Abstract
Objective
To compare the impact of nortriptyline to sertraline on change in cognitive functioning in depressed older adults.
Methods
We used pre-post neuropsychological data collected as part of a 12-week medication trial comparing sertraline to nortriptyline in the treatment of older adults with non-psychotic, unipolar major depression to examine change in cognitive functioning. Neuropsychological assessments included mental status (Mini-Mental Status Exam), psychomotor speed (Purdue Pegboard), attention (Continuous Performance Test; Trail Making Test A), executive functioning (Stroop Color/Word Test; Trail Making Test B), and memory (Buschke Selective Reminding Test).
Results
Within treatment groups, patients treated with sertraline improved only on verbal learning. This change did not depend on responder status. Between treatment groups, patients treated with sertraline improved more in verbal learning compared to patients treated with nortriptyline. Looking at change in cognition as a function of medication condition and responder status revealed that sertraline responders improved more in verbal learning compared to nortriptyline responders but not more than sertraline non-responders or nortriptyline non-responders. Nortriptyline responders were the only treatment by responder status group to show no improvement in verbal learning from baseline to endpoint.
Conclusions
Unexpectedly, nortriptyline responders showed no improvement in verbal learning as compared to patients treated with sertraline or nortriptyline non-responders. However, given the small sample sizes and number of statistical tests (potential for type 1 error), replication is warranted.
Keywords: cognitive functioning, cognitive impairment, depression, nortriptyline, sertraline
Cognitive impairment is common in geriatric depression. Cognitive domains reported to be affected include memory (Gallassi, et al. 2006; Kramer-Ginsberg, et al. 1999; Salloway, et al. 1996), visuospatial functioning (Butters, et al. 2004; Kramer-Ginsberg et al. 1999), information processing speed (Lesser, et al. 1996; Nebes, et al. 2000), and executive functioning (Lesser et al. 1996; Lockwood, et al. 2002). The conversion rate of late-life depression into dementia among those with cognitive impairment is much higher than those without cognitive impairment (Modrego and Ferrandez 2004). Antidepressant medication is the first line of treatment for depression, particularly in the older adult community where primary care doctors provide the majority of treatment. Therefore, it is important to understand the effects of antidepressant medication on cognition among the depressed elderly, a cognitively vulnerable population.
When examining the effect of antidepressant medication on cognition in depressed older adults, two interrelated sets of questions arise. The first set of questions focuses on within-treatment changes in cognitive functioning: a) does cognitive functioning change from pre- to post-treatment? and b) is there a differential impact of medication on cognition depending on whether or not the patient responds? Within the second set of questions, the focus is on between-treatment changes in cognitive functioning: a) is there a differential impact of antidepressant medication on cognitive functioning depending on medication class (e.g., SSRIs, SNRIs, TCAs, MAOs)? b) does change in cognitive functioning between treatment conditions depend on responder status? Of course, the concept of cognitive functioning is broad and consists of a number of functions including but not limited to memory, attention, processing speed, executive functioning, and visuospatial/visuoconstructional skills. Therefore, it is important to examine each of the above questions within the context of a multi-domain assessment to address how antidepressant medication (including whether or not the patient responds) differentially affects each of these cognitive domains.
A number of studies have examined the impact of antidepressant medication on cognition but have not fully addressed the two interrelated sets of questions we have outlined (Bhalla, et al. 2006; Bondareff, et al. 2000; Butters, et al. 2000; Doraiswamy, et al. 2003; Gallassi et al. 2006; Nebes, et al. 2003; Raskin, et al. 2007). For example, some studies did not address whether change in cognition depends on response to treatment (Bondareff et al. 2000; Raskin et al. 2007). Other studies included only responders in their analyses rather than comparing change in cognition among responders and non-responders, which would have helped to differentiate the impact of medication versus response on change in cognitive functioning (Bhalla et al. 2006; Butters et al. 2000; Gallassi et al. 2006; Nebes et al. 2003). Finally, another study used a composite cognitive score in its analyses and thus did not address the differential impact of medication on different cognitive domains (Doraiswamy et al. 2003).
