Abstract
Bioactive n-3 polyunsaturated fatty acids (PUFA), abundant in fish oil, have potential for treating symptoms associated with inflammatory and metabolic disorders; therefore, it is essential to determine their fundamental molecular mechanisms. Recently, several labs have demonstrated the n-3 PUFA docosahexaenoic acid (DHA) exerts anti-inflammatory effects by targeting the molecular organization of plasma membrane microdomains. Here we briefly review the evidence that DHA reorganizes the spatial distribution of microdomains in several model systems. We then emphasize how models on DHA and plasma membrane microdomains can be applied to mitochondrial membranes. We discuss the role of DHA acyl chains in regulating mitochondrial lipid-protein clustering, and how these changes alter several aspects of mitochondrial function. In particular, we summarize effects of DHA on mitochondrial respiration, electron leak, permeability transition, and mitochondrial calcium handling. Finally, we conclude by postulating future experiments that will augment our understanding of DHA-dependent membrane organization in health and disease.
Introduction
Fish oil is highly enriched in n-3 polyunsaturated fatty acids (PUFA). The major bioactive n-3 PUFAs of fish oil are eicosapentaenoic (EPA) and docosahexaenoic acids (DHA). Consumption of n-3 PUFAs is known to provide benefits for a number of diseases and disorders, although the cellular mechanisms underlying PUFA-mediated improvements are still a matter of investigation and debate (1). To translate n-3 PUFAs for treating a wide variety of afflictions, an understanding of their fundamental molecular mechanisms is essential.
Several pathways, which work in concert, have been proposed by which n-3 PUFAs target cellular function in inflammatory and metabolic diseases. These include manipulation of eicosanoid metabolism, production of neuroprotectin/resolvins, alterations in signaling pathways and gene expression, and spatial redistribution of lipids and proteins in the plasma membrane (1). Very recently, several studies ranging from the atomic level to animals have emerged to show that DHA, in particular, targets the physical organization of plasma membrane microdomains (2). Here we briefly review this evidence and then apply models developed with these studies toward understanding how DHA acyl chains could manipulate mitochondrial membrane organization and thereby impact disease endpoints.
DHA and models of plasma membrane molecular organization
Studies over the past decade have shown that DHA can manipulate the molecular organization of plasma membrane microdomains known as lipid rafts (2). Lipid rafts are operationally defined as nanoscale fluctuations composed of sphingolipids and cholesterol that coalesce into larger signaling assemblies in response to stimulation (i.e. antigen bindings its receptor) (3, 4). Although lipid rafts have remained controversial for various reasons, recent lipidomic and high-resolution imaging approaches have provided more compelling evidence for their existence (4).
Studies on DHA and lipid microdomains have relied on three types of model systems, each with its own weaknesses and strengths (Table 1). The first model system has investigated how DHA impacts the molecular organization of liquid ordered raft-like domains using lipid vesicles of defined composition (5). These studies have revealed several novel findings. One, DHA acyl chains, due to their high degree of conformational flexibility (6), can impart unique effects on numerous membrane properties to regulate lipid microdomain formation (5, 7). Second, phosphatidylethanolamines containing DHA prefer to avoid interactions with cholesterol and sphingolipid acyl chains (5). However, when heteroacid phosphatidylcholines containing DHA are forced to interact with cholesterol, molecular order of the phospholipid containing DHA increases (5, 8).
Table 1. Model systems used to study the effects of n-3 PUFAs on lipid microdomain organization.
