Abstract
This proteomic study investigates the widely observed clinical phenomenon, that after comparable brain injuries, geriatric patients fare worse and recover less cognitive and neurologic function than younger victims. Utilizing a rat traumatic brain injury model, sham surgery or a neocortical contusion was induced in 3 age groups. Geriatric (21 months) rats performed worse on behavioral measures than young adults (12–16 weeks) and juveniles (5– 6 weeks). Motor coordination and certain cognitive deficits showed age-dependence both before and after injury. Brain proteins were analyzed using silver-stained two-dimensional electrophoresis gels. Spot volume changes (>2-fold change, p<0.01) were identified between age and injury groups using computer-assisted densitometry. Sequences were determined by mass spectrometry of tryptic peptides. The 19 spots identified represented 13 different genes that fell into 4 general age- and injury-dependent expression patterns. Fifteen isoforms changed differentially with respect to both age and injury (p<0.05). Further investigations into the nature and function of these isoforms may yield insights into the vulnerability of older patients and resilience of younger patients in recovery after brain injuries.
Keywords: Age, Geriatric brain injury, Adult brain injury, Pediatric brain injury, Proteomics
1. Introduction
Clinical experience has shown that advancing age negatively impacts brain injury mortality and morbidity. Geriatric patients endure worse and more long-lasting neurologic deficits after brain injuries than do younger adults or juveniles with comparable injuries (Goldstein and Levin, 2001; Stuss et al., 1989; Vollmer et al., 1991; Yager et al., 2006;Yager and Thornhill, 1997). In addition, millions of people worldwide suffer from traumatic brain injury (TBI) each year and have an increased risk of premature cognitive decline, dementia and neurodegenerative disease in the ensuing years (Jellinger, 2004; Klein et al., 1996; Oka and Takashima, 1997; Sendroy-Terrill et al., 2010). Unfortunately, the underlying causes for increased vulnerability of the aging brain to trauma and other injuries are not well understood (Baek et al., 2001; Casolini et al., 2002; Erdincler et al., 2002; Godbout and Johnson, 2004; Hamm et al., 1991; Hamm et al., 1992; Kyrkanides et al., 2001; Leong et al., 1981; Zhu et al., 2003).
Candidate mechanisms include age-related structural deterioration, diminished stem cell complement, or attenuation of neuroplastic responses. This laboratory is studying yet another set of candidate mechanisms, dysregulated neuroinflammatory and neuroimmune responses. These have been documented in aging humans and animals (Dilger and Johnson, 2008; Floyd and Hensley, 2002; Gemma and Bickford, 2007; Peila and Launer, 2006; Smith et al., 2005; Sparkman and Johnson, 2008; Wilson et al., 2002), and materially contribute to age-related changes that may result in cognitive decline (Chung et al., 2001; Godbout et al., 2005; McGeer and McGeer, 2004).
Inflammatory responses in the brain are induced rapidly after TBI (Csuka et al., 2000; Maskin et al., 2001; Ott et al., 1994; Scherbel et al., 1999; Soares et al., 1995; Strauss et al., 2000; Whalen et al., 1997). For example, we previously demonstrated that the proinflammatory activity, cyclooxygenase-2 is elevated acutely and for at least 72 hours in the injured brain (Strauss et al., 2000). Interestingly, anti-inflammatory treatments improved functional recovery, even when initiated several hours after injury (Gopez et al., 2005; Malik et al., 2003).
The current study determines age- × injury-related neurologic and proteomic differences after focal brain injury to the somatosensory cortex in juvenile, young adult, and geriatric rats. The goal of the study was to discover protein isoforms that were differentially regulated with both TBI and aging. In designing this study, we anticipated that acute differences in the rate of change between subjects would create much variability, possibly obscuring age- × injury-related differences. Thus, this study examines subacute changes after TBI. Subacute and chronic inflammatory or neurotoxic responses have been well documented after brain injuries (Funk et al., 2001; Holmin et al., 1997, 1998; Itoh et al., 2007; Scherbel et al., 1999; Strauss et al., 2000; Swartz et al., 2001; Weaver et al., 2000). Moreover, studying neuroproteomic changes at 72 hours postinjury permitted the evaluation of functional recovery in the same animals, ensuring that subjects included in the proteomic study were representative of their age and treatment groups from a behavioral standpoint.
2. Methods
2.1. Experimental design
Given the large size of the proteomic study (see below), it was decided to combine 2 neocortical brain regions, known to be associated with functional deficits after brain injury. Although combining the hippocampus and parietal cortex ipsilateral to injury might mask some changes, this would serve to bring out the most robust differences in neocortex that could be characterized further by selective immunoblot analyses.
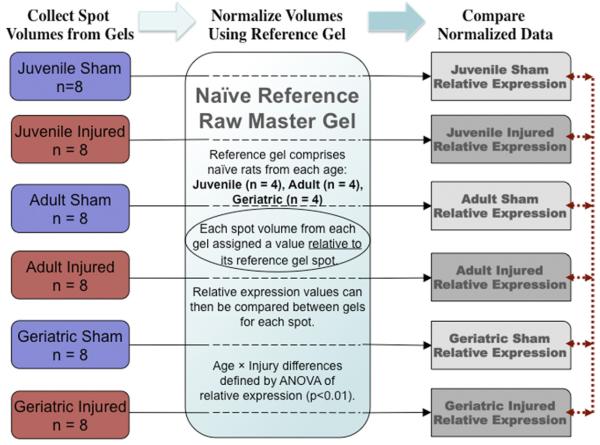
As the goal was to identify changes with both age × traumatic brain injury, naive brain proteins were not directly compared between age groups because several experimental manipulations were required to induce TBI; injured animals were compared with shams rather than naïves. The gels from naive animals of all age groups were combined in a composite “raw master gel” and used in this study as a reference. This allowed more spots from experimental animals to be accurately compared with each other, indirectly through their cognate spots on the reference gel (Fig. 1).
Fig. 1.
Proteomic Research Design. The variability in response to injury as well as potential age-related differences between individuals required a large number of animals per group (n ≥ 8). Each animal was represented by the composite of duplicate gels (3 per animal, best 2 used in analysis). To facilitate the comparisons, a single composite reference gel (raw master gel, RMG) was constructed using 12 naive animals (4 juveniles, 4 adults, 4 geriatrics). Gels from each experimental group were compared directly with the naive reference gel (black lines) to generate a relative expression (RE) for each spot on the experimental gels compared to the reference gel. These RE values could then be compared to each other by virtue of “normalizationp” through the reference gel using Z3 software to calculate relative expression values and significance of the comparisons. Spots that occurred in experimental samples but not in naive samples were mapped to the identical region of the reference gel. Each of the experimental groups, comprising 8 animals, was also composited into a raw master gel. Coefficients of variation for each spot were calculated from the 7 RMGs.
2.2. Animals and surgery
Animals were handled according to protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (Harlan Laboratories, Indianapolis), purchased at age 4 weeks (juvenile, n = 24), 12 weeks (adult, n = 24), and 20 months (geriatric, n = 30) were used in 2 treatment groups. Each age group consisted of naive (n = 4), sham operated (n = 10), and brain injured (n = 10) rats.
Brain injury was induced over the somatosensory cortex in an established model of lateral controlled cortical impact brain injury (Strauss et al., 2000). Briefly, isoflurane anesthesia was used with a nose cone and rats were fixed on a stereotaxic platform. The scalp was incised and reflected, underlying connective tissue cleared, and a 6-mm craniotomy was made extending from the lateral ridge to the central suture halfway between lambda and bregma using a trephine, leaving the dura intact. For the injury, a pneumatic piston (5 mm rounded) impacted the dura for 100 ms at 4 m/s to a depth of 2.7 mm. Sham operated rats were surgerized without the piston impact. Hemostasis was achieved, the skin approximated, and the incision closed with clips. Animals that did not recover pinna, corneal and righting reflexes within 9 minutes were excluded from the study. Of the geriatric rats, five of the surgerized animals were excluded and one naive animal perished (20% mortality), but there were no mortalities among the other age groups. Three days following surgery, shortly after assessing functional recovery, conscious animals were decapitated using a sharpened guillotine.
Brains were rapidly removed, snap-frozen on powdered dry ice, coronally sectioned at −8 °C into thick (300 μm) slices, briefly thaw mounted, and stored at −80 °C for microdissection at −20 °C. These studies were performed using neocortex (combined cortex and hippocampus); and selected confirmatory studies were performed using each tissue individually. Both parietal cortex and hippocampus ipsilateral to injury were microdissected (from bregma −2.8 to −4.0 mm, according to the coordinates of Paxinos and Watson, 1986) from fresh-frozen thick sections in order to observe neocortical proteomic changes after TBI.
2.3. Two-dimensional gel electrophoresis and mass spectrometry (MALDI-TOF MS)
To minimize variability derived from systematic errors or intrinsic differences, the top and bottom performing rats in each age group (see Cognitive and Motor Outcome Measures, below) were excluded, leaving 8 injured and 8 sham for protein analyses (see Fig. 1 legend). Software (Z3, Compugen) was used to evaluate 1.5–2.2 × 103 spots per gel on 120 gels, representing 60 animals; 8 in each of 6 treatment groups, and 4 animals in each naive age group, with duplicates for each animal. Proteins were extracted immediately after microdissection by sonication in ice cold lysis buffer (9M urea, 4% CHAPS, 2% dithiothreitol, 0.1% sodium dodecyl sulfate [SDS]), then frozen at −80 °C. Two-dimensional gel electrophoresis was performed essentially as described (Eismann et al., 2009). Proteins (100 μg, determined by urea compatible assay, G-Biosciences, St. Louis, MO) were loaded onto IPG strips (pI 3–10, Iso-Phor, Amersham/GE Healthcare, Piscataway, NJ) by rehydration overnight using a urea-thiourea solubilizing agent, and sepa-rated by isoelectric focusing. IPG strips were equilibrated with running buffer, embedded on the single well of a 10% SDS-polyacrylamide gel (22 × 22 cm, 1.5 mm thick; Tris-Tricine-SDS running buffer, Genomic Solutions, Ann Arbor, MI) and electrophoretically separated by size. Gels were stained using a destainable silver kit (Genomic Solutions) and scanned as high resolution TIFF files. Each sample was run in triplicate and the best duplicates were used for comparisons.
Next, a “raw master gel” (RMG) was generated for each sample using computer-assisted spot comparison software (Z3, Compugen, Tel Aviv, Israel). This study was designed and powered to determine differences between injured animals at different ages, thus naive animals were included solely as a reference source of homologous proteins to cross-compare as many protein isoforms as possible across age and treatment groups. Therefore, all of the naive brain samples (4 juvenile, 4 adult, and 4 geriatric rats) were combined into a single RMG and used as a reference gel (Fig. 1) for subsequent comparisons. After adjustments for background, the Z3 software assigned each spot an integrated density value (volume) corresponding to its proportion of the total volume of spots on each gel. Gels of neocortex proteins from 6 experimental groups, namely juvenile sham, juvenile injured, adult sham, adult injured, geriatric sham, geriatric injured (n = 8 per group) were also analyzed using Z3 software.