To our knowledge, few studies have fully tested the impact of medication on cognitive function in depressed older adults. For example, in an 8-week randomized placebo-controlled trial of citalopram (Culang, et al. 2009), antidepressant non-response was associated with cognitive decline in verbal learning and psychomotor speed. Although responders on medication improved on some domains (psychomotor speed and visuospatial functioning), their improvement did not exceed the expected practice effect observed among patients randomized to placebo. This study, therefore, shows decline in some domains and improvement in other domains, and is an example of a differential impact of medication on cognition. In another study, nortriptyline and phenelzine produced no change in any cognitive domain assessed (verbal learning, psychomotor speed, visual memory) in depressed older adults when compared to placebo, and this effect did not depend on responder status (Georgotas, et al. 1989). In a third study, an uncontrolled trial in depressed older adults showed no change in any cognitive domain assessed (processing speed, executive functioning, verbal and visual memory, visuoperceptive functioning, and attention) over 12 months of treatment when compared to age-matched controls (Portella, et al. 2003). Furthermore, there were no differences in change among remitted and non-remitted patients.
The purpose of this study was to examine the impact of a TCA and SSRI on cognition and to determine whether change in cognition depends on response to treatment and cognitive domain. To accomplish this aim, we used pre-post neuropsychological data on global cognitive functioning, verbal learning, attention, psychomotor speed, and executive functioning (the switching and response inhibition components), collected as part of a 12-week, randomized, double-blind, parallel-group design comparing sertraline and nortriptyline in the treatment of depressed older adults. In the context of this multi-domain assessment, we addressed the two sets of questions outlined above: 1.a) whether there were pre- to post-treatment changes in cognitive functioning within the sertraline and nortriptyline conditions, 1.b) whether change in cognitive functioning within each treatment condition depended on responder status, 2.a) whether there were differences in change in cognitive functioning between the nortriptyline and sertraline conditions, and 2.b) whether change in cognitive functioning between treatment conditions depended on responder status.
METHOD
Study Procedures
This study was a double-blind, randomized, 12-week clinical trial comparing nortriptyline to sertraline in depressed older adults. Patients were recruited by radio and newspaper advertisements and/or through referral from other physicians. At the initial visit, a comprehensive psychiatric evaluation, including a Structured Clinical Interview for DSM-IV, 24-item Hamilton Rating Scale for Depression (HRSD), Mini-Mental Status Examination (MMSE), Newcastle I scale for the assessment of melancholia, and a medical history were performed. If the patient met inclusion criteria and signed informed consent, a physical examination, ECG, CBC, chemistries, electrolytes, and thyroid panel were performed.
Inclusion criteria were 1) age > 45; 2) unipolar depression, single or recurrent, nonpsychotic, by DSM-IV criteria; 3) HRSD ≥ 16 at the initial visit and at the end of 1 week of placebo; 4) MMSE score ≥ 24; and 5) willing and able to give informed consent. Exclusion criteria were 1) current or history of obsessive-compulsive disorder, psychotic disorder, or substance dependence within the past year (other than nicotine) by DSM-IV criteria; 2) judged to be a current suicide risk or serious suicide attempt within the past year; 3) patients status post myocardial infarction, coronary artery bypass, or angioplasty, or with a positive history of angina or positive stress test; 4) QRS interval greater than 0.12 sec or QTc interval ≥ 46 msec; 5) treatment with coumadin, heparin or type 1 antiarrhythmic medications; 6) diagnosis of narrow angle glaucoma; 7) stroke, epilepsy or Parkinson’s disease; 8) acute, severe or unstable medical condition; 9) positive urine toxicology screen for drugs of abuse including amphetamine, barbiturates, cocaine, marijuana, methadone, methaqualone, opioids, and PCP; 10) treatment in the current episode of depression with either nortriptyline with a plasma level between 50 and 150 ng/ml, desipramine or imipramine with a plasma level of 250 ng/ml or greater, paroxetine 40mg, fluoxetine 40mg, or sertraline 200mg for at least 4 weeks.