Extensive explanation of the results from these model systems is described in a recent review (2).
| Lipid Vesicles | Cell culture | Animals | |
|---|---|---|---|
| Description of model system | Vesicles of defined size and composition relying on DHA-containing phospholipids | Lymphocytes Hepatocytes Splenocytes Cardiomyocytes Macrophages Cancer cells |
C57BL/6 mice Fat-1 transgenic mice Rats |
| Recent results | Cholesterol avoids DHA acyl chains; however, when cholesterol is forced to interact with DHA, there is an ordering effect on the phospholipid. | EPA/DHA infiltrate raft-like membranes EPA/DHA differentially modify cholesterol distribution EPA/DHA differentially modify lipid microdomain size |
Fish oil increases B and T lymphocyte microdomain size on a micron scale and molecular order EPA/DHA infiltrate raft-like membranes in differing cell types |
| Advantages | Atomic to nanoscale measurements Address mechanisms |
Determine bioactivity of EPA vs. DHA Address mechanisms |
Physiological relevance |
| Disadvantages | Physiological relevance | EPA/DHA membrane incorporation does not completely model the diet | Difficult to address membrane-based mechanisms in vivo |
The second model system has relied on treating various cell types, often lymphocytes, with fatty acids to assess changes in “raft” composition (Table 1). These studies have revealed EPA and DHA incorporate directly into raft-like membranes and thereby influence protein lateral organization and activity (9–12). Very recent studies from cell culture experiments have provided novel mechanistic insight into how DHA could manipulate raft organization; that is, n-3 PUFA acyl chains could be altering the distribution of cholesterol between rafts and non-rafts. This would be highly consistent with model membrane studies (5). However, more studies are needed in this area since the results are discrepant. One lab showed DHA treatment of SH-SY5Y cells promoted cholesterol to move from rafts to nonrafts while another lab showed EPA treatment of hepatocytes promoted cholesterol to move from nonrafts to rafts (13, 14). Perhaps these differences are due to the unique bioactivity of EPA versus DHA.
The third model system is animals fed diets enriched in n-3 PUFAs or the fat-1 transgenic mouse (Table 1). These studies suggest that n-3 PUFAs increase the size and molecular order of lipid rafts on a micron scale. For example, the Shaikh lab showed that cholera toxin induced raft clustering of B220+ B cells was diminished in different n-3 PUFA diet models accompanied by suppression of B cell mediated antigen presentation (15, 16). Furthermore, DHA, but not EPA, increased membrane order upon cross-linking rafts relative to no cross-linking (16). Similarly, Chapkin and co-workers have established n-3 PUFAs promoted the formation of ordered immunological synapses to suppress CD4+ T cell activation (17). Overall, changes in raft size and order impact protein activity and thereby downstream signaling and gene expression (2).
Integrating models on DHA and lipid microdomains
The differing model systems must be effectively integrated to generate complete mechanisms by which n-3 PUFAs manipulate microdomain molecular organization (Table 1). While lipid vesicles and cells allow very direct mechanistic studies, physiological application toward animals and humans is limited. Similarly, although studies at the animal level are highly physiologically relevant, dissecting the effects of fatty acids from the diet on membrane organization is difficult. Irrespective of the model system used to study n-3 PUFAs and membrane domains, more studies are needed at the nanoscale, which is the relevant size scale.
Taken together, the model that emerges from the differing experimental systems is that DHA, more than EPA, acyl chains incorporate directly into ordered lipid microdomains to manipulate protein organization (Fig. 1). These effects appear to be driven biophysically and biochemically by DHA (2). Changes in the composition and structure of lipid microdomains with DHA then impacts protein clustering, activity, and signaling. Specifically, in vivo studies suggest DHA diminishes raft clustering and increases membrane molecular order upon formation of lipid microdomains, which could promote protein clustering or de-clustering (15–17). Ultimately, changes in cellular function are influenced by events that were initiated on the surface of the cell by DHA.
Figure 1. Generalized model on how the underlying lipid environment in a membrane can regulate protein clustering and thereby downstream function.
