms of MT1-MMP and MMP-9 have been reported (35, 39–41). These studies show that MT1-MMP forms a homodimer through noncovalent interactions in the hemopexin-like domains (39). Likewise, MMP-9 forms a homodimer through hydrophobic interactions and a salt bridge, which are mediated by the hemopexin-like domain (36). MMP-9 also forms heterodimers with various molecules, including TIMP-1 and TIMP-3 (27), MMP-1 (42), gelatinase B-associated lipocalin (43), and proteoglycans (41), through covalent or noncovalent interactions via the hemopexin-like domain. MMP-2 is known to form heterodimers with TIMPs and glycosaminoglycans via the hemopexin-like domain of the enzyme (26–28), but its homodimerization has not been reported. In this study, we have shown that the hemopexin-like domain of MMP-2 is not involved in its homodimerization, unlike in MT1-MMP and MMP-9. Another difference is that MMP-2 forms a homodimer through an intermolecular disulfide bond formed between Cys102 and the neighboring Cys102. This cysteine residue plays a role in inhibition of catalytic activity through a cysteine-zinc ion pairing (4). Our observation that a cysteine residue in the cysteine-zinc ion pairing is involved in the disulfide bond formation is, to our knowledge, a novel finding in the protease field.
Several studies have shown that protein dimerization is regulated by phosphorylation. Dimerization of receptor tyrosine kinases is induced by tyrosine phosphorylation of the cytosolic domains (44). Moreover, homodimerization of Mel-18, a polycomb group protein, is inhibited by PKC-meditated phosphorylation (45). However, our experimental data show that homodimerization of MMP-2 is not associated with PKC-mediated phosphorylation of MMP-2, although we cannot exclude the possibility that structural changes in MMP-2 by PKC-mediated phosphorylation may affect the homodimerization.
The disulfide bond is not likely to be formed between Cys102 and the neighboring Cys102 because this cysteine residue interacts with the catalytic Zn2+ ion via its side chain thiol group (4). Thus, we suggest that the coordination of Cys102 with the catalytic Zn2+ ion may be prevented by Ca2+ ion-induced conformational changes in MMP-2 that result in the formation of the disulfide bond. MMP-2 can bind two Ca2+ ions in the catalytic domain, one within the S-shaped loop and the other bound by two peripheral loops (46). There are several reports showing Ca2+ ion-dependent protein dimerization. However, unlike homodimerization of MMP-2, Ca2+ ion-mediated dimerization of these proteins occurs mainly through noncovalent interactions. For example, Ca2+ ion promotes dimerization of a subtilisin-like protease from a Bacillus subtilis strain through electrostatic interactions (47). Ca2+ ion is also involved in homodimerization of E-cadherin through hydrogen bonds (48). Through interactions with amino acid residues in the linker region of E-cadherin, Ca2+ ion structures a large component of the 2-fold symmetric dimer interface (48). Ca2+ ions are also implicated in the folding and disulfide bond formation of several proteins (49–51). In the absence of Ca2+ ions, negligible amounts of α-lactalbumin are refolded, whereas substantial renaturation is attained in the presence of Ca2+ ions (49). Moreover, folding intermediates of α-lactalbumin contain only two native disulfide bonds in the presence of Ca2+ ions and subsequently form the native structure when Ca2+ ions bind to the β-sheet calcium-binding domain (50). In the case of α-amylase, Ca2+ ion acts as an anti-aggregatory agent (51). Taken together, these data suggest that Ca2+ is a major mediator in protein folding and dimerization.
Cell signaling is initiated by dimerization of receptor tyrosine kinases (44). MT1-MMP forms a homodimer that keeps MT1-MMP molecules close together to facilitate pro-MMP-2 activation (39). The subtilisin-like serine protease from the B. subtilis strain maintains its stability and catalytic activity through dimerization (47). As described above, protein dimerization is essential for various biological functions of proteins. Therefore, we also investigated the biological role of MMP-2 homodimerization. Thrombin preferentially cleaves pro-MMP-2 immediately downstream of Arg101, resulting in MMP-2 activation (7). However, this cleavage site is likely to be protected from thrombin cleavage by adjacent cysteine-zinc ion pairing. This hypothesis is supported by our experimental data showing that MMP-2 monomers from calphostin C-treated cells are very slowly activated by thrombin (data not shown). Moreover, the MMP-2 homodimer does not possess catalytic activity against large substrates, although it catalyzes the proteolysis of small peptides. Thus, we suggest that one of the biological functions for MMP-2 homodimerization is enhancement of thrombin-mediated MMP-2 activation.
There are a number of small biological peptides (e.g. calcitonin gene-related peptide) that are cleaved by MMP-2 (52). Thus, the fact that the MMP-2 homodimer can cleave low molecular weight peptides suggests an interesting pathophysiological function of the MMP-2 homodimer without cleavage of the propeptide.
