Summary
Legionella pneumophila, the causative agent of Legionnaires’ disease, survives in macrophages by altering the endocytic pathway of its host cell. To accomplish this, the bacterium utilizes a type IVB secretion system to deliver effector molecules into the host cell cytoplasm. In a previous report, we performed an extensive characterization of the L. pneumophila type IVB secretion system that resulted in the identification of a critical five-protein subcomplex that forms the core of the secretion apparatus. Here we describe a second Dot/Icm protein subassembly composed of the type IV coupling protein DotL, the apparatus proteins DotM and DotN, and the secretion adaptor proteins IcmS and IcmW. In the absence of IcmS or IcmW, DotL becomes destabilized at the transition from the exponential to stationary phases of growth, concurrent with the expression of many secreted substrates. Loss of DotL is dependent on ClpA, a regulator of the cytoplasmic protease ClpP. The resulting decreased levels of DotL in the icmS and icmW mutants exacerbates the intracellular defects of these strains and can be partially suppressed by overproduction of DotL. Thus, in addition to their role as chaperones for Legionella T4SS substrates, IcmS and IcmW perform a second function as part of the Dot/Icm type IV coupling protein subcomplex.
Introduction
Legionella pneumophila survives and replicates within a wide variety of phagocytic cells, including protozoa and macrophages (Fields et al., 2002, Horwitz, 1983). After uptake by a phagocytic cell, Legionella is contained within a vacuole (LCV), which avoids the endocytic pathway and instead fuses with endoplasmic reticulum-derived vesicles. This process results in the formation of an unique intracellular compartment, termed the replicative phagosome, where the bacteria multiply to high numbers (reviewed in (Isberg et al., 2008)).
Alteration of the host endocytic pathway is mediated by effector proteins translocated into the host cell cytoplasm via the L. pneumophila type IVB secretion system (T4SS), which is encoded by twenty-six dot/icm genes (Vogel et al., 1998, Segal et al., 1998). Over the last decade, significant progress has been made in the characterization of the L. pneumophila Dot/Icm system (Isberg et al., 2008). For example, an extensive biochemical and genetic analysis of the Dot/Icm system revealed the existence of a major subassembly called the core-transmembrane subcomplex that is composed of five proteins (DotC, DotD, DotF (IcmG), DotG (IcmE), and DotH (IcmK)) (Vincent et al., 2006b). DotH, presumed to form the outer membrane pore, requires the lipoproteins DotC and DotD for its insertion in the outer membrane (Vincent et al., 2006b, Nakano et al., 2010). DotF and DotG are inner membrane proteins that interact with DotC/DotD/DotH in the outer membrane (Luo & Isberg, 2004, Vincent et al., 2006b). DotG, based on its homology to the Agrobacterium tumefaciens VirB10 protein, likely transduces energy from the inner membrane to the outer membrane similar to the action of TonB (Cascales & Christie, 2004).
In addition to the core-transmembrane complex, preliminary data from Vincent et al implicated the existence of a second subcomplex composed of the proteins DotL (IcmO), DotM (IcmP), DotN (IcmJ), IcmS, and IcmW (Vincent et al., 2006b). This second subcomplex is of particular interest because DotL has been proposed to function as the type IV coupling protein (T4CP) for the Dot/Icm secretion system (Buscher et al., 2005, Sexton et al., 2004, Sexton et al., 2005). Coupling proteins are key components of T4SSs and are thought to serve two essential roles in substrate secretion. First, they function as the inner membrane receptor linking substrates to the secretion machinery (Atmakuri et al., 2003, Atmakuri et al., 2004, Cabezon et al., 1997, Gilmour et al., 2003, Hamilton et al., 2000, Llosa et al., 2003, Schroder et al., 2002, Szpirer et al., 2000). Second, they are thought to catalyze the hydrolysis of ATP, thereby directly providing energy for substrate translocation (Tato et al., 2005). As a result, any subcomplex containing DotL will likely perform a critical role in the L. pneumophila IV secretion system.
Little is known about DotM and DotN other than their association with the inner membrane. In contrast to DotM and DotN, more is known about the type IV adaptor proteins IcmS and IcmW as they are thought to function analogously to type III secretion chaperones. These factors are typically small, acidic proteins that bind substrates and perform diverse roles including the stabilization of substrates, prevention of substrate aggregation, and maintenance of substrates in a secretion competent state (Bennett & Hughes, 2000, Parsot et al., 2003). Three putative adaptor proteins, IcmS, IcmW, and LvgA, have been identified in L. pneumophila (Coers et al., 2000, Vincent & Vogel, 2006, Cambronne & Roy, 2007). The best characterized are IcmS and IcmW, which are required for the export of a wide variety of Dot/Icm substrates including SdeA, SidA, SidB, SidC, SidD, SidE, SidG, and SidH (Bardill et al., 2005, Cambronne & Roy, 2007). However, the substrates RalF and SidF do not require IcmS and IcmW for their export, indicating that a subset of substrates can be secreted in a type IV adaptor-independent manner (Cambronne & Roy, 2007). Although IcmS and IcmW bind certain Dot/Icm substrates, they have not been shown to interact with any components of the T4SS apparatus.
In this report, we confirm the existence of a second Dot/Icm protein subcomplex consisting of DotL, DotM, and DotN. In addition, we describe for the first time an interaction between the secretion adaptor proteins IcmS and IcmW and proteins that are not T4SS substrates. Furthermore, we found that in the absence of IcmS or IcmW, DotL becomes destabilized in a ClpA-dependent manner at the transition from exponential to stationary phase, correlating with the expression of many Dot/Icm substrates. These findings indicate a second, previously unknown role for the secretion adaptor proteins IcmS and IcmW as part of the type IV coupling protein subcomplex of the L. pneumophila Dot/Icm T4SS.
Results
Stability Effects Between Members of the DotL T4CP Subcomplex
It was previously hypothesized that the L. pneumophila type IV coupling protein DotL may exist in a subcomplex with two components of the secretion apparatus, DotM and DotN, and two soluble adaptor proteins, IcmS and IcmW (Vincent et al., 2006b). The existence of this subcomplex was proposed based on destabilization effects observed in stationary phase in strains lacking individual components of this putative subcomplex. This interpretation was complicated by the fact that dotL, dotM, and dotN are required for the viability of the L. pneumophila strain Lp02 (Buscher et al., 2005), thus necessitating their construction in a strain containing a suppressor mutation. In that study, we used a ΔdotA mutation as a suppressor, as inactivation of the type IV secretion system can alleviate the lethality associated with deletions of dotL, dotM, or dotN (Buscher et al., 2005). However, the absence of an otherwise functional Dot/Icm complex in these strains raised concerns as to whether the stability effects observed were specifically due to inactivation of dotL, dotM, and dotN or whether they were due to a combinatorial effect involving multiple dot mutants.
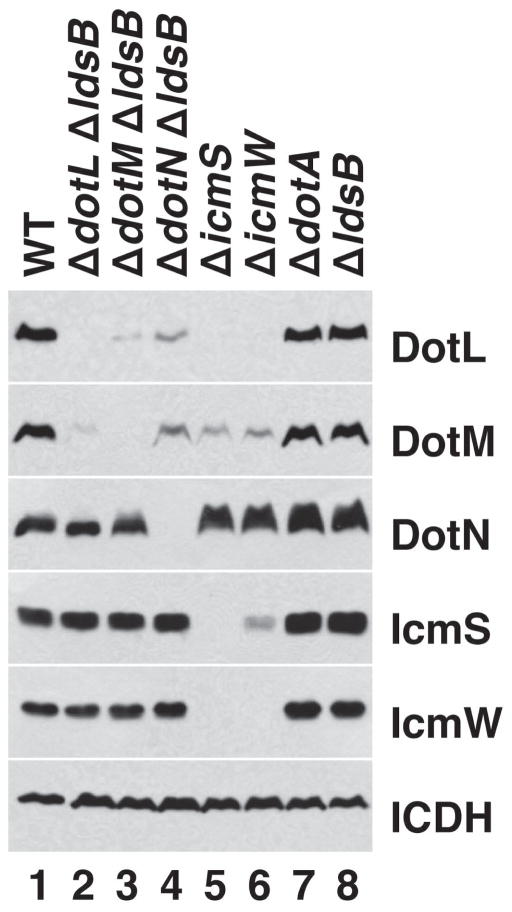
To address this concern, we re-examined the stability effects in a strain background that contains a mutation in ldsB, a non-dot/icm suppressor of ΔdotL lethality (Vincent et al., 2006a). ldsB (lethality of ΔdotL suppressor) encodes a putative inner membrane protein that is not part of the Dot/Icm T4SS, not required for the expression of any of the Dot/Icm proteins, nor necessary for the proper function of the T4SS, including growth within host cells. Utilizing the suppressive activity of the ΔldsB mutation, strains lacking each of the components of this second putative subcomplex were grown to early stationary phase, when L. pneumophila strains become virulent, and whole cell extracts were analyzed by Western blotting. As shown in Figure 1, the levels of the coupling protein DotL were greatly reduced in the ΔdotM ΔldsB and ΔdotN ΔldsB strains (lanes 3 and 4) and were undetectable in the ΔicmS and ΔicmW strains (lanes 5 and 6). The destabilization of DotL observed in the ΔdotM ΔldsB and ΔdotN ΔldsB strains was not due to the ΔldsB mutation, as the ΔldsB mutant exhibited normal levels of DotL (Figure 1, lane 8). Inactivation of another dot/icm gene, dotA, also did not significantly affect the levels of DotL (Figure 1, lane 7) (Vincent et al., 2006b), demonstrating the result was specific to the inactivation of a subset of dot/icm genes.