Patients who met inclusion/exclusion criteria and signed informed consent were given one week of single-blind placebo. If patients still met inclusion/exclusion criteria at the end of the placebo week and did not reduce their HRSD score by 25%, they were randomized. The assessments performed at the end of the placebo week and every visit thereafter included the HRSD, the Montgomery-Åsberg Depression Rating Scale (MADRS), the Beck Depression Inventory (BDI), and the Clinical Global Impression (CGI) of severity and improvement. The Hamilton Anxiety Rating Scale was performed at baseline and at the end of weeks 2, 4, and 8 of treatment; the Medical Outcomes Study 36-Item Short-Form Health Survey and the MMSE were performed at baseline and at the end of week 12 or upon early termination. The Cumulative Illness Rating Scale for Geriatrics (CIRS-G) was also administered at baseline. Stratification of the sample was based on diagnosis of melancholia by DSM-IV criteria (questions resolved by case conference). Randomization was done using permuted blocks of ten.
Participants randomized to sertraline received 50 mg for one week and then 100 mg for the next 4 weeks. If the patient did not meet criteria for remission (HRSD < 10) by week 5, the dose was increased to 150 mg/day. If the patient did not show evidence of response by week 9, the dose was increased to 200 mg/day. The nortriptyline dose was calculated at 1 mg/kg; 1/3 of that dose was given days 1 through 3, 2/3 on days 3 through 6, and the full dose of medication was given on day 7. A plasma level was drawn 7 days later and the dose of nortriptyline was adjusted so that the plasma level was within 80-120 ng/ml. The New York State Psychiatric Institute IRB approved this study.
Neuropsychological Test Battery
The test battery was designed to assess a number of cognitive functions pertinent to aging and major depression including mental status, psychomotor speed, attention, and memory. The tests included the 30-item Folstein MMSE (Folstein, et al. 1975) to estimate global cognitive functioning, the Purdue Pegboard (both hands) (Tiffin and Asher 1948) as a measure of psychomotor speed, the 4-digits fast condition of the Continuous Performance Test (CPT) – Identical Pairs (Cornblatt, et al. 1988) and Trail Making Test A (TMT A) (Reitan and Wolfson 1985) to assess attention, the Stroop Color/Word Test (MacLeod 1991) and Trail Making Test B (TMT B) (Reitan and Wolfson 1985) to assess the response inhibition and switching components of executive functioning, respectively, and the Buschke Selective Reminding Test (SRT) (Buschke and Fuld 1974) as a measure of verbal learning. Two of the tests (CPT and Stroop) were presented on a Macintosh laptop computer and were written in the PsyScope programming language (Cohen, et al. 1993). Performance on the CPT was summarized by d-prime, a sensitivity index that represents the standardized difference between hit and false alarm rates. Percent interference (percent change in median reaction time to color/word versus color responses) was used as the outcome measure on the Stroop. The other five tests (MMSE, SRT, TMT A and B, and Purdue Pegboard) were administered by hand. Alternate forms of the CPT and SRT were used in an attempt to eliminate the problem of practice effects.
Missing Data
One hundred and twelve patients were randomized to treatment with either nortriptyline or sertraline. Forty-nine patients were missing all neuropsychological data across the two time-points (baseline and week 12) and were excluded from this study. No differences between those patients with neuropsychological data (n=63) and those without neuropsychological data (n=49) were detected on any clinical or demographic variable. To accommodate missing data for the remaining sample (n=63), we used the multiple imputation (Schafer and Olsen 1998) procedure in SPSS. Multiple imputation replaces missing data with a set of plausible values based on all variables in the working dataset, which includes demographic, clinical outcome, and neuropsychological test variables. To capture the uncertainty in the estimated values, multiple imputation is conducted several times yielding similar but not identical datasets. This report is based on five imputed data sets, which is sufficient to obtain excellent results unless rates of missing data are exceptionally high (Schafer 1999). The five imputed data sets are analyzed separately using standard statistical analyses. Results from the analyses are then combined using Rubin’s rules (Schafer and Graham 2002; Schafer and Olsen 1998) to generate valid statistical inferences that reflect uncertainty due to missing values and improve the accuracy of the results.
Statistical Analyses
Prior to testing for differences in change in neuropsychological test performance, we used simple and logistic regression in SPSS to test for differences at baseline between the two treatment conditions as well as the four treatment condition by responder status groups (see below). There were no differences on age, education, gender, baseline depression severity, responder status, or on any of the neuropsychological tests when comparing the two (treatment condition) and four (responder status by treatment condition) patient groups. Therefore, we did not adjust for demographic, clinical, or neuropsychological tests in the subsequent analyses.