In conclusion, we propose a novel regulatory mechanism for thrombin-mediated activation of pro-MMP-2 that is modulated through homodimerization of MMP-2. Ca2+ ions may induce conformational changes in MMP-2 that result in prevention of cysteine-zinc ion pairing and subsequent formation of the disulfide bond. Finally, this homodimerization results in the proteolysis of small peptide substrates without cleavage of the propeptide and enhances thrombin-mediated activation of pro-MMP-2 for proteolysis of large substrates.
This work was supported by the Future-based Technology Development Program through the National Research Foundation of Korea (NRF) funded by Ministry of Education, Science, and Technology Grant 2009-0081760.
- MMP
- matrix metalloproteinase
- TIMP
- tissue inhibitor of metalloproteases
- MT1-MMP
- membrane-type 1 MMP
- PMA
- phorbol 12-myristate 13-acetate
- MCA
- (7-methoxycoumarin-4-yl)acetyl
- DPA
- N-3-(2,4-dinitrophenyl)-l-α,β-diaminopropionyl.
REFERENCES
- 1. Somerville R. P., Oblander S. A., Apte S. S. (2003) Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 4, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woessner J. F., Jr. (1994) The family of matrix metalloproteinases. Ann. N.Y. Acad. Sci. 732, 11–21 [DOI] [PubMed] [Google Scholar]
- 3. Okada Y., Morodomi T., Enghild J. J., Suzuki K., Yasui A., Nakanishi I., Salvesen G., Nagase H. (1990) Matrix metalloproteinase-2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymatic properties. Eur. J. Biochem. 194, 721–730 [DOI] [PubMed] [Google Scholar]
- 4. Ra H. J., Parks W. C. (2007) Control of matrix metalloproteinase catalytic activity. Matrix Biol. 26, 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bescond A., Augier T., Chareyre C., Garçon D., Hornebeck W., Charpiot P. (1999) Influence of homocysteine on matrix metalloproteinase-2: activation and activity. Biochem. Biophys. Res. Commun. 263, 498–503 [DOI] [PubMed] [Google Scholar]
- 6. Baramova E. N., Bajou K., Remacle A., L'Hoir C., Krell H. W., Weidle U. H., Noel A., Foidart J. M. (1997) Involvement of PA/plasmin system in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 405, 157–162 [DOI] [PubMed] [Google Scholar]
- 7. Koo B. H., Park M. Y., Jeon O. H., Kim D. S. (2009) Regulatory mechanism of matrix metalloprotease-2 enzymatic activity by factor Xa and thrombin. J. Biol. Chem. 284, 23375–23385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson M. T., Smith M. M., Smith S. M., Jackson C. J., Xue M., Little C. B. (2009) Activation of cartilage matrix metalloproteinases by activated protein C. Arthritis Rheum. 60, 780–791 [DOI] [PubMed] [Google Scholar]
- 9. Chen J. M., Dando P. M., Stevens R. A., Fortunato M., Barrett A. J. (1998) Cloning and expression of mouse legumain, a lysosomal endopeptidase. Biochem. J. 335, 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strongin A. Y., Collier I., Bannikov G., Marmer B. L., Grant G. A., Goldberg G. I. (1995) Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 270, 5331–5338 [DOI] [PubMed] [Google Scholar]
- 11. Morrison C. J., Butler G. S., Bigg H. F., Roberts C. R., Soloway P. D., Overall C. M. (2001) Cellular activation of MMP-2 (gelatinase A) by MT2-MMP occurs via a TIMP-2-independent pathway. J. Biol. Chem. 276, 47402–47410 [DOI] [PubMed] [Google Scholar]
- 12. Nakada M., Yamada A., Takino T., Miyamori H., Takahashi T., Yamashita J., Sato H. (2001) Suppression of membrane-type 1 matrix metalloproteinase (MMP)-mediated MMP-2 activation and tumor invasion by testican 3 and its splicing variant gene product, N-Tes. Cancer Res. 61, 8896–8902 [PubMed] [Google Scholar]
- 13. Pei D. (1999) Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J. Biol. Chem. 274, 8925–8932 [DOI] [PubMed] [Google Scholar]
- 14. Nie J., Pei D. (2003) Direct activation of pro-matrix metalloproteinase-2 by leukolysin/membrane-type 6 matrix metalloproteinase/matrix metalloproteinase 25 at the Asn109–Tyr bond. Cancer Res. 63, 6758–6762 [PubMed] [Google Scholar]
- 15. Kahan C., Seuwen K., Meloche S., Pouysségur J. (1992) Coordinate, biphasic activation of p44 mitogen-activated protein kinase and S6 kinase by growth factors in hamster fibroblasts. Evidence for thrombin-induced signals different from phosphoinositide turnover and adenylyl cyclase inhibition. J. Biol. Chem. 267, 13369–13375 [PubMed] [Google Scholar]