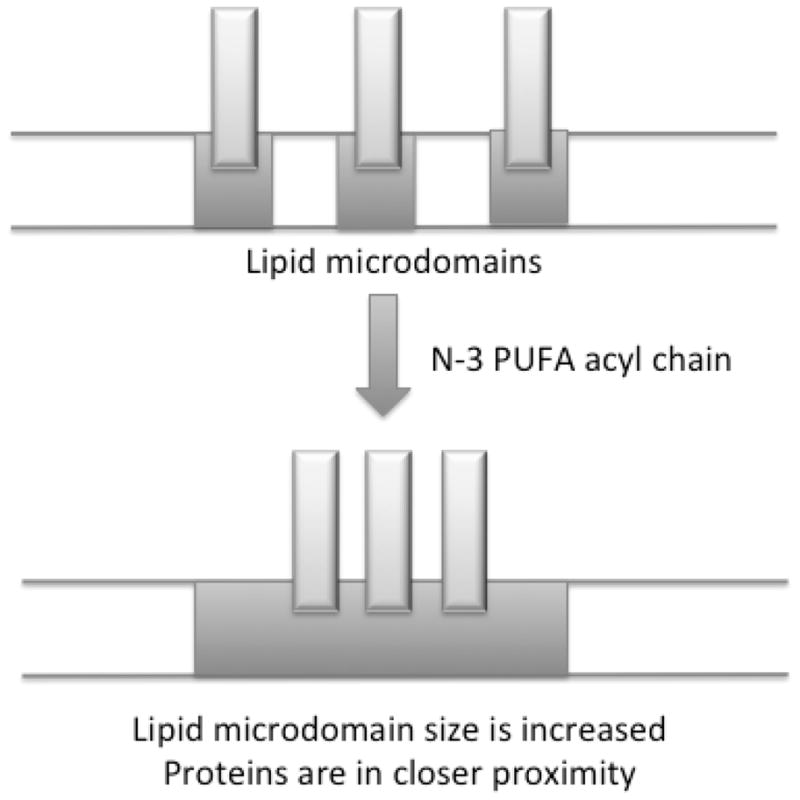
Studies on the plasma membrane suggest that incorporation of n-3 PUFA acyl chains, DHA in particular, modify the composition and size of lipid microdomains. As a consequence of this change, the ability of proteins to cluster, communicate and signal is modified. For simplicity, this model shows n-3 PUFA acyl chains increasing protein clustering but could also do the opposite; that is, de-cluster proteins. This model is applicable to endomembranes such as the mitochondrial membrane.
Mitochondrial membranes as a target of n-3 PUFAs
While there is increasing evidence that DHA disrupts plasma membrane molecular organization, far less is known about the impact of DHA on endomembrane organization. This gap in knowledge stems from the fact that microdomain organization of lipids and proteins of endomembranes is poorly studied (18). Here we focus on the mitochondrial membranes as an example of endomembranes that may be a major target of n-3 PUFAs with profound functional consequences.
Mitochondria are obligatory for cellular survival/function and their membranes are susceptible to modulation by dietary and pharmacological interventions. Lipid-protein interactions are particularly important in mitochondrial inner membranes, which have significantly higher protein content than plasma membranes. Some estimates of the inner membrane protein:lipid ratios are as high as 3:1 (19). Among the population of inner membrane proteins, a significant portion is comprised of proteins associated with the electron transport chain. Vectorial electron transfer along inner mitochondrial membrane respiratory complexes creates an electrochemical proton gradient, and the energy liberated by this gradient is normally coupled to the phosphorylation of ADP during ATP synthesis. The efficiency of electron flow along the chain is critically dependent on the redox potential and spatial proximity of adjacent complexes, sometimes referred to as the ‘respirasome’ (20). Given that bioenergetic abnormalities are observed in a wide array of diseases including cardiovascular diseases, cancer, Alzheimer’s, diabetes, and immune disorders, PUFA-mediated improvements in health likely involve improved mitochondrial respiration. Indeed, proton leak from the respiratory chain decreases following n-3 PUFA treatment, and this was directly related to incorporation of the PUFAs into membrane phosphatidylcholine (PC) phosphatidylethanolamine (PE), phosphatidylinositol (PI) and cardiolipin (CL) fractions (21). Of course, it is important to note that the effects of n-3 PUFAs are highly cell specific. For instance, DHA increases proton leak in some cancer cells (22).
