Abstract
Retinoic acid (RA) is used to treat leukemia and other cancers through its ability to promote cancer cell differentiation. Strategies to enhance the anti-cancer effects of RA could deepen and broaden its beneficial therapeutic applications. In this study, we describe a receptor crosstalk system that addresses this issue. RA effects are mediated by RAR/RXR receptors that we show are modified by interactions with the aryl hydrocarbon receptor (AhR), a protein functioning both as a transcription factor and a ligand-dependent adaptor in an ubiquitin ligase complex. RAR/RXR and AhR pathways crosstalk at the levels of ligand-receptor and also receptor - promoter interactions. Here we assessed the role of AhR during RA-induced differentiation and an hypothesized convergence at Oct4, a transcription factor believed to maintain stem cell characteristics. RA upregulated AhR and downregulated Oct4 during differentiation of HL-60 promyelocytic leukemia cells. AhR overexpression in stable transfectants downregulated Oct4 and also decreased ALDH1 activity, another stem cell associated factor, enhancing RA-induced differentiation as indicated by cell differentiation markers associated with early (CD38 and CD11b) and late (neutrophilic respiratory burst) responses. AhR overexpression also increased levels of activated Raf1, which is known to help propel RA-induced differentiation. RNAi-mediated knockdown of Oct4 enhanced RA-induced differentiation and G0 cell cycle arrest relative to parental cells. Consistent with the hypothesized importance of Oct4 downregulation for differentiation, parental cells rendered resistant to RA by biweekly high RA exposure displayed elevated Oct4 levels that failed to be downregulated. Together, our results suggested that therapeutic effects of RA-induced leukemia differentiation depend on AhR and its ability to downregulate the stem cell factor Oct4.
Keywords: AhR, Oct4, differentiation, neutrophil, HL-60
Introduction
Because malignant cell transformation is often associated with a maturational block, mechanisms of overcoming the differentiation block have engendered therapeutic interest. Retinoids have been shown to induce differentiation and have antiproliferative activities against skin, head and neck, breast, uterine, cervical and liver cancer, although the most effective activity is against acute promyelocytic leukemia (1–3). Retinoic acid (RA) is known to induce cell differentiation through RAR/RXR nuclear receptor activation. Aryl-hydrocarbon receptor (AhR) is another nuclear receptor with a proposed role in differentiation. Recently, AhR has been shown to propel breast cancer (4) and liver cancer (5) cell differentiation. AhR has been found to be expressed in all tissues analyzed. It is present in the cytosol and in the nuclei. Two AhR functions are known, both being ligand dependent. It is a basic helix-loop-helix/Per-Arnt-Sim (bHLH-PAS) transcription factor (6), and also an adaptor in the cullin 4B ubiquitin ligase complex (7). The transcriptional activity is the most studied, especially in the regulation of detoxification enzymes such as CYP1A1 (8). The role in the ubiquitin complex is emerging and has been found to be important for estrogen receptor α and androgen receptor degradation (9).
It has been shown that a limited number of transcription factors are needed to induce the self-renewing pluripotent stem cell state (10–12). Yamanaka and coworkers proposed Oct4, SOX2, KLF4 and c-Myc (10), whereas Thomson and coworkers proposed Oct4, SOX2, NANOG and Lin28 (11), as essential factors for inducing the self-renewal stem cell state. A number of subsequent publications showed that, under certain conditions, the number can be further reduced to Oct4, SOX2, NANOG (13), or only Oct4 (14). Thus, Oct4 is the only currently known essential regulator/inducer of induced pluripotent stem cells (iPS) amongst the Yamanaka/Thomson factors. As such, Oct4 becomes a prominent candidate as a regulator of cell differentiation caused by the embryonic morphogen, retinoic acid.
There are reasons to suspect that RAR/RXR, AhR and Oct4 controlled pathways are inter-related. The RAR/RXR and AhR pathways are known to crosstalk. For example, these receptors compete for SMRT protein and are upregulated by the same chemicals (15). In the case of the estrogen receptor (ER), another nuclear receptor which can also form heterocomplexes with RXR, AhR binds to ER response elements in target gene promoters (16). AhR can also target ER for degradation, a manifestation of the AhR cytosolic function (7). There may ergo also be multiple levels of crosstalk between AhR and RAR /RXR. Some findings suggest that cross-talk between the AhR and RA pathways occurs during cell differentiation. Teratogenic effects such as cleft palate and hydronephrosis can be induced by retinoids (17) and also by an AhR agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (18). In fish, RA and its receptors are required both for AhR transcription and embryonic development of blood vessels and bones (19).
We conducted an in silica search for putative response elements in the Oct4 promoter/enhancer region using Genomatix software. We have found very close putative response elements for AhR/ARNT(225 to 249+) and RAR/RXR (230 to 254+) in Oct4B 5’ sequences, and also AhR/ARNT(82 to 106+) and RAR/RXR (108 to 132+) in Oct4A, the form with stem cell promoting properties, 5’ sequences. There was also another AhR site in each promoter region and another four RXR and seven RXR sites for Oct4B and Oct4A respectively. It is indeed known that the Oct4 promoter contains retinoic acid response elements responsible for Oct4 repression (20), but this might occur in cooperation with AhR in view of the sequence analysis, as well as the coincidence of teratogenic effects driven by activating the RA or AhR pathways.
The present report shows that RA upregulates AhR expression and downregulates Oct4 when inducing the myeloid differentiation of HL-60 human leukemia cells. If these changes are of functional significance, we expect cells overexpressing AhR to differentiate faster than the parent cell line after RA treatment. Likewise we expect cells expressing less Oct4 (Oct4 knockdown cells) to differentiate faster than their parent cell line. We created these stable transfectants and found that this was the case. AhR overexpression downregulated Oct4 and increased the amount of activated Raf1, which is known to drive RA-induced differentiation; and differentiation was enhanced. Differentiation was measured by the CD38 and CD11b cell surface markers and by the functional differentiation marker, inducible oxidative metabolism, namely the respiratory burst characteristic of mature myeloid cells. Oct4 siRNA knockdown enhanced RA-induced differentiation, too. The results suggest that RA-induced differentiation depends on AhR and the mechanism involves downregulation of Oct4. The HL-60 cell line, a human myeloid (FAB M2) precursor cell capable of induced myeloid or monocytic differentiation was used as an experimental system for RA-induced myeloid differentiation. It is an archetype model for the mechanism of action of RA that has been highly studied with many specific features of the RA-induced differentiation process very well defined. It is also a PML-RARα negative system still responsive to RA, and therefore it has the potential to highlight pathways involved in RA-induced differentiation that might be exploited in cancers other than APL.
