Abstract
Background
Alanine aminotransferase (ALT) is commonly used to measure liver injury in resource limited settings. Elevations in ALT are predictive of increased mortality from liver disease and may influence choice of first line antiretroviral therapy (ART).
Methods
A cross-sectional analysis of the prevalence and predictors of elevated ALT (defined as > 40 IU/L) was conducted. ART naïve, HIV-infected adults with a baseline ALT who were enrolled in any of the 18 HIV care and Treatment clinics in Dar es Salaam, Tanzania November 2004 and December 2009 were included. Median values were calculated and log-binomial regression models were used to examine predictors of elevated ALT.
Results
During the study period, 41,891 adults had a baseline ALT performed. The prevalence of ALT greater than 40 IU/L, 120 IU/L and 200 IU/L was 13%, 1% and 0.3%, respectively. In multivariate analyses, male sex, CD4+ T lymphocyte count <200 cells/mm3 and higher WHO clinical stages were associated with a significantly higher risk of ALT >40 IU/L (all p <0.01). Hypertryglyceridemia, hyperglycemia and hepatitis B co-infection (HbsAg+) were significantly associated with higher risk of elevated ALT. Pregnancy, anemia, LDL>130 mg/dl and current TB treatment were associated with significantly reduced risk for elevated ALT.
Conclusion
In this HIV-infected, ART-naïve, Tanzanian population extreme elevations in ALT were infrequent but minor elevations were not uncommon. Antiretrovirals with potentially hepatotoxic side effects should be initiated with caution in males, patients with hepatitis B co-infection, advanced immunosuppression and components of the metabolic syndrome.
Keywords: ALT, HIV, ARV naïve
Introduction
In 2008, an estimated 22.4 million people were living with HIV in Sub-Saharan Africa (SSA)[1]. As a result of considerable efforts by national governments and international organizations in scaling up HIV Care and Treatment services in SSA, approximately 3 million HIV infected patients were receiving antiretroviral therapy (ART) by the end of 2008[1]. Since the large scale rollout of ART, significant declines in HIV- related morbidity and mortality have been observed [2]. Following improved life expectancy with ART, non-AIDS defining diseases are now emerging as leading causes of death in HIV- infected populations [3, 4], with liver disease as the most frequent cause of death in developed countries [5]. Similar changes in patterns of mortality can be expected to occur in SSA as access to ART improves.
Alanine aminotransferase (ALT) is a liver enzyme commonly used to measure liver disease in resource-limited settings. Elevated ALT is a highly specific indicator for liver injury and has been shown to be associated with deaths from liver disease in non-HIV infected populations [6]. Since it is often the only marker used to monitor liver disease in HIV-infected individuals in resource-limited settings, understanding the prevalence and risks associated with elevations in ALT in these settings is important. Liver enzyme elevation is common in HIV patients in SSA [7, 8] and various risk factors have been described mainly in Europe and North America including; male sex, HIV itself, viral hepatitis, most antiretrovirals, anti-tuberculosis and lipid lowering drugs; alcohol, and metabolic syndrome [7, 9–17].
In SSA, few studies have examined the prevalence of elevated ALT and risk factors associated with elevations in ALT in HIV-infected individuals, particularly mild elevations of ALT or ALT elevations in the absence of ART exposure. Such studies are necessary as HIV-infected individuals may be at much higher risk of liver injury in SSA due to additional competing risks of liver disease specific to these settings including presence of more advanced immunosuppression, co-infections and exposure to aflatoxins [18, 19]. In addition, elevations prior to ART initiation may impact responses to treatment with ART.
In this study, we report the prevalence of elevated ALT levels and associated risk factors in a cohort of ART naïve HIV-infected patients enrolled in a large urban HIV Care and Treatment program in Dar es Salaam, Tanzania.
Methods
This cross-sectional study was conducted among ART naïve HIV-infected individuals at the time of enrollment at 18 MDH-PEPFAR supported HIV Care and Treatment Clinics in Dar es Salaam, Tanzania, between November 2004 and December 2009. The MDH HIV Care and Treatment Program was established in 2004 and provides infrastructure, laboratory and technical support in HIV related care to the 3 municipalities of Dar es Salaam: Temeke, Ilala and Kinondoni. In this study, we included all HIV patients enrolling at MDH supported sites aged >15 years who had not yet been initiated ART.