For spots found to be differentially expressed, identification was accomplished by dissecting and combining the same spot from multiple gels, destaining, tryptic digestion, purification of peptides by solid phase extraction (C18 ZipTips, Millipore, Bedford, MA), and subsequent matrix-assisted laser desorption/ionization mass spectrometry (MALDI-Time-Of-Flight/ Time-Of-Flight instrument operated in reflector-positive mode, Applied Biosystems, Foster City, CA) exactly as described (Eismann et al., 2009). Peptide sequence analyses were performed using the MASCOT algorithm (Perkins et al., 1999) with a minimum of two MS/MS spectra.
2.4. Analyses and statistics
Differences in protein isoform abundance on 2D gels were analyzed as follows. Each experimental RMG was compared with the naive reference gel. In this way, relative expression (RE) values were generated, permitting comparisons of changes in spot volume (vs. the reference gel) inone group with fold-changes in other groups. RE was “calculated based on all of the differential expression values related to that spot. . . [which] is more accurate, robust, and reliable than spot quantity because RE is based on the full analysis of corresponding areas on separate gel images” (Z3 User’s Manual). Spots with the highest probability of differential expression with age × injury were determined by first ordering the data using age as the independent variable (in the order: juvenile-sham, juvenile-injured, adult-sham, adult-injured, geriatric-sham, geriatric-injured) and then repeating the analysis using injury as the independent variable (order: juvenile-sham, adult-sham, geriatric-sham, juvenile-injured, adult-injured, geriatric-injured). To increase the specificity, and reduce the chances of false-positives, we chose a 2-fold change in relative expression (recommended in the Z3 user guide). The software calculated relative expression, standard deviation, and p-values including corrections for multiple comparisons based upon the number of biological replicates, number of experimental groups, and number of spot comparisons. Finally, each spot’s calculated RE was compared between groups by two-way ANOVA with Tukey’s Honestly Significant Difference post hoc test for all mean comparisons, using SAS software (Cary, NC).
Other results were expressed as group means ± standard error of the mean (SEM). Differences in group means were evaluated by analysis of variance with post hoc Dunnett and Scheffé tests. A p < 0.05 was considered necessary to reject the null hypothesis that the group means were equivalent.
2.5. Immunoblots
Soluble proteins from brain regions of interest were extracted by sonication in ice cold buffer H (10 mM potassium phosphate, pH 7.4, 10 mM EDTA, 1 mM dithiothreitol, Cømplete® protease inhibitor cocktail (Roche)) and clarified (20 minutes at 13 k xg, 4 °C). Clarified supernatants (protein concentrations were determined by Bradford microassay, Bio-Rad) were mixed with 0.25 volumes of 5× Laemmli buffer, boiled for 4 minutes, and frozen at −80 °C. Proteins (25 μg) were separated by denaturing SDS-polyacrylamide gel (6%) electrophoresis and transferred to nitrocellulose membranes. Multiple gels/blots were processed in parallel throughout the process for optimal comparisons. Blots were blocked (4% BLOTTO, 0.5% Tween 20), incubated with primary antiserum (shaking overnight at 4 °C), washed repeatedly in TRIS-buffered saline, 0.1% Tween 20 (TBST), incubated with the appropriate peroxidase-conjugated secondary antibodies (1 hour at room temperature), washed again, and visualized by chemiluminescence (Supersignal WestPico, Pierce Biotech, Rockford, IL). Loadingcontrol proteins (e.g., antisera for β-actin, 1:10,000, Pierce) were determined subsequently by stripping membranes with Restore (Pierce) for 15 minutes at 37 °C, reblocking and reprobing. Densitometry software (AlphaEase, Alpha Innotech, San Leandro, CA) was used to quantify the intensity of the appropriate bands for semiquantitative comparisons. Primary antibodies used were total CRMP2 (1:2000) and phospho-Thr514-CRMP2 (1:1000, Cell Signaling Technology, Danvers, MA); T-kininogen 1/2 (1:1000, sc-103887) and oxoglutarate dehydrogenase (αKGD, 1:1000, sc-67238, Santa Cruz Biotechnology, Inc, Santa Cruz, CA).
2.6. Cognitive and motor outcome measures
Animals were handled briefly in the days prior to training to acclimate them to the investigator. The Morris water maze, beam walk, neurological and exploratory behavior tests were carried out according to established procedures (Hamm et al., 1992a; Hamm et al., 1992b; Malik et al., 2003; Smith et al., 1991). Results of multiple trials were averaged for each animal, except where noted (e.g., average “best” performance).
In the Morris water maze, a test of working spatial memory, the rat was placed in a circular tank (2-m diameter, containing water at 19 –21 °C) with a submerged platform (125 cm2, placed halfway between the wall and center) to swim for 2 minutes. During the acquisition period, if the submerged platform was not found, the animal was placed on the platform, remaining there for 1 minute to orient to extramaze visual cues. Two blocks of 4 trials were done on the day before surgery and 2 blocks completed 90 minutes prior to surgery. Videotaped probe trials (2 minutes, no platform) were performed at 3 days postinjury to measure retention. Outcome measures from image analysis software (Ethovision, Noldus, Netherlands) included latency to platform, number of platform crossings, distance from platform, swim speed, and time spent within 2 diameters of the platform (Gopez et al., 2005; Malik et al., 2003).
The beam walk, a test of complex motor coordination, was performed on a 1-m long, 2.5-cm wide beam, 1-m above the ground. A black box with an entrance adjoining the beam was at one end, a bright lamp and 90-decibel noise generator at the other end. Initially, rats were placed near the box with the light and noise turned on. Upon entering the box to escape, the aversive stimuli were terminated. After a 1 minute resting period, the procedure was repeated, starting further back on the beam, until the animal completed the task from the far end of the beam. This pretraining was performed the day before surgery. The day of surgery, each rat was started at the far end of the beam in 2 blocks of 3 runs. The outcome measures were latency to enter the box, and number of (cushioned) falls from the beam, measured 3 days after surgery (Gopez et al., 2005; Malik et al., 2003).
Neurological reflexes (forelimb flexion, hind limb extension, lateral pulsion) were tested before and 3 days after surgery as described (Gopez et al., 2005; Malik et al., 2003). For each side, flexion (forelimbs) or extension (hind limbs) was graded on a 4 point scale (1– 4, 4 being best). Each rat was placed on the bench top and pushed laterally to flip the animal onto its back. The resistance to this pulsion was graded on a 4 point scale for each side. Neuroscore comprised the sum of the scores assigned (6 –24 points). Exploratory behavior was evaluated in a behavior box (50 × 30 cm). The activity score was the total number of gridline crossings (10 × 10 cm partitions) and rearings (Gopez et al., 2005; Malik et al., 2003).
3. Results
3.1. Age-related vulnerability to behavioral deficits
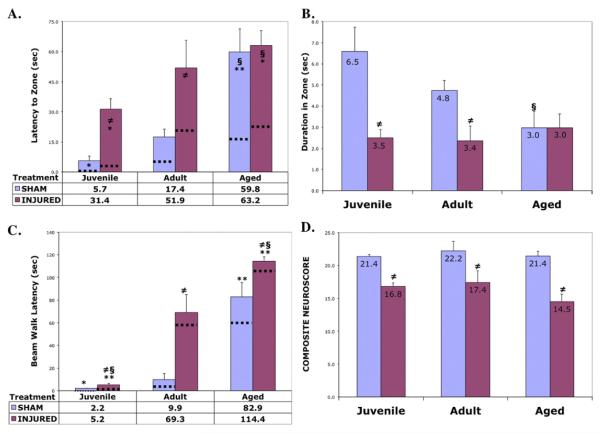
Geriatric rats (20 –21 months) performed worse on cognitive and neurologic tasks than young adult (14 –16 weeks) and juvenile (4 –5 weeks) rats. Except for limb reflexes, behavioral performance before surgery (data not shown) and among shams was worse in geriatrics than in the younger groups (Fig. 2, shams). Geriatric rats performed worse before surgery in our test of working spatial memory (Morris water maze). After training in the water maze, the least squares mean distance from the platform was 54.1 ± 1.8 cm for juveniles, 52.9 ± 1.7 cm for adults, and 63.6 ± 2.8 cm for geriatrics (p < 0.05, Scheffé vs. juveniles and adults). Aged rats were impaired and required more training to acquire the complex motor coordination beam walk task, possibly due to their increased size, compared to the younger groups. There were no age-related differences, however, in open field activity or water maze swim speed between age groups.
Fig. 2.
Differential Age- and Injury-Related Cognitive/Neurologic Deficits. Age-related differences in memory performance in the Morris water maze probe trials; (A) latency to platform, (B) duration in platform zone (n = 8 per group of sham or injured). (C) Age-related differences in complex motor coordination on the beam walk task (latency to target box). (D) No detectable age-related differences in baseline or injury-induced deficits on limb reflexes (neuroscore). Results are mean ± SEM. Mean values are presented in or below the histograms. Dotted lines indicate the mean of the best latencies in each group. Two-way ANOVA, watermaze: F = 6.44, p < 0.0001; beam walk: F = 28.7, p < 0.0001; §p < 0.05, Scheffé (vs. Adults, within treatment); **p < 0.01, *p<0.05, Dunnett (vs. Adult control within treatment); ≠p < 0.05, Dunnett (Injured vs. Sham control, within age group).
Functional deficits were assessed at 3 days after traumatic brain injury (or sham surgery). Geriatric animals showed greater deficits both in terms of working spatial memory (Fig. 2A) and beam walk (Fig. 2C) latencies (p < 0.05, Dunnett vs. adults, and p < 0.05, Scheffé vs. juveniles). All ages showed comparable deficits on water maze duration in the target zone (Fig. 2B) and limb reflexes (Fig. 2D).
Intriguingly, although juveniles had the greatest relative energy and proportional volume of brain tissue displaced with controlled cortical impact injury, juveniles had less functional deficits compared to older rats (Fig. 2A,C). Another surprising observation, neuroworsening was actually lower in the geriatric group, possibly since baseline function was impaired in these rats. The average “best” performance in water maze and beam walk latencies (Fig. 2A,C dotted lines) revealed interesting trends. Whereas the differences in average “best” for water maze latency hardly changed for juvenile and geriatric groups, neuroworsening was greatest in the young adults. On the beam walk task, again little neuroworsening was observed in the juveniles, however, adult and geriatric rats showed much greater neuroworsening. Interestingly, on both these measures the geriatric shamgroup appeared to perform similarly to the brain injured adult group (Fig. 2A,C).