To test whether antidepressant medication (sertraline or nortriptyline) had an impact on cognition, pre- and post-treatment performance on each of the neuropsychological tests were compared using paired t-tests. Independent samples t-tests were used to determine whether change in cognitive performance within each treatment condition depended on responder status; in this model, change scores were computed for each neuropsychological test (t2-t1) and used as the outcome variable and responder status was treated as the independent variable. We next tested for differences in change in neuropsychological test performance between the two treatment conditions using an independent samples t-test. In these analyses, treatment condition (nortriptyline=0, sertraline=1) was treated as the independent variable and the change score as the outcome variable. Finally, to test whether change in neuropsychological test performance between treatment conditions depended on responder status, we used a dummy-coded variable to designate the four patient groups (sertraline responders, sertraline non-responders, nortriptyline responders, and nortriptyline non-responders). Multiple regression was used and the neuropsychological test change scores were again treated as the outcome variable.
The partial or regressed change approach to two time point data is often recommended (Cohen, et al. 2003). In this procedure, the endpoint neuropsychological test score is treated as the outcome variable and the baseline test score is treated as a covariate. This effectively removes all correlation of the endpoint score from the baseline score and represents an improvement over simple change scores (subtracting baseline from endpoint) which tend to overcorrect the endpoint score by the baseline score due to unreliability of measurement (Cohen et al. 2003). We used a change score model in order to be consistent throughout our statistical analyses and to facilitate the presentation of results. Furthermore, conducting the analyses using both strategies did not yield substantively different findings. Throughout our analyses, significance tests were evaluated at the 5% level.
RESULTS
Descriptive Statistics
Table 1 presents baseline demographic and clinical characteristics of the total sample (n=63) and sertraline and nortriptyline subgroups. The average study participant was 64 years old and completed about 4 years of college. Approximately 60% of the sample were women, average baseline depression severity was 24.37 on the 24-item HRSD, and 43% of the sample were classified as responders. The average MMSE score of the sample at baseline was 27.71.
Table 1.
Baseline Clinical and Demographic Characteristics of the total sample, the sertraline and nortriptyline conditions, and the four patients groups classified by treatment condition and responder status (complete case data).
| Variable | Total Sample (n=63) |
Sertraline (n= 33) |
Nortriptyline (n= 30) |
Sertraline Responders (n=11) |
Sertraline Non-responders (n=22) |
Nortriptyline Responders (n=16) |
Nortriptyline Non-responders (n=14) |
|---|---|---|---|---|---|---|---|
| Age | 64.19 (8.47) | 64.85 (8.83) | 63.47 (8.15) | 65.82 (9.19) | 64.36 (8.82) | 63.25 (8.94) | 63.71 (7.47) |
| Women (%) | 60 | 61 | 60 | 45 | 68 | 63 | 57 |
| Education, Yr | 16.17 (2.42) | 16.29 (2.14) | 16.04 (2.73) | 17.40 (.52) | 15.67 (2.45) | 16.23 (2.35) | 15.83 (3.19) |
| HRSD Baseline | 24.37 (4.87) | 23.91 (4.38) | 24.87 (5.39) | 23.18 (4.56) | 24.27 (4.36) | 24.81 (4.45) | 24.93 (6.47) |
| CGI-Severity Baseline | 4.35 (.93) | 4.38 (.71) | 4.33 (1.12) | 4.30 (.82) | 4.41 (.67) | 4.63 (.72) | 4.00 (1.41) |
| CIRS-G | 3.11 (2.14) | 3.52 (2.22) | 2.70 (2.0) | 3.30 (1.83) | 3.60 (2.48) | 2.25 (1.69) | 3.21 (2.26) |
Table 2 presents complete case pre- and post-treatment data for all neuropsychological tests by medication group and responder status. As can be seen from Table 2, test scores remained relatively stable in both treatment conditions and in the treatment condition by responder status groups with the exception of scores on the Buschke SRT, CPT and TMT B. Qualitatively, nortriptyline responders showed no improvement on the Buschke SRT as compared to patients treated with sertraline or nortriptyline non-responders. On the TMT B, nortriptyline responders seemed to decline as compared to the improvement seen among nortriptyline non-responders. Finally, both nortriptyline and sertraline responders appeared to improve on the CPT whereas non-responders across treatment conditions declined. To formally test these apparent differences in change based on complete case data, we ran a series of analyses using change scores on each test as the outcome variable and accommodated missing data using multiple imputation.