Materials and Methods
Cell culture and treatments
HL-60 human myeloblastic leukemia cells were grown in RPMI 1640 supplemented with 5% heat-inactivated fetal bovine serum (both: Invitrogen, Carlsbad, CA) and 1x antibiotic/antimycotic (Sigma, St. Louis, MO) in a 5% CO2 humidified atmosphere at 37°C. The cell lines were derived from the original isolates and were a generous gift of Dr. Robert Gallagher and maintained in this laboratory. Retinoic acid resistant cells (RH) were created by maintaining the cells in high density cultures (above 2×106 cells/ml) and treating them with 9 µM RA once every two weeks. For treatments, all trans retinoic acid (RA) (Sigma, St. Louis, MO) was added from a 0.5 mM stock solution in ethanol with a final concentration of 1 µM in culture. Valproic acid (VPA) was added to a final concentration if 1 mM. The VPA treatment was 4 h before the RA treatment, and all the times of indicated endpoints are with respect to RA treatment as the start. Experimental cultures were initiated at a density of 0.1 × 106 cells/ml. The RH cells were kept at normal density and no RA for a week before the experiments. Viability was monitored by 0.2% trypan blue (Invitrogen, Calsbad, CA) exclusion and routinely exceeded 95%. All reagents were purchased from Sigma (St Louis, MO) unless otherwise mentioned.
Ectopic expression
The AhR overexpressor in pCMV6-XL4 vector was obtained from OriGene (Rockville, MD). The Oct4 siRNA in U6.1/Neo was purchased from GenScript (Piscataway, NJ). For transfection in HL- 60, 50 µg plasmid and 50 µl lipofectamine (2000CD, Invitrogen, Calsbad, CA) were incubated in 450 µl serum free RPMI on ice for 15 min, then used to resuspend 10 × 106 cells and electroporated immediately at 300 mV.
CD38, CD11b quantification
Expression of cell surface differentiation markers was quantified by flow cytometry. 0.5 × 106 cells were collected from cultures and centrifuged at 1000 rpm in a microfuge for 5 min. Cell pellets were resuspended in 200 µl 37° C PBS containing 2.5 µl of antibody, APC or PE conjugated CD11b or CD38, as indicated in Results section (all from BD Biosciences, San Jose, CA). Following 1 h incubation at 37°C, cell surface expression levels were analyzed by flow cytometry (BD LSRII flow cytometer, BD Biosciences, San Jose, CA). APC fluorescence is excited at 633 nm and collected with a 660/20 band pass filter. PE fluorescence is excited at 488 nm and collected with a 576/26 band pass filter. Undifferentiated control cells were used to determine the fluorescence intensity of cells negative for the respective surface antigen. The gate to determine percent increase of expression was set to exclude 95% of the control population.
Respiratory burst quantification
Respiratory burst, a functional differentiation marker, was measured by flow cytometry. 1 × 106 cells were collected and centrifuged at 1000 rpm for 5 min in a microfuge. Cell pellets were resuspended in 500 µl 37°C PBS containing 5 µM 5-(and-6)-chloromethyl-2’,7’-dichlorodihydro–fluorescein diacetate acetyl ester (H2-DCF, Molecular Probes, Eugene, OR) and 0.2 µg/ml 12-o-tetradecanoylphorbol-13-acetate (TPA, Sigma, St. Louis, MO). Both, H2-DCF and TPA stock solutions were made in DMSO at concentrations of 0.2 mg/ml and 5 mM, respectively. A control group incubated in H2-DCF and DMSO only was included. Cells were incubated for 20 min at 37° C prior to analysis by flow cytometry (BD LSRII). Oxidized DCF was excited by a 488 nm laser and emission collected with a 530/30 nm band pass filter. The shift in fluorescence intensity in response to TPA was used to determine the percentage of cells capable of inducible oxidative metabolism (21).Gates to determine percent positive cells were set to exclude 95% of control cells not stimulated with TPA.
Cell cycle quantification
1 × 106 cells were collected by centrifugation and resuspended in 200 µl of cold propidium iodide (PI) hypotonic staining solution containing 50 µg/ml propidium iodine, 1 µl/ml Triton X-100, and 1 mg/ml sodium citrate. Cells were incubated at room temperature for 1 h and analyzed by flow cytometry (BD LSRII) using 488-nm excitation and emission collected with a 576/26 band-pass filter. Doublets were identified by a PI signal width versus area plot and excluded from the analysis (21, 22).
Nuclear Oct4 quantification
1 × 106 cells were collected from cultures and centrifuged at 1000 rpm in a microfuge for 5 min, resuspended in 200 µl of cold propidium iodide hypotonic solution as previously described for nuclei isolation (23), incubated on ice 15 min, centrifuged again at 1000 rpm for 5 min, fixed by resuspension in 100 µl of PBS with 2% paraformaldehyde (Alfa Aesar, Ward Hill, MA), incubated at room temperature for 10 min followed by addition of 900 µl of ice cold methanol to obtain a 90% methanol solution. Following an incubation for 1 h at -20° C samples were washed 2 times in hypotonic solution and resuspended in 200 µl propidium iodide hypotonic solution containing 2.5 µl of Oct4 (Santa Cruz, CA) primary antibody. Following a 4° C over night incubation period, nuclei were washed once with 1 ml propidium iodide hypotonic solution and stained with secondary AlexaFluor 350 goat anti-rabbit antibody for 1 h and then analyzed by flow cytometry (BD LSRII) after one wash in PBS. Excitation was at 325 nm and emission was collected with a 440/40 band-pass filter. Nuclei unstained with the primary antibody but incubated with the secondary antibody were used to generate the background signal. Logic gates were set to include all the nuclei and the results are given as percentage of the mean fluorescence intensity of untreated nuclei.
Aldehyde dehydrogenase enzymatic activity assay
ALDH1 enzymatic activity was measured using the Aldefluor kit (Stem Cell Technologies, Durham, NC) as described by Ginestier et. al. (24), with the modifiication that the cells were incubated with the substrate at 37°C for 50 min instead of 40 min.
Protein detection by Western blot
2 × 107 cells were lysed for total lysate using 200 µL lysis buffer (Pierce) and lysates were cleared by centrifugation at 13,000 rpm for 20 min at 4° C after 3 cycles of freeze-thaw. For detection of nuclear proteins, 100 µL hypotonic lysis buffer was used to obtain the nuclei which were then lysed in RIPA buffer (Sigma) as previously described (23). For AhR detection, 25µg protein (total and nuclear lysate ) was resolved on a 7.5% polyacrylamide gel. The same amount of protein was used on 12% gels for the other proteins. The electro-transfer was for 1 h at 300 mA. The membranes were incubated with the indicated primary antibody at 4° C overnight. Anti AhR antibody was from Santa Cruz Biotechnology. All the others were from Cell Signaling. Horseradish peroxidase–linked, anti-mouse (for ERK1/2) anti-rabbit (for all others) IgG secondary antibodies (Cell Signaling) and ECL (GE Healthcare, Pittsburg, PA) were used for detection. All blots were repeated 3 times.
Statistical analysis
Statistical analyses were performed using SYSTAT 8.0 software. Means of treatment groups of interest were compared using the Paired-Samples T Test. All treatment groups were compared to control cells at the same time point. The data represents the means of three repeats ± S.E.M. A p-value of < 0.05 was considered significant.