We excluded patients whose ALT measurements at baseline were not available. At MDH supported sites patients have the following screening laboratory tests at their baseline visit prior to ART initiation: CD4+ T cell count (Beckton Dickiton (BD) FACS Calibur), hemoglobin, white cell count and platelets (Beckman Coulter ACT5 DIFF hematology analyzer), low density lipoproteins (LDL), high density lipoproteins (HDL), triglycerides (TG) blood glucose and ALT, bilirubin (Roche Cobas intergra 400 plus Chemistry Analyzer) and pulmonary tuberculosis (PTB) screening with a chest X-ray and sputum smear for acid fast bacilli using florescent microscopy. Hepatitis B and hepatitis C serostatus are determined using SD Bioline antigen and antibodies respectively. Viral load measurements are not performed routinely. Patients are initiated on ART according to Tanzanian National AIDS Control Program (NACP) ART initiation criteria: CD4 cell count<200 cells/ mm3 or clinical WHO stage IV or clinical WHO stage III with CD4 cell count of <350 cells/ mm3 (6). Antiretroviral drugs and co-trimoxazole are provided free of charge by the Tanzanian government. The study was approved by the Muhimbili University of Health and Allied Sciences and the Harvard School of Public Health ethical boards.
Data collection and management
Patient demographic, clinical, laboratory and therapeutic data are collected by physicians and nurses on standard case report forms and National Care and Treatment Centre forms at enrollment and at each follow up visit. Demographic and anthropometric measurements, clinical examination findings, including presence or absence of jaundice, hepatospleenomegaly and WHO HIV clinical stage were included in this study. Laboratory data included: ALT, CD4+ T cell counts, Hemoglobin (Hgb), high density lipoprotein (HDL) low density lipoprotein (LDL), triglyceride (TG), HbsAg, HCV antibody and a fasting or random blood glucose. Data reviewers are stationed at each clinic to ensure adequacy and completeness of data recording by the healthcare workers. Data collected is then entered into a secure computerized database designed solely for the purpose of data collection and analysis. Unique patient identifiers are used. The database is updated daily by professional data entry clerks. Weekly quality assurance checks of the database are performed by the data management team to ensure data accuracy. The primary outcomes of interest was elevated ALT defined as an ALT greater than 40IU/L taken between 0 to 7 days of enrollment to the HIV clinics and before ART was initiated.
Statistical methods
Statistics were conducted using SAS version 9.1 (Cary, NC) statistical software. We used means (SD) for continuous variables and proportions were used to describe the basic characteristics of the study population at the time of enrollment. Log-binomial regression models were used to obtain point and interval estimates of prevalence ratios for elevated ALT and to obtain P-values. Ordinal score tests were used to obtain p-values for ordinal categorical variables [20, 21]. Variables with P values of less than 0.05 were considered significant.
Results
Between November 2004 and December 2009, a total of 66,609 adult patients enrolled in the MDH program. After exclusion of patients with missing baseline ALT values and patients on ART at baseline, 41,891 were eligible for inclusion in our analysis. Compared to the excluded patients, those who were included in this analysis had, on average, a significantly somewhat lower CD4 cell count (242 cells/mm3 in study vs. 297 not in the study), had a significantly higher proportion of patients in WHO HIV stage 4 (20.% Stage 4 in study, and 16.8% not in the study), had enrolled at a significantly somewhat later point in calendar time (mean 2008.3 in study, vs. 2007.2 not in the study), and were more likely to be male (30.5% male in study, and 26.8% not) and differed slightly by district of enrollment.
Baseline characteristics of the study population are presented in table 1. The mean age was 36 (±10) years and more than two thirds (71%) were females. The majority of patients were severely immunosuppressed at baseline: 54% patients had a CD4+ cell count < 200cells/µl and 61% of patients were WHO HIV clinical stage III or IV. Approximately 27% of patients had BMI < 18.5kg/m2, 6% were obese and 16% of patients were on TB therapy at the time of enrollment.
Table 1.