Table 2.
Unadjusted neuropsychological test performance scores at baseline and endpoint for the sertraline and nortriptyline subsamples and the four patient groups classified according to treatment condition and responder status (complete case data)
| NP Test | Sertraline (n=33) |
Nortriptyline (n=30) |
Sertraline Responders (n=11) |
Sertraline Non-responders (n=22) |
Nortriptyline Responders (n=16) |
Nortriptyline Non-responders (n=14) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| MMSE | 27.85 (2.56) | 28.19 (2.16) | 27.55 (2.87) | 28.32 (2.50) | 28.45 (2.07) | 28.55 (2.34) | 27.55 (2.77) | 27.80 (1.99) | 27.07 (3.37) | 28.00 (2.85) | 28.07 (2.24) | 29.17 (.75) |
| TMT A | 48.16 (36.39) | 48.55 (24.27) | 49.86 (20.64) | 51.01 (20.65) | 38.30 (9.83) | 36.75 (12.09) | 53.10 (43.54) | 61.54 (28.08) | 50.23 (21.53) | 55.09 (21.68) | 49.46 (20.44) | 40.13 (13.68) |
| TMT B | 106.37 (52.53) | 101.80 (49.38) | 109.49 (63.89) | 126.15 (80.73) | 89.55 (48.96) | 90.24 (39.45) | 114.79 (53.31) | 115.92 (58.62) | 118.16 (72.68) | 142.95 (87.37) | 100.20 (54.08) | 81.35 (34.06) |
| CPT | 1.74 (1.02) | 1.57 (.97) | 1.51 (.56) | 1.48 (.90) | 1.75 (1.11) | 1.96 (1.05) | 1.72 (.98) | 1.12 (.68) | 1.49 (.52) | 1.71 (.89) | 1.56 (.74) | .95 (.78) |
| Purdue Pegboard | 10.11 (3.11) | 10.02 (3.13) | 10.66 (3.06) | 11.18 (3.84) | 10.91 (3.30) | 10.14 (3.48) | 9.70 (3.00) | 9.90 (2.88) | 11.00 (3.36) | 10.94 (4.02) | 10.29 (2.79) | 11.83 (3.54) |
| Buschke SRT | 102.16 (16.38) | 121.55 (16.35) | 108.96 (23.18) | 114.27 (21.43) | 105.36 (15.14) | 122.70 (19.85) | 100.48 (17.11) | 120.40 (12.93) | 112.07 (17.96) | 112.19 (24.13) | 105.62 (28.13) | 119.83 (11.44) |
| Stroop | .54 (.34) | .55 (.29) | .45 (.34) | .43 (.27) | .45 (.30) | .43 (.23) | .59 (.35) | .67 (.31) | .43 (.41) | .43 (.30) | .47 (.27) | .43 (.18) |
Note. Table values are means and standard deviations.
MMSE=Mini Mental Status Exam, total number correct; Stroop=Color/Word Test, interference effect; CPT=Continuous Performance Test, d-prime; TMT=Trail Making Test, seconds; Buschke SRT=Buschke Selective Reminding Test, immediate recall, total number correct
Hypothesis Testing
We first compared pre- and post-treatment neuropsychological test scores within each treatment condition to address whether antidepressant medication has an impact on cognitive functioning. Within the sertraline condition, significant change occurred on the Buschke SRT [t(2082)=-6.30, p=.001]. No change was observed on the other neuropsychological tests. Within the nortriptyline condition, no significant change was observed on any of the neuropsychological tests. These results are graphically displayed in Figure 1, which depicts pre- to post-treatment change in cognitive performance within each treatment condition across the seven neuropsychological tests.
Figure 1.