Results
RA-induced differentiation correlates with increased AhR levels and decreased Oct4 nuclear levels
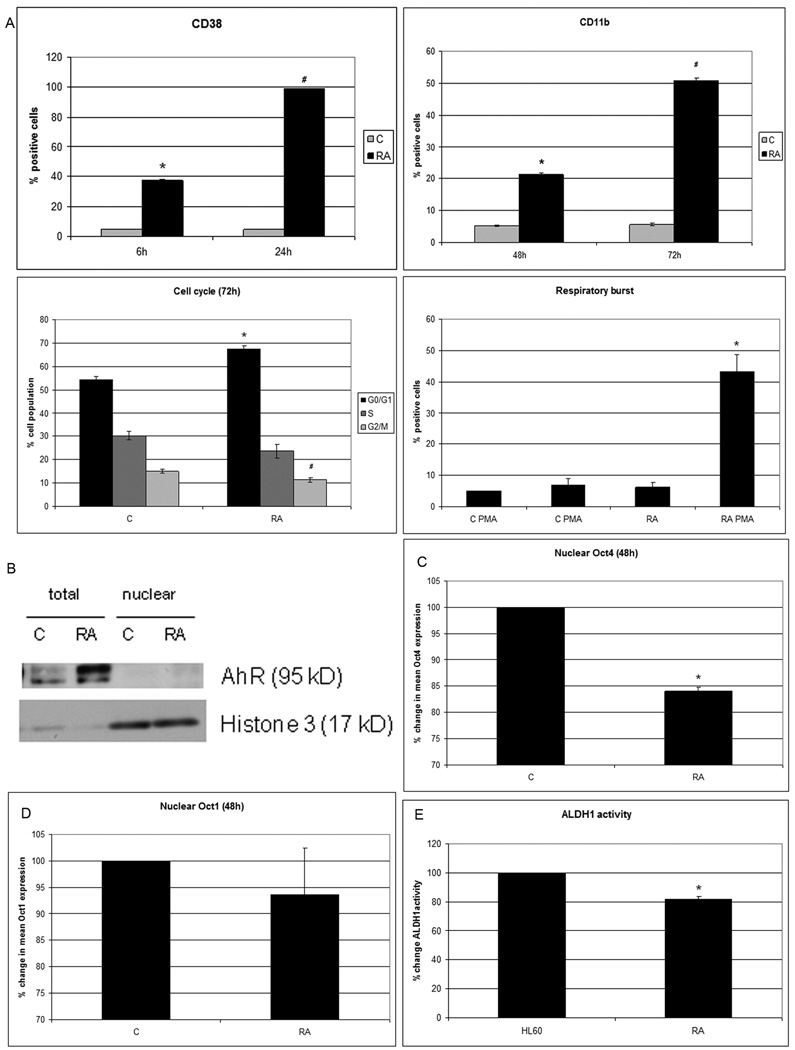
RA treatment of HL-60 cells induces expression of a series of phenotypic markers consistent with induced differentiation. CD38 receptor expression can be detected within 6 hours of treatment, and 100% of the cells express it within 24 hours. CD38 is a type II transmembrane glycoprotein, expressed on several leukocytes and early hematopoietic precursor cells (25), that can signal through Raf and ERK activation(26). Expression of CD11b, an integrin receptor subunit that is also a marker for myeloid differentiation, occurs with slower kinetics than CD38 post RA treatment. After expression of these markers G0 cell cycle arrest becomes apparent by approximately 72 hours of RA treatment. Likewise inducible oxidative metabolism, a functional marker that is the antimicrobial respiratory burst of neutrophils, also finally becomes apparent (Fig.1A). As RA induces this phenotypic conversion, AhR expression is upregulated without nuclear translocation (Fig.1B) and nuclear Oct4 protein abundance is downregulated (Fig.1C). The upper band on the AhR detected on the 7.5% gel may reflect phosphorylation induced gel mobility retardation since AhR is well known to be phosphorylated upon activation (27). In contrast to Oct4, Oct1 is not downregulated (Fig.1D). We have previously shown that BLR1 receptor expression is essential for RA-induced differentiation toward neutrophils and that BLR1 expression depends on the transcriptional activity of Oct1 (28). While Oct1 and Oct4 have structural and functional similarities, only Oct4 confers pluripotency (29), consistent with their differential regulation observed here. As expected for differentiating cells (24, 30), and consistent with previous reports showing that RA down-regulates ALDH (31), we found that ALDH1 enzymatic activity is downregulated by 1µM RA treatment, assessed 48 hours posttreatment as cells undergo differentiation (Fig.1E). In sum RA-induced phenotypic conversion to a differentiated cell is associated with AhR upregulation and Oct4, but not Oct1, down regulation.
Figure 1. RA induces differentiation, AhR upregulation and Oct4 downregulation.
A.CD38 expression (assessed with PE-conjugated antibody) at 6 and 24h post-treatment is significantly induced by RA (P<0.0005). CD11b expression (assessed with APC-conjugated antibody) at 48 and 72h post-treatment is significantly induced by RA (P<0.0005). Flow cytometric assay of live cells was performed setting the logical gate to exclude 95% of the untreated cells (CD38 and CD11b detection). RA induces a significant (p<0.05) G0 cell cycle arrest compared to control. G2/M accumulation is significantly decreased by RA (p=0.01) as shown by flow cytometry of DNA stained nuclei. Inducible oxidative metabolism is increased by RA (p=0.03) Flow cytometric assay of live cells was performed setting the logical gate to exclude 95% of the untreated cells. B. AhR protein is upregulated and post-transcriptional modified by RA treatment as showed 72h post treatment by Western blot. AhR appear to largely reside in the cytosol. C. Nuclear Oct4 protein of G0 nuclei is significantly decreased (p<0.005, 48h after treatment). D. Nuclear Oct1 protein level is unchanged by RA (as shown at 48h). The results of flow cytometric analysis of paraformaldehyde fixed cells are expressed as mean fluorescence of the entire population (C and D). E. Aldehyde dehydrogenase1 activity is significantly lower (p=0.01) in the cells treated with RA compared to control cells, The results of flow cytometric analysis of live cells are expressed as mode fluorescence of the entire population.