Distribution of baseline characteristics of HIV patients enrolled for treatment at MDH-PEPFAR sites in Dar es Salaam, Tanzania’ (N=41891)
| Variable | Proportion (%) / mean (SD) |
|---|---|
| Age categories (years) | |
| <30 | 27 |
| 30–<40 | 43 |
| 40–<50 | 21 |
| 50 + | 9 |
| Male Sex | 29 |
| Pregnancy Status (Yes) | 30 |
| BMI categories (kg/m2) | |
| < 18.5 | 27 |
| 18.5–<25 | 53 |
| 25–<30 | 15 |
| 30+ | 6 |
| WHO Stage | |
| I | 21 |
| II | 18 |
| III | 41 |
| IV | 20 |
| CD4 categories (cells/mm3) | |
| 0–<50 | 20 |
| 50–<100 | 13 |
| 100–<200 | 21 |
| 200+ | 46 |
| Hemoglobin categories (g/dL) | |
| <7.5 | 12 |
| 7.5–<8.5 | 10 |
| 8.5–<11 | 39 |
| 11+ | 39 |
| Lymphocyte count (K/µL) | 2 (1) |
| Current TB treatment (yes) | 16 |
| Hypertension (yes) SBP ≥140mmHg and/or DBP≥90mmHg |
12 |
| Hyperglycemia (yes) RBG ≥200mg/dl or FBG≥ 126 mg/dl |
3 |
| LDL >130 mg/dl | 13 |
| HDL <40 mg/dl | 69 |
| Triglycerides >150 mg/dl | 30 |
| Hepatitis B | 6 |
| Hepatitis C | 2 |
Elevated ALT > 40IU was found in 5301 (13%) patients. ALT greater than three and five times upper limit of normal (ULN = 40IU) were observed in 457 (1%) and 141 (0.3%) patients respectively.
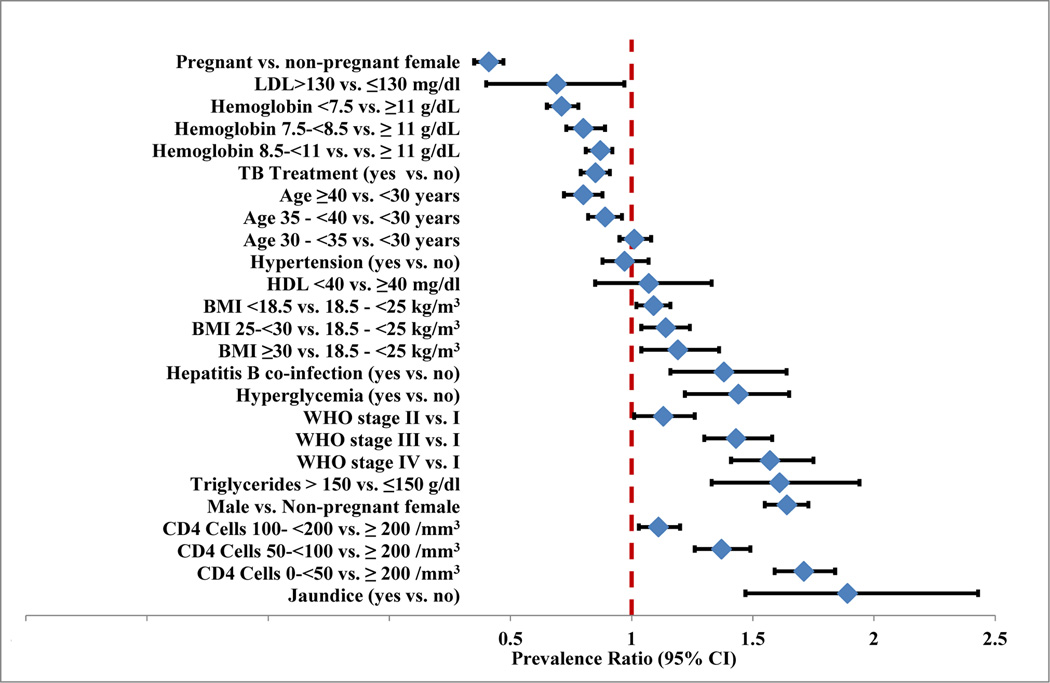
In multivariate analyses, patients aged 40 years and above had a significantly lower risk of elevated ALT compared to patients <30 yrs. Pregnant females had a significantly lower prevalence of elevated ALT compared to non-pregnant females (prevalence ratio (PR)= 0.41; 95%CI 0.35, 0.47). Males had an increased prevalence of elevated ALT than females (PR = 1.64; 95% CI 1.55, 1.73). Patients with lower CD4+ cell counts compared to those with CD4+ cell counts >200 cells/µl had a significantly higher prevalence of elevated ALT. The prevalence of elevated ALT was 71% higher in patients with CD4+ cell count <50 cells/µl compared to patients with CD4+ cell count >200 cells/µl. Similarly, the prevalence of elevated ALT was significantly higher in patients with WHO Stage 2, 3 and 4 disease compared to stage 1; patients with WHO stage 4 had a 57% higher prevalence of elevated ALT compared to patients with WHO stage 1. Patients who were underweight, overweight or obese had a significantly higher prevalence of elevated ALT compared to patients with normal BMI. Those, with BMI <18.5 kg/ m2 had a 9% increased prevalence of elevated ALT compared to those with BMI 18.5- <25kg/m2. Patients with obesity had a 19% increased prevalence of elevated ALT (PR= 1.19; 95%CI 1.04, 1.36). Hyperglycemia, (PR = 1.42; 95%CI 1.22, 1.65), but not hypertension was significantly associated with an increased prevalence of elevated ALT. Anemia was significantly associated with reduced prevalence for elevated ALT. A hemoglobin of <7.5g/dl was associated with a 29% lower prevalence (PR = 0.71; 95%CI 0.65, 0.78) of elevated ALT. Current TB treatment was associated with 15% lower prevalence for elevated ALT (RR= 0.85; 95% CI 0.79, 0.91).