Mitochondrial composition and microdomain formation
The bulk composition of the mitochondrial membranes is PC >PE > CL ~ PI, which respectively represent about ~ 40, 30, and 15% of the total phospholipids (23). Unlike the plasma membrane, the levels of sphingolipids and cholesterol in the mitochondria are very low (24). Therefore, there is some debate if mitochondria form lipid raft-like domains. Very recent proteomic data from Zhen et al. show that mitochondria do not contain rafts (25). However, some labs have proposed that CL does form raft-like domains in mitochondria (26). One intriguing study showed that edelfosine, a synthetic anti-tumor ether lipid, exerts its effects on mitochondrial function by redistributing plasma membrane rafts to the mitochondria (27).
The debate on rafts in the mitochondria does not rule out the notion that lipids and proteins are forming microdomains or clusters, likely on a nanometer scale. Indeed, very recent data using STED microscopy have revealed that proteins exist as clusters in the mitochondrial membrane. For example, Singh et al. showed that cytochrome c oxidase subunit 2 existed in clusters of ~28 nm and similarly a voltage dependent anion channel also displayed clustering on different length scales (28). In another study, prohibitin complexes in the mitochondria appeared to be potential nucleating centers for lipids and proteins in the inner mitochondrial membrane, supporting the notion of localized membrane domains (29, 30).
DHA in cardiolipin
One major fate of DHA in endomembranes is CL, which has a highly novel structure. The glycerol base has two attached phosphatidates, which renders it highly inflexible (31). Therefore, it is hypothesized that the small headgroup and relative overall inflexibility makes CL more susceptible to interactions with proteins. CL was originally hypothesized to be located in the inner membrane, but more recent studies show its presence in the outer leaflet (23).
We propose that the highly disorder structure of DHA incorporating into CL would dramatically change the molecular properties of cardiolipin and thereby its ability to interact with neighboring lipids and proteins. In particular, localized lipid and protein clusters would be altered and ultimately this would impact mitochondrial function. CL, similar to PE, has the ability to form inverted hexagonal phases in model membranes due to its cone shaped structure (31). Indeed, Shaikh et al. previously showed that DHA incorporated into PEs promoted hexagonal phase (32). Thus, it is intriguing to speculate that DHA, a highly flexible structure, incorporating into CL, an inflexible structure, would have very unique effects on mitochondrial membrane structure. Similar to the plasma membrane model (Fig. 1), one can hypothesize that biophysical changes with DHA in the inner membrane of mitochondria would regulate protein activity.
We are finding in preliminary studies that DHA incorporates into the mitochondria of lymphocytes and cardiomyocytes (Fig. 2). As an example, EL4 lymphomas acutely treated with a Bodipy labeled DHA shows significant co-localization with the mitochondria (Teague et al., manuscript under review). Similarly, in neonatal rat cardiomyocytes, we find that radiolabeled DHA incorporates into CL to a greater extent than other major phospholipids. This raises the exciting possibility that DHA can target the molecular organization of mitochondrial lipid-protein clusters in numerous cell types.
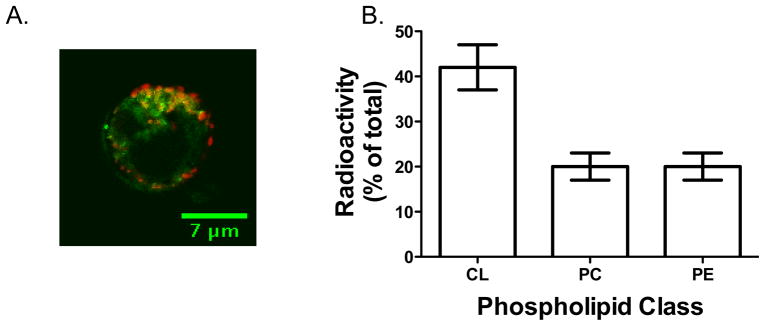
Figure 2. DHA incorporates into the mitochondria of lymphocytes and cardiomyocytes.