AhR upregulation enhances RA-induced differentiation, increases Raf pS621 phosphorylation and decreases levels of nuclear Oct4
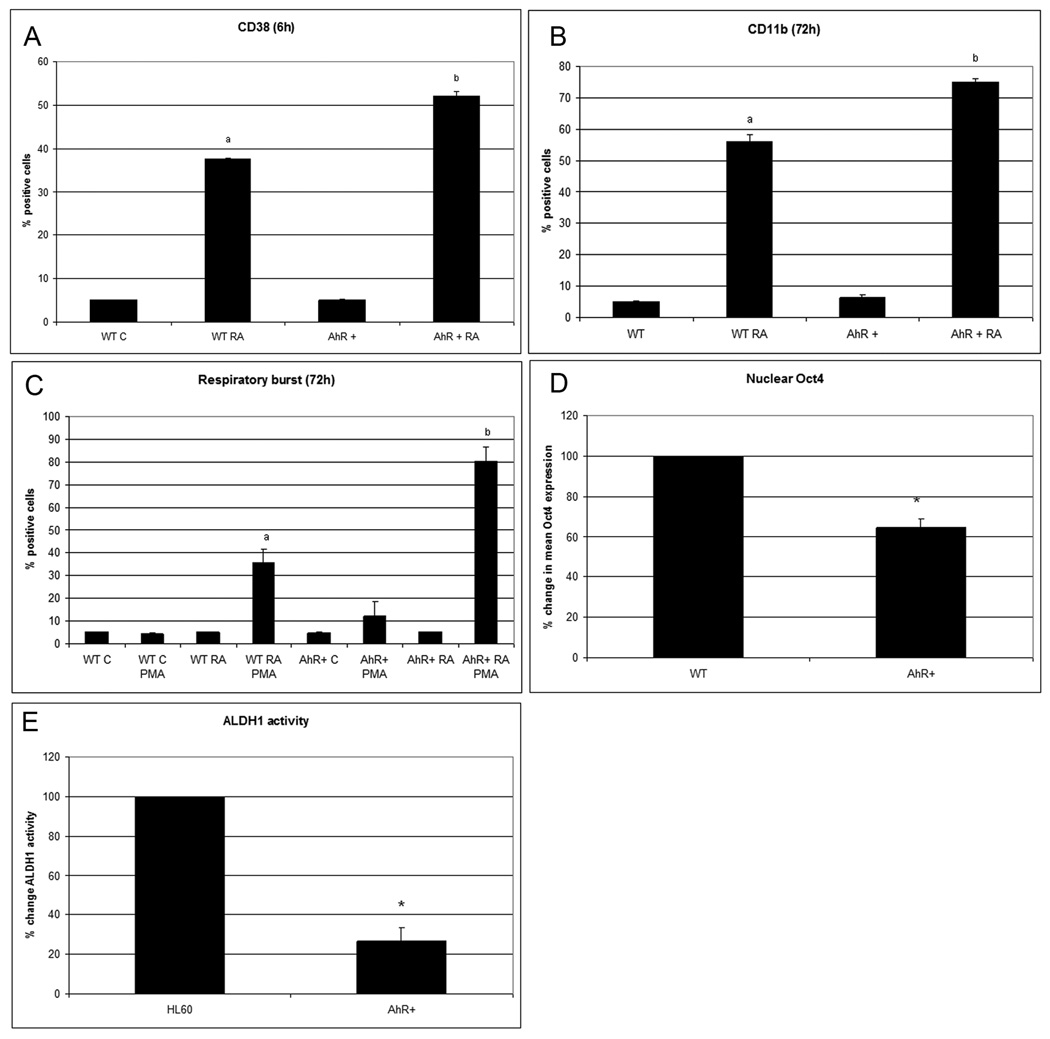
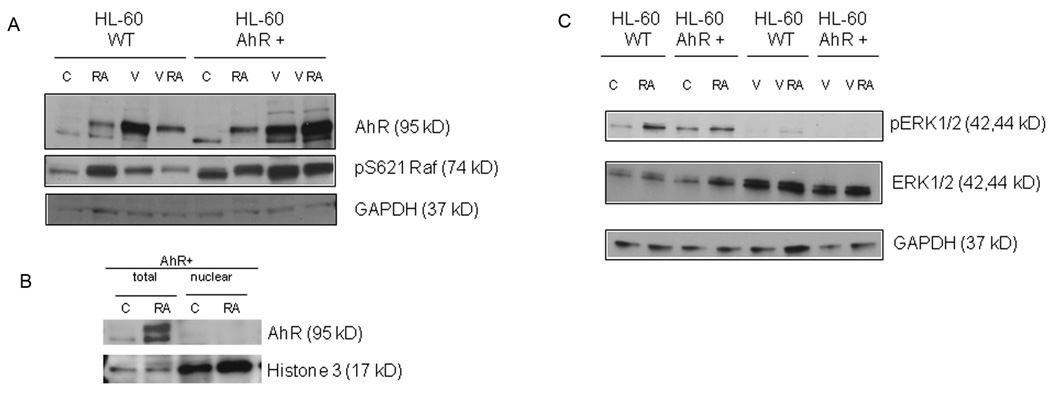
To determine if RA-induced AhR expression has functional significance for differentiation, AhR stable transfectants were created. Overexpression of AhR in stable transfectants enhances RA-induced differentiation. There is a significant increase of RA-induced CD38 and CD11b expression compared to parental cells (Fig.2A–B).Inducible oxidative metabolism, the respiratory burst functional differentiation marker is also enhanced (Fig.2C). AhR expression by itself decreases nuclear Oct4 protein levels (Fig.2D). Consistent with our hypothesis that AhR upregulation propels differentiation, ALDH1 activity is downregulated (p=0.0079) in AhR overexpressors compared to wild type cells (Fig.2E). Figure 3A shows a Western blot confirming the overexpression of AhR in the AhR stable transfectants (control wt HL-60 vs. control AhR transfected HL-60). AhR is well known to be phosphorylated upon activation (27), and resolution of the protein on a 7.5% gel shows phosphorylation induced gel retardation consistent with this. AhR protein in AhR overexpressors, as in wild type cells, did not translocate in to the nucleus (Fig.3B). AhR has a known cytosolic function acting as a ligand dependent adaptor in the cullin 4B ubiquitin ligase whereby it has been found to decrease estrogen receptor expression and might decrease nuclear Oct4 levels in a similar way (7).
Figure 2. AhR upregulation correlates with propelled differentiation and decreased nuclear Oct4 levels.
AhR overexpressors treated with RA present A. CD38 (6 h; p<0.005), B.CD11b (72h p=0.012), C. respiratory burst (72h p=0.02) faster than wild type cells treated with RA. Flow cytometric assay of live cells was performed setting the logical gate to exclude 95% of the untreated cells (A–C). D. Nuclear Oct4 protein levels are downregulated in AhR overexpressors compared to wild type in cultures at the same cell density (p<0.005). The results of flow cytometric analysis of paraformaldehyde fixed cells are expressed as mean fluorescence of the entire population. E. Aldehyde dehydrogenase1 enzymatic activity is significantly lower (p=0.0079) in AhR overexpressors compared to wild type cells.
Figure 3. AhR upregulation leads to increased Raf pS621 phosphorylation and decreased ERK1/2 phosphorylation.
Cell lysates collected 48h after RA treatment (52 h after valproic acid (V)treatment) were resolved on 7.5% gel for AhR detection and 12% gel for all the other proteins. 25 µg protein was loaded per well. For treatment, RA final concentration was 1 µM and V was 1 mM. A. AhR protein levels and pS621 Raf are increased in overexpressors nontreated and in RA and/or V treated cells. B. AhR protein is upregulated and post-transcriptional modified by RA treatment as showed 72h post treatment by Western blot. AhR appear to largely reside in the cytosol. C. Valproic acid antagonizes ERK phosphorylation.