We performed additional multivariate analyses in the subset of patients (n = 8037) with available hepatitis B status at enrollment. Patients who were HBsAg positive had a 38% increased prevalence of elevated ALT compared to those who were HBsAg negative (PR= 1.38; 95%CI 1.16, 1.64). In the subgroup of 3331 patients with non missing HDL, LDL and triglycerides data, TG greater than 150mg/dl (PR= 1.61; 95%CI: 1.33, 1.94) were significantly associated with an increased risk of elevated ALT. LDL > 130 mg/dl was associated with 31% reduced prevalence for elevated ALT (PR= 0.69; 95%CI 0.49, 0.97) and no independent effect of HDL was observed.
Discussion
This study is, to our knowledge the first and one of the largest studies to examine the prevalence and risk factors associated with elevated ALT in HIV-infected individuals prior to ART initiation in an African setting. Three notable findings include the relatively high prevalence of ALT elevations >40IU/L; the significant association between elevated ALT and male sex, immunosupression and components of the metabolic syndrome (elevated triglycerides, hyperglycemia, and obesity) and finally the interesting finding of a protective effect of pregnancy, anemia and current TB treatment. It should be noted that all these associations are independent of treatment with ART.
The prevalence of elevated ALT (13%) found in our study is lower than that reported among pre-ART HIV- infected individuals [7, 13, 16, 17, 22] in Europe and North America, where rates vary between 19–29%, but similar to reports of studies from Africa [8, 23]. In a multi-national study of HIV-infected patients in Kenya, Zambia and Thailand; baseline ALT greater than 40IU was present in about 14% of 812 HIV-infected patients [23]. Similar figures were reported in a rural community in Uganda [8]. There are several reasons for this difference. First, in the studies from Europe and North/South America, males represented between 63–94% of the study population compared to our study where females accounted for 71% of our study population [7, 13, 16, 17, 22]. Second, an inverse relationship between black ethnicity and chronic ALT elevation has been reported in several studies of HIV-infected patients [5, 14]. Third, a lower prevalence of viral hepatitis B and C infections, obesity and dyslipidemia was observed in our study population, all of which have been shown to be associated with elevations in ALT [13–14].
In multivariate analyses, we found a number of factors associated with elevated ALT>40IU/L. Two notable findings were the significant associations between elevated ALT and reduced CD4+ cell count, worsening WHO clinical HIV stage; and components of the metabolic syndrome including elevated BMI, hypertriglyceridemia and hyperglycemia. Our finding of an increased risk for elevated ALT among people with severe immune depression is similar to findings from a study by Sterling et al, where HIV-infected patients with CD4<200cells/µl had a 57% excess risk for elevated ALT compared to those with CD4 ≥ 200cell/µl [13]. Mechanisms underlying this association are not clear, although direct immune activation and pro-apoptotic effects of HIV on hepatocytes as explained by a correlation with higher viral loads has been hypothesized [10].