(A) Sample fluorescence image showing co-localization between a DHA labeled fluorophore and mitochondria of EL4 cells. Green indicates DHA-Bodipy and red is mito-tracker. (B) Lipid analysis reveals radiolabeled DHA incorporates into cardiolipin (CL) of neonatal rat cardiomyocytes to a greater than phosphatidylcholines (PC) and phosphatidylethanolamines (PE). Cardiomyocytes were treated with 5μM DHA spiked with [3H]DHA for 24 hours, the lipids were extracted, and CL/PC/PE fractions were separated on high performance thin layer chromatography plates. Radioactivity was measured using a scintillation counter. Data are representative of 2–3 independent experiments.
Physiological consequences of targeting membrane organization
The functional implications of manipulating membrane organization are starting to emerge. For example, in immunological studies, it is now clear that EPA and/or DHA suppress CD4+ T cell activation, B cell mediated antigen presentation, or macrophage activation by targeting the molecular organization of lipid microdomains and their associated proteins (11, 16, 17, 33–36). This has profound therapeutic value for suppressing inflammation and autoimmune responses associated with a variety of diseases (36). For instance, suppressing macrophage activation could reduce pro-inflammatory cytokine production in adipose tissue, which then targets pathways associated with insulin sensitivity (37).
A number of studies have investigated the effect of altered CL side chain content on mitochondrial function. CL associates with just about every major protein complex within the mitochondrial inner membrane, most notably the complexes involved in the electron transport chain (38). CL dramatically alters the formation of mitochondrial respiratory supercomplexes, which is required for optimal electron transfer along the complexes (39, 40). Altered CL acyl chain composition directly influences cellular respiration. Given that n-3 PUFAs can partition within CL (Fig. 2), a number of studies have demonstrated improved mitochondrial physiology with changed CL composition. Disease models demonstrate clear shifts in the acyl chain composition of CL, typically with a reduction in 18:2 that ultimately results in diminished CL content (41). Although the consequence of this CL loss is speculative, these changes may decrease bioenergetic capacity by impairing the formation of respiratory supercomplexes (the ‘respirasome’) (20). Future studies will advance our understanding of how CL side chains (particularly those incorporating n-3 PUFAs) influence the formation of respiratory supercomplexes.
In addition to the respiratory chain, another mitochondrial protein complex that appears to be very sensitive to altered acyl chain composition is the permeability transition pore (PTP). Although the molecular identity of the pore is still a matter of debate (42), the opening of the PTP collapses mitochondrial energetics and promotes apoptotic/necrotic cell death in a number of cell types (43, 44).
The influence of n-3 PUFAs on the opening of the permeability transition pore has recently been investigated in different models, although the findings are somewhat divergent. Several studies using cancer cell lines have determined the influence of n-3 PUFAs (DHA in particular) on PTP opening. Ng et al. demonstrated that colonic cells displayed an increased propensity for PTP opening when treated with DHA, a property that was postulated to underlie the anti-cancer properties of DHA (45). These findings were supported by another report from the same group where DHA was administered alongside butyrate (46). To the contrary, Wu et al. found that PTP opening in lung adenocarcinoma cells was prevented with DHA treatment, ultimately leading to better maintenance of mitochondrial energetics and improved cell survival (47). While there are clear differences in the experimental models used (DHA administration varied between 30 minutes and 3 days in the studies discussed), more research in cell culture models will improve our understanding of PTP modulation by DHA.
Several recent studies by Stanley’s group indicate that DHA may have cardioprotective effects by delaying calcium-induced PTP opening. In a series of studies, these investigators used dietary PUFAs to determine if the fatty acid composition influenced PTP opening (48–50). These studies showed that DHA in particular delayed PTP opening, ostensibly by targeting the phospholipid composition of the mitochondria. This study was intriguing in that EPA did not have a similar effect. This is highly consistent with plasma membrane models in which DHA, but not EPA, disrupts lipid microdomain organization in lymphocytes (10, 16). Future studies examining the direct influence of mitochondrial lipid composition on PTP complex assembly (and subsequent open probability) will significantly advance our understanding of n-3 PUFAs in mitochondrial physiology.