- 16. Chen D., Carpenter A., Abrahams J., Chambers R. C., Lechler R. I., McVey J. H., Dorling A. (2008) Protease-activated receptor 1 activation is necessary for monocyte chemoattractant protein 1-dependent leukocyte recruitment in vivo. J. Exp. Med. 205, 1739–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ozaki Y., Nishimura M., Sekiya K., Suehiro F., Kanawa M., Nikawa H., Hamada T., Kato Y. (2007) Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 16, 119–129 [DOI] [PubMed] [Google Scholar]
- 18. McNamara C. A., Sarembock I. J., Gimple L. W., Fenton J. W., 2nd, Coughlin S. R., Owens G. K. (1993) Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J. Clin. Invest. 91, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henderson N., Markwick L. J., Elshaw S. R., Freyer A. M., Knox A. J., Johnson S. R. (2007) Collagen I and thrombin activate MMP-2 by MMP-14-dependent and -independent pathways: implications for airway smooth muscle migration. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L1030–L1038 [DOI] [PubMed] [Google Scholar]
- 20. Wang Z., Kong L., Kang J., Morgan J. H., 3rd, Shillcutt S. D., Robinson J. S., Jr., Nakayama D. K. (2009) Thrombin stimulates mitogenesis in pig cerebrovascular smooth muscle cells involving activation of pro-matrix metalloproteinase-2. Neurosci. Lett. 451, 199–203 [DOI] [PubMed] [Google Scholar]
- 21. Galis Z. S., Kranzhöfer R., Fenton J. W., 2nd, Libby P. (1997) Thrombin promotes activation of matrix metalloproteinase-2 produced by cultured vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 17, 483–489 [DOI] [PubMed] [Google Scholar]
- 22. Lafleur M. A., Hollenberg M. D., Atkinson S. J., Knäuper V., Murphy G., Edwards D. R. (2001) Activation of pro-matrix metalloproteinase-2 (pro-MMP-2) by thrombin is membrane-type MMP-dependent in human umbilical vein endothelial cells and generates a distinct 63-kDa active species. Biochem. J. 357, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazzieri R., Masiero L., Zanetta L., Monea S., Onisto M., Garbisa S., Mignatti P. (1997) Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 16, 2319–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen M., Arkell J., Jackson C. J. (1999) Thrombin rapidly and efficiently activates gelatinase A in human microvascular endothelial cells via a mechanism independent of active MT1 matrix metalloproteinase. Lab. Invest. 79, 467–475 [PubMed] [Google Scholar]
- 25. Koo B. H., Han J. H., Yeom Y. I., Kim D. S. (2010) Thrombin-dependent MMP-2 activity is regulated by heparan sulfate. J. Biol. Chem. 285, 41270–41279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iida J., Wilhelmson K. L., Ng J., Lee P., Morrison C., Tam E., Overall C. M., McCarthy J. B. (2007) Cell surface chondroitin sulfate glycosaminoglycan in melanoma: role in the activation of pro-MMP-2 (pro-gelatinase A). Biochem. J. 403, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagase H., Visse R., Murphy G. (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 69, 562–573 [DOI] [PubMed] [Google Scholar]
- 28. Crabbe T., Ioannou C., Docherty A. J. (1993) Human pro-gelatinase A can be activated by autolysis at a rate that is concentration-dependent and enhanced by heparin bound to the C-terminal domain. Eur. J. Biochem. 218, 431–438 [DOI] [PubMed] [Google Scholar]
- 29. Newton A. C., Johnson J. E. (1998) Protein kinase C: a paradigm for regulation of protein function by two membrane-targeting modules. Biochim. Biophys. Acta 1376, 155–172 [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi E., Nakano H., Morimoto M., Tamaoki T. (1989) Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 159, 548–553 [DOI] [PubMed] [Google Scholar]
- 31. Sariahmetoglu M., Crawford B. D., Leon H., Sawicka J., Li L., Ballermann B. J., Holmes C., Berthiaume L. G., Holt A., Sawicki G., Schulz R. (2007) Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 21, 2486–2495 [DOI] [PubMed] [Google Scholar]
- 32. Oishi K., Yamaguchi M. (1994) Effect of phorbol 12-myristate 13-acetate on Ca2+-ATPase activity in rat liver nuclei. J. Cell Biochem. 55, 168–172 [DOI] [PubMed] [Google Scholar]