3.2. Neocortex proteomics shows 15 protein isoforms with age × injury-related changes
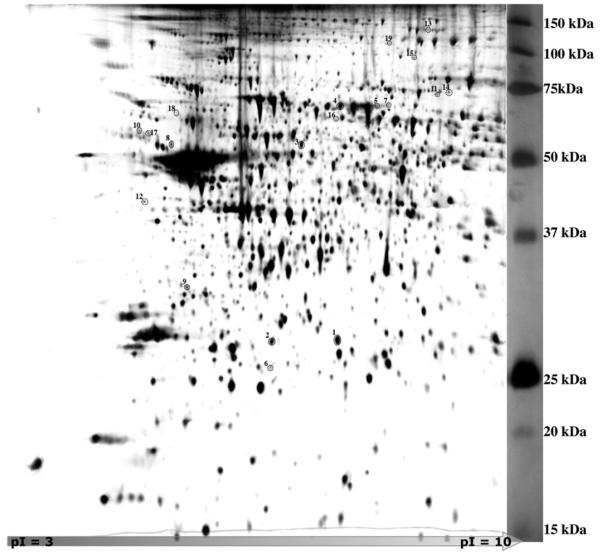
To facilitate the maximum number of spot comparisons for detection of combined age × injury-related differences, the naive brain gels (4 juvenile, 4 adult, and 4 geriatric rats) were combined into a single RMG and used as a reference gel for subsequent comparisons. This also minimized the number of spot comparisons necessary to determine relative expression (RE) values, i.e., each spot on each gel was compared with its cognate spot on the reference gel, and thus could be compared with each other, through the reference gel (Fig. 1). Nineteen spots (Fig. 3, Tables 1, 1–19) were identified by the Z3 software as having greater than 2-fold differences in relative expression with respect to both age and injury (p < 0.01). Characterization of these spots by tryptic peptide analysis using mass spectrometry and sequence matching (Supplementary Data I) identified 14 distinct gene products.
Fig. 3.
Proteomic Two-Dimensional Raw Master Gel. The hippocampus, parietal and perirhinal cortex were microdissected, homogenized in lysis buffer containing 9M urea, loaded onto IPGphor strips, separated by isoelectric point (pI 3–10) then separated by molecular size on 10% polyacrylamide denaturing gels and silver stained (see Methods). Duplicate gel scans for each specimen were chosen for analysis using Z3 Software, and a composite “raw master gel” was generated for each animal. Further composites were generated for all the naive animals (reference gel shown here) and for each treatment group (not shown). Spots with greater than 2-fold difference from the naive reference gel (p<0.01) are circled.
Table 1.
Protein isoforms differentially expressed with age or traumatic brain injury
| Spot# | Gene name | Gene ID | Calculated mass (Mr) | Calculated pI |
|---|---|---|---|---|
| 1 | HSP27 | 149063018 | 22891 | 6.12 |
| 2 | HSP27 | 149063018 | 22891 | 6.12 |
| 3 | CRMP2 (also known as DPYL2, DPR2) | 40254595 | 62273 | 5.92 |
| 4 | SAP | 124028612 | 68686 | 6.09 |
| 5 | Serum albumin precursor | 124028612 | 68686 | 6.09 |
| 6 | Serum albumin precursor | 124028612 | 68686 | 6.09 |
| 7 | Serum albumin precursor | 124028612 | 68686 | 6.09 |
| 8 | Alpha-2-HS-glycoprotein (α2-HS-GP) | 77416365 | 38001 | 6.32 |
| 9 | Apolipoprotein E (Apo E) | 37805241 | 35741 | 5.23 |
| 10 | Serine protease inhibitor precursor (Serpin1) |
32563565 | 68180 | 5.31 |
| 11 | Translocase of outer mitochondrial membrane 70 homolog A (TOM70A) |
47058988 | 67402 | 7.44 |
| 12 | Vimentin | 14389299 | 53729 | 4.86 |
| 13 | Oxoglutarate dehydrogenase (lipoamide) (αKGD) |
62945278 | 116221 | 6.30 |
| 14 | Moesin (could also be radixin or ezrin) | 13540688 | 67733 | 6.11 |
| 15 | Loss of heterozygosity, 11, chromosomal region 2, gene A homolog (LOH11CR2A) |
38454250 | 91425 | 6.18 |
| 16 | Serum albumin | 124028612 | 68686 | 6.09 |
| 17 | Serum albumin (bovine, BSA) | 162647 | 69287 | 5.76 |
| 18 | T-kininogen I LMW precursor | 205085 | 47674 | 6.29 |
| 19 | Oxoglutarate dehydrogenase (lipoamide) | 62945278 | 116221 | 6.30 |
The nineteen genes listed here correspond to the protein isoforms identified by two-dimensional gels and mass spectroscopic analyses of spot tryptic digests. Gene ID is the number assigned to each gene by the National Center for Bioinformatics on the Entrez web site (www.ncbi.nlm.nih.gov/Entrez); Mass and pI are the estimated molecular weight and the estimated isoelectric point, based on primary sequence information, as calculated by MacVector software (MacVector, Inc.)
A synopsis of sham and injury trends for all 19 spots is shown in Supplementary Figure 1. Two-way analysis of variance was performed on relative expression values of the 19 spots from each experimental gel (see Supplmentary Data III for n-fold changes between groups). Differential expression with age × injury was found in 15 spots (Table 2, interaction p < 0.05, Tukey HSD). At least 8 of the 19 spots showed potential sham effects (see Supplementary Data IV). Based upon the coefficients of variation (Table 2) and the disparity from naive values, it appeared that 2 serum albumins (#7, 17) were highly suppressed in shams, and T-kininogen (#18) was highly induced in shams.
Table 2.
Protein spot relative expression with age and brain injury: two-way analysis of variance
| GENE | SPOT# | Trend |
CV
% |
JUVENILE (J) |
ADULT (A) |
GERIATRIC (G) |
A. Sham vs. Injured | B. (J) vs. (A) vs. (G) | A. × B. INTERACTION | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Injured | Sham | Injured | Sham | Injured | p-value | p-value | p-value | ||||
| HSP27 | 1 | A | 48.7 | 42% | 135% | 26% | 144% | 107% | 146% | 0.0290 | 0.1900 | 0.0383 |
| CRMP2 | 3 | A | 58.7 | 26% | 141% | 44% | 167% | 59% | 164% | 0.0011 | 0.3600 | 0.0625 |
| SAP | 4 | A | 33.1 | 54% | 158% | 73% | 117% | 108% | 90% | 0.3100 | 0.4700 | 0.0293 |
| SAP | 5 | A | 28.1 | 48% | 167% | 47% | 145% | 96% | 98% | 0.0620 | 0.5000 | 0.0238 |
| T-kininogen 1 | 18 | A | 88.5 | 37% | 34% | 21% | 61% | 234% | 213% | 0.9800 | 3.2 × 10−5 | 0.1092 |
| HSP27 | 2 | B | 50.8 | 70% | 189% | 64% | 141% | 40% | 96% | 0.0270 | 0.2300 | 0.0339 |
| SAP | 6 | B | 79.0 | 13% | 231% | 44% | 159% | 27% | 126% | 4.2 × 10−6 | 0.3800 | 0.0715 |
| α2-HS-GP | 8 | B | 79.2 | 28% | 180% | 69% | 236% | 41% | 46% | 0.0024 | 0.0035 | 0.0011 |
| Apolipoprotein E | 9 | B | 41.9 | 64% | 180% | 68% | 116% | 61% | 111% | 0.0700 | 0.3100 | 0.0297 |
| Moesin | 14 | B | 66.3 | 38% | 230% | 49% | 125% | 52% | 106% | 0.0027 | 0.1600 | 0.0155 |
| LOH11CR2A | 15 | B | 68.4 | 43% | 244% | 59% | 117% | 65% | 72% | 0.0180 | 0.0970 | 0.0126 |
| BSA | 17 | B | 126.6 | 33% | 382% | 43% | 52% | 63% | 27% | 0.0028 | 6.8 × 10−6 | 0.0005 |
| SAP | 7 | C | 118.6 | 78% | 356% | 61% | 89% | 3.7% | 14% | 0.0035 | 6.7 × 10−9 | 0.0006 |
| Serpin1 | 10 | C | 79.1 | 42% | 252% | 86% | 146% | 15% | 60% | 0.0035 | 0.0110 | 0.0014 |
| Vimentin | 12 | C | 76.5 | 14% | 216% | 81% | 147% | 0.2% | 142% | 2.6 × 10−5 | 0.4100 | 0.0594 |
| SAP | 16 | C | 104.3 | 42% | 190% | 71% | 287% | 0.4% | 9.2% | 0.0003 | 3.4 × 10−15 | 0.0001 |
| TOM70A | 11 | D | 58.1 | 32% | 62% | 114% | 88% | 217% | 88% | 0.2200 | 0.0065 | 0.0173 |
| αKGD | 13 | D | 76.4 | 57% | 14% | 107% | 87% | 258% | 77% | 0.0170 | 0.0005 | 0.0020 |
| αKGD | 19 | D | 44.8 | 90% | 141% | 88% | 67% | 135% | 78% | 0.7200 | 0.0610 | 0.0238 |
Relative Changes of Age- and Brain Injury-related Protein Isoforms. Two-way analysis of variance showed effects of age, injury, and age × injury among 3 age groups and 2 treatment groups. Spot volumes from each gel were first compared with cognate spot volumes on the reference RMG, then relative expression (RE) values were compared between groups. Percentages represent the proportion of the mean RE value over all groups. Values presented are (Mean RE for the group/Mean RE across groups) × 100%. CV(%) is coefficient of variation of the spot volumes on the naive and 6 experimental RMGs. Protein gene names and spot numbers as in Table 1. Trend is the apparent pattern of expression over the lifespan (see Discussion). p-values for the ANOVAs are in the columns to the right. p-values < 0.05 (Tukey HSD) indicate significant interactions of both age and injury to the observed differences. For direct group comparisons, see Supplementary Data III.
Two isoforms of heat-shock protein 27 (HSP27, Table 1 #1,2) appeared to differ in isoelectric point (Fig. 3). These 2 isoforms did not appear to change with age alone, but both showed upregulation in response to injury (Table 2, p < 0.03) as well as age × injury interactions. HSP27 (#1) showed an interaction (p < 0.03) in which geriatric shams > adult shams ≈ juvenile shams (Table 2). In contrast, while HSP27 (#2) also showed increases in response toinjury, the interaction (p < 0.03) indicated a reduced induction with increasing age.
Like HSP27, collapsin-response mediator protein 2 (CRMP2, Table 1 #3) did not change with age alone, and was upregulated after injury (Table 2, p < 0.03), with an apparent trend by age × injury (p = 0.0625). Among injureds, juveniles appeared to induce more than young adult or geriatric rats (see Supplementary Data III for n-fold changes between groups).