Figure 1.
Stability effects caused by inactivation of dotL, dotM, dotN, icmS, and icmW. Total protein extracts from L. pneumophila strains grown to early stationary phase were analyzed by SDS-PAGE followed by Western blotting with antibodies specific for DotL, DotM, DotN, IcmS, and IcmW. The protein isocitrate dehydrogenase (ICDH) served as a loading control. Genotypes of the strains used are shown at the top and the antibody used for western analysis is shown at the right. Lane 1 is wild-type L. pneumophila (Lp02), lane 2 is ΔdotL ΔldsB (JV5976), lane 3 is ΔdotM ΔldsB (JV6019), lane 4 is ΔdotN ΔldsB (JV5972), lane 5 is ΔicmS (JV1962), lane 6 is ΔicmW (JV3598), lane 7 is ΔdotA (JV2064), and lane 8 is ΔldsB (JV5775).
The levels of DotM were reduced in strains lacking dotL, dotN, icmS, and icmW, although a low amount of DotM was detectable in each of these strains. Similar to DotL, the individual ΔdotA and ΔldsB mutations had no effect on DotM. In contrast, the levels of DotN were not significantly decreased in the ΔdotL ΔldsB and ΔdotM ΔldsB mutants nor were they affected by deletions in icmS or icmW. As previously seen (Ninio et al., 2005, Vincent & Vogel, 2006), IcmS and IcmW required each other for their stability (Figure 1, lanes 5–6) but they were not noticeably affected by the absence of dotL, dotM, or dotN (Figure 1, lanes 2–4). Together, these data indicate that the destabilization results are due to loss of a component of the proposed subcomplex and not due to indirect effects from the suppressor mutation used in constructing the ΔdotL, ΔdotM, and ΔdotN strains.
Interactions Between DotL and Components of the Inner Membrane Apparatus
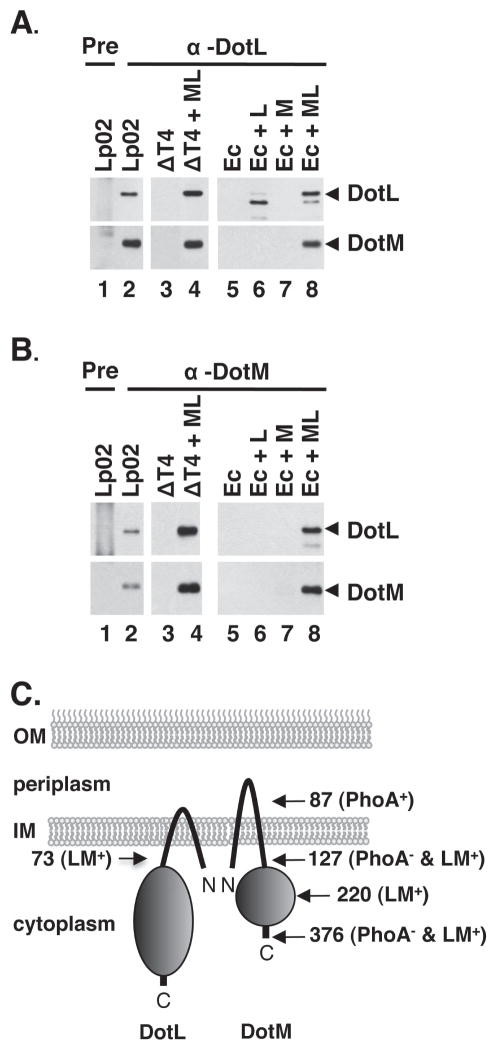
The multiple stability effects observed in the dotL, dotM, dotN, icmS, and icmW deletions suggested that these proteins might interact to form a subcomplex. To investigate this possibility, we first examined if DotL and DotM associate by performing immunoprecipitations using DotL- and DotM-specific antibodies followed by Western blotting to identify co-precipitated proteins. As shown in Figure 2, DotM could be co-immunoprecipitated from the wild-type L. pneumophila strain Lp02 using a DotL specific antibody (Figure 2A, lane 2). Neither protein was seen in control reactions using pre-immune serum (Figure 2A, lane 1) or when DotL serum and lysates from a L. pneumophila strain missing all of the dot/icm genes (lane 3) or an E. coli strain (lane 5) were used. The immunoprecipitations were specific as an unrelated cytoplasmic protein, isocitrate dehydrogenase (ICDH), was not precipitated with the DotL- or DotM- specific antisera (data not shown). Notably, the interaction appeared to be direct, as DotM could be co-precipitated from a L. pneumophila strain or an E. coli strain expressing only DotL and DotM and none of the remaining dot/icm genes (Figure 2A, lane 4 and 8).
Figure 2. Interactions between DotL, DotM, IcmS and IcmW demonstrated by co-immunoprecipitation.
(A) DotM co-immunoprecipitates with DotL. Immunoprecipitations using L. pneumophila or E. coli lysates (genotypes shown at top) were performed using pre-immune serum (“pre”) or DotL-specific antiserum followed by Western blotting using antibodies specific to DotL or DotM, as indicated to the right of each blot (arrowheads designate full-length protein). Lysates were made from the following strains: lanes 1 and 2 are wild-type Legionella (Lp02), lane 3 is Δdot/icm + vector (JV4191), lane 4 is Δdot/icm + dotML (JV4192), lane 5 is E. coli + vector (JB3188), lane 6 is E. coli + dotL (JB3189), lane 7 is E. coli + dotM (JB3190), and lane 8 is E. coli + dotML (JB3191).
(B) DotL co-immunoprecipitates with DotM. Same as in (A) except DotM-specific antiserum was used (lanes 2–8).
(C) Schematic representation of the orientation of and interaction between DotL and DotM in the inner membrane. Sites of PhoA and two-hybrid fusions are indicated by arrows and the final amino acid in each fusion. PhoA positive and negative fusions are shown by a plus or a minus sign. Fusions that were positive in the two-hybrid assay are indicated by an LM+ designation.
Similar results were obtained when an antibody specific to DotM was used to co-immunoprecipitate DotL (Figure 2B). Both proteins were precipitated from the wild-type L. pneumophila strain (Figure 2B, lane 2) when DotM-specific serum was used, although neither DotM nor DotL were precipitated with pre-immune serum (lane 1). Again, both proteins could be immunoprecipitated when expressed from a plasmid in the L. pneumophila dot/icm deletion strain (Figure 2B, lane 4) or in E. coli (lane 6), but were not observed when this plasmid was not present (lanes 3 and 5). We were unable to establish an interaction between these proteins and DotN utilizing this technique, due to instability of the DotN protein under these lysis conditions (data not shown).
To confirm that DotL and DotM directly interact, we examined their membrane association and interaction using a bacterial two-hybrid system (Karimova et al., 1998). Based on sequence analysis and the homology of DotL to a type IV coupling protein (Buscher et al., 2005), it likely possesses two transmembrane domains near the N-terminus and a large cytoplasmic C-terminal domain that contains the Walker box nucleotide binding motif. Similarly, DotM also possesses two predicted transmembrane domains (amino acids 17–39 and 92–114) and a medium sized cytoplasmic C-terminal domain. The localization of DotM was determined by analyzing fusions to alkaline phosphatase (PhoA) (Figure S1), confirming a similar topology of DotM to DotL (data summarized in the schematic shown in Figure 2C).
Although a two-hybrid interaction could not be detected using fusions to full length DotL and DotM, a positive interaction was observed between fusions to the first 73 amino acids of DotL and full length DotM (Figure S2). Moreover, a robust interaction could also be detected using the DotL fragment and fusions containing only the amino-terminal 220 or 127 amino acids of DotM (Figure S2). Since a positive signal could be detected in E. coli with the amino-terminal 73 amino acids of DotL and the first 127 amino acids of DotM, regions that primarily contain the transmembrane domains of each protein, this suggests that DotL and DotM directly interact at least through their transmembrane domains (Figure 2C).