Dietary PUFAs are also known to influence mitochondrial calcium transport. Although the molecular identification of the mitochondrial calcium uniporter was only recently discovered (51, 52), the ability of n-3 PUFAs to modulate mitochondrial calcium flux has been known for over 10 years (53). Pepe et al. found that rats fed a diet rich in n-3 PUFAs displayed resistance to mitochondrial calcium overload, implying that mitochondrial calcium flux is susceptible to modulation by dietary PUFAs (53). As with the other mitochondrial proteins discussed, future experiments are needed to determine how the inner membrane lipid composition influences the assembly/clustering of mitochondrial calcium channel subunits. Given the important role of mitochondrial calcium fluxes in health and disease, such insight may foster novel treatments to improve mitochondrial function in a number of disease states where bioenergetics is affected.
Conclusions
Recent studies from the atomic scale to cells and animals are providing a mechanistic view of how DHA regulates plasma membrane organization. A central theme is that DHA reorganizes the molecular organization of lipid microdomains to alter protein clustering and thereby cellular function. We propose that DHA acyl chains may also exert similar effects on lipid-protein clusters of mitochondrial membranes to regulate metabolic and inflammatory activity. Overall, determining the effects of DHA on endomembrane organization will aid in the translation of the fatty acid into clinical trials.
Acknowledgments
The research was supported in part by grants from the NIH (R15AT006152) to S.R.S. and Stealth Peptides to D.A.B.
Footnotes
Author disclosures: no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 2.Shaikh SR. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem. 2012;23:101–105. doi: 10.1016/j.jnutbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike LJ. Rafts defined: A report on the keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Lingwood DD, Simons K. Lipid rafts as a membrane-organizing principle. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 5.Wassall SR, Stillwell W. Polyunsaturated fatty acid–cholesterol interactions: Domain formation in membranes. Biochim Biophys Acta. 2009;1788:24–32. doi: 10.1016/j.bbamem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Gawrisch KK, Soubias O. Structure and dynamics of polyunsaturated hydrocarbon chains in lipid bilayers—significance for GPCR function. Chem Phys Lipids. 2008;153:64–75. doi: 10.1016/j.chemphyslip.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soni SP, LoCascio DS, Liu Y, et al. Docosahexaenoic acid enhances segregation of lipids between raft and nonraft domains: 2H-NMR study. Biophys J. 2008;95:203–214. doi: 10.1529/biophysj.107.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihailescu M, Soubias O, Worcester D, et al. Structure and dynamics of cholesterol-containing polyunsaturated lipid membranes studied by neutron diffraction and NMR. J Membr Biol. 2011;239:63–71. doi: 10.1007/s00232-010-9326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapkin RS, Wang N, Fan Y, et al. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaikh SR, Rockett BD, Salameh M, et al. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 11.Wong SW, Kwon M, Choi AMK, et al. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–553. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 13.Grimm MOW, Kuchenbecker J, Groesgen S, et al. Docosahexaenoic acid reduces amyloid β production via multiple, pleiotropic mechanism. J Biol Chem. 2011;286:14028–14039. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliche-Djoudi F, Podechard N, Chevanne M, et al. Physical and chemical modulation of lipid rafts by a dietary n-3 polyunsaturated fatty acid increases ethanol-induced oxidative stress. Free Radic Biol Med. 2011;51:2018–2030. doi: 10.1016/j.freeradbiomed.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Rockett BD, Franklin A, Harris M, et al. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonrafts of EL4 and B cells. J Nutr. 2011;141:1041–1048. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockett BD, Teague H, Harris M, et al. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012 doi: 10.1194/jlr.M021782.. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim W, Fan YY, Barhoumi R, et al. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaikh SR, Edidin MA. Membranes are not just rafts. Chem Phys Lipids. 2006;144:1–3. doi: 10.1016/j.chemphyslip.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Nichols DG, Ferguson SJ. Bioenergetics. 3. Academic Press; London: 2002. [Google Scholar]