Similar to other studies, overweight, obesity, increasing triglycerides and hyperglycemia were significantly associated with increased risk for elevated ALT. These conditions are significant risk factors for non-alcoholic fatty liver disease and non-alcoholic steato-hepatitis (NASH) which these elevations in ALT could represent [7, 13–15]. Non-alcoholic steato-hepatitis (NASH) or fatty liver, associated with metabolic syndrome, diabetes mellitus and hypertriglyceridemia have been reported to be cause of the abnormal liver enzymes in HIV-infected patients in several recent studies [14, 15]. Contrary to other studies, we found a reduced risk of elevated ALT with LDL cholesterol > 130mg/dl [13–15]. Although, non fasting measurement of lipids may lead to this contradictory finding, the relationship of LDL and elevated ALT among ART naïve HIV is still unclear and deserves further investigation in the absence of ART exposure.
In this study, we noted an interesting gender difference with respect to the risk of elevated ALT. Compared to non-pregnant women, men had an increased risk of ALT>40 IU/L while pregnant women had a reduced risk of elevated ALT in adjusted analyses. This gender discrepancy is similar to a finding by Weidle et al. who observed men to have a 55% higher risk of elevated baseline liver enzymes than their female counterparts [8]. The reasons for this gender discrepancy are not clear. One potential confounder that was not examined in our study and could have contributed to the excess risk of ALT elevation is alcohol consumption, which has been shown to be significantly higher in men compared to women in urban Tanzanian settings [24]. Interestingly, pregnant HIV -infected patients were at a reduced risk for elevated baseline ALT compared to non-pregnant women. There have been contradictory reports demonstrating the risk of elevated liver enzymes among pregnant HIV-infected women exposed to ART particularly NVP [22 – 24]. There is no data however to suggest the same risk applies to pregnant women in the absence of ART [25] and therefore deserves further study. However, physiological changes during a normal pregnancy have been associated with lower than normal liver enzymes, including ALT [26].
Surprisingly, we found a reduced risk for elevated ALT among HIV patients who were on anti-Tb therapy at the time of recruitment to HIV clinics. Few data exists on hepatotoxicity of TB drugs among HIV patients prior to ARV initiation. Two studies by Hoffman and Weidle et al. documented an increased risk of hepatotoxicity in patients on concomitant ART and anti TB drugs [7, 8]. Similarly, Coca et al. reported an increased risk for hepatotoxicity secondary to TB therapy among hospitalized HIV patients; however, the effect of concomitant ART could not be assessed in this retrospective study of hospital records [22]. In studies from other African settings hepatotoxicity from TB therapy is reported to be low [27, 28]. In Tanzania, the prevalence of hepatotoxicity was only 0.9% at two months of TB therapy [27]. Likewise, in Malawi, among HIV- infected ART naïve patients during TB treatment; only five (1.3%) developed grade 2 hepatotoxicity (defined as ALT =126–250 IU/l), three (0.9%) developed grade 3 hepatotoxicity (defined as 251–500 IU/l) and there was no grade 4 hepatotoxicity (defined as ALT > 500IU/l)) [28]. Breen et al. found serious adverse events of TB therapy in 40% of HIV-infected patients, 71% of whom were on concomitant ART; as opposed to only 26% of HIV un-infected patients reporting (p = 0.008). However, the rates of hepatotoxicity was comparable between the two groups [29]. Therefore, it is likely that the risk of hepatotoxicity with anti-TB therapy observed among HIV-infected individuals is through the interaction or confounding with other risk factors such as hepatitis C, B or ART treatment and not HIV infection per se as has been previously suggested [30].