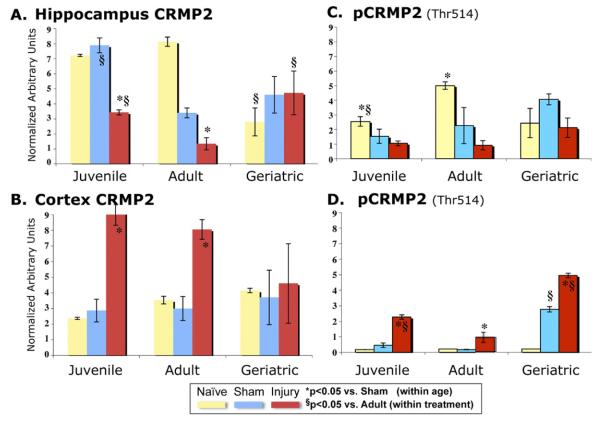
Immunoblots were performed to examine CRMP2 levels in the hippocampus and cortex individually. Age- and injury-related changes were observed in both brain regions (Fig. 4A,B), though these would not be expected to reproduce exactly the proteomic findings of isoform-specific changes in combined neocortex. In the hippocampus, naive juveniles and adults exhibited more CRMP2 than geriatric rats (Fig. 4A, P < 0.05), whereas in the cortex (Fig. 4B) there was no significant difference. Decreased CRMP2 was seen in injured hippocampus in juveniles and adults but not in geriatrics at 3 days postinjury (Fig. 4A, P < 0.05). Conversely, in the injured cortex, total CRMP2 was highly induced in the juveniles and adults (Fig. 4B, P < 0.05) but not in the geriatrics.
Fig. 4.
Age- and Injury-related CRMP2 Changes Differed With Brain Region and Epitope Detected by Immunoblot (A) Hippocampal CRMP2 decreased with injury in juvenile and adult, but not in geriatric brains. (B) Cortex CRMP2 increased in juvenile and adult, but not in geriatric brains. (C) Hippocampal phospho-Thr514-CRMP2 (pCRMP2, Thr514-CRMP2 phospho-specific antibody, Methods) appeared to decrease with injury (p < 0.05, all ages combined). (D) Cortex pCRMP2 increased in each age group compared to shams. Data normalized to β-actin in each sample and presented as mean ± SEM. Immunoblots (n = 4–5 per group) were arranged by age (all juvenile specimens on one gel, etc.) and repeated arranged by same treatment (shams of all ages on one gel, etc.); results were comparable for both approaches. No significant changes in the β–actin internal control were observed. Two-way ANOVAs [Hippocampus FCRMP2 = 11.12, p < 0.0001; FpCRMP2 = 6.40, p < 0.0002; Cortex FCRMP2 = 11.12, p < 0.0001; FpCRMP2 = 3.77, p < 0.004] were followed by one-way ANOVA with Dunnett post hoc tests; *p < 0.05 vs. Sham (within age group); §p < 0.05 vs. Adult (within treatment group).
CRMP2 has at least 9 putative phosphorylation sites (Cid et al., 2007; Ni et al., 2008; Yoshimura et al., 2005), of which, phospho-Thr514-CRMP2 (pCRMP2) was the only isoform for which we found a commercially available antibody. Immunoblots of hippocampus showed decreased pCRMP2 in shams compared to naïves (Fig. 4C, juveniles and adults, p < 0.05). TBI appeared to further decrease this isoform (did not reach statistical significance for individual age groups, but p < 0.05, all ages combined). Conversely, pCRMP2 levels were barely detectable in uninjured cortex (Fig. 4D; although note the increase in geriatric shams, p < 0.05), whereas TBI induced cortical pCRMP2 levels in juveniles, adults, and particularly in the geriatric injury groups. In fact, the fold induction of cortex pCRMP2 (Fig. 4D) closely matched that observed for the isoform identified by proteomics in the combined neocortex (Table 2). Thus, the cortex total CRMP2 and pCRMP2 immunoblot results were comparable to the proteomic findings, where both injury-related increases and age × injury interactions were observed.
Serum albumin proteins (SAP) were identified in 6 spots (SAP, Table 1 #4, 5, 6, 7, 16, 17) each with different expression patterns. SAP #4 had the highest brain levels and showed changes with age × injury (Table 2), but not individually with age or injury. SAP #5 showed comparable patterns with age × injury, and not with age or injury alone. The pattern for shams was geriatric > adult > juvenile; whereas the pattern for injureds was the opposite, juvenile > adult > geriatric. Both SAP #4 and #5 were induced by injury in juvenile and adult but not in geriatric brains.
SAP #6 was a low molecular weight, low abundance serum albumin isoform that showed no changes with age alone (Table 2). SAP #6 was induced by injury (p < 0.0001), but showed only a trend (p < 0.10) for interaction of age × injury. SAP #7 isoform was decreased with age (juvenile ≈ adult » geriatric), induced by injury, and showed an age × injury interaction (p < 0.005, p < 0.0001, p < 0.001, respectively, Table 2). SAP #7 levels were most highly expressed in juveniles, and there was much less injury-induced change in adult and geriatric brains. SAP #16 also showed age-related differences (adult > juvenile » geriatric), was induced by injury, and showed an age × injury interaction (p < 0.0003, p < 0.0001, p < 0.0001, respectively, Table 2). Both SAP #7 and #16 were observed only at very low levels in geriatric sham and injured brains.
SAP #17 was deduced to be of bovine not rat origin (see Supplementary Data I, Notes). This bovine isoform appeared to increase with age in shams (geriatric ≥ adult ≈ juvenile), was increased by injury in juveniles only, and showed an age × injury interaction (p < 0.003, p < 0.0001, p < 0.0005, respectively, Table 2). No change was seen in SAP #17 in injured adults, however, injured geriatric levels of this serum albumin isoform appeared to be about half those of geriatric shams.
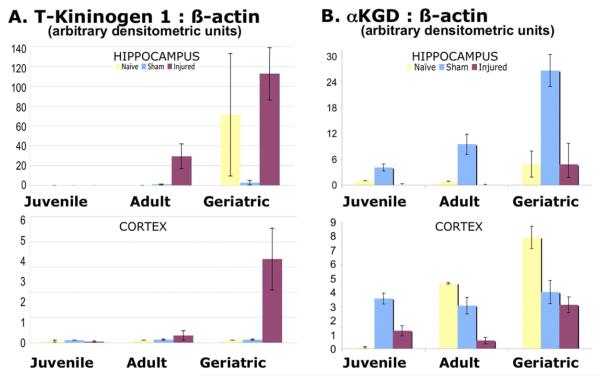
T-kininogen 1 low molecular weight precursor (Tkininogen 1, Table 1 #18) had an observed mass ~68 kDa but its genomic form was only 48 kDa. Interestingly, the peptides identified by MALDI-TOF MS were consistent with several rat proteins, all mapping back to the same genetic locus (Supplementary Data I). This isoform showed upregulation in the geriatric sham and injured brains (p < 0.0001, Table 2) but exhibited neither injury-related nor age × injury-related changes.
On immunoblot, antisera to rat T-kininogen 1/2 showed increases in injured hippocampus (geriatrics > adults » juveniles, Fig. 5A top), as well as injured cortex (geriatrics » adults » juveniles, Fig. 5A bottom). As in the proteomic study, most of the T-kininogen detected on immunoblot was in the geriatric specimens. Note the near absence of T-kininogen 1 in the geriatric shams and the highly variable signal in geriatric naïves; these differences from the proteomic results may be due to that isoform not being detected by the antisera, or as with CRMP2, could indicate a potential suppression of brain T-kininogen 1 (likely by anesthesia), even 3 days after surgery.
Fig. 5.
Age- and Injury-related Changes in T-kininogen 1 and α-ketoglutarate dehydrogenase by Immunoblot Detection (A) Hippocampal T-kininogen 1 (top) showed geriatric levels higher than juvenile and adult (p < 0.05), injured levels greater than sham (p < 0.05), and geriatric injured greater than sham (p < 0.05, Scheffé), but no other interaction within age groups. Cortex T-kininogen 1 (bottom) showed the same relationships as hippocampus. Combining values for both tissues, again showed the same relationships. (B) Hippocampal αKGD (top) showed increased levels in geriatric shams compared to all other groups (p < 0.05, Scheffé). Cortex αKGD (bottom) showed naive geriatrics and adults greater than juveniles (p < 0.05, Scheffé); no difference between sham groups; and geriatric injured greater than adult injured (p < 0.05, Scheffé). Combining values for both tissues, geriatrics showed αKGD levels higher than adult or juveniles (p < 0.05); over all age groups, injureds showed the lowest values (p < 0.05); and within age groups, sham was greater than injured in juvenile, adult, and geriatric rats (p < 0.05, Scheffé). Two-way ANOVAs [Hippocampus FαKGD = 8.09, p < 0.0001; FT-K1 = 7.56, p < 0.0001; Cortex FαKGD = 11.70, p < 0.0001; FT-K1 = 4.82, p < 0.0009] were followed by one-way ANOVA with Scheffé post hoc tests. Results were derived from the same immunoblots as in Fig 4 after being stained for β-actin and restripped (see Methods).
Alpha-2-HS-glycoprotein (α2-HS-GP, Table 1 #8) had an observed mass greater than 50 kDa (Fig. 3) but its genomic form was calculated at only 38 kDa. This α2-HS-GP isoform showed changes with age, injury, with a significant age × injury interaction (p < 0.003, p < 0.004, p < 0.002, respectively, Table 2). In sham-operated rats, there appeared to be greater levels of α2-HS-GP in the adults vs. juvenile or geriatric shams. Injury-related induction was observed in juvenile (~6-fold) and adult (~3-fold) but not in the geriatric brains.
Apolipoprotein E (ApoE, Table 1 #9) isoform levels in sham neocortex appeared unaffected by age, and showed only a trend toward upregulation with injury (p = 0.07, Table 2), but demonstrated a significant age × injury interaction (p < 0.03). This isoform of ApoE showed increased levels in all injured animals, with greater increases in the juveniles (2.8-fold increase vs. 1.7–1.8-fold increases in adults and geriatrics).
Isoforms of moesin (Table 1 #14) and LOH11CR2A (loss of heterozygosity, chromosome 11, region 2, gene A homolog, Table 1 #15) showed very similar expression trends. Though there was no significant difference by age alone, moesin and LOH11CR2A increased in response to injury (p < 0.003, p < 0.02, respectively, Table 2), and both isoforms showed age × injury interactions (p < 0.02). Their fold-induction after TBI appeared to decrease with age (Supplementary Data III), i.e., injured juveniles > injured adults > injured geriatrics. Though the moesin isoform was increased in geriatric brains after TBI, no change was observed in the LOH11CR2A isoform.
Serine protease inhibitor precursor (serpin1, Table 1 #10), a ~68 kDa isoform was either the protein precursor or a modified form that “regained” its mass via N-glycosylations or other post-translational modifications. Serpin1 displayed significant changes with age, injury, and an interaction of age × injury (p < 0.004, p < 0.02, p < 0.002, respectively, Table 2). Increases after TBI were observed in juveniles, adults, and geriatrics (6-, 1.7-, and 4-fold induction, respectively). As with α2-HS-GP and vimentin, sham levels of this serpin1 isoform in adults were highest (adult > juvenile > geriatric). This isoform was identified by only 2 peptides, and could also have come from other closely related serpin family members (see Supplementary Data I).