Interaction between DotM/DotL and the adaptors IcmS/IcmW
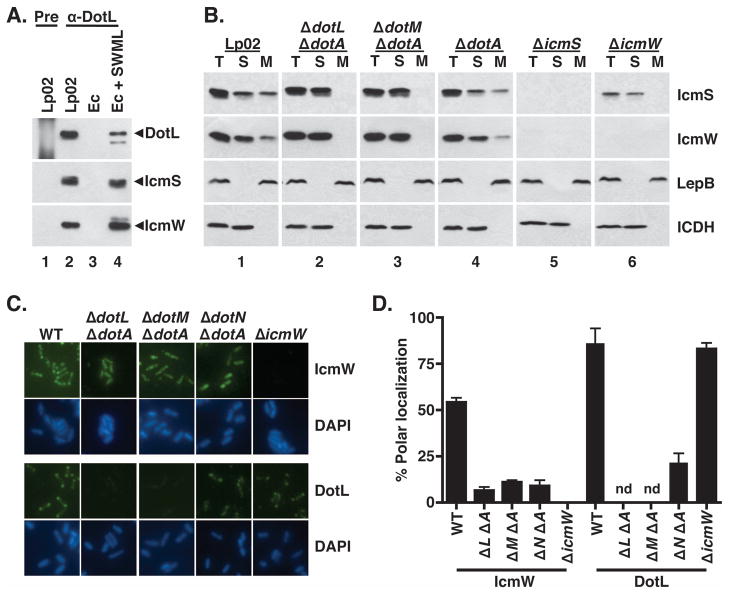
In order to determine if DotL interacts with additional Dot/Icm proteins, we further analyzed the DotL immunoprecipitation reactions using antibodies that recognize nineteen other Dot/Icm proteins. Only two other proteins, the adaptor proteins IcmS and IcmW, could be detected in these immunoprecipitations (data not shown and Figure 3A, lane 2). The reactions were specific since IcmS and IcmW were not precipitated when pre-immune serum was used (Figure 3A, lane 1). Co-precipitation of the adaptor proteins did not require any Dot/Icm proteins other than DotL and DotM as the complex could be precipitated from an E. coli strain expressing only icmS, icmW, dotL, and dotM (Figure 3A, lane 4). IcmS and IcmW could also be precipitated using an antibody specific to DotM (data not shown). Since DotL and DotM require each other for stability, we were unable to differentiate in these experiments whether the IcmS/IcmW heterodimer binds directly to DotL, DotM, or both proteins.
Figure 3. IcmS and IcmW are cytoplasmic proteins that associate with the bacterial membrane at the poles in a DotL/DotM/DotN-dependent manner.
(A) IcmS and IcmW co-immunoprecipitate with DotL. Immunoprecipitations were performed using pre-immune serum (“pre”, lane 1) or DotL-specific antiserum (lanes 2–4) and samples were analyzed by Western blotting using antibodies to DotL, IcmS, and IcmW. Lysates were made from the following strains: lanes 1 and 2 are wild-type (Lp02), lane 3 is E. coli + vector (JB3188), and lane 4 is E. coli + icmS + icmW + dotML (JB4728).
(B) L. pneumophila strains were grown to exponential phase, fractionated, followed by Western blotting with antibodies specific to IcmS, IcmW, DotL, the inner membrane control protein LepB, and the cytoplasmic control protein ICDH (indicated on the right). Fractions are labeled as follows: (T) total cellular protein, (S) soluble proteins, and (M) membrane proteins. Panel 1 is wild type (Lp02), panel 2 is ΔdotL ΔdotA (JV2422), panel 3 is ΔdotM ΔdotA (JV5361), panel 4 is ΔdotA (JV2064), panel 5 is ΔicmS (JV1962), and panel 6 is ΔicmW (JV3598).
(C and D) Polar localization of DotL and IcmW are dependent on the subcomplex. L. pneumophila strains were grown to exponential phase, and immunofluorescence microscopy analysis was conducted as described. Affinity purified DotL and IcmW antibodies were used to probe wild type (Lp02), ΔdotL ΔdotA, ΔdotM ΔdotA, ΔdotN ΔdotA, and ΔicmW strains. DNA was stained with DAPI. Polar staining of DotL and IcmW was quantified from three independent experiments with one hundred cells scored in each experiment. Polar localization of DotL was not determined (nd) for the ΔdotL and ΔdotM strains due to the absence of detectable antigen. Error bars represent the standard deviation from the mean.
Because the L. pneumophila adaptors IcmS and IcmW are predicted to be soluble, cytoplasmic proteins, we envisaged that their interaction with DotL/DotM might target them to the type IV membrane complex. This would be consistent with the proposed role of DotL as a type IV coupling protein (T4CP) (Buscher et al., 2005), which serves as the inner membrane receptor for T4SS substrates. To test this hypothesis, lysates from wild type and several L. pneumophila Δdot strains were fractionated into total, soluble, and membrane fractions and analyzed by Western blotting to determine the subcellular localization of various proteins. In these experiments, ΔdotA was used to suppress the lethality of the ΔdotL and ΔdotM mutants since we had shown that the mechanism of suppression was not relevant (Figure 1). In wild-type cells (Lp02), a significant amount of IcmS and IcmW were found associated with the membrane fraction (Figure 3B, panel 1). The control proteins LepB (type I signal peptidase) and ICDH (isocitrate dehydrogenase) localized exclusively to the membrane and soluble fractions, respectively, demonstrating that localization of IcmS and IcmW to the membrane fraction was not due to poor separation of membrane and cytoplasmic proteins.
When localization of IcmS and IcmW was examined in a ΔdotL ΔdotA strain, neither IcmS nor IcmW could be detected in the membrane fraction (Figure 3B, panel 2). Similarly, no membrane localization of IcmS or IcmW was observed in the ΔdotM ΔdotA strain (Figure 3B, panel 3). The ΔdotA mutation, in contrast, did not alter the localization of IcmS and IcmW, indicating DotA is not itself involved in targeting IcmS/IcmW to the membrane (Figure 3B, panel 4). Although IcmW was not observable in the ΔicmS strain (Figure 3B, panel 5), a small amount of IcmS could be detected in the ΔicmW strain, however none of it was associated with the membrane (Figure 3B, panel 6). This suggested that membrane localization of IcmS requires IcmW in addition to DotL and DotM. In summary, these findings support a model in which DotL/DotM functions as the inner membrane receptor that targets the adaptor proteins to the Dot/Icm complex.
Based on our recent observation that components of the Legionella Dot/Icm core-transmembrane subcomplex localize to the poles of the bacterial cell (Kwang Cheol Jeong and Joseph Vogel, unpublished data), we examined if the type IV adaptor proteins displayed a similar localization pattern. Immunofluoresence staining using an IcmW-specific antibody revealed that a significant proportion of cells have IcmW specifically targeted to both poles of the bacterium (Figure 3C and 3D). Attempts to localize IcmS by immunofluorescence were unsuccessful due to poor detection by our IcmS-specific antibody but likely resemble IcmW as the two proteins interact. The observed immunofluorescence signal using the IcmW antiserum was due to the presence of the IcmW protein, as no signal was seen in an icmW deletion or in an icmS mutant in which IcmW is degraded. Strikingly, IcmW did not localize to the bacterial poles in the ΔdotL ΔdotA, ΔdotM ΔdotA, and ΔdotN ΔdotA mutants (Figure 3C), indicating that these proteins might mediate polar recruitment of the adaptors. IcmW localization was not dependent on the presence of DotA (data not shown) indicating that its mislocalization was specifically due to the absence of DotL, DotM, or DotN proteins.
To test the hypothesis that DotL might be responsible for the localization of IcmW, we examined if DotL also localized to the poles. Using an affinity purified DotL antibody, we observed that DotL localizes primarily to the poles of the bacteria in wild-type L. pneumophila cells (approximately 85%) (Figure 3D). No fluorescence was observed in a ΔdotL ΔdotA mutant, demonstrating that the immunofluorescence signal was specific to DotL. In addition, no DotL fluorescence was detectable in the ΔdotM ΔdotA (Figure 3C), consistent with the decreased stability of DotL in this strain. DotL was faintly detectable in the ΔdotN strain but with decreased polar localization, indicating that DotN may be involved in the proper positioning of DotL within the bacterial cell. Similar to the ΔdotM and ΔdotN mutants, DotL could not be detected in stationary phase cells lacking icmW or icmS (data not shown). However, during exponential phase growth, polar localization of DotL was not affected by the absence of icmW (Figure 3C) or icmS (data not shown). Taken together, these results demonstrate that DotL/DotM/DotN are capable of targeting the IcmS/IcmW adaptors to the membrane secretion apparatus located at the poles of the bacterium.