Our study had several limitations. First, we did not collect data on illicit drugs or alcohol consumption which are important risks for elevated ALT. Second, we were unable to include 37% of patients otherwise eligible in our program, due to either being non ARV naïve at enrollment (10%) or because of missing baseline ALT measurements (27%). Patients included in this analysis were sicker with more advanced HIV infection. Our study and others published in the literature have found that the risk of elevated ALT is higher in patients with more advanced HIV. Thus, the prevalence of elevated ALT may be somewhat overestimated in this report. However, there was also a small significant difference in the distribution by district, but is not clear how district would affect the prevalence estimates. Regardless of the district, all clinics included in this analysis are supported by the same program, MDH-PEPFAR which and offer similar care to patients. It is important to emphasize that these small differences in baseline characteristics between the patients included in this analysis and those excluded are not expected interfere with the internal validity of this analysis, particularly as concerns the risk factors identified in Table 2. Third, because this study was cross-sectional, the temporal sequence of exposure and outcome cannot be ascertained. A longitudinal design would allow for a more precise determination of predictors of elevated ALT. Use of a laboratory surrogate marker (i.e. elevated ALT level) as a sign for hepatopathy is less sensitive than other non- invasive and invasive measures of detecting liver disease such as Fibroscan © and liver biopsy. However, these investigations are neither available nor feasible in study setting. Moreover, elevated ALT level is considered to be a highly specific indicator of liver injury and is a commonly used marker for acute and chronic liver diseases. Only one ALT per individual was measured and significant intra-individual variability with a single measure is likely; however, such misclassification is likely to be non-differential with respect to the other factors we have considered and potentially under-estimates of the associations noted. Finally, we did not have the power to assess higher ALT elevations which might be of more clinical significance in liver disease.
Table 2.
Risk factors for ALT > 40 IU/ L among HIV patients enrolled for treatment at MDH-PEPFAR sites in Dar es Salaam, Tanzania’ (5301 events, N=41891)
| Variable | Univariate PR (95% CI) |
P | Multivariate PR (95% CI) |
P |
|---|---|---|---|---|
| Age (years) | ||||
| <30 | Reference | Reference | ||
| 30– <35 | 1.39 (1.30, 1.49) | 1.01 (0.95, 1.08) | <.003 | |
| 35–<40 | 1.47 (1.36, 1.58) | 0.89 (0.82, 0.96) | <.003 | |
| 40 + | 1.34 (1.21, 1.49) | <0.0001 | 0.80 (0.72, 0.88) | <.0001 |
| Pregnancy | ||||
| Non-pregnant female | Reference | Reference | ||
| Pregnant female | 0.32 (0.28, 0.36) | <0.0001 | 0.41 (0.35, 0.47) | <0.0001 |
| Male | 1.73(1.64, 1.82) | <.0001 | 1.64 (1.55, 1.73) | <.0001 |
| BMI categories (kg/m2) | ||||
| <18.5 | 1.40 (1.32, 1.48) | <0.0001 | 1.09 (1.02, 1.16) | 0.006 |
| 18.5–<25 | Reference | Reference | ||
| 25–<30 | 0.87 (0.80, 0.95) | 1.14 (1.04, 1.24) | 0.004 | |
| 30 + | 0.81 (0.71, 0.93) | 1.19 (1.04, 1.36) | 0.01 | |
| WHO Stage | <0.0001 | <0.0001 | ||
| I | Reference | Reference | ||
| II | 1.50 (1.20, 1.87) | 1.13 (1.01, 1.26) | ||
| III | 2.18 (1.81, 2.63) | 1.43 (1.30, 1.58) | ||
| IV | 3.05 (2.51, 3.71) | 1.57 (1.41, 1.75) | ||
| CD4 categories (cells/mm 3) |
<0.0001 | |||
| 0–<50 | 2.38 (2.23, 2.54) | 1.71 (1.59, 1.84) | <0.0001 | |
| 50–<100 | 1.74 (1.60, 1.88) | 1.37 (1.26, 1.49) | <0.0001 | |
| 100–<200 | 1.29 (1.19, 1.39) | 1.11 (1.03, 1.20) | 0.0062 | |
| 200+ | Reference | Reference | ||