Vimentin (Table 1 #12) appeared as a ~35 kDa fragment of a calculated 54 kDa protein. There were no significant changes with age alone, but a large injury effect and a trend toward age × injury interaction. Among shams, the levels of this fragment appeared greatest in adults (» juvenile » geriatric, not significant, likely due to the high variance within groups). This vimentin was increased by injury (p < 0.0001, Table 2) and showed a trend with age × injury(16-fold induction in juvenile injured, 1.8-fold in adult injured, and 644-fold in geriatric injured, p < 0.06).
Translocase of outer mitochondrial membrane protein 70 homolog A (TOM70A, Table 1 #11) appeared as a ~50 kDa fragment of a predicted 67 kDa protein (Fig. 3) and was identified with only 2 tryptic peptides (Supplementary Data I). There was no significant difference between the injured groups, likely due to high variance within groups. However, TOM70A did show age-related changes and age injury interactions (p < 0.007, p < 0.02, respectively, Table 2). The TOM70A isoform increased over the lifespan (juvenile » adult < geriatric) in shams but responded differentially to injury. Whereas juveniles appeared to induce this isoform 2-fold, decreases were seen in both adult and geriatric injured neocortex (23% and 60%, respectively).
Oxoglutarate dehydrogenase (α-ketoglutarate dehydrogenase) was identified in 2 spots (αKGD, Table 1 #13, 19). Interestingly, peptides identified by MALDI-TOF MS in spot #13 represented 2 distinct genetic loci of αKGD (see Supplementary Data I). αKGD (spot #19) was a different isoform of one of these 2 genes. αKGD (#13) showed upregulation with increasing age, a contrasting downregulation with injury, and significant age × injury interactions (p < 0.02, p < 0.005, p < 0.002, respectively, Table 2). αKGD (#13) showed decreases after TBI, with a diminished injury response in adults (only ~20% decrease, compared with a 3- to 4-fold reduction in juvenile or geriatric neocortex). The second isoform, αKGD (#19) showed no significant changes with respect to age or injury. Nonetheless, an interaction of age × injury (p < 0.03, Table 2) showed a 57% increase after injury in juveniles, contrasted with injury-related decreases in geriatrics and in adults (Supplemental Fig. 1, Supplemental Data III).
Immunoblots using antisera to amino acids 1−61 of human αKGD (Fig. 5B) showed results comparable to αKGD (#13). There were age × injury interactions in both hippocampus and cortex (p < 0.0001 and p < 0.04, respectively). In sham animals, αKGD increased with age in the hippocampus (geriatric > adult ≈ juvenile, p < 0.01, Tukey HSD). As with CRMP2 and T-kininogen 1, there appeared to be sham effects; shams levels of αKGD were higher than naïves in the hippocampus across all age groups, and in the cortex juvenile shams also showed increased levels, whereas geriatric shams showed decreased αKGD levels (p < 0.05, Tukey test for all comparisons). Decreases were seen in all injured hippocampus compared to shams in each age group (p < 0.01, Tukey HSD). In the cortex, levels increased with age in naïves (p < 0.01, Tukey HSD), however, no age-related differences were observed among shams. Brain injury decreased αKGD levels in juvenile, adult, but not in geriatric cortex (p < 0.01, Tukey HSD). Thus, the proteomic results for αKGD (#13) in neocortex resemble the combination of both hippocampus and cortex on immunoblot (compare Fig. 5B and Supplementary Fig. 1D).
Finally, redox proteomics have shown that a variety of proteins, including dihydropyrimidinase-like 2 (CRMP2) are oxidatively regulated in aging brain (Kuhla et al., 2007; Sultana et al., 2006; Vaishnav et al., 2007). In this study the following proteins showed evidence of oxidative changes on mass spectrometric analyses: SAPs #5, 6, 7, 16; α2HS-GP #8; CRMP2 #3; vimentin #12; and LOH11CR2A #15 (Supplementary Data I).
4. Discussion
Older rats performed worse than young adults and juveniles on selected cognitive and motor measures (Morris water maze and beam walk, respectively), independent of brain injury. Geriatric sham-operated rats performed worse on spatial working memory (in latency to, total distance from, and total duration near the water maze target), and were far more impaired than adults or juveniles on the beam walk (but not neuroscore). Brain-injured geriatrics had worse deficits than their younger counterparts on 2 of the 3 cognitive measures (latency to and total distance from the target), and on the beam walk (but not neuroscore). Exploratory activity and swim speed were not affected by age in this study.
Interestingly, the geriatric shams’ “best” performance was comparable to the mean of adult shams (Fig. 2A,C, dotted lines), implying older animals could not sustain their best behavior throughout the training trials, analogous to decreased stamina in elderly humans. Surprisingly, cognitive neuroworsening appeared to be lower in the geriatric group; this was likely due to their low baseline performance and advanced age (30% mortality between 20 and 21 months). Thus, a younger geriatric cohort would be needed to observe potential differences in neuroworsening or delayed functional recovery. Nonetheless, these age-related differences in behavior and recovery appear analogous to clinical differences observed in juvenile, adult, and elderly populations (Elwan et al., 2003; Goldstein and Levin, 2001; McGeer and McGeer, 2004; Pentland et al., 1986; Vollmer et al., 1991; Yager and Thornhill, 1997).
We used the brains of these animals, euthanized at 3 days after surgery to identify brain protein isoforms that might underlie the observed ageand injury-related differences in behavioral deficits. Our hypothesis that neuroinflammatory associated proteins would be most highly represented among the differentially expressed protein isoforms was not supported by the results, although clearly limited in its examination of a single subacute time point at 3 days postinjury. Nonetheless, the subacute and chronic changes revealed in this study may serve as prospective targets for further investigations.
The 19 spots identified in this study represent 13 known genes and as yet undetermined protein isoforms. It should be noted that these results could support a variety of conclusions, depending upon what splice variant(s), posttrans-lational modification(s), or degradation products were represented in the spots. Thus, certain isoforms might represent activation, others inactivation, yet others target the protein for membrane insertion, proteolytic processing or degradation, etc.
Not surprisingly, HSP27 previously has been shown to be regulated with either age or brain injury. Heat shock proteins were shown to be induced by a variety of brain insults (Allen and Chase, 2001; Blumenfeld et al., 1992; Brown et al., 1989; Brown, 1991; Heurteaux et al., 1993; Plumier et al., 1997; Raghupathi et al., 1995; Tanno et al., 1993), and with age (Krueger-Naug et al., 2002). In this study, age-related differences were seen in shams (HSP27 #1, greatest in geriatrics) and after TBI (#2, juveniles > adults > geriatric) at 3 days postsurgery (Table 2).
Over half of the age- and injury-regulated proteins identified in this study are derived from serum proteins. Brain tissue was not perfused before collection to avoid potential confounds of post-mortem changes, however, care was taken in the dissections to avoid patently bloody or necrotic tissue. These include the serum albumin proteins (SAP# 4,5,6,7,16,17), T-kininogen 1 (#16), serpin 1 (#10) and α2-HS-glycoprotein (#8). Moreover, 7 of these proteins that would usually be found in the blood were of hepatic origin (spots # 4,5,6,7,8,16,18). However, taken together, the findings indicate that observed changes were not simply indicative of blood volumes codissected with neocortex. SAP’s #4 and #5 were increased in the neocortex with age (geriatric > adult > juvenile) while SAP’s #7 and #16 decreased with age. In the injured brains, SAP’s #4 and #5 showed distinct age-related declines, and again SAP’s #6, #7, and #16 decreased with age. This was counter to expectations that aged animals would have greater blood– brain barrier compromise after TBI (Goldman et al., 1992; Knox et al., 1980; Mayhan, 1990; Mayhan, 2001; Mooradian, 1988; Sankar et al., 1983). Examination of concurrent changes did not reveal any obvious correlations that might lead to a hypothesis about age- or injury-related significance (e.g., appearance of the low molecular weight SAP#6 in tandem with disappearance of other isoforms).
Serum albumins act not only as osmotic/oncotic regulators but also transport bilirubin, hemin, thyroxine, and bind proportionally high amounts of small hydrophobic molecules (e.g., steroids, fatty acids). In fact, SAPs have been proposed to play a significant role in buffering the concentration of these and drug molecules in the blood (Peters, 1995). Thus, the observed increases of selected SAP isoforms in the injured juvenile brain might represent a neuroprotective response, for example, transport of beneficial molecules from the blood or binding of toxic molecules produced after TBI.
On the other hand, one piece of evidence points to a contribution of extravasation of serum albumins after TBI. One SAP was found to be bovine serum albumin (SAP #17, Supplementary Data I). It is very unlikely that this result reflects sample contamination as specimens were dissected, processed, and separated in random order, under stringently clean conditions, and on a variety of days. Another explanation might be that this is a novel rat albumin, or a heretofore unknown set of mutations (Supplementary Data I, Notes). But there may have been bovine products in the rat chow (M. Kurtzman DVM, personal communication), so however unlikely, this could indicate the entry of ingested proteins into the peripheral circulation and/or neocortex of these animals. Surprisingly, the finding that injured juvenile rats exhibited greatly increased albumin levels at 3 days postinjury suggests a greater blood-brain barrier compromise in this group than in older rats or that their brain cells may produce albumin or other serum proteins (Kalmovarin et al., 1991) under adverse conditions.
T-kininogen 1 (#16), another serum protein, increased with age in the neocortex and was induced with injury in adult and geriatric brain. The immunoblot results differed from the proteomics study in that very little of the protein was observed in geriatric shams, however, the naive geriatric hippocampus showed highly variable levels (Fig. 5A top). Increased variability might be due to differences in sample preparation (all proteins in the former vs. soluble proteins only in the latter), or in the slightly different brain regions microdissected from adjacent frozen thick sections for later studies. T-kininogens are plasma glycoproteins comprised of 3 cysteine protease inhibitor domains, as well as the bradykinin peptide that affects vascular permeability, blood pressure, and smooth muscle contraction when cleaved from the precursor T-kininogen (Furuto-Kato et al., 1985; Greenbaum, 1992). This gene plays an important role in vascular pathophysiology, particularly in cellular adhesion and inflammatory responses in blood vessels as well as solid tissue (Kageyama et al., 1985; Sainz et al., 2007). In particular, kininogens facilitate white blood cell migration to and stabilization at sites of local inflammation and injury. Its upregulation in geriatric groups may indicate that a greater inflammatory load may exist and persist in the aged brain and after injury.
Another serum constituent, α2-HS-glycoprotein (#8, also known as fetuin (Brown et al., 1992)) is a liver-derived protein thought to function as an inhibitor of tyrosine kinases (Arnaud and Kalabay, 2002). LOH11CR2A (#15) is another serum glycoprotein, a von Willebrand factor A-containing protein thought to function as a tumor suppressor (Monaco et al., 1997). Moesin, α2-HS-GP and LOH11CR2A previously have been suggested to be deficient in various pathologies, and/or decreasing over the lifespan (Kalabay et al., 2007; Masseguin et al., 2005; Molvarec et al., 2009; Puchades et al., 2003; Sironi et al., 2001; Yoshida et al., 1999). In fact, it has been proposed that treatment with α2-HS-GP may benefit the injured brain (Wang et al., 2010). Thus, the weak induction of these isoforms in the geriatric injured group might point to a potential therapeutic modality in elderly patients.