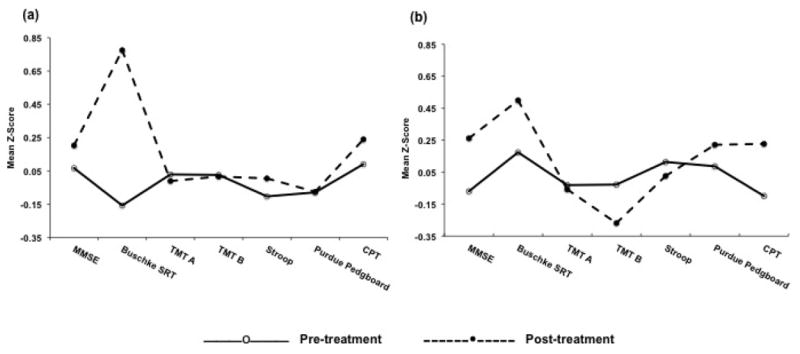
Change in cognitive performance from pre to post-treatment in the (a) sertraline condition and (b) nortriptyline condition across seven neuropsychological tests.
Next, we examined whether change in cognitive functioning within treatment depended on response. In the sertraline condition, 33% of patients responded compared to 53% in the nortriptyline condition (χ2(1)=2.57, p=.11). Change in cognitive performance from baseline to endpoint on the Buschke SRT did not depend on response to sertraline [t(40)=-0.54, p=.60]. Although there were no statistically significant changes in cognition within the nortriptyline condition, we nevertheless examined whether there were differences depending on response; however, no significant findings were observed.
We next examined whether there were differences in change in cognitive functioning between the nortriptyline and sertraline conditions. There was a statistically significant difference between the sertraline and nortriptyline conditions at endpoint on the Buschke SRT. Although both treatment conditions improved on the Buschke SRT from baseline to endpoint, patients treated with sertraline improved significantly more than patients treated with nortriptyline [t(8803)=-2.44, p=0.02]. No other comparisons between sertraline and nortriptyline were statistically significant.
Finally, we examined whether change in cognitive functioning between treatment conditions depended on responder status. Patients who responded on sertraline improved significantly more on the Buschke SRT than patients who responded on nortriptyline [B=-15.73, SE=6.14, t=-2.56, p=0.01] but no more than sertraline non-responders or nortriptyline non-responders. No other comparisons were statistically significant.
DISCUSSION
The purpose of the present study was to examine the impact of nortriptyline and sertraline on change in cognitive functioning of depressed older adults using data from a twelve-week, double-blind, randomized clinical trial. Within this multi-domain assessment, we addressed two interrelated sets of questions: 1) Within treatment condition, does cognitive functioning change from pre- to post-treatment and does it depend on medication response? 2) Between treatment conditions, is there a differential effect of medication on change in cognition and does it depend on medication response?
We found that patients treated with sertraline showed a significant change in verbal learning from pre- to post-treatment, but this change did not depend on responder status. Therefore, taking sertraline improved memory regardless of whether the patient responded to the medication. Of course, this effect could be because of sertraline or non-specific factors associated with participating in a clinical trial. However, improvement in memory (or any other cognitive domain) was not observed in the nortriptyline condition. Therefore, we can infer that memory improvement is likely to be associated with taking sertraline because we did not see a similar effect in the nortriptyline condition, which shared the same non-specific factors. This finding is consistent with previous reports showing improvement in memory on sertraline in non-depressed older adults (Furlan, et al. 2001; Schmitt, et al. 2001).
We next compared change in cognitive functioning between the nortriptyline and sertraline conditions and found that patients treated with sertraline showed significantly more improvement in verbal learning compared to patients treated with nortriptyline. This finding is consistent with previous studies comparing the impact of sertraline to nortriptyline on the cognitive functioning of older adults. For example, treatment with sertraline in the geriatric depressed led to greater improvement in verbal learning (as measured by the Shopping List Task (SLT)) when compared to treatment with nortriptyline (Doraiswamy et al. 2003). In another study of depressed older adults, sertraline treatment led to improvement in verbal learning (as measured by the SLT) whereas nortriptyline treatment led to a mild decline over 12 weeks of treatment (Finkel, et al. 1999).
Finally, we compared change in cognition as a function of medication condition and responder status and found that sertraline responders showed significantly more improvement in verbal learning compared to nortriptyline responders but no more than sertraline non-responders or nortriptyline non-responders. To our surprise, nortriptyline responders were the only treatment by responder status group to show no improvement in verbal learning from baseline to endpoint.