| Hemoglobin categories (g/dL) | <0.0001 | <0.0001 | ||
| <7.5 | 0.95 (0.87, 1.03) | 0.71 (0.65, 0.78) | ||
| <7.5–<8.5 | 0.92 (0.84, 1.01) | 0.80 (0.73, 0.89) | ||
| 8.5–<11 | 0.86 (0.81, 0.92) | 0.87 (0.81, 0.92) | ||
| 11 + | Reference | Reference | ||
| Current TB Treatment | ||||
| No | Reference | Reference | ||
| Yes | 1.16 (1.09, 1.25) | <.0001 | 0.85 (0.79, 0.91) | <.0001 |
| Jaundice | ||||
| No | Reference | Reference | ||
| Yes | 2.62 (2.05, 3.36) | <.0001 | 1.89 (1.47, 2.43) | <.0001 |
| Hypertension | ||||
| No | Reference | Reference | ||
| Yes | 0.91 (0.83, 1.01) | 0.07 | 0.97 (0.88, 1.07) | 0.5 |
| Hyperglycemia | ||||
| No | Reference | Reference | ||
| Yes | 1.67 (1.43, 1.95) | <0.001 | 1.44 (1.22, 1.65) | <.0001 |
| Hepatitis B* | ||||
| No | Reference | Reference | ||
| Yes | 1.7 (1.43, 2.02) | <0 .0001 | 1.38 (1.16, 1.64) | 0.0002 |
| HDL** <40 mg/dl | ||||
| No | Reference | Reference | ||
| Yes | 1.43 (1.16, 1.78) | 0.001 | 1.07 (0.85, 1.33) | 0.6 |
| LDL**>130 mg/dl | ||||
| No | Reference | Reference | ||
| Yes | 0.65 (0.46, 0.91) | 0.01 | 0.69 (0.49, 0.97) | 0.03 |
| Triglycerides**> 150 g/dl | ||||
| No | Reference | Reference | ||
| Yes | 1.19 (1.15, 1.23) | <0.0001 | 1.61 (1.33, 1.94) | <0.0001 |
Additionally adjusted for; year of recruitment, district of residence, lymphocytes count and platelet count.
N = 8037,
N =3331
Notwithstanding the limitations, our study has significant strengths. Its large sample size allows for very accurate estimates of the relative risks for elevated ALT among ART naïve HIV-infected individuals in comprehensive multivariate models. We were able to consider a large set of variables as determinants for elevated ALT, after adjusting for many potential confounders. Patients in the study were recruited from all the three municipalities in Dar es Salaam, increasing the external generalizability of the study. This is a first pre-ART investigation of factors associated with ALT elevations among HIV patients in resource-limited setting, and the findings will contribute to improved patient management in the settings.
In conclusion, modest elevations of ALT among ART naïve HIV patients are not uncommon in Tanzania. These elevations are more likely to occur among males, immunocompromised patients and those with components of the metabolic syndrome. These findings have important implications for long-term outcomes among HIV-infected individuals, given the known association between elevations in ALT and liver related morbidity and mortality [6]. Longer follow up is needed to assess the effect of elevations in ALT at baseline on morbidity and mortality in this cohort, as well as closer monitoring of ALT after initiation of ART especially with potentially hepatotoxic therapies.
Figure 1. Risk factors for ALT > 40 IU/L among anti-retroviral Naïve HIV-positive adults enrolled for treatment at MDH-PEPFAR sites in Dar es Salaam, Tanzania (5301 events, N=41891).
Abbreviations: LDL = low density lipoprotein, TB = Tuberculosis, HDL = high density lipoprotein, BMI = body mass index, WHO = World Health Organization, CI = Confidence interval.
Estimates are from a multivariate log-binomial regression model with high vs. low ALT as the dependent variable. All variables shown as well as year of recruitment, district of residence, lymphocyte cell count and platelet count (data not shown) were adjusted for.
Acknowledgment
We are grateful to the patients who agreed participated in this study. We also acknowledge the efforts all the personnel who contributed to completion of this study. HIV clinics in this study were funded in collaboration by the Government of Tanzania and the US Presidents Emergency Program for AIDS relief (PEPFAR). The Author was supported by NIH-Fogarty scholar program grant no 5R24TW007988.