The serine protease inhibitor 1 isoform (#10) increased in adults vs. juveniles, but was barely detectable in geriatric shams. Moreover, this serpin1 was upregulated after TBI, particularly in the juvenile group. Serpins are a family of evolutionarily conserved acidic inhibitor proteins that bind the active site of serine proteases and become covalently attached upon cleavage (Horvath et al., 2004; Ohkubo et al., 1991; Pages et al., 1990). They are heavily glycosylated and become downregulated in the liver upon inflammatory challenge (Ohkubo et al., 1991; Pages et al., 1990). Thus, the low levels of this serpin1 in geriatric neocortex might be explained by chronic inflammation in this group.
As a whole, the serum protein results were inconsistent with the idea that the blood– brain barrier becomes generally more “leaky” with age; apparently selected blood proteins are better able to penetrate this barrier in juveniles. Thus, while blood products might leave distinct non-neural signatures, these might yet be of significance as biomarkers of age-related differences in brain perfusion, blood– brain barrier integrity, or liver function over the lifespan. Moreover, it would be straightforward to determine the neural origin of these products by messenger ribonucleic acid studies, e.g., in situ hybridization histochemistry.
Several of the genes identified in this study are known or suspected to contribute to neuroplastic changes, either developmentally or after injury. The structural proteins vimentin (#12) and moesin (#14) (in the ezrin/radixin/moesin family (Fehon et al., 2010)) are intermediate filaments with microtubule associations required in the organization or rebuilding of neural substrates. Interestingly, virtually none of the vimentin isoform was observed in geriatric sham (or naive, not shown) neocortex; this may represent a deficit in the aged brain’s ability to rebuild damaged neural structures. However, both moesin and vimentin isoforms were strongly induced in the injured brain, especially in juveniles. This may represent the juvenile’s superior plasticity and ability to recuperate.
In addition, 2 other proteins identified here have been implicated in neuron-specific growth or regrowth, namely, CRMP2 (role in somal polarity and neuronal branching) and Apo E (glial-mediated neuronal membrane organization, lipid transport, etc.). Collapsin response-mediator protein 2 (CRMP2, #3), also known as dihydropyrimidinase-like 2 (DRP-2, DPYSL2, TOAD64, et al.), is constitutively expressed in the developing nervous system (Hamajima et al., 1996) and is critical for establishing polarity and neuronal branching by bringing tubulin components to the microtubule assembly (Fukata et al., 2002; Gu and Ihara, 2000; Yoshimura et al., 2005). Both CRMP2 and moesin associate with microtubules to bring them into proximity with the cell membrane. CRMP2 is regulated in the brain during development and aging (Cnops et al., 2004; Cnops et al., 2006; Fountoulakis et al., 2000; Hamajima et al., 1996; Poon et al., 2004; Poon et al., 2006; Veyrac et al., 2005), as well as in a variety of neuropathologic conditions (Bretin et al., 2006; Carrette et al., 2006; Castegna et al., 2002; Chen et al., 2007; Cid et al., 2007; Cole et al., 2004; Cole et al., 2007; Fujisawa et al., 2008; Iwazaki et al., 2007; Lin and Hsueh, 2008; Putman et al., 1997; Ryan and Pimplikar, 2005; Sultana et al., 2007; Vuaillat et al., 2008; Yoshida et al., 1998; Zhang et al., 2007; Zhao et al., 2006). Yet there is much still to be learned about the appearance and function of this highly regulated mediator of neuroplasticity in the injured and aging brain.
CRMP2 is activated via phosphorylation at Ser522 and Thr518, and inactivated at Thr514. It may also be phosphorylated and oxidized at other sites, being regulated by various kinases and phosphatases (e.g., CDK5, GSK-3β, PI3-kinase/Akt (Cid et al., 2007; Ni et al., 2008; Yoshimura et al., 2005)). The apparent increase of CRMP2-Thr514 phosphorylation in the cortex of injured geriatric rats, together with increases of total CRMP2 in juveniles and adults in response to TBI (Fig. 4B) as well as tissue-specific differences (compare Fig. 4A vs. 4B, and Fig. 4C vs. 4D), suggest a role for this isoform in the brain’s response to trauma. CRMP2 also has been found to be differentially modified in Alzheimer’s and Down’s syndrome brain (Castegna et al., 2002; Weitzdoerfer et al., 2001; Yoshida et al., 1998). Thus, aberrant regulation of CRMP2 is a potential contributing factor in age- and injury-related cognitive deficits of the central nervous system.
Apo E-containing lipoproteins are synthesized and secreted by glia. ApoE contributes to repair and regeneration of neuronal membranes, and can also act at neuronal lipoprotein receptors to activate pathways involved in axonal growth, as well as neuronal survival (Vance and Hayashi, 2010). However, intensive study of this gene with respect to changes of vulnerability in a variety of neurologic and other pathologic disorders have yielded associations but no firm conclusions about the significance of its functionality (Bu, 2009; Lanterna and Biroli, 2009; Laskowitz et al., 2006; Laskowitz and Vitek, 2007; Sun and Jiang, 2008; Vance and Hayashi, 2010). Nonetheless, the stronger increase observed in the injured juvenile rats may represent an advantage in neuroplasticity and recovery in this age group.
Oxoglutarate dehydrogenase is α-ketoglutarate dehydrogenase, part of the tricarboxylic acid cycle complex that catalyzes regeneration of the 4-carbon substrate succinylCoA, producing CO2 and NADH+H+ in the process (2.5 adenosine triphosphate equivalents). The 2 energy-related isoforms (αKGD #13, 19) were differently regulated with age and injury (Supplementary Fig. 1D). First, αKGD #13 contained the products of 2 genetic loci (gene IDs 62945278 and 157819765) that increased with increasing age and were downregulated by TBI, perhaps more so in the juvenile rats. Second, αKGD #19 (gene ID 62945278) was a more acidic (perhaps phosphorylated) isoform induced by injury only in the juveniles (and showed a trend toward elevated levels in geriatric shams). These changes may reflect age-dependence in the metabolic mismatch or energy challenge thatoccurs in the injured brain (Glenn et al., 2003; Hattori et al., 2003; Prins and Hovda, 2009). Interestingly, as αKGD #13 decreased in the juveniles, concomitantly αKGD #19 increased (both gene ID 62945278). This suggests the possibility that one form of the protein may be converted to the other, and that replenishment of the more basic isoform (#13, Fig. 3) might be helpful to minimize brain energy deficits in adults.
TOM70A (#11) increased over the lifespan (geriatric > adult > juvenile) but may actually have been suppressed after TBI (Table 2, Supplementary Fig. 1D). It was discovered as the rat homolog to a fungal component of the translocase of the outer mitochondrial membrane (TOM) complex (Alvarez-Dolado et al., 1999). Interestingly, amyloid precursor protein in Alzheimer’s brains was found to impede protein transport into mitochondria by obstructing its human analog, TOM40 (Devi et al., 2006; Hansson Petersen et al., 2008). TOM70A was found to be regulated by thyroid hormone in specific regions of the rat brain, in particular, the striatum (Alvarez-Dolado et al., 1999). Also, relevant to both aging and brain injuries, this mitochondrial transporter was shown to be regulated by vascular endothelial growth factor (VEGF) in an Akt3-dependent manner (Wright et al., 2008). It has been proposed that impaired mitochondrial production and repair in aged skeletal muscle may be due to reduced outer mitochondrial membrane translocase assembly complex formation (Rapaport, 2005). It may be postulated that the observed decreases in VEGF activity over the lifespan (Gennaro et al., 2003; Pola et al., 2004; Rivard et al., 1999; Swift et al., 1999), as well as changes in thyroid hormone with age (Peeters, 2008) affect energy production by impairing mitochondrial TOM expression. Thus, the observed increase of TOM70A (necessary but not sufficient for mitochondrial protein translocation) in geriatric sham brains might be a compensatory response, and the suppression of its induction after TBI an age-dependent vulnerability factor. As with αKGD, increased abundance of this isoform over the lifespan might be a compensatory response to metabolic insufficiency (in geriatric brain) or a developmental feature (in juvenile brain). The relative decreases in both TOM70A and αKGD observed in the brain after TBI could have implications for increased vulnerability of the aging brain to metabolic mismatch and delayed recovery.
In conclusion, a plurality of the protein isoforms were highly induced by injury in juvenile and adult, but not in geriatric rats (Trends B,C). These gene products might be replaced in elderly patients to reduce their vulnerability and impaired recovery. Several isoforms appeared to be overexpressed in the geriatric brain (Trends A, D), and also induced with injury (Trend A). These may be indicators that the aged brain behaves like an injured brain (amplified inflammatory or compensatory responses). These findings open potential new avenues of investigation into differences in the brain’s response to injury over the lifespan. More in-depth studies of how the “aged” brain differs from younger brains may point to novel therapeutic modalities for brain injuries and neurodegenerative disorders in all age groups.
Supplementary Material
Acknowledgments
This work was supported in part by the Greater Cincinnati Foundation, Elizabeth P. Lips Memorial Award and NINDS R01-NS054890, NINDS R01-NS038654. The authors wish to acknowledge the excellent technical assistance of Michael Wyder, Shawn Lewis, Aixiang Xue, Raquel Moore, Mingfu Zhou, Jason Dudley and Nathan Kuhn. Thanks also to Detlef Schumann and Kenneth Greis for valuable discussions on experimental design, and to Jack Lipton for suggestions on the manuscript.