The most cogent explanation for this unexpected finding is that memory improvement is blocked by the anticholinergic effect of nortriptyline. Tricyclic antidepressants have five times the anticholinergicity of SSRIs in older adults (Pollock, et al. 1998). More specifically, sertraline has been found to produce no anticholinergic activity at therapeutic doses whereas nortriptyline demonstrates a moderate anticholinergic activity (5-15 pmol/ml) (Chew, et al. 2008). Furthermore, drug-induced anticholinergic activity has been associated with cognitive impairment in older adults (Oxman 1996); greater anticholinergic effect was significantly (negatively) associated with endpoint cognitive improvement (in verbal learning and processing speed) in depressed older adults (Doraiswamy et al. 2003). In another study of the geriatric depressed, higher plasma nortriptyline concentration over 6 weeks of treatment was associated with poorer free recall but better affective outcome (Young, et al. 1991) indicating that the therapeutic and cognitive effects of nortriptyline may have different mechanisms. Even very low anticholinergic activity has been associated with specific cognitive deficits. In one study, depressed elderly subjects with serum anticholinergic activity performed more poorly in verbal learning than did those without anticholinergic activity (Nebes, et al. 1997). However, plasma drug levels of nortriptyline were blood-controlled in the present study, and it is unlikely that the anticholinergic effect differentially impacted the cognitive functioning of responders and non-responders on nortriptyline.
Another possible explanation for the unexpected finding is that the nortriptyline responder group had a disproportionately high number of cognitively impaired patients. However, the average MMSE score at baseline for the sample was 27.01, which is within normal limits and there was no significant difference in MMSE score between the four treatment by responder status groups at baseline. There were also no differences between the four groups in age or education. It is also possible that the overall medical burden was higher among nortriptyline responders compared to the other three groups. The interaction between medical illness and antidepressant medication could adversely affect cognitive functioning. However, there were no significant differences in medical burden (as assessed by the Cumulative Illness Rating Scale for Geriatrics) between the four groups.
This study should be interpreted in the context of several limitations. First, the sample size was relatively small and this study was not specifically powered to detect between drug differences in cognitive function by responder status. The responder analyses should therefore be interpreted with caution. Furthermore, the small sample size did not allow for a test of the interaction between depressive subtype, responder status, and treatment condition on change in cognitive function nor did it allow us to take into account differences due to depressive subtype (melancholia vs. non-melancholia). Second, the findings of this study may have been only a statistical anomaly. These were post-hoc analyses involving a multiplicity of statistical tests. Therefore, the findings are intended to be hypothesis generating only and are clearly in need of replication. Third, although multiple cognitive domains were examined, the assessment within each domain was relatively limited. Fourth, there was no placebo control group making it difficult to determine whether the observed improvement in verbal learning was nothing more than a practice effect. However, treatment with sertraline led to an improvement on the Buschke SRT that exceeded the improvement observed in patients randomized to placebo in our previous study (Culang et al. 2009) (¾ and ¼ of a standard deviation, respectively), suggesting there was significant change from pre- to post-treatment beyond a practice effect.
Acknowledgments
This research was supported by a grant from National Institute of Mental Health grants R01 MH55716 (Steven P. Roose) and K23 MH075006 (Joel R. Sneed).