RA-induces MAPK signaling which is necessary to propel differentiation (32, 33), The MAPK signaling utilizes Raf, activation of which is necessary to induce terminal myeloid differentiation (34).One might anticipate from the present results that AhR expression resulting in enhanced RA-induced CD38 expression would also result in enhanced Raf activation betrayed by S621 phosphorylation. Western analysis confirms that the AhR transfected cells had enhanced Raf activation (Fig. 3A, control wt HL-60 versus control AhR transfected HL-60). Surprisingly enhanced Raf activation due to AhR overexpression does not result in increased ERK phosphorylation compared to basal levels in parental wild type controls. RA treatment has been found to cause phosphorylation of ERK1/2 (32, 33), which is confirmed here (Fig.3C). But AhR overexpression suppresses that, suggesting a potential negative regulatory relationship between AhR and ERK. Interestingly the reverse has been reported by Chen at al (35) who showed that upon ERK kinase inhibition, AhR protein level accumulates. The results thus show that AhR expression enhances Raf activation, which is consistent with previously reported results on Raf propulsion of differentiation (34). They also suggest the conjecture that Raf can do this independent of activating ERK, possibly reflecting Raf functions other than activation of MEK/ERK such as its nuclear translocation (23) and association with nuclear proteins such as Rb (36), which is a target downregulated by RA (37, 38)
Valproic acid (VPA), an HDAC inhibitor, is known to induce expression of cytochrome family members that are transcriptionally upregulated by AhR (39). One might thus anticipate that VPA could cause AhR expression and enhance RA-induced differentiation. Figure 3A confirms AhR upregulation by VPA, alone or in combination with RA. VPA causes enhanced Raf activation (Fig. 3A). However, as for AhR overexpression VPA suppresses ERK activation in the absence or the presence of RA, although total ERK protein is upregulated (Fig.3C). VPA thus causes AhR overexpression with corresponding signaling effects previously found associated with AhR upregulation.
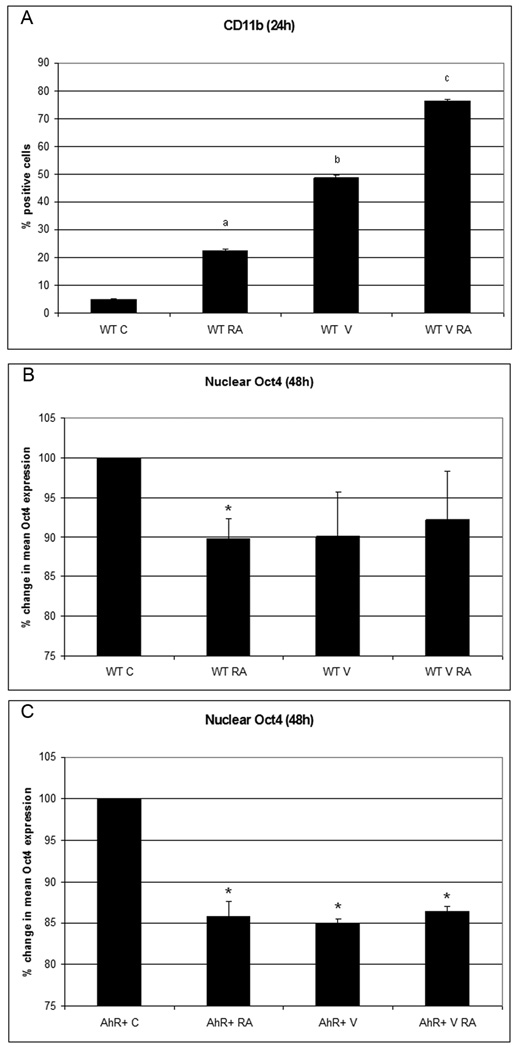
The ability of VPA to upregulate AhR motivated testing its effects on differentiation. VPA treatment induced the expression of the CD11b cell surface differentiation marker early after treatment (24h). RA-induced CD11b expression was also enhanced by co-treatment with VPA at 24h after treatment. (Fig.4A). VPA also caused downregulation of nuclear Oct4 expression (Fig.4B, C). RA, AhR, and VPA thus all cause downregulation of Oct4, motivating the conjecture that Oct4 downregulation facilitates differentiation. Interestingly, all conditions achieved a similar amount of Oct4 downregulation, possibly reflecting tight control of Oct4 expression.
Figure 4. AhR chemically induced propels differentiation and blunts Oct4 levels in HL-60 cells.
A. Valproic acid by itself (P<0.005 compared to non treated cells) and with RA (p<0.005 compared to RA treated cells) augments integrin receptor CD11b (24h post treatment). Flow cytometric assay of live cells was performed setting the logical gate to exclude 95% of the untreated cells. Valproic acid by itself blunts Oct4 expression at a level comparable to RA –downregulated Oct4 levels (as shown 48h post-treatment), both in the wild type (panel B) and AhR overexpressors (panel C). The results of flow cytometric analysis of paraformaldehyde fixed cells are expressed as mean fluorescence of the entire population.
Decreased levels of nuclear Oct4 propel RA-induced differentiation
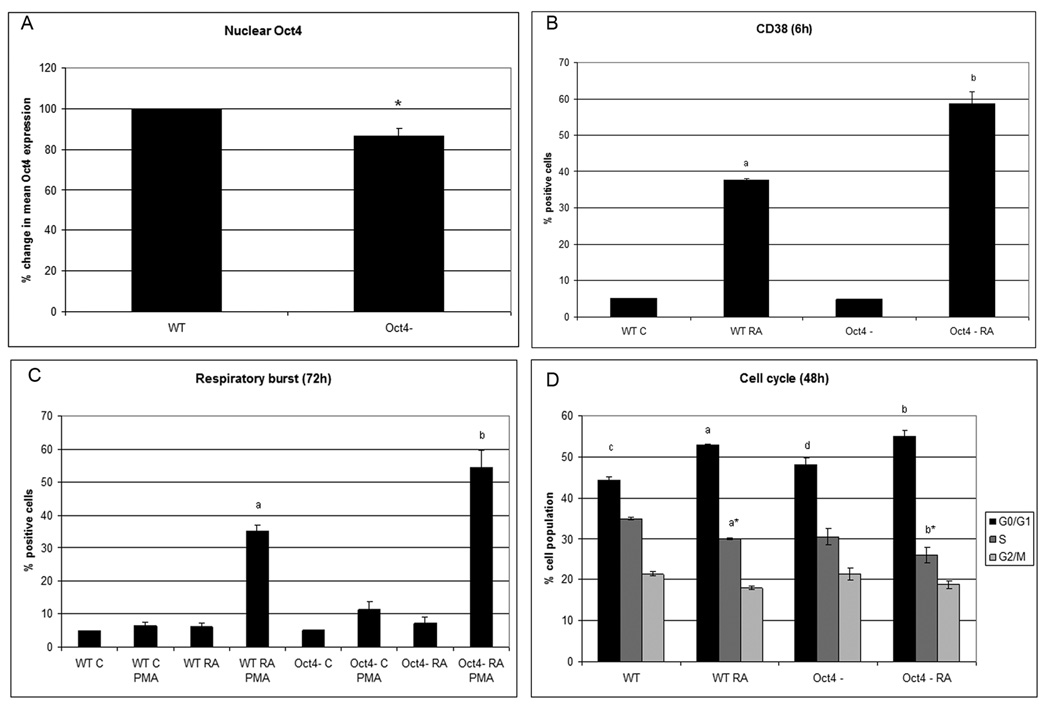
RA and increased AhR expression caused Oct4 downregulation. To determine if Oct4 downregulation is functionally significant and facilitates RA-induced differentiation, Oct4 siRNA knockdown stable transfectants were created. The decreased nuclear Oct4 in stable transfectants is confirmed by flow cytometry (Fig. 5A) with a p value of 0.013. The knockdown achieved was comparable to the RA-induced reduction in expression. Although the Oct4 knockdown by itself does not precipitate differentiation, as evidenced by lack of CD38 or inducible respiratory burst, it does significantly (p<0.05) enhance RA-induced CD38 expression, inducible oxidative metabolism (Figs.5B and C), and increase in the percentage of cells in G1/0, p=0.015 (Fig.5D). It is noteworthy that the siRNA knockdown was demonstrable and statistically significant, but was modest; hence even a relatively small apparent reduction in Oct4 can have demonstrable effects on facilitating RA-induced differentiation.