References
- Allen GV, Chase T. Induction of heat shock proteins and motor function deficits after focal cerebellar injury. Neuroscience. 2001;102:603–614. doi: 10.1016/s0306-4522(00)00519-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, González-Moreno M, Valencia A, Zenke M, Bernal J, Muñoz A. Identification of a mammalian homologue of the fungal Tom70 mitochondrial precursor protein import receptor as a thyroid hormone-regulated gene in specific brain regions. J. Neurochem. 1999;73:2240–2249. doi: 10.1046/j.1471-4159.1999.0732240.x. [DOI] [PubMed] [Google Scholar]
- Arnaud P, Kalabay L. Alpha2-HS glycoprotein: a protein in search of a function. Diabetes/Metab. Res. Rev. 2002;18:311–314. doi: 10.1002/dmrr.315. [DOI] [PubMed] [Google Scholar]
- Blumenfeld KS, Welsh FA, Harris VA, Pesenson MA. Regional expression of c-fos and heat shock protein-70 mRNA following hypoxia-ischemia in immature rat brain. J. Cereb. Blood Flow Metab. 1992;12:987–995. doi: 10.1038/jcbfm.1992.136. [DOI] [PubMed] [Google Scholar]
- Bretin S, Rogemond V, Marin P, Maus M, Torrens Y, Honnorat J, Glowinski J, Prémont J, Gauchy C. Calpain product of WT-CRMP2 reduces the amount of surface NR2B NMDA receptor subunit. J. Neurochem. 2006;98:1252–1265. doi: 10.1111/j.1471-4159.2006.03969.x. [DOI] [PubMed] [Google Scholar]
- Brown IR. Expression of heat shock genes (hsp70) in the mammalian nervous system. Results Probl. Cell Differ. 1991;17:217–229. doi: 10.1007/978-3-540-46712-0_15. [DOI] [PubMed] [Google Scholar]
- Brown IR, Rush S, Ivy GO. Induction of a heat shock gene at the site of tissue injury in the rat brain. Neuron. 1989;2:1559–1564. doi: 10.1016/0896-6273(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Brown WM, Christie DL, Dziegielewska KM, Saunders NR, Yang F. The rat protein encoded by clone pp63 is a fetuin/alpha 2-HS glycoprotein-like molecule, but is it the tyrosine kinase inhibitor pp63? Cell. 1992;68:7–8. doi: 10.1016/0092-8674(92)90200-v. [DOI] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrette O, Burgess JA, Burkhard PR, Lang C, Cote M, Rodrigo N, Hochstrasser DF, Sanchez JC. Changes of the cortex proteome and Apolipoprotein E in transgenic mouse models of Alzheimer’s Disease. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006;840:1–9. doi: 10.1016/j.jchromb.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J. Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Chen A, Liao WP, Lu Q, Wong WSF, Wong PTH. Upregulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats—a proteomics approach. Neurochem. Int. 2007;50:1078–1086. doi: 10.1016/j.neuint.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann. N. Y. Acad. Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- Cid C, Garcia-Bonilla L, Camafeita E, Burda J, Salinas M, Alcazar A. Proteomic characterization of protein phosphatase 1 complexes in ischemia-reperfusion and ischemic tolerance. Proteomics. 2007;7:3207–3218. doi: 10.1002/pmic.200700214. [DOI] [PubMed] [Google Scholar]
- Cnops L, Hu T-T, Burnat K, Van der Gucht E, Arckens L. Age-dependent alterations in CRMP2 and CRMP4 protein expression profiles in cat visual cortex. Brain Res. 2006;1088:109–119. doi: 10.1016/j.brainres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Cnops L, Van de Plas B, Arckens L. Age-dependent expression of collapsin response mediator proteins (CRMPs) in cat visual cortex. Eur. J. Neurosci. 2004;19:2345–2351. doi: 10.1111/j.0953-816X.2004.03330.x. [DOI] [PubMed] [Google Scholar]
- Cole AR, Knebel A, Morrice NA. GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J Biol. Chem. 2004;279:50176–50180. doi: 10.1074/jbc.C400412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AR, Noble W, van Aalten L, Plattner F, Meimaridou R, Hogan D, Taylor M, LaFrancois J, Gunn-Moore F, Verkhratsky A, Oddo S, LaFerla F, Giese KP, Dineley KT, Duff K, Richardson JC, Yan SD, Hanger DP, Allan SM, Sutherland C. Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer’s disease progression. J. Neurochem. 2007;103:1132–1144. doi: 10.1111/j.1471-4159.2007.04829.x. [DOI] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J. Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eismann T, Huber N, Shin T, Kuboki S, Galloway E, Wyder M, Edwards MJ, Greis KD, Shertzer HG, Fisher AB, Lentsch AB. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G266–G274. doi: 10.1152/ajpgi.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwan O, Madkour O, Elwan F, Mostafa M, Helmy A. Abbas, Abdel-Naseer M, Shafy S. Abdel, El Faiuomy N. Brain aging in normal Egyptians: cognition, education, personality, genetic and immunological study. J. Neurol. Sci. 2003;211:15–22. doi: 10.1016/s0022-510x(03)00032-7. [DOI] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Fountoulakis M, Hardmaier R, Schuller E, Lubec G. Differences in protein level between neonatal and adult brain. Electrophoresis. 2000;21:673–678. doi: 10.1002/(SICI)1522-2683(20000201)21:3<673::AID-ELPS673>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Fujisawa H, Ohtani-Kaneko R, Naiki M, Okada T, Masuko K, Yudoh K, Suematsu N, Okamoto K, Nishioka K, Kato T. Involvement of post-translational modification of neuronal plasticityrelated proteins in hyperalgesia revealed by a proteomic analysis. Proteomics. 2008;8:1706–1719. doi: 10.1002/pmic.200700928. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Itoh TJ, Kimura T, Ménager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 2002;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- Furuto-Kato S, Matsumoto A, Kitamura N, Nakanishi S. Primary structures of the mRNAs encoding the rat precursors for bradykinin and T-kinin. Structural relationship of kininogens with major acute phase protein and alpha 1-cysteine proteinase inhibitor. J. Biol. Chem. 1985;260:12054–12059. [PubMed] [Google Scholar]
- Gemma C, Bickford PC. Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev. Neurosci. 2007;18:137–148. doi: 10.1515/revneuro.2007.18.2.137. [DOI] [PubMed] [Google Scholar]
- Gennaro G, Ménard C, Michaud SE, Rivard A. Age-dependent impairment of reendothelialization after arterial injury: role of vascular endothelial growth factor. Circulation. 2003;107:230–233. doi: 10.1161/01.cir.0000050652.47145.4c. [DOI] [PubMed] [Google Scholar]
- Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, Hovda DA, Bergsneider M, Hillered L, Martin NA. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Goldman H, Berman RF, Gershon S, Murphy S, Morehead M, Altman HJ. Cerbrovascular permeability and cognition in the aging rat. Neurobiol. Aging. 1992;13:57–62. doi: 10.1016/0197-4580(92)90009-m. [DOI] [PubMed] [Google Scholar]
- Goldstein FC, Levin HS. Cognitive outcome after mild and moderate traumatic brain injury in older adults. J. Clin. Exp. Neuropsychol. 2001;23:739–753. doi: 10.1076/jcen.23.6.739.1028. [DOI] [PubMed] [Google Scholar]
- Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, Strauss KI. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum LM. The T-kininogen, T-kinin system of the rat. Agents Actions suppl. 1992;36:215–222. [PubMed] [Google Scholar]
- Gu Y, Ihara Y. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J. Biol. Chem. 2000;275:17917–17920. doi: 10.1074/jbc.C000179200. [DOI] [PubMed] [Google Scholar]
- Hamajima N, Matsuda K, Sakata S, Tamaki N, Sasaki M, Nonaka M. A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene. 1996;180:157–163. doi: 10.1016/s0378-1119(96)00445-3. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992b;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, White-Gbadebo DM, Lyeth BG, Jenkins LW, Hayes RL. The effect of age on motor and cognitive deficits after traumatic brain injury in rats. Neurosurgery. 1992a;31:1072–1077. doi: 10.1227/00006123-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Petersen C.A. Hansson, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Huang SC, Wu HM, Yeh E, Glenn TC, Vespa PM, McArthur D, Phelps ME, Hovda DA, Bergsneider M. Correlation of regional metabolic rates of glucose with glasgow coma scale after traumatic brain injury. J. Nucl. Med. 2003;44:1709–1716. [PubMed] [Google Scholar]
- Heurteaux C, Bertaina V, Widmann C, Lazdunski M. K+ channel openers prevent global ischemia-induced expression of c-fos, c-jun, heat shock protein, and amyloid beta-protein precursor genes and neuronal death in rat hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9431–9435. doi: 10.1073/pnas.90.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath AJ, Forsyth SL, Coughlin PB. Expression patterns of murine antichymotrypsin-like genes reflect evolutionary divergence at the Serpina3 locus. J. Mol. Evol. 2004;59:488–497. doi: 10.1007/s00239-004-2640-9. [DOI] [PubMed] [Google Scholar]
- Iwazaki T, McGregor IS, Matsumoto I. Protein expression profile in the striatum of rats with methamphetamine-induced behavioral sensitization. Proteomics. 2007;7:1131–1139. doi: 10.1002/pmic.200600595. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Kitamura N, Ohkubo H, Nakanishi S. Differential expression of the multiple forms of rat prekininogen mRNAs after acute inflammation. J. Biol. Chem. 1985;260:12060–12064. [PubMed] [Google Scholar]
- Kalabay L, Gráf L, Vörös K, Jakab L, Benko Z, Telegdy L, Fekete B, Prohászka Z, Füst G. Human serum fetuin A/alpha2HSglycoprotein level is associated with long-term survival in patients withalcoholic liver cirrhosis, comparison with the Child-Pugh and MELD scores. BMC Gastroenterol. 2007;7:15. doi: 10.1186/1471-230X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmovarin N, Friedrichs WE, O’Brien HV, Linehan LA, Bowman BH, Yang F. Extrahepatic expression of plasma protein genes during inflammation. Inflammation. 1991;15:369–379. doi: 10.1007/BF00917353. [DOI] [PubMed] [Google Scholar]
- Knox CA, Yates RD, Chen I, Klara PM. Effects of aging on the structural and permeability characteristics of cerebrovasculature in normotensive and hypertensive strains of rats. Acta Neuropathol. 1980;51:1–13. doi: 10.1007/BF00688844. [DOI] [PubMed] [Google Scholar]
- Krueger-Naug AM, Plumier JC, Hopkins DA, Currie RW. Hsp27 in the nervous system: expression in pathophysiology and in the aging brain. Prog. Mol. Subcell. Biol. 2002;28:235–251. doi: 10.1007/978-3-642-56348-5_13. [DOI] [PubMed] [Google Scholar]
- Kuhla B, Kuhla S, Rudolph PE, Albrecht D, Metges CC. Proteomics analysis of hypothalamic response to energy restriction in dairy cows. Proteomics. 2007;7:3602–3617. doi: 10.1002/pmic.200700248. [DOI] [PubMed] [Google Scholar]
- Lanterna LA, Biroli F. Significance of apolipoprotein E in subarachnoid hemorrhage: neuronal injury, repair, and therapeutic perspectives—a review. J. Stroke Cerebrovasc. Dis. 2009;18:116–123. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Fillit H, Yeung N, Toku K, Vitek MP. Apolipoprotein E-derived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta Neurol. Scand. Suppl. 2006;185:15–20. doi: 10.1111/j.1600-0404.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Vitek MP. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–969. doi: 10.2217/14622416.8.8.959. [DOI] [PubMed] [Google Scholar]
- Lin Y-L, Hsueh Y-P. Neurofibromin interacts with CRMP-2 and CRMP-4 in rat brain. Biochem. Biophys. Res. Commun. 2008;369:747–752. doi: 10.1016/j.bbrc.2008.02.095. [DOI] [PubMed] [Google Scholar]