DotL Stability Effects are Growth Phase Dependent
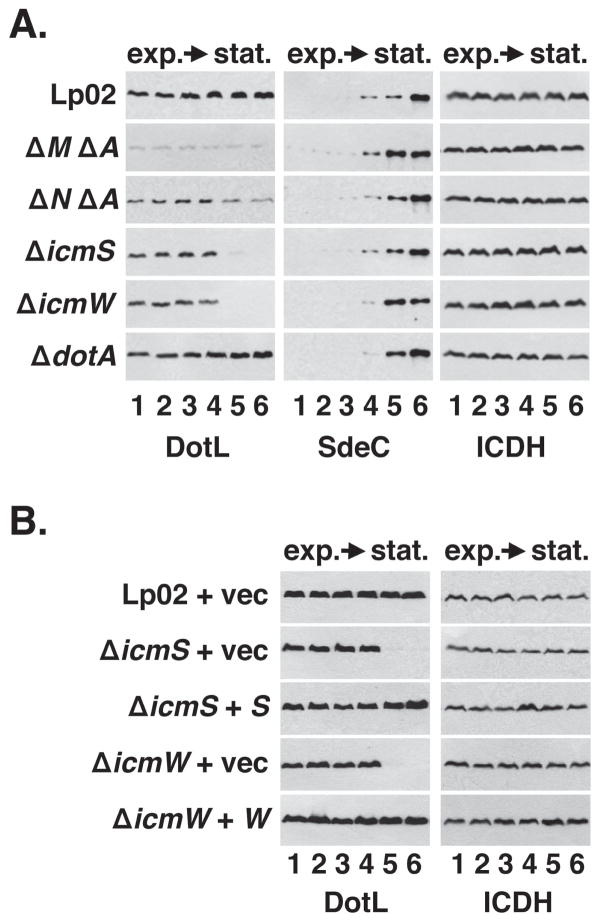
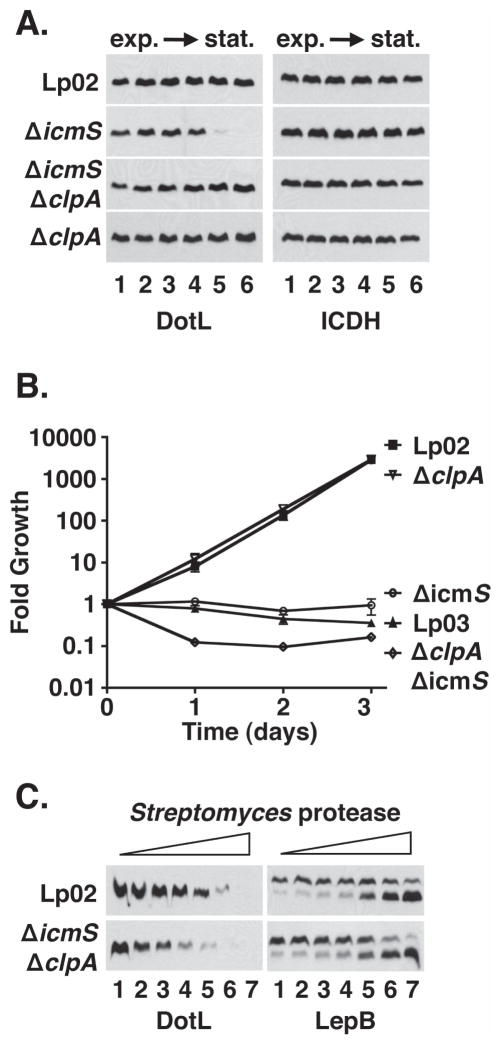
Although the recruitment of IcmS/IcmW by DotL/DotM/DotN to the secretion apparatus is consistent with the role of DotL as a type IV coupling protein, the loss of DotL in the ΔicmS and ΔicmW mutants was particularly intriguing as dotL is essential for the growth of the L. pneumophila strain Lp02 (Buscher et al., 2005). The presence of DotL-specific immunofluorescence in the ΔicmW mutant during exponential phase (Figure 3C) suggested a possible explanation for why this strain is viable, and prompted us to investigate the growth phase dependence of the stability effects observed in Figure 1. Because we had initially examined the stability of DotL only during early stationary phase, the point at which L. pneumophila becomes virulent (Byrne & Swanson, 1998, Hammer & Swanson, 1999), we reexamined this result during different phases of growth. In wild-type cells, DotL levels remained fairly constant throughout the growth cycle in vitro (Figure 4A), typical of most Dot/Icm proteins (Vincent et al., 2006b). In contrast, DotL levels rapidly decreased at the transition into stationary phase in the ΔicmS and ΔicmW mutants, although they were similar to wild type during exponential phase (Figure 4A), thus possibly explaining why these strains are able to grow. In the ΔdotM and ΔdotN mutants, DotL levels were uniformly decreased throughout the growth cycle, whereas a deletion of dotA did not affect DotL levels (Figure 4A). Interestingly, the destabilization of DotL in the ΔicmS and ΔicmW mutants closely correlated with the induced expression of many Dot/Icm substrates, such as RalF and SdeC, that is seen in the transition from exponential growth to stationary phase (Figure 4A and data not shown) (Ninio & Roy, 2007). The destabilization of DotL in early stationary phase was expressly due to inactivation of icmS and icmW since complementation of these mutants restored DotL to wild-type levels in stationary phase (Figure 4B). Thus, inactivation of icmS and icmW leads to the destabilization of DotL at a defined point in growth, coincident with the time when L. pneumophila becomes virulent by inducing the synthesis of T4SS effectors.
Figure 4. Destabilization of DotL in the ΔicmS and ΔicmW strains occurs during the transition to stationary phase and correlates with expression of secreted substrates.
(A) L. pneumophila strains were grown in broth and cells were collected at various stages of growth. Whole-cell extracts were analyzed by Western blotting using antibodies specific to the proteins listed beneath each panel. Extracts were made from cultures at the following OD600: lane 1, early exponential phase (OD600 ~1.0), lane 2, exponential phase (OD600 ~ 2.0), lane 3, exponential phase (OD600 ~ 2.5), lane 4, late exponential phase (OD600 ~ 3.0), lane 5, early stationary phase (OD600 ~ 3.2), lane 6, stationary phase (OD600 ~ 3.5). Strains used were: wild-type (Lp02), ΔdotM ΔdotA (JV5361), ΔdotN ΔdotA (JV3719), ΔicmS (JV1962), ΔicmW (JV3598), and ΔdotA (JV2064).
(B) Complementation of the stationary phase DotL instability in the ΔicmS and ΔicmW strains. Strains used were: wild-type plus vector (JV1139), ΔicmS plus vector (JV5156), ΔicmS plus icmS (JV5157), ΔicmW plus vector (JV3658), and ΔicmW plus icmW (JV3649). In both (A) and (B), Western blots for ICDH served as a loading control. Results shown are representative of three independent experiments.
Although the destabilization of DotL in the absence of the adaptors IcmS/IcmW correlated with the synthesis of L. pneumophila Dot/Icm substrates, these two events may not be connected. For example, loss of DotL could be due an unrelated change that occurs in Gram-negative bacteria as they transition from exponential phase into stationary phase. To differentiate between these possibilities, we examined the stability of DotL in an E. coli strain expressing DotM, DotL, and DotN in the absence or presence of IcmS/IcmW. In contrast to the situation in Legionella, DotL was stable throughout at all phases of growth in the presence and absence of IcmS and IcmW (Figure S3). Thus, the DotL destabilization result was not simply due to conserved growth phase changes in Gram-negative bacteria, but is L. pneumophila specific. This is consistent with the hypothesis that production of effectors causes the decreased levels of DotL in a Legionella icmS mutant strain.
ClpA is Responsible for DotL Cleavage in the Absence of IcmS
Because DotL is present during exponential phase, but not in stationary phase of an ΔicmS mutant, the disappearance of DotL is most likely due to proteolysis. In order to confirm this hypothesis, we attempted to identify the protease involved in this process. DotL stability was examined in over two dozen Legionella mutants defective for a specific protease or regulator of protease activity (Table S2). Each mutant was constructed in an ΔicmS background and DotL stability was examined during stationary phase growth (Sexton & Vogel, 2004). Inactivation of only one of twenty-seven genes, clpA, restored the levels of DotL in the ΔicmS mutant (Figure 5A and data not shown). ClpA is an ATP-dependent regulator of the cytoplasmic protease ClpP and is conserved in most bacteria, including L. pneumophila (Zolkiewski, 2006). Unfortunately we were unable to isolate a ΔclpP mutation in the L. pneumophila strain Lp02, suggesting that the gene may be essential (data not shown). A ΔclpP mutant has been reported in JR32, a related L. pneumophila strain, although it appeared to be sick as it exhibited a number of cell division defects (Li et al., 2010). Interestingly, inactivation of clpA had no effect on the levels of DotL in the wild-type strain Lp02 (Figure 5A), suggesting that removal of DotL in the absence of IcmS may be a stress response mediated by ClpAP.
Figure 5. ClpA is responsible for the decreased level of DotL observed in the absence of IcmS/IcmW.
(A) Stability of DotL in the absence of IcmS and ClpA. L. pneumophila strains were grown in broth and cells were collected at various stages of growth. Whole cell extracts were analyzed by Western blotting using antibodies specific to the proteins listed beneath each panel. Optical densities (600 nm) of the cultures were similar to those described in Figure 4. Strains used were: wild-type plus vector (JV1139), ΔicmS plus vector (JV5156), ΔicmS ΔclpA::CmR plus vector (JV6296) and the ΔclpA::CmR plus vector (JV6503). Western blots for ICDH served as a loading control.