- 20.Rosca MG, Vazquez EJ, Kerner J, et al. Cardiac mitochondria in heart failure: Decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pehowich DJ. Thyroid hormone status and membrane n-3 fatty acid content influence mitochondrial proton leak. Biochim Biophys Acta. 1999;1411:192–200. doi: 10.1016/s0005-2728(99)00041-9. [DOI] [PubMed] [Google Scholar]
- 22.Fan YY, Ran Q, Toyokuni S, et al. Dietary fish oil promotes colonic apoptosis and mitochondrial proton leak in oxidatively stressed mice. Cancer Prev Res. 2011;4:1267–1274. doi: 10.1158/1940-6207.CAPR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osman CC, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng YZ, Berg KB, Foster LJ. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J Lipid Res. 2009;50:988–998. doi: 10.1194/jlr.M800658-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim BW, Lee JW, Choo HJ, et al. Mitochondrial oxidative phosphorylation system is recruited to detergent-resistance lipid rafts during myogenesis. Proteomics. 2010;10:2498–2515. doi: 10.1002/pmic.200900826. [DOI] [PubMed] [Google Scholar]
- 27.Mollinedo F, Fernández M, Hornillos V, et al. Involvement of lipid rafts in localization and dysfunction effect of the antitumor ether phospholipid edelfosine in mitochondria. Cell Death Dis. 2011;19:e158. doi: 10.1038/cddis.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh H, Lu R, Rodriguez PF, et al. Visualization and quantification of cardiac mitochondrial protein clusters with STED microscopy. Mitochondrion. 2011 doi: 10.1016/j.mito.2011.09.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkwirth C, Langer T. Prohibitin function within mitochondria: Essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Osman C, Merkwirth C, Langer T. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci. 2009;122:3823–3830. doi: 10.1242/jcs.037655. [DOI] [PubMed] [Google Scholar]
- 31.Lewis RN, McElhaney RN. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim Biophys Acta. 2009;1788:2069–2079. doi: 10.1016/j.bbamem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Shaikh SR, Brzustowicz MR, Stillwell W, et al. Formation of inverted hexagonal phase in SDPE as observed by solid-state (31)P NMR. Biochem Biophys Res Commun. 2001;286:758–763. doi: 10.1006/bbrc.2001.5454. [DOI] [PubMed] [Google Scholar]
- 33.Bonilla DL, Ly LH, Fan YY, et al. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to mycobacterium tuberculosis. PLoS One. 2010;5:e10878. doi: 10.1371/journal.pone.0010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaikh SR, Edidin M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J Lipid Res. 2007;48:127–138. doi: 10.1194/jlr.M600365-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Weatherill AR, Lee JY, Zhao LL, et al. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 36.Chapkin RS, Kim W, Lupton JR, et al. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prost Leuk Essent Fatty Acid. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver E, McGillicuddy FC, Harford KA, et al. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between LC n-3 PUFA. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.06.014. In press. [DOI] [PubMed] [Google Scholar]
- 38.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer K, Gohil V, Stuart RA, et al. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 41.Sparagna GC, Chicco AJ, Murphy RC, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 45.Ng Y, Barhoumi R, Tjalkens RB, et al. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolar SS, Barhoumi R, Lupton JR, et al. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–5568. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 47.Wu S, Xing D, Gao X, et al. High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol. 2009;218:603–611. doi: 10.1002/jcp.21636. [DOI] [PubMed] [Google Scholar]
- 48.O’Shea KM, Khairallah RJ, Sparagna GC, et al. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol. 2009;47:819–827. doi: 10.1016/j.yjmcc.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khairallah RJ, Sparagna GC, Khanna N, et al. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta. 2010;1797:1555–1562. doi: 10.1016/j.bbabio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khairallah RJ, O’Shea KM, Brown BH, et al. Treatment with docosahexaenoic acid, but not eicosapentaenoic acid, delays Ca2+-induced mitochondria permeability transition in normal and hypertrophied myocardium. J Pharmacol Exp Ther. 2010;335:155–162. doi: 10.1124/jpet.110.170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Stefani D, Raffaello A, Teardo E, et al. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pepe S, Tsuchiya N, Lakatta EG, et al. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–58. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]