- 33. Zhu D. M., Narla R. K., Fang W. H., Chia N. C., Uckun F. M. (1998) Calphostin C triggers calcium-dependent apoptosis in human acute lymphoblastic leukemia cells. Clin. Cancer Res. 4, 2967–2976 [PubMed] [Google Scholar]
- 34. Thastrup O., Cullen P. J., DrøbakØ B. K., Hanley M. R., Dawson A. P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olson M. W., Bernardo M. M., Pietila M., Gervasi D. C., Toth M., Kotra L. P., Massova I., Mobashery S., Fridman R. (2000) Characterization of the monomeric and dimeric forms of latent and active matrix metalloproteinase-9. Differential rates for activation by stromelysin 1. J. Biol. Chem. 275, 2661–2668 [DOI] [PubMed] [Google Scholar]
- 36. Cha H., Kopetzki E., Huber R., Lanzendörfer M., Brandstetter H. (2002) Structural basis of the adaptive molecular recognition by MMP-9. J. Mol. Biol. 320, 1065–1079 [DOI] [PubMed] [Google Scholar]
- 37. Nikai T., Taniguchi K., Komori Y., Masuda K., Fox J. W., Sugihara H. (2000) Primary structure and functional characterization of bilitoxin-1, a novel dimeric P-II snake venom metalloproteinase from Agkistrodon bilineatus venom. Arch. Biochem. Biophys. 378, 6–15 [DOI] [PubMed] [Google Scholar]
- 38. Cominetti M. R., Ribeiro J. U., Fox J. W., Selistre-de-Araujo H. S. (2003) BaG, a new dimeric metalloproteinase/disintegrin from the Bothrops alternatus snake venom that interacts with α5β1 integrin. Arch. Biochem. Biophys. 416, 171–179 [DOI] [PubMed] [Google Scholar]
- 39. Itoh Y., Takamura A., Ito N., Maru Y., Sato H., Suenaga N., Aoki T., Seiki M. (2001) Homophilic complex formation of MT1-MMP facilitates pro-MMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 20, 4782–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. (1985) Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J. Biol. Chem. 260, 2493–2500 [PubMed] [Google Scholar]
- 41. Winberg J. O., Kolset S. O., Berg E., Uhlin-Hansen L. (2000) Macrophages secrete matrix metalloproteinase-9 covalently linked to the core protein of chondroitin sulfate proteoglycans. J. Mol. Biol. 304, 669–680 [DOI] [PubMed] [Google Scholar]
- 42. Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. (1992) Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J. Biol. Chem. 267, 4583–4591 [PubMed] [Google Scholar]
- 43. Kjeldsen L., Johnsen A. H., Sengeløv H., Borregaard N. (1993) Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 268, 10425–10432 [PubMed] [Google Scholar]
- 44. Schlessinger J. (2000) Cell signaling by receptor tyrosine kinases. Cell 103, 211–225 [DOI] [PubMed] [Google Scholar]
- 45. Fujisaki S., Ninomiya Y., Ishihara H., Miyazaki M., Kanno R., Asahara T., Kanno M. (2003) Dimerization of the polycomb group protein Mel-18 is regulated by PKC phosphorylation. Biochem. Biophys. Res. Commun. 300, 135–140 [DOI] [PubMed] [Google Scholar]
- 46. Morgunova E., Tuuttila A., Bergmann U., Isupov M., Lindqvist Y., Schneider G., Tryggvason K. (1999) Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science 284, 1667–1670 [DOI] [PubMed] [Google Scholar]
- 47. Mikhailova E. O., Mardanova A. M., Balaban N. P., Rudenskaya G. N., Ilyinskaya O. N., Sharipova M. R. (2009) Biochemical properties of Bacillus intermedius subtilisin-like proteinase secreted by a Bacillus subtilis recombinant strain in its stationary phase of growth. Biochemistry 74, 308–315 [DOI] [PubMed] [Google Scholar]
- 48. Nagar B., Overduin M., Ikura M., Rini J. M. (1996) Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 380, 360–364 [DOI] [PubMed] [Google Scholar]
- 49. Rao K. R., Brew K. (1989) Calcium regulates folding and disulfide bond formation in α-lactalbumin. Biochem. Biophys. Res. Commun. 163, 1390–1396 [DOI] [PubMed] [Google Scholar]
- 50. Chang J. Y., Li L. (2002) Pathway of oxidative folding of α-lactalbumin: a model for illustrating the diversity of disulfide folding pathways. Biochemistry 41, 8405–8413 [DOI] [PubMed] [Google Scholar]
- 51. Yazdanparast R., Khodarahmi R. (2005) The combined effects of two anti-aggregatory agents, α-cyclodextrin and Ca2+, on the refolding process of denatured α-amylase. Biotechnol. Appl. Biochem. 41, 157–162 [DOI] [PubMed] [Google Scholar]
- 52. Fernandez-Patron C., Stewart K. G., Zhang Y., Koivunen E., Radomski M. W., Davidge S. T. (2000) Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ. Res. 87, 670–676 [DOI] [PubMed] [Google Scholar]