(B) Deletion of ClpA does not suppress the intracellular growth defect of the ΔicmS mutant in U937 cells. Strains used were wild-type plus vector (JV1139, filled squares), ΔclpA::CmR plus vector (JV6503, open inverted triangles), ΔicmS plus vector (JV5156, open circles), Lp03 plus vector (JV1139, open triangles), and ΔicmS ΔclpA::CmR plus vector (JV6296, open diamonds). Each time point represents the mean and standard deviation of colony forming units (CFU) recovered from triplicate wells. Growth curves are representative of three independent experiments.
(C) Protease susceptibility of DotL in the absence of IcmS and ClpA. L. pneumophila strains wild-type (Lp02) and ΔicmS ΔclpA::CmR (JV339) were grown to late stationary phase in broth, cells were sonicated, and treated with protease from Streptomyces griseus (Sigma) at the following concentrations: lane 1 (0 μM), lane 2 (5 μM), lane 3 (10 μM), lane 4 (20 μM), lane 5 (40 μM), lane 6 (80 μM), and lane 7 (160 μM). Samples were analyzed by Western blotting using antibodies specific to the proteins listed beneath each panel. Strains used are listed on the left. Results shown are representative of three independent experiments.
Since DotL is required for replication within host cells, decreased levels of DotL in the ΔicmS and ΔicmW mutants would likely lead to diminished intracellular growth by these mutants (Buscher et al., 2005). Consistent with this, icmS and icmW mutants have been documented to be severely attenuated for intracellular multiplication, although this defect has been proposed to be due solely to decreased secretion of effectors that bind the adaptors IcmS/IcmW (Coers et al., 2000, Vincent & Vogel, 2006, Zuckman et al., 1999, Ninio et al., 2005). To determine if decreased levels of DotL contribute to the severity of the growth defect of these mutants, we compared the intracellular growth of an ΔicmS mutant, which has decreased levels of DotL, with an ΔicmS ΔclpA double mutant, which has close to wild-type levels of DotL.
Intracellular growth was assayed in the human monocytic U937 cell line, which is permissive for growth of Legionella (Figure 5B). The wild-type strain Lp02 was able to grow ~3,000-fold over 72 hours, while the dotA mutant strain Lp03, which contains a non-functional Dot/Icm complex, was completely defective for intracellular growth. An icmS mutant was almost as defective for growth as the Lp03 strain (Figure 5B). Interestingly, the ΔicmS ΔclpA strain remained completely defective for growth, even though it contains normal levels of DotL in stationary phase (Figure 5A). This failure to grow was not due to an indirect effect caused by the ΔclpA mutation, as inactivation of only clpA resulted in a strain capable of wild-type growth.
Although the ΔicmS ΔclpA mutant accumulates wild-type amounts of DotL, it is plausible the protein is not functional, perhaps because it is misfolded or inappropriately complexed with other proteins. To test this premise, we performed limited proteolysis on lysates from various strain and then assayed for differential cleavage between samples. A difference in cleavage patterns can indicate that the protein is misfolded or in a different conformation making it more accessible to protease digestion (Cascales & Christie, 2004). Lysates prepared from stationary phase Lp02 (wild-type strain) and from the ΔicmS ΔclpA mutant were incubated with increasing concentrations of Streptomyces griseus protease (Figure 5C). Although DotL present in Lp02 was susceptible to degradation by high amounts of the protease, DotL produced in the icmS, clpA double mutant was clearly sensitive to lower amounts of protease (Figure 5C). This result was specific to DotL as LepB, a conserved inner membrane protein, was degraded at similar rates in both strains (Figure 5C, right panel). As a result, inactivation of clpA was able to restore wild-type levels of DotL but was not able to suppress the intracellular growth defect of the ΔicmS mutant likely because DotL was misfolded or inappropriately bound by another protein.
Suppression of ΔicmS and ΔicmW Phenotypes by Overproduction of DotL
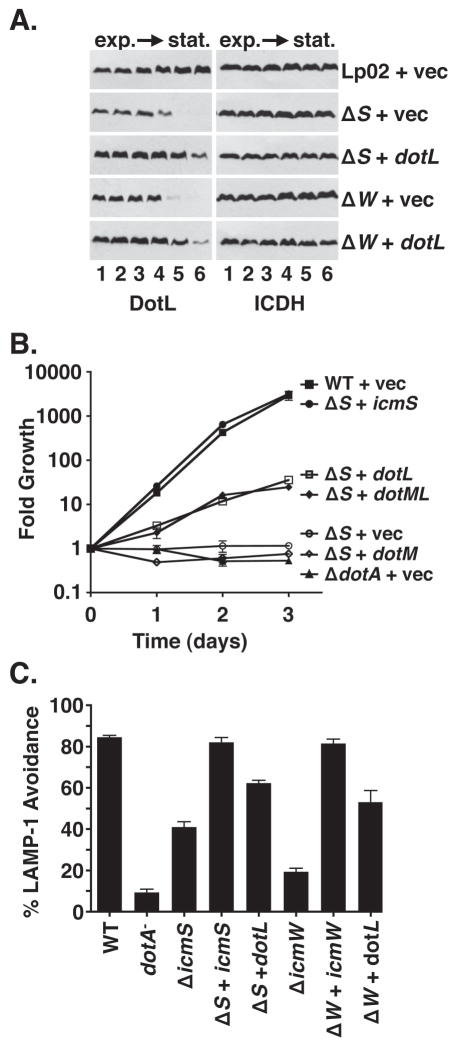
As protein instability can sometimes be suppressed by increased production of the protein, we assayed the effect of overproducing DotL in the ΔicmS and ΔicmW mutants. Increased synthesis of DotL, accomplished by using a multi-copy plasmid containing the dotL gene under the control of the robust Ptac promoter, remarkably restored wild-type levels of DotL protein in the ΔicmS and ΔicmW mutants (Figure 6A). Moreover, in contrast to the result observed with the ΔclpA ΔicmS double mutant, over-production of DotL resulted in enhanced levels of intracellular growth when compared to the ΔicmS mutant (Figure 6B) or the ΔicmW mutant (Figure S4). Suppression of the growth defect of these mutants by increased synthesis of DotL was not complete, although the defects of these mutants could be fully complemented by expression of the corresponding gene, eliminating the possibility of a secondary mutation in these strains. Over-production suppression was specific to DotL as increased synthesis of DotM did not improve growth of the ΔicmS mutant (Figure 6B). Therefore, the destabilization effect caused by the absence of the adaptors appears to be mediated directly through DotL, and not indirectly through DotM’s effect on DotL stability.
Figure 6. Overexpression of DotL partially suppresses the intracellular growth defects of the ΔicmS and ΔicmW strains.
(A) Destabilization of DotL in the ΔicmS and ΔicmW strains can be partially overcome by overexpression of DotL. L. pneumophila strains were grown in broth and cells were collected at various stages of growth. Whole cell extracts were analyzed by Western blotting using antibodies specific to the proteins listed beneath each panel. Optical densities (600 nm) of the cultures were similar to those described in Fig. 4. Strains were: wild-type plus vector (JV1139), ΔicmS plus vector (JV5156), ΔicmS plus dotL (JV5565), ΔicmW plus vector (JV3658), and ΔicmW plus dotL (JV5570). Western blots for ICDH served as a loading control.
(B) Partial complementation of the intracellular growth defect of the ΔicmS mutant in U937 cells by overexpression of DotL. Strains used were: wild-type plus vector (JV1139, filled squares),ΔicmS plus icmS (JV5157, filled circles), ΔicmS plus dotM + dotL (JV5566, filled diamonds),ΔicmS plus dotL (JV5565, open squares), ΔicmS plus dotM (JV5809, open diamonds) ΔicmS plus vector (JV5156, open circles), and ΔdotA plus vector (JV3029, filled triangles). Fold growth was calculated by dividing the number of colony forming units (CFU) recovered each day by the number of CFU recovered immediately after infection (day 0). Each time point represents the mean and standard deviation of colony forming units (CFU) recovered from triplicate wells. Growth curves are representative of three independent experiments.
(C) Co-localization of L. pneumophila-containing phagosomes with the endocytic marker LAMP-1. Mouse bone marrow-derived macrophages were infected for 1 hour at 37° C, fixed, stained for LAMP-1 and intracellular versus extracellular L. pneumophila, and examined by immunofluoresence microscopy. Strains used were: wild-type plus vector (JV1139), ΔdotA plus vector (JV3029), ΔicmS plus vector (JV5156), ΔicmS plus icmS (JV5157), ΔicmS plus dotL (JV5565), ΔicmW plus vector (JV3658), ΔicmW plus icmW (JV3649), and ΔicmW plus dotL (JV5570). Avoidance of LAMP-1 containing vesicles is shown as the average and standard deviation of four sets of 100 bacteria scored for each strain. Results are representative of two independent experiments.