Footnotes
Conflict of interest
There is no conflict of interest
References
- 1.World Health Organisation. (WHO) and United Nations Programme on HIV/AIDS (UNAIDS). AIDS epidemic update. Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO); 2009. pp. 6–11. [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Out patient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. 26. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Katlama C, Johnson AM, et al. AIDS across Europe, 1994–98: the EuroSIDA study. Lancet. 2000;356(9226):291–296. doi: 10.1016/s0140-6736(00)02504-6. 22. [DOI] [PubMed] [Google Scholar]
- 4.Martinez E, Milinkovic A, Buira E, et al. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007;8(4):251–258. doi: 10.1111/j.1468-1293.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 5.The DAD group. Liver-related deaths in persons infected with the human immunodeficiency virus. Arch Intern Med. 2006;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 6.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136(2):477–485. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21(10):1301–1308. doi: 10.1097/QAD.0b013e32814e6b08. 19. [DOI] [PubMed] [Google Scholar]
- 8.Weidle PJ, Moore D, Mermin J, et al. Liver enzymes improve over twenty-four months of first-line non-nucleoside reverse transcriptase inhibitor-based therapy in rural Uganda. AIDS Patient Care STDs. 2008;22(10):787–795. doi: 10.1089/apc.2008.0020. [DOI] [PubMed] [Google Scholar]
- 9.Gao S, Gui XE, Deng L, et al. Antiretroviral therapy hepatotoxicity: Prevalence, risk factors, and clinical characteristics in a cohort of Han Chinese. Hepatol Res. 2010;40(3):287–294. doi: 10.1111/j.1872-034X.2009.00608.x. [DOI] [PubMed] [Google Scholar]
- 10.Mata-Marín JA, Gaytán-Martínez J, Grados-Chavarría BH, Fuentes-Allen JL, Arroyo-Anduiza CI, Alfaro-Mejía A. Correlation between HIV viral load and aminotransferases as liver damage markers in HIV infected naive patients: a concordance cross-sectional study. Virol J. 2009;6:181. doi: 10.1186/1743-422X-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar MA, Mathieson K, Arey B, et al. Hepatic histopathology and clinical characteristics associated with antiretroviral therapy in HIV patients without viral hepatitis. Eur J Gastroenterol Hepatol. 2008;20(12):1194–1204. doi: 10.1097/MEG.0b013e328305b9e0. [DOI] [PubMed] [Google Scholar]
- 12.Cote HC, Brumme ZL, Craib KJ, et al. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med. 2002;346(11):811–820. doi: 10.1056/NEJMoa012035. [DOI] [PubMed] [Google Scholar]
- 13.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C co-infections. Dig Dis Sci. 2008;53(5):1375–1382. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovari H, Ledergerber B, Battegay M, et al. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis b or c virus co-infection. Clin Infect Dis. 2010;50(4):502–511. doi: 10.1086/649922. [DOI] [PubMed] [Google Scholar]
- 15.Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50(5):464–473. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich DT, Robinson PA, Love J, Stern JO. Drug-Induced Liver Injury Associated with the Use of Non-nucleoside Reverse-Transcriptase Inhibitors. Clinical Infectious Diseases. 2004;38(S2):S80–S89. doi: 10.1086/381450. [DOI] [PubMed] [Google Scholar]
- 17.Ofotokun I, Smithson SE, Lu C, Easley KA, Lennox JL. Liver enzymes elevation and immune reconstitution among treatment-naïve HIV-infected patients instituting antiretroviral therapy. Am J Med Sci. 2007;334(5):334–341. doi: 10.1097/MAJ.0b013e31811ec780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hizza PR. Epidemiological Study of Carcinoma of Liver in Dodoma Region in Tanzania. J Nat Med Assoc. 1979;71(6):585–587. [PMC free article] [PubMed] [Google Scholar]
- 19.Nagu TJ, Bakari M, Matee M. Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health. 2008;8:416. doi: 10.1186/1471-2458-8-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences (Invited Brief Commentary) American Journal of Epidemiology. 2005;162:199. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 21.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. American Journal of Epidemiology. 1986;123:174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 22.Coca NS, Oliveira MS, Voieta I, Antunes CM, Lambertucci JR. Antituberculosis drug-induced hepatotoxicity: a comparison between patients with and without human immunodeficiency virus seropositivity. Rev Soc Bras Med Trop. 2010;43(6):624–628. doi: 10.1590/s0037-86822010000600004. [DOI] [PubMed] [Google Scholar]
- 23.Peters PJ, Stringer J, McConnell MS, et al. Nevirapine-associated hepatotoxicity was