Figure 5. Oct4 is instrumental in propelling differentiation.
A. confirms Oct4 downregulation in Oct4 knockdown cells. The results of flow cytometric analysis of paraformaldehyde fixed cells are expressed as mean fluorescence of the entire population. B. 6 h after RA treatment a significantly greater number of cells (P<0.05) expressed CD38. C. Oxidative metabolism is enhanced in Oct4 knockdowns (p= 0.03). Flow cytometric assay of live cells was performed setting the logical gate to exclude 95% of the untreated cells (B. and C.) D. Oct4 downregulation increases the percentage of cells in G0 -with no treatment p =0.01 (c vs. d) or with RA treatment p=0.015 (a vs. b) at 48h. In RA treated cells, S is significantly lower in Oct4 knockdowns. (flow cytometry of PI labeled nuclei).
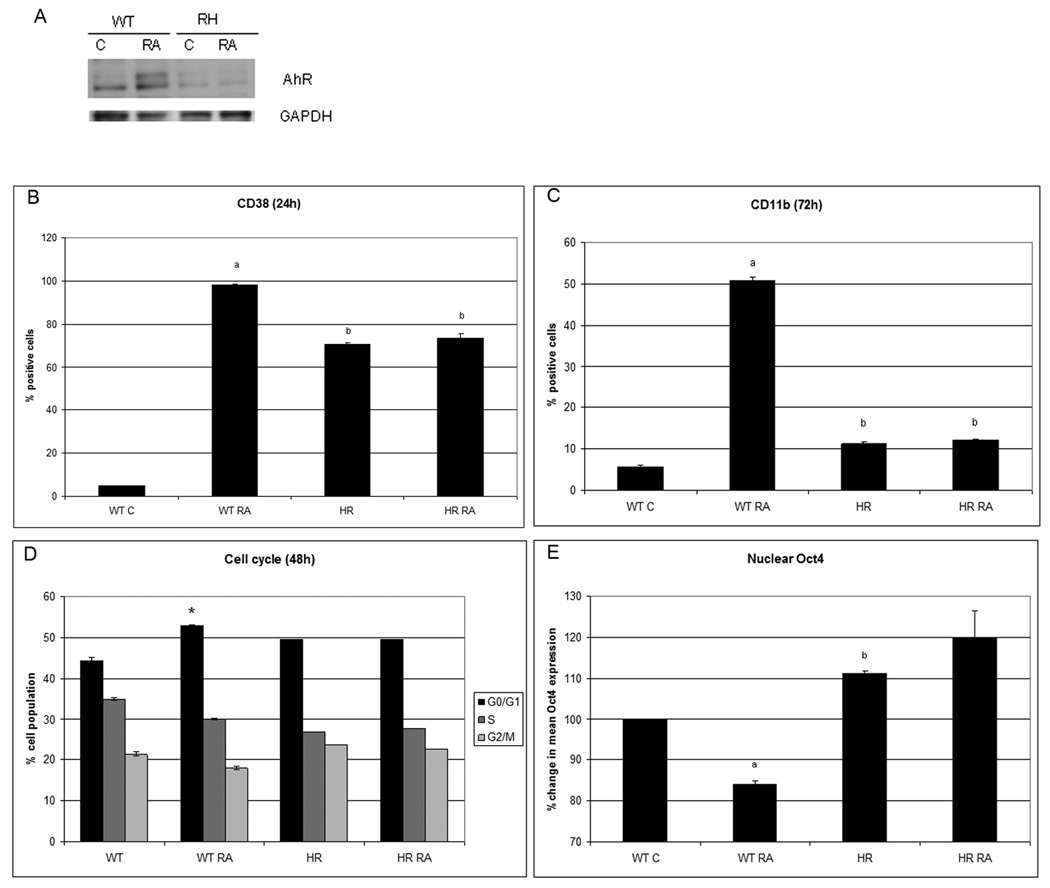
RA resistant cells have increased levels of nuclear Oct4 after RA treatment
If Oct4 is essential for maintaining cellular stem-like properties - such as self renewal and immature phenotype - and decreasing Oct4 levels facilitate RA-induced differentiation, then the failure of RA resistant cells to differentiate may be associated with a failure to decrease expression of nuclear Oct4 after RA treatment. An RA-resistant derivative of the parental cells was created by subjecting cells maintained at high density to repeated high doses of RA. The resulting resistant cells grown from such treatment had lower levels of AhR that failed to be up regulated by RA (Fig. 6A), Although the RA resistant cells have higher basal expression levels of CD38 before RA treatment than wild type cells, RA treatment failed to increase expression levels of CD38 (Fig.6B). RA-induced CD11b expression was also blocked in the resistant cells (Fig.6C). Consistent with this, there is no RA-induced G0 block, and the G1/0, S and G2 distribution is unaffected by RA treatment (Fig. 6D). The resistant cells had higher basal levels of Oct4 (p=0.0043) (Fig. 6E) and treated with RA failed to downregulate Oct4 levels. The significantly higher nuclear Oct4 levels in RA-resistant cells compared to wild type cells is consistent with the hypothesis that Oct4 downregulation promotes cell differentiation.
Figure 6. RA resistant cells.
had low levels of AhR and RA treatment did not upregulate AhR (A), did not change the CD38 at 24h (B) and CD11b at 72h (C) levels, neither the cell cycle phase distribution (D) and present higher nuclear Oct4 basal levels than wild type cells (E) (flow cytometry).