Footnotes
Disclosures: SPR has received consultant fees from Medtronics and Orexigen
References
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF, Becker JT. Persistence of Neuropsychologic Deficits in the Remitted State of Late-Life Depression. American Journal of Geriatric Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Alpert M, Friedhoff AJ, Richter EM, Clary CM, Batzar E. Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. American Journal of Psychiatry. 2000;157:729–736. doi: 10.1176/appi.ajp.157.5.729. [DOI] [PubMed] [Google Scholar]
- Buschke H, Fuld P. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF., III Changes in Cognitive Functioning Following Treatment of Late-Life Depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollack BG, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, Kirshner MA, Sorisio DA, Bies RR, Gharabawi G. Anticholinergic activity of 107 medications commonly used by older adults. Journal of the American Geriatrics Society. 2008;56:1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation for the behavioral sciences. Mahwah, NJ: Lawrence Erlbaum Publishers; 2003. [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavioral Research, Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Culang M, Sneed J, Keilp J, Rutherford B, Pelton GH, Devanand DP, Roose SP. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am J Geriatr Psychiatry. 2009;17:881–888. doi: 10.1097/jgp.0b013e3181b4bf4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy PM, Krishnan KR, Oxman T, Jenkyn LR, Coffey DJ, Burt T, Clary CM. Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci. 2003;58:M1137–1144. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- Finkel SI, Richter EM, Clary CM, Batzar E. Comparative efficacy of sertraline vs. fluoxetine in patients age 70 or over with major depression. American Journal of Geriatric Psychiatry. 1999;7:221–227. doi: 10.1097/00019442-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the state of patients for the clincian. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Furlan PM, Kallan MJ, Ten Have T, Pollock BG, Katz I, Lucki I. Cognitive and psychomotor effects of paroxetine and sertraline on healthy elderly volunteers. Geriatr Psychiatry. 2001;9 [PubMed] [Google Scholar]
- Gallassi R, Di Sarro R, Morreale A, Amorec M. Memory impairment in patients with late-onset major depression: The effect of antidepressant therapy. Journal of Affective Disorders. 2006;91:243–250. doi: 10.1016/j.jad.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Reisberg B, Ferris SH, Nagachandran N, Chang I, Mir P. The Effects of Mood Changes and Antidepressants on the Cognitive Capacity of Elderly Depressed Patients. International Psychogeriatrics. 1989;1:135–143. doi: 10.1017/s1041610289000141. [DOI] [PubMed] [Google Scholar]
- Kramer-Ginsberg E, Greenwald BS, Krishnan KRR, Christiansen B, Hu J, Ashtari M, Patel M, Pollack S. Neuropsychological Functioning and MRI Signal Hyperintensities in Geriatric Depression. Am J Psychiatry. 1999;156:438–444. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- Lesser I, Boone K, Mehringer C, Wohl M, Miller B, Berman N. Cognition and white matter hyperintensities in older depressed patients. American Journal of Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. American Journal of Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. see comment. [DOI] [PubMed] [Google Scholar]
- MacLeod C. A half-century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, G PB, Zmuda MD, Houck PR, Reynolds CF., III Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Medicine. 2000;30:679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF., III Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of Psychiatric Research. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Mulsant BH, Kirshner MA, Halligan E, Zmuda M, Reynolds CFr. Low-level serum anticholinergicity as a source of baseline cognitive heterogeneity in geriatric depressed patients. Psychopharmacology Bulletin. 1997;33:715–720. [PubMed] [Google Scholar]
- Oxman TE. Antidepressants and cognitive impairment in the elderly. Journal of Clinical Psychiatry. 1996;57:38–44. [PubMed] [Google Scholar]
- Pollock BG, Mulsant BH, Nebes R, Kirshner MA, Begley AE, Mazumdar S, Reynolds CFr. Serum anticholinergicity in elderly depressed patients treated with paroxetine or nortriptyline. American Journal of Psychiatry. 1998;155:1110–1112. doi: 10.1176/ajp.155.8.1110. [DOI] [PubMed] [Google Scholar]
- Portella MJ, Marcos T, Rami L, Navarro V, Gasto C, Salamero M. Residual cognitive impairment in late-life depression after a 12-month period follow-up. International J Geriatric Psychiatry. 2003;18:571–576. doi: 10.1002/gps.895. [DOI] [PubMed] [Google Scholar]
- Raskin J, Wiltse CG, Siegal A, Sheikh J, Xu J, Dinkel JJ, Rotz BT, Mohs RC. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164:900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, Tung G, Richardson E, Thomas C, Westlake R. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Multiple imputation: A primer. Statistical Methods in Medical Research. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analyst’s perspective. Multivariate Behavioral Research. 1998;33:545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- Schmitt JAJ, Kruizinga MJ, Riedel WJ. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. Journal of Psychopharmacology. 2001;15:173–179. doi: 10.1177/026988110101500304. [DOI] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Young RC, Mattis S, Alexopoulos GS, Meyers BS, Shindledecker RD, Dhar AK. Verbal memory and plasma drug concentrations in elderly depressives treated with nortriptyline. Psychopharmacology Bulletin. 1991;27:291–294. [PubMed] [Google Scholar]