Since the intracellular growth defects of the ΔicmS and ΔicmW mutants has been shown to be due to fusion of the LCV with the host endocytic pathway (Coers et al., 2000, Vincent & Vogel, 2006, Zuckman et al., 1999), we investigated whether over-expression of DotL in these mutants also restored their ability to alter the host endocytic pathway. In this assay, wild-type L. pneumophila is able to efficiently avoid fusion with lysosomes (85%) as assayed by co-localization of Legionella-containing vacuoles with the endocytic marker LAMP-1. In contrast, a dotA mutant was defective for this process (9% of ΔdotA mutant phagosomes were LAMP-1 negative) (Figure 6C). The ΔicmS mutant exhibited an intermediate phenotype (41% LAMP-1 negative) and could be fully complemented by the icmS-complementing clone. Expression of DotL from a plasmid significantly increased the ability of the ΔicmS mutant to avoid LAMP-1 acquisition (62% LAMP-1 negative). Similar results were observed for icmW (19% of the ΔicmW mutant were LAMP-1 negative whereas expression of DotL increased the percentage of LAMP-1 negative to 53%). Consequently, overproduction of DotL suppresses the growth defect of the adaptor mutants likely by partially restoring their ability to alter the endocytic pathway of the host cell.
Restoration of Secretion of IcmS-Independent Effectors in ΔicmS by Over-Expression of DotL
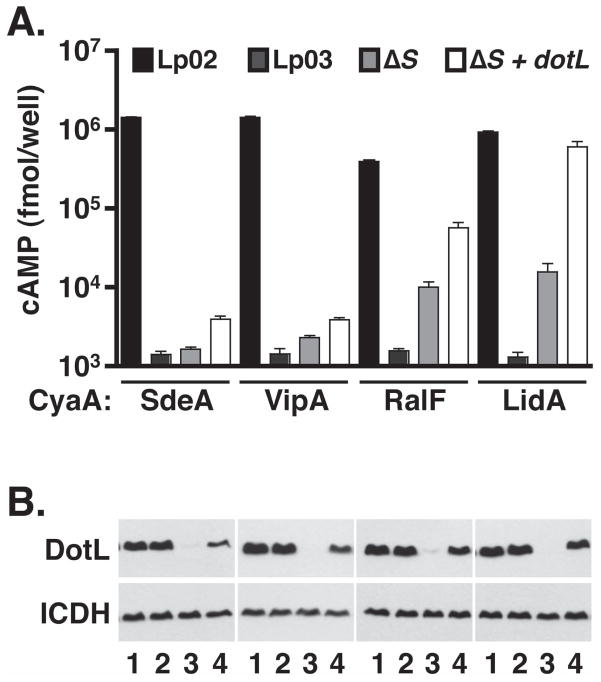
Limited suppression of the intracellular growth defect of the ΔicmS and ΔicmW mutants suggests that they may be defective for multiple functions, including a failure to properly chaperone IcmS/IcmW-dependent substrates and decreased levels of DotL. As a result of this pleiotropy, it is possible that export of some Dot/Icm substrates may appear to be partially dependent on the adaptors when this is actually caused by an indirect effect on DotL. To test this theory, we compared the export of substrates that are completely dependent on IcmS (SdeA and VipA) with substrates whose secretion has been reported to be IcmS-independent (RalF and LidA) (Bardill et al., 2005, Cambronne & Roy, 2007). Export was assayed in a wild-type strain, a dotA mutant that fully inactivates the T4SS, an ΔicmS mutant, and an ΔicmS strain that overproduces DotL (Figure 7). In each case, export was measured using fusions to Bordetella adenylate cyclase (CyaA), a well-established reporter for translocation of proteins into host cells (Karimova et al., 1998).
Figure 7. Increased export of IcmS-independent effectors by overexpression of DotL in an ΔicmS mutant.
(A) Secretion of SdeA, VipA, RalF, LidA was assayed using adenylate cyclase (CyaA) fusions. DotL overproduction was accomplished by expressing dotL from the CyaA fusion plasmid. CyaA fusions were expressed in a wild-type strain (Lp02), a dotA mutant (Lp03), and an ΔicmS mutant (JV1962). These strains were used to infect U937 cells and calmodulin-induced cAMP production was measured via an ELISA assay. cAMP assays were conducted in triplicate. Error bars represent the standard deviation from the mean.
(B) Westerns blots were performed in order to measure levels of DotL and of the control protein ICDH.
All four fusions (SdeA, VipA, RalF, and LidA) were secreted into host cells by the wild-type strain Lp02 but were not exported by the dotA mutant strain Lp03, demonstrating that their export is dependent on the L. pneumophila T4SS. As previously reported (Bardill et al., 2005, Cambronne & Roy, 2007), export of SdeA and VipA was severely diminished in an ΔicmS mutant (Figure 7). In contrast, RalF and LidA were exported in the ΔicmS mutant, although at significantly lower levels than in the wild-type strain, thus leading to some confusion as to whether they are exported in a truly IcmS-independent manner. Overproduction of DotL in the ΔicmS mutant only slightly affected the export of SdeA and VipA, whereas it resulted in a dramatic increase in the secretion of RalF and LidA to near wild-type levels. Therefore, overproduction of DotL in an ΔicmS mutant can be used to clearly differentiate IcmS-dependent substrates from those that are exported via a mechanism that does not absolutely require the type IV adaptor proteins. In addition, these results clearly indicates that the phenotype(s) of an ΔicmS mutant is due to multiple defects and therefore the adaptor proteins IcmS and IcmW perform separate roles in binding and stimulating the export of certain effector proteins and in preventing destabilization of DotL.
Discussion
We report here the existence of a second major subcomplex of the Legionella pneumophila type IV secretion system. This subcomplex consists of DotL, the type IV coupling protein, the Dot/Icm apparatus proteins DotM and DotN, and the cytoplasmic type IV adaptor proteins IcmS and IcmW. In the absence of IcmS and IcmW, DotL is destabilized and degraded in a ClpA-dependent manner during stationary phase when many Dot/Icm substrates are synthesized. The decreased levels of DotL observed in the ΔicmS and ΔicmW mutants could be restored by overproduction of DotL and this was sufficient to partially suppress the intracellular growth defect of the adaptor mutants. Thus, in addition to their proposed roles in the recognition of Dot/Icm substrates, IcmS and IcmW perform a second role involving the stabilization of DotL.
Based on an extensive biochemical and genetic analysis of the Dot/Icm apparatus, we have now identified two major subcomplexes, each consisting of five proteins. The first, named the “core-transmembrane subcomplex”, is made up of DotC, DotD, DotF, DotG and DotH (Vincent et al., 2006b). The second L. pneumophila T4SS subcomplex reported here consists of DotL, DotM, DotN, IcmS and IcmW. DotL, the predicted type IV coupling protein for the Dot/Icm secretion system, has been proposed to interact with the secretion apparatus and with secreted substrates (Buscher et al., 2005). DotL directly binds with the inner membrane protein DotM, consistent with previous reports demonstrating interactions between coupling proteins and components of type IVA secretion machineries (Atmakuri et al., 2004, Gilmour et al., 2003, Llosa et al., 2003). While the exact molecular function of DotM is not known, DotL and DotM are each unstable in the absence of each other, suggesting that DotM may play a structural role in the Dot/Icm complex and/or could regulate the putative ATPase activity of DotL. DotN, a small cysteine-rich protein, is required for the stability of DotL and DotM, although the precise mechanism of action of DotN is not known. Consistent with the idea that these three proteins function together as a subcomplex, dotL, dotM, and dotN are the only dot/icm genes required for the viability of the L. pneumophila strain Lp02 (Buscher et al., 2005).
Taken together, the two major L. pneumophila Dot/Icm subcomplexes account for ten of the twenty-six known dot/icm genes (Segal et al., 2005, Isberg et al., 2008). Although we do not have evidence that the two subcomplexes directly interact, they are likely to do so based on known interactions in type IVA secretion systems. Specifically, coupling proteins from these systems are known to bind to homologs of the energy transducing protein VirB10 (Atmakuri et al., 2004, Cascales & Christie, 2004, Gilmour et al., 2003, Llosa et al., 2003). Since DotL has been proposed to function as the Legionella Dot/Icm T4CP, and DotG contains a domain with homology to VirB10, it is possible that the two T4SS subcomplexes might be linked via a DotL:DotG interaction. Our inability to observe a direct interaction between these subcomplexes by co-immunoprecipitations may be due to the inherent insolubility of the core subcomplex in a number of detergents (data not shown).
Interestingly, we have obtained multiple pieces of data indicating that DotL/DotM interact with the type IV adaptors IcmS and IcmW. These data includes stability effects (Figure 1 and 4), co-immunoprecipitations (Figure 3A), membrane fractionation (Figure 3B), and polar localization (Figure 3C). Consistent with our results, the A. tumefaciens T4CP, VirD4, was previously shown to interact with the chaperone VirE1 in the presence and absence of the substrate VirE2 (Atmakuri et al., 2003). In addition to T4SSs, a similar result has been observed in a T3SS where the enteropathogenic E. coli CesT chaperone was capable of directly binding the inner membrane T3SS ATPase EscN without and with its substrate Tir (Gauthier & Finlay, 2003, Thomas et al., 2005). Although it is plausible that some of the observed interaction between IcmS/IcmW and DotL/DotM is indirect and is mediated by a substrate(s), we believe that DotL/DotM can also directly bind IcmS/IcmW in the absence of substrates. This is supported by the fact that an association between DotL/DotM and IcmS/IcmW can be observed in exponential phase L. pneumophila cultures, when the majority of Dot/Icm substrates are not synthesized. More convincing, an interaction was observed when DotLMN and IcmS/IcmW were expressed in E. coli, which lacks any known Dot/Icm substrate. It is not known at this time whether IcmS/IcmW binds DotL and/or DotM.
In addition to our data demonstrating the existence of the T4CP subcomplex, we observed a profound effect on DotL stability in stationary phase cells lacking IcmS/IcmW. This result can be explained by at least two scenarios. In the first, IcmS/IcmW may function as a direct chaperone for DotL, perhaps assisting in the assembly or proper folding of DotL. Although conceivable, we do not favor this model as DotL is expressed stably during exponential phase growth of Legionella ΔicmS and ΔicmW strains and during all phases of growth when DotLMN are expressed in E. coli. An alternative model, which we favor, is based on the concurrent induction of synthesis of many Dot/Icm substrates and DotL destabilization in the absence of the type IV adaptors. In this scenario, degradation of DotL could be caused by aberrant interactions between DotL and secreted substrates. Specifically, IcmS/W-dependent substrates might interact with DotL incorrectly in the absence of the type IV adaptors causing them to “jam” the secretion machinery. This misfolded receptor/substrate complex would then be detected by the cell and targeted for degradation by the ClpAP stress response system.
In either case, the specific role of the type IV adaptors remains unknown. One possibility is that IcmS/IcmW could be actively involved in binding substrates in the cytoplasm and delivering/targeting them to DotL/DotM/DotN at the inner membrane. We do not favor this as SidG was shown to bind IcmS/IcmW via a domain distinct from the carboxy-terminal signal sequence and removal of this domain resulted in export of the protein in a type IV adaptor-independent manner (Cambronne & Roy, 2007), a result inconsistent with the adaptors playing a critical role in targeting substrates to the export apparatus. Alternatively, based on the proposed function of type III secretion chaperones, type IV adaptors may act to maintain certain substrates in a secretion competent state while in the cytoplasm of the bacteria. Since the adaptor proteins do not appear to be secreted, the second Dot/Icm subcomplex, consisting of DotL/DotM/DotN and IcmS/IcmW, likely plays an essential role in mediating T4SS substrate export. Further characterization of this subcomplex, in particular determining which Dot/Icm protein is bound by the adaptors, will be critical to understanding the molecular mechanisms used by Legionella to survive and replicate within host cells.
Materials and Methods
Strains, media and antibodies
Bacterial strains, plasmids, and primers used in this study are listed in Table S1. All L. pneumophila strains used were derived from Lp02 (thyA, hsdR, rpsL), a derivative of the clinical isolate Philadelphia-1 (serogroup 1) (Berger & Isberg, 1993). L. pneumophila strains were cultured in ACES buffered yeast extract broth (AYE) or on ACES-buffered charcoal yeast extract agar (CYE) supplemented with 100 μg/ml thymidine as necessary. E. coli strains were cultured in Luria-Bertani (LB) broth or on LB agar plates, supplemented with 150 μg/ml ampicillin as needed. The following polyclonal rabbit antibodies were used: antibodies recognizing DotL (Buscher et al., 2005), DotM and DotN (Vincent et al., 2006b), IcmS, IcmW, LepB and RalF (Vincent & Vogel, 2006), and SdeC (Bardill et al., 2005). ICDH antibodies were obtained from Dr. Linc Sonenshein (Tufts University) and anti-alkaline phosphatase antibodies from Chemicon.
Cell culture
U937 cells (Pearlman et al., 1988) were cultured in RPMI-1640 containing 10% FBS, 2 mM glutamine and penicillin/streptomycin (100 U penicillin/ml, 100μg streptomycin/ml) (Hyclone). U937 cells were differentiated prior to infections by incubation with phorbol 12-myristate 13-acetate (Sigma) for approximately 40 hrs. Mouse bone marrow-derived macrophages (BMM) were isolated from the femurs of A/J mice (Celada et al., 1984, Swanson & Isberg, 1995) and cultured in RPMI-1640 containing 20% heat-inactivated FBS, 1.6 mM glutamine, 30% L-cell culture medium, and penicillin/streptomycin (100 U penicillin/ml, 100 μg streptomycin/ml) for 8 days prior to plating in 24-well tissue culture dishes.
Protein fractionations
Soluble and membrane proteins were isolated from exponential phase cultures of L. pneumophila as described (Vincent et al., 2006b). Cells were resuspended in lysis buffer (50 mM Tris pH 8) with protease inhibitor cocktail (Sigma P2714) and 0.2 mg/ml lysozyme (Fisher BP535) prior to lysis by French press (14,000 PSI). Unlysed cells were then removed by centrifugation at 10,000 × g for 10 minutes at 4° C. The whole-cell lysate was then centrifuged at 100,000 × g for 60 min at 4° C to pellet membrane proteins. Total and soluble protein fractions were diluted with 2X Laemmli sample buffer. Membrane proteins were resuspended in 1X sample buffer. All samples were loaded proportionally on SDS-PAGE gels and analyzed by Western blotting. The purity of the protein fractions was assessed by Western blotting for the cytoplasmic control protein isocitrate dehydrogenase (ICDH) and the inner membrane control protein LepB (type I signal peptidase).
Immunoprecipitations
Cells were grown to exponential phase in AYE. Bacteria (Legionella and E. coli) were harvested and resuspended to a volume of 7 OD600 units per ml in lysis buffer containing 50 mM Tris pH8, 150 mM NaCl, 1% Triton X-100, and protease inhibitor cocktail (Sigma). Cells were lysed by French press (14,000 PSI) precleared by centrifugation at 15,000 × g for 20 minutes at 4° C. Lysates were incubated with preimmune serum or DotL-specific antibody at 4° C overnight with slow agitation. Samples were then incubated for 1 hour at 4° C with slow agitation with a 20 μL bed volume of Protein A-Sepharose CL-4B beads (Pharmacia). The Protein A-Sepharose CL-4B beads were then pelleted by centrifugation, the lysate was removed, and the beads were washed three times with 1 mL of lysis buffer. Beads were then resuspended in 50 μL of Laemmli 2X sample buffer, boiled for 5 minutes, and analyzed by SDS-PAGE followed by immunoblot analysis.
Intracellular growth assays
Intracellular growth of L. pneumophila was assayed as described previously (Swanson & Isberg, 1995, Vincent & Vogel, 2006) and additional details are provided in the Supplementary Materials section.
Intracellular targeting assays
Intracellular targeting of L. pneumophila strains was assayed as described (Swanson & Isberg, 1996, Vincent & Vogel, 2006) and additional details are provided in the Supplementary Materials section.
Immunofluorescence microscopy
Immunofluorescence microscopy (IFM) was carried out as described previously (Hiraga et al., 1998). In brief, about 10×106 stationary phase L. pneumophila cells were fixed in methanol, resuspended in phosphate buffered saline (PBS), and adhered to poly-L-lysine (Sigma) coated microscope slides. The cells were permeabilized with a lysozyme solution (3 mg/ml in 25 mM Tris-HCl, pH 8.0, 50 mM glucose, 10 mM EDTA), washed with PBS, and incubated with affinity purified DotL, DotM, IcmS and IcmW antibodies for 1 hour, respectively. The cells were decorated with Oregon Green-conjugated goat anti-rabbit IgG (Molecular Probes) and stained with DAPI. Fluorescence anti-fade reagent was used to observe immunostained cells by fluorescence microscope (Olympus, 100X objective). All images were captured and further analyzed with IPLab software (BD Bioscience). Polar localization was scored visually using images acquired by IPLab software. In most cases, the staining was clearly polar or cytoplasmic. In minor cases, were staining was present in both locations, a positive polar location was scored if the staining at the poles was significantly greater than the staining in the cytoplasm.
Adenylate cyclase secretion assay
U937 cells were plated into 24-well tissue culture plates at 2.5×106/well. Legionella cultures grown to stationary phase were harvested and 5×106 bacteria were added to each well. Infections were conducted for 1 hr, the cells were washed three times with cold PBS to remove non-adherent cells, and lysed in 200 μl of lysis buffer (50 mM HCl and 0.1% Triton X-100) on ice. The lysates were transferred to Eppendorf tubes, boiled for 5 min, and 12 μl of 0.5 M NaOH was added to neutralize the samples. cAMP was extracted using 2 volume of ethanol and collected by centrifugation at 12,000 × g for 5 min and then lyophilized. Total cAMP concentration was measured using an ELISA kit (GE health, RPN-225) (Sory & Cornelis, 1994).
Protease susceptibility assay
Legionella cultures were grown to late stationary phase, pelleted, resuspended in 20mM Tris pH 8.0, treated with 60 μM lysozyme and sonicated for one minute. The appropriate amount of protease (5 to 160 μM in 2-fold steps) or a mock treatment from Streptomyces griseus (Sigma) was added to an equivalent amount of each culture, and incubated on ice for 15 minutes (Cascales & Christie, 2004). An equal volume of 2X Laemmli’s buffer was added and each sample was boiled for 10 minutes before loading on an SDS PAGE gel and analyzing by Western.
Supplementary Material
Acknowledgments
We thank Dr A.L. Sonenshein for the ICDH antibody. We also thank Ms. Emily Buford for technical assistance. This work was funded by NIH Grant AI48052 to J.P.V.
Abbreviations
- T4SS
type IV secretion system
- T4CP
type IV coupling protein
- LCV
Legionella containing vacuole
- BMM
bone marrow derived macrophage
References
- Atmakuri K, Cascales E, Christie PJ. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol Microbiol. 2004;54:1199–1211. doi: 10.1111/j.1365-2958.2004.04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmakuri K, Ding Z, Christie PJ. VirE2, a type IV secretion substrate, interacts with the VirD4 transfer protein at cell poles of Agrobacterium tumefaciens. Mol Microbiol. 2003;49:1699–1713. doi: 10.1046/j.1365-2958.2003.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. doi: 10.1111/j.1365-2958.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Bennett JC, Hughes C. From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol. 2000;8:202–204. doi: 10.1016/s0966-842x(00)01751-0. [DOI] [PubMed] [Google Scholar]
- Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Buscher BA, Conover GM, Miller JL, Vogel SA, Meyers SN, Isberg RR, Vogel JP. The DotL protein, a member of the TraG-coupling protein family, is essential for viability of Legionella pneumophila strain Lp02. J Bacteriol. 2005;187:2927–2938. doi: 10.1128/JB.187.9.2927-2938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Swanson MS. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezon E, Sastre JI, de la Cruz F. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol Gen Genet. 1997;254:400–406. doi: 10.1007/s004380050432. [DOI] [PubMed] [Google Scholar]
- Cambronne ED, Roy CR. The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation. PLoS Pathog. 2007;3:e188. doi: 10.1371/journal.ppat.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E, Christie PJ. Agrobacterium VirB10, an ATP energy sensor required for type IV secretion. Proc Natl Acad Sci U S A. 2004;101:17228–17233. doi: 10.1073/pnas.0405843101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers J, Kagan JC, Matthews M, Nagai H, Zuckman DM, Roy CR. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol Microbiol. 2000;38:719–736. doi: 10.1046/j.1365-2958.2000.02176.x. [DOI] [PubMed] [Google Scholar]
- Fields BS, Benson RF, Besser RE. Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier A, Finlay BB. Translocated intimin receptor and its chaperone interact with ATPase of the type III secretion apparatus of enteropathogenic Escherichia coli. J Bacteriol. 2003;185:6747–6755. doi: 10.1128/JB.185.23.6747-6755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour MW, Gunton JE, Lawley TD, Taylor DE. Interaction between the IncHI1 plasmid R27 coupling protein and type IV secretion system: TraG associates with the coiled-coil mating pair formation protein TrhB. Mol Microbiol. 2003;49:105–116. doi: 10.1046/j.1365-2958.2003.03551.x. [DOI] [PubMed] [Google Scholar]
- Hamilton CM, Lee H, Li PL, Cook DM, Piper KR, von Bodman SB, Lanka E, Ream W, Farrand SK. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J Bacteriol. 2000;182:1541–1548. doi: 10.1128/jb.182.6.1541-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer BK, Swanson MS. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol. 1999;33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- Hiraga S, Ichinose C, Niki H, Yamazoe M. Cell cycle-dependent duplication and bidirectional migration of SeqA-associated DNA-protein complexes in E. coli. Mol Cell. 1998;1:381–387. doi: 10.1016/s1097-2765(00)80038-6. [DOI] [PubMed] [Google Scholar]
- Horwitz MA. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg RR, O’Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol. 2008 doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci U S A. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Zeng YL, Gao Y, Zheng XC, Zhang QF, Zhou SN, Lu YJ. The ClpP protease homologue is required for the transmission traits and cell division of the pathogen Legionella pneumophila. BMC Microbiol. 2010;10:54. doi: 10.1186/1471-2180-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa M, Zunzunegui S, de la Cruz F. Conjugative coupling proteins interact with cognate and heterologous VirB10-like proteins while exhibiting specificity for cognate relaxosomes. Proc Natl Acad Sci U S A. 2003;100:10465–10470. doi: 10.1073/pnas.1830264100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci U S A. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Kubori T, Kinoshita M, Imada K, Nagai H. Crystal structure of Legionella DotD: insights into the relationship between type IVB and type II/III secretion systems. PLoS Pathog. 2010:6. doi: 10.1371/journal.ppat.1001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio S, Roy CR. Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 2007;15:372–380. doi: 10.1016/j.tim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Ninio S, Zuckman-Cholon DM, Cambronne ED, Roy CR. The Legionella IcmS-IcmW protein complex is important for Dot/Icm-mediated protein translocation. Mol Microbiol. 2005;55:912–926. doi: 10.1111/j.1365-2958.2004.04435.x. [DOI] [PubMed] [Google Scholar]
- Parsot C, Hamiaux C, Page AL. The various and varying roles of specific chaperones in type III secretion systems. Curr Opin Microbiol. 2003;6:7–14. doi: 10.1016/s1369-5274(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Pearlman E, Jiwa AH, Engleberg NC, Eisenstein BI. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microbial pathogenesis. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Schroder G, Krause S, Zechner EL, Traxler B, Yeo HJ, Lurz R, Waksman G, Lanka E. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J Bacteriol. 2002;184:2767–2779. doi: 10.1128/JB.184.10.2767-2779.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G, Feldman M, Zusman T. The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol Rev. 2005;29:65–81. doi: 10.1016/j.femsre.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci U S A. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JA, Pinkner JS, Roth R, Heuser JE, Hultgren SJ, Vogel JP. The Legionella pneumophila PilT homologue DotB exhibits ATPase activity that is critical for intracellular growth. J Bacteriol. 2004;186:1658–1666. doi: 10.1128/JB.186.6.1658-1666.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JA, Vogel JP. Regulation of hypercompetence in Legionella pneumophila. J Bacteriol. 2004;186:3814–3825. doi: 10.1128/JB.186.12.3814-3825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JA, Yeo HJ, Vogel JP. Genetic analysis of the Legionella pneumophila DotB ATPase reveals a role in type IV secretion system protein export. Mol Microbiol. 2005;57:70–84. doi: 10.1111/j.1365-2958.2005.04667.x. [DOI] [PubMed] [Google Scholar]
- Sory MP, Cornelis GR. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect Immun. 1996;64:2585–2594. doi: 10.1128/iai.64.7.2585-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer CY, Faelen M, Couturier M. Interaction between the RP4 coupling protein TraG and the pBHR1 mobilization protein Mob. Mol Microbiol. 2000;37:1283–1292. doi: 10.1046/j.1365-2958.2000.02077.x. [DOI] [PubMed] [Google Scholar]
- Tato I, Zunzunegui S, de la Cruz F, Cabezon E. TrwB, the coupling protein involved in DNA transport during bacterial conjugation, is a DNA-dependent ATPase. Proc Natl Acad Sci U S A. 2005;102:8156–8161. doi: 10.1073/pnas.0503402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NA, Deng W, Puente JL, Frey EA, Yip CK, Strynadka NC, Finlay BB. CesT is a multi-effector chaperone and recruitment factor required for the efficient type III secretion of both LEE- and non-LEE-encoded effectors of enteropathogenic Escherichia coli. Mol Microbiol. 2005;57:1762–1779. doi: 10.1111/j.1365-2958.2005.04802.x. [DOI] [PubMed] [Google Scholar]
- Vincent CD, Buscher BA, Friedman JR, Williams LA, Bardill P, Vogel JP. Identification of non-dot/icm suppressors of the Legionella pneumophila ΔdotL lethality phenotype. J Bacteriol. 2006a doi: 10.1128/JB.00937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent CD, Friedman JR, Jeong KC, Buford EC, Miller JL, Vogel JP. Identification of the core transmembrane complex of the Legionella Dot/Icm type IV secretion system. Mol Microbiol. 2006b doi: 10.1111/j.1365-2958.2006.05446.x. [DOI] [PubMed] [Google Scholar]
- Vincent CD, Vogel JP. The Legionella pneumophila IcmS-LvgA protein complex is important for Dot/Icm-dependent intracellular growth. Mol Microbiol. 2006;61:596–613. doi: 10.1111/j.1365-2958.2006.05243.x. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- Zolkiewski M. A camel passes through the eye of a needle: protein unfolding activity of Clp ATPases. Mol Microbiol. 2006;61:1094–1100. doi: 10.1111/j.1365-2958.2006.05309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckman DM, Hung JB, Roy CR. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol Microbiol. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.