Discussion
The present studies show that when RA induces myeloid differentiation of lineage uncommitted human myelo-monocytic leukemia cells, HL-60, it causes upregulation of AhR and downregulation of nuclear Oct4. The functional significance of this was tested. Ectopic overexpression of AhR enhanced Raf activation, known to propel differentiation; downregulated Oct4, suggesting a downstream function of Oct4; and enhanced RA-induced differentiation indexed by cell surface markers and functional differentiation. Furthermore, VPA induced AhR upregulation and also enhanced RA-induced differentiation. Downregulation of Oct4 in siRNA stable transfectants also enhanced RA-induced differentiation, confirming the anticipated downstream role of Oct4 downregulation in facilitating differentiation. Interestingly the modulation of AhR and Oct4 expression levels to have these effects apparently does not have to be large. Consistent with a suggested role for Oct4 downregulation in differentiation, RA resistant HL-60 cells created by high dose RA treatment had elevated Oct4 levels that did not downregulate. In sum we have found that RA induces AhR upregulation and Oct4 downregulation, where AhR expression negatively regulates Oct4, and both occurrences promote RA-induced differentiation.
The finding that AhR overexpression and knockdown of Oct4 expression enhanced the induction of differentiation by RA is novel but consistent with other findings. Oct4 is known to support de-differentiated stem cells (14, 29). RA, which induces differentiation, was reported to repress Oct4 protein expression (40), and we show here that AhR is involved in Oct4 downregulation by RA. RAR, RXR, AhR and ER are members of nuclear receptor family of transcription factors binding as heterodimers and known for their cross-talk (7, 9, 15). In the case of AhR and ER it has been shown that AhR can bind to ER response elements and augment ER responses (41). In HL-60 cells estradiol at low doses stimulates proliferation and at high doses inhibits proliferation, similar to the dose response effects of retinoic acid, suggesting the possibility of cross talk between estrodiol and RA (42). Furthermore estradiol/ER signaling has been found to enhance RA-induced HL-60 cell differentiation (43). Crosstalk between estrogen and RA has also been demonstrated at the nuclear level, where for instance overlapping ERE and RARE occurs as for the lactoferrin gene (44). ER could potentially thus be involved in relating AhR and RAR signaling and crosstalk. In the case of the Oct4A promoter, the AhR and RAR response elements are very close, hence multiple ways of cross talk could be possible, for example by cooperation in protein-DNA binding, in recruiting cofactors, and inducing chromatin changes responsible for modulation of Oct4 gene expression. There are thus a number of reasons to anticipate that RAR, AhR, and Oct4 might be inter-related, and that anticipation is borne out in the currently reported findings.
It has also been shown that AhR participates in ER and AR degradation by functioning as a ligand dependent substrate recognizing component of an ubiquitin ligase complex (7). Although it is beyond the scope of our present study to elucidate the mechanism of Oct4 downregulation by AhR, the fact that AhR was reported to downregulate other protein levels supports our report.
We found that AhR expression and its possible phosphorylation drive up Raf pS621 levels. Raf as part of the Raf/MEK/ERK MAPK signaling module has been found to be needed in RA-induced differentiation (34). ERK activation has been found with RA-induced differentiation. But the role of Raf, although important, is not fully mechanistically defined. We have previously shown that Raf pS621 accumulates in the nuclei during RA-induced differentiation and others have shown that AhR phosphorylation increases its nuclear activity. The fact that VPA increases Raf pS612 levels, but inhibits occurrence of ERKpTEpY, and that AhR overexpression due to ectopic expression also blunts RA-induced ERK activation suggests that ERK activation is not absolutely necessary. Of relevance, ERK has been found capable of acting as a scaffold to facilitate signaling without actual activation (45). The cumulative evidence points to the importance of Raf activation in contributing propulsion to RA-induced differentiation.
The current statistics associated with APL continue to show a relatively high recurrence rate together with a poor survival despite a very high rate of remission after the standard RA-induced differentiation therapy. The results presented here are of potential clinical significance. They show that in the standard RA-induced differentiation induction therapy the downregulation of stem cell promoting factor, Oct4, is important in overcoming the maturation block and that this process is AhR dependent. Furthermore VPA can promote aspects of this process.
Acknowledgments
Supported by grants from NIH (R01 CA033505(AY), U54 CA143876 (Craighead) ), NYSTEM NY Dept. Health. RPB was supported in part by a Center for Vertebrate Genomics Scholarship.
References
- 1.Hansen LA, Sigman CC, Andreola F, Ross SA, Kelloff GJ, De Luca LM. Retinoids in chemoprevention and differentiation therapy. Carcinogenesis. 2000;21(7):1271–1279. [PubMed] [Google Scholar]
- 2.Hittelman WN, Liu DD, Kurie JM, et al. Proliferative changes in the bronchial epithelium of former smokers treated with retinoids. Journal of the National Cancer Institute. 2007;99(21):1603–1612. doi: 10.1093/jnci/djm205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moriwaki H, Shimizu M, Okuno M, Nishiwaki-Matsushima R. Chemoprevention of liver carcinogenesis with retinoids: Basic and clinical aspects. Hepatol Res. 2007;37 Suppl 2:S299–S302. doi: 10.1111/j.1872-034X.2007.00201.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall JM, Barhoover MA, Kazmin D, McDonnell DP, Greenlee WF, Thomas RS. Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation. Mol Endocrinol. 2010;24(2):359–369. doi: 10.1210/me.2009-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer research. 2010;70(1):212–220. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper PA, Giannone JV, Okey AB, Denison MS. In vitro transformation of the human Ah receptor and its binding to a dioxin response element. Mol Pharmacol. 1992;42(4):603–612. [PubMed] [Google Scholar]
- 7.Ohtake F, Baba A, Takada I, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446(7135):562–566. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q. Induction of CYP1A1. The AhR/DRE paradigm: transcription, receptor regulation, and expanding biological roles. Current drug metabolism. 2001;2(2):149–164. doi: 10.2174/1389200013338603. [DOI] [PubMed] [Google Scholar]
- 9.Ohtake F, Fujii-Kuriyama Y, Kato S. AhR acts as an E3 ubiquitin ligase to modulate steroid receptor functions. Biochem Pharmacol. 2009;77(4):474–484. doi: 10.1016/j.bcp.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 12.Utikal J, Maherali N, Kulalert W, Hochedlinger K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci. 2009 doi: 10.1242/jcs.054783. jcs.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orkin SH, Wang J, Kim J, et al. The Transcriptional Network Controlling Pluripotency in ES Cells. Cold Spring Harbor symposia on quantitative biology. 2008 doi: 10.1101/sqb.2008.72.001. [DOI] [PubMed] [Google Scholar]