- Malik AS, Narayan RK, Wendling WW, Cole RW, Pashko LL, Schwartz AG, Strauss KI. A novel dehydroepiandrosterone analog improves functional recovery in a rat traumatic brain injury model. J. Neurotrauma. 2003;20:463–476. doi: 10.1089/089771503765355531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masseguin C, LePanse S, Corman B, Verbavatz JM, Gabrion J. Aging affects choroidal proteins involved in CSF production in Sprague-Dawley rats. Neurobiol. Aging. 2005;26:917–927. doi: 10.1016/j.neurobiolaging.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Disruption of blood– brain barrier during acute hypertension in adult and aged rats. Am. J. Physiol. 1990;258:H1735–H1738. doi: 10.1152/ajpheart.1990.258.6.H1735. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Regulation of blood– brain barrier permeability. Microcirculation. 2001;8:89–104. doi: 10.1111/j.1549-8719.2001.tb00160.x. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann. N. Y. Acad. Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Kalabay L, Derzsy Z, Szarka A, Halmos A, Stenczer B, Arnaud P, Karádi I, Prohászka Z, Rigó J., Jr Preeclampsia is associated with decreased serum alpha(2)-HS glycoprotein (fetuin-A) concentration. Hypertens. Res. 2009;32:665–669. doi: 10.1038/hr.2009.79. [DOI] [PubMed] [Google Scholar]
- Monaco C, Negrini M, Sozzi G, Veronese ML, Vorechovsky I, Godwin AK, Croce CM. Molecular cloning and characterization of LOH11CR2A, a new gene within a refined minimal region of LOH at 11q23. Genomics. 1997;46:217–222. doi: 10.1006/geno.1997.5036. [DOI] [PubMed] [Google Scholar]
- Mooradian AD. Effect of aging on the blood– brain barrier. Neurobiol. Aging. 1988;9:31–39. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- Ni MH, Wu CC, Chan WH, Chien KY, Yu JS. GSK-3 mediates the okadaic acid-induced modification of collapsin response mediator protein-2 in human SK–N–SH neuroblastoma cells. J. Cell. Biochem. 2008;103:1833–1848. doi: 10.1002/jcb.21575. [DOI] [PubMed] [Google Scholar]
- Ohkubo K, Ogata S, Misumi Y, Takami N, Ikehara Y. Molecular cloning and characterization of rat contrapsin-like protease inhibitor and related proteins. J. Biochem. 1991;109:243–250. [PubMed] [Google Scholar]
- Pages G, Rouayrenc JF, Cam G. Le, Mariller M, Cam A. Le. Molecular characterization of three rat liver serine-protease inhibitors affected by inflammation and hypophysectomy. Protein and mRNA analysis and cDNA cloning. Eur. J. Biochem. 1990;190:385–391. doi: 10.1111/j.1432-1033.1990.tb15587.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Peeters RP. Thyroid hormones and aging. Hormones. 2008;7:28–35. doi: 10.14310/horm.2002.1111035. [DOI] [PubMed] [Google Scholar]
- Peila R, Launer LJ. Inflammation and dementia: epidemiologic evidence. Acta Neurol. Scand. Suppl. 2006;185:102–106. doi: 10.1111/j.1600-0404.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- Pentland B, Jones PA, Roy CW, Miller JD. Head injury in the elderly. Age Ageing. 1986;15:193–202. doi: 10.1093/ageing/15.4.193. [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probabilitybased protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Peters T. All About Albumin: Biochemistry, Genetics and Medical Applications. Academic Press; San Diego: 1995. [Google Scholar]
- Plumier JC, Hopkins DA, Robertson HA, Currie RW. Constitutive expression of the 27-kDa heat shock protein (Hsp27) in sensory and motor neurons of the rat nervous system. J. Comp. Neurol. 1997;384:409–428. [PubMed] [Google Scholar]
- Pola R, Aprahamian TR, Bosch-Marcé M, Curry C, Gaetani E, Flex A, Smith RC, Isner JM, Losordo DW. Age-dependent VEGF expression and intraneural neovascularization during regeneration of peripheral nerves. Neurobiol. Aging. 2004;25:1361–1368. doi: 10.1016/j.neurobiolaging.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Poon HF, Castegna A, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA. Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice brain. Neuroscience. 2004;126:915–926. doi: 10.1016/j.neuroscience.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Poon HF, Vaishnav RA, Getchell TV, Getchell ML, Butterfield DA. Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiol. Aging. 2006;27:1010–1019. doi: 10.1016/j.neurobiolaging.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Prins ML, Hovda DA. The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. J. Neurotrauma. 2009;26:1083–1093. doi: 10.1089/neu.2008.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchades M, Hansson SF, Nilsson CL, Andreasen N, Blennow K, Davidsson P. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer’s disease. Brain Res. Mol. Brain Res. 2003;118:140–146. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Putman CW, Rotteveel JJ, Wevers RA, van Gennip AH, Bakkeren JA, De Abreu RA. Dihydropyrimidinase deficiency, a progressive neurological disorder? Neuropediatrics. 1997;28:106–110. doi: 10.1055/s-2007-973681. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Welsh FA, Lowenstein DH, Gennarelli TA, McIntosh TK. Regional induction of c-fos and heat shock protein-72 mRNA following fluid-percussion brain injury in the rat. J. Cereb. Blood Flow Metab. 1995;15:467–473. doi: 10.1038/jcbfm.1995.58. [DOI] [PubMed] [Google Scholar]
- Rapaport D. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J. Cell Biol. 2005;171:419–423. doi: 10.1083/jcb.200507147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- Ryan KA, Pimplikar SW. Activation of GSK-3 and phosphorylation of CRMP2 in transgenic mice expressing APP intracellular domain. J. Cell Biol. 2005;171:327–335. doi: 10.1083/jcb.200505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein-kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thromb. Haemost. 2007;98:77–83. [PubMed] [Google Scholar]
- Sankar R, Blossom E, Clemons K, Charles P. Age-associated changes in the effects of amphetamine on the blood– brain barrier of rats. Neurobiol. Aging. 1983;4:65–68. doi: 10.1016/0197-4580(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Sironi L, Tremoli E, Miller I, Guerrini U, Calvio AM, Eberini I, Gemeiner M, Asdente M, Paoletti R, Gianazza E. Acutephase proteins before cerebral ischemia in stroke-prone rats: identification by proteomics. Stroke. 2001;32:753–760. doi: 10.1161/01.str.32.3.753. [DOI] [PubMed] [Google Scholar]
- Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J. Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- Smith RG, Betancourt L, Sun Y. Molecular endocrinology and physiology of the aging central nervous system. Endocr. Rev. 2005;26:203–250. doi: 10.1210/er.2002-0017. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KI, Barbe MF, Marshall RM, Raghupathi R, Mehta S, Narayan RK. Prolonged cyclooxygenase-2 induction in neurons and glia following traumatic brain injury in the rat. J. Neurotrauma. 2000;17:695–711. doi: 10.1089/089771500415436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Proteomics analysis of the Alzheimer’s disease hippocampal proteome. J. Alzheimers Dis. 2007;11:153–164. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Sun XC, Jiang Y. Genetic susceptibility to traumatic brain injury and apolipoprotein E gene. Chin. J. Traumatol. 2008;11:247–252. doi: 10.1016/s1008-1275(08)60051-6. [DOI] [PubMed] [Google Scholar]
- Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab. Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- Tanno H, Nockels RP, Pitts LH, Noble LJ. Immunolocalization of heat shock protein after fluid percussive brain injury and relationship to breakdown of the blood– brain barrier. J. Cereb. Blood Flow Metab. 1993;13:116–124. doi: 10.1038/jcbfm.1993.14. [DOI] [PubMed] [Google Scholar]
- Vaishnav RA, Getchell ML, Poon HF, Barnett KR, Hunter SA, Pierce WM, Klein JB, Butterfield DA, Getchell TV. Oxidative stress in the aging murine olfactory bulb: redox proteomics and cellular localization. J. Neurosci. Res. 2007;85:373–385. doi: 10.1002/jnr.21130. [DOI] [PubMed] [Google Scholar]
- Vance JE, Hayashi H. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta. 2010;1801:806–818. doi: 10.1016/j.bbalip.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Giannetti N, Charrier E, Reymond-Marron I, Aguera M, Rogemond V, Honnorat J, Jourdan F. Expression of collapsin response mediator proteins 1, 2 and 5 is differentially regulated in newly generated and mature neurons of the adult olfactory system. Eur. J. Neurosci. 2005;21:2635–2648. doi: 10.1111/j.1460-9568.2005.04112.x. [DOI] [PubMed] [Google Scholar]
- Vollmer D, Torner J, Jane J, Sadovnic B, Charlebois D, Foulkes M, Marmarou A, Marshall L. Age and outcome following traumatic coma: why do older patients fare worse? J. Neurosurg. 1991;75:S37–S49. [Google Scholar]
- Vuaillat C, Varrin-Doyer M, Bernard A, Sagardoy I, Cavagna S, Chounlamountri I, Lafon M, Giraudon P. High CRMP2 expression in peripheral T lymphocytes is associated with recruitment to the brain during virus-induced neuroinflammation. J. Neuroimmunol. 2008;193:38–51. doi: 10.1016/j.jneuroim.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Wang H, Li W, Zhu S, Li J, D’Amore J, Ward MF, Yang H, Wu R, Jahnen-Dechent W, Tracey KJ, Wang P, Sama AE. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J. Cereb. Blood Flow Metab. 2010;30:493–504. doi: 10.1038/jcbfm.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzdoerfer R, Fountoulakis M, Lubec G. Aberrant expression of dihydropyrimidinase related proteins–2, –3 and –4 in fetal Down syndrome brain. J. Neural Transm. Suppl. suppl. 2001;95:107. doi: 10.1007/978-3-7091-6262-0_8. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J. Am. Geriatr. Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wright GL, Maroulakou IG, Eldridge J, Liby TL, Sridharan V, Tsichlis PN, Muise-Helmericks RC. VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J. 2008;22:3264–3275. doi: 10.1096/fj.08-106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci. Biobehav. Rev. 1997;21:167–174. doi: 10.1016/s0149-7634(96)00006-1. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Watanabe A, Ihara Y. Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer’s disease. J. Biol. Chem. 1998;273:9761–9768. doi: 10.1074/jbc.273.16.9761. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Suzuki Y, Yamamoto K, Sinohara H. cDNA sequencing of guinea pig alpha 2-HS glycoprotein, its expression in various tissues and acute phase expression. Biol. Chem. 1999;380:95–99. doi: 10.1515/BC.1999.013. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ottens AK, Sadasivan S, Kobeissy FH, Fang T, Hayes RL, Wang KKW. Calpain-mediated collapsin response mediator protein-1, -2, and -4 proteolysis after neurotoxic and traumatic brain injury. J. Neurotrauma. 2007;24:460–472. doi: 10.1089/neu.2006.0078. [DOI] [PubMed] [Google Scholar]
- Zhao X, Tang R, Xiao Z, Shi Y, Feng G, Gu N, Shi J, Xing Y, Yan L, Sang H, Zhu S, Liu H, Chen W, Liu J, Tang W, Zhang J, He L. An investigation of the dihydropyrimidinase-like 2 (DPYSL2) gene in schizophrenia: genetic association study and expression analysis. Int. J Neuropsychopharmcol. 2006;9:705–712. doi: 10.1017/S1461145705006267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.