- 14.Kim JB, Greber B, Arauzo-Bravo MJ, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009 doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 15.Soprano DR, Soprano KJ. Pharmacological doses of some synthetic retinoids can modulate both the aryl hydrocarbon receptor and retinoid receptor pathways. The Journal of nutrition. 2003;133(1):277S–281S. doi: 10.1093/jn/133.1.277S. [DOI] [PubMed] [Google Scholar]
- 16.Ohtake F, Baba A, Fujii-Kuriyama Y, Kato S. Intrinsic AhR function underlies cross-talk of dioxins with sex hormone signalings. Biochemical and biophysical research communications. 2008;370(4):541–546. doi: 10.1016/j.bbrc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Shenefelt RE. Gross congenital malformations. Animal model: treatment of various species with a large dose of vitamin A at known stages in pregnancy. The American journal of pathology. 1972;66(3):589–592. [PMC free article] [PubMed] [Google Scholar]
- 18.Mimura J, Yamashita K, Nakamura K, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2(10):645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashida Y, Kawamura T, Hori-e R, Yamashita I. Retionic acid and its receptors are required for expression of aryl hydrocarbon receptor mRNA and embryonic development of blood vessel and bone in the medaka fish, Oryzias latipes. Zoological science. 2004;21(5):541–551. doi: 10.2108/zsj.21.541. [DOI] [PubMed] [Google Scholar]
- 20.Pikarsky E, Sharir H, Ben-Shushan E, Bergman Y. Retinoic acid represses Oct-3/4 gene expression through several retinoic acid-responsive elements located in the promoter-enhancer region. Molecular and cellular biology. 1994;14(2):1026–1038. doi: 10.1128/mcb.14.2.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiterer G, Yen A. Platelet-derived growth factor receptor regulates myeloid and monocytic differentiation of HL-60 cells. Cancer research. 2007;67(16):7765–7772. doi: 10.1158/0008-5472.CAN-07-0014. [DOI] [PubMed] [Google Scholar]
- 22.Reiterer G, Yen A. Inhibition of the janus kinase family increases extracellular signal-regulated kinase 1/2 phosphorylation and causes endoreduplication. Cancer research. 2006;66(18):9083–9089. doi: 10.1158/0008-5472.CAN-06-0972. [DOI] [PubMed] [Google Scholar]
- 23.Smith J, Bunaciu RP, Reiterer G, et al. Retinoic acid induces nuclear accumulation of Raf1 during differentiation of HL-60 cells. Experimental cell research. 2009;315(13):2241–2248. doi: 10.1016/j.yexcr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson DG, Bell JI. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144(7):2811–2815. [PubMed] [Google Scholar]
- 26.Zubiaur M, Izquierdo M, Terhorst C, Malavasi F, Sancho J. CD38 ligation results in activation of the Raf-1/mitogen-activated protein kinase and the CD3-zeta/zeta-associated protein-70 signaling pathways in Jurkat T lymphocytes. J Immunol. 1997;159(1):193–205. [PubMed] [Google Scholar]
- 27.Pongratz I, Stromstedt PE, Mason GG, Poellinger L. Inhibition of the specific DNA binding activity of the dioxin receptor by phosphatase treatment. The Journal of biological chemistry. 1991;266(25):16813–16817. [PubMed] [Google Scholar]
- 28.Wang J, Yen A. A novel retinoic acid-responsive element regulates retinoic acid-induced BLR1 expression. Molecular and cellular biology. 2004;24(6):2423–2443. doi: 10.1128/MCB.24.6.2423-2443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J, Shakya A, Tantin D. Stem cells, stress, metabolism and cancer: a drama in two Octs. Trends in biochemical sciences. 2009;34(10):491–499. doi: 10.1016/j.tibs.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104(6):1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 31.Moreb JS, Gabr A, Vartikar GR, Gowda S, Zucali JR, Mohuczy D. Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde. J Pharmacol Exp Ther. 2005;312(1):339–345. doi: 10.1124/jpet.104.072496. [DOI] [PubMed] [Google Scholar]
- 32.Yen A, Roberson MS, Varvayanis S. Retinoic acid selectively activates the ERK2 but not JNK/SAPK or p38 MAP kinases when inducing myeloid differentiation. In vitro cellular & developmental biology. 1999;35(9):527–532. doi: 10.1007/s11626-999-0063-z. [DOI] [PubMed] [Google Scholar]
- 33.Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer research. 1998;58(14):3163–3172. [PubMed] [Google Scholar]
- 34.Wang J, Yen A. A MAPK-positive feedback mechanism for BLR1 signaling propels retinoic acid-triggered differentiation and cell cycle arrest. The Journal of biological chemistry. 2008;283(7):4375–4386. doi: 10.1074/jbc.M708471200. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Operana T, Bonzo J, Nguyen N, Tukey RH. ERK kinase inhibition stabilizes the aryl hydrocarbon receptor: implications for transcriptional activation and protein degradation. The Journal of biological chemistry. 2005;280(6):4350–4359. doi: 10.1074/jbc.M411554200. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Ghosh RN, Chellappan SP. Raf-1 physically interacts with Rb and regulates its function: a link between mitogenic signaling and cell cycle regulation. Molecular and cellular biology. 1998;18(12):7487–7498. doi: 10.1128/mcb.18.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen A, Williams M, Platko JD, Der C, Hisaka M. Expression of activated RAF accelerates cell differentiation and RB protein down-regulation but not hypophosphorylation. European journal of cell biology. 1994;65(1):103–113. [PubMed] [Google Scholar]
- 38.Chen YH, Lavelle D, DeSimone J, Uddin S, Platanias LC, Hankewych M. Growth inhibition of a human myeloma cell line by all-trans retinoic acid is not mediated through downregulation of interleukin-6 receptors but through upregulation of p21(WAF1) Blood. 1999;94(1):251–259. [PubMed] [Google Scholar]
- 39.Rogiers V, Akrawi M, Vercruysse A, Phillips IR, Shephard EA. Effects of the anticonvulsant, valproate, on the expression of components of the cytochrome-P-450-mediated monooxygenase system and glutathione S-transferases. European journal of biochemistry / FEBS. 1995;231(2):337–343. doi: 10.1111/j.1432-1033.1995.tb20705.x. [DOI] [PubMed] [Google Scholar]
- 40.Schoorlemmer J, van Puijenbroek A, van Den Eijnden M, Jonk L, Pals C, Kruijer W. Characterization of a negative retinoic acid response element in the murine Oct4 promoter. Molecular and cellular biology. 1994;14(2):1122–1136. doi: 10.1128/mcb.14.2.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohtake F, Takeyama K, Matsumoto T, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 42.Danel L, Cordier G, Revillard JP, Saez S. Presence of estrogen binding sites and growth-stimulating effect of estradiol in the human myelogenous cell line HL60. Cancer research. 1982;42(11):4701–4705. [PubMed] [Google Scholar]
- 43.Kauss MA, Reiterer G, Bunaciu RP, Yen A. Human myeloblastic leukemia cells (HL-60) express a membrane receptor for estrogen that signals and modulates retinoic acid-induced cell differentiation. Experimental cell research. 2008;314(16):2999–3006. doi: 10.1016/j.yexcr.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MO, Liu Y, Zhang XK. A retinoic acid response element that overlaps an estrogen response element mediates multihormonal sensitivity in transcriptional activation of the lactoferrin gene. Molecular and cellular biology. 1995;15(8):4194–4207. doi: 10.1128/mcb.15.8.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong SK, Yoon S, Moelling C, Arthan D, Park JI. Noncatalytic function of ERK1/2 can promote Raf/MEK/ERK-mediated growth arrest signaling. The Journal of biological chemistry. 2009;284(48):33006–33018. doi: 10.1074/jbc.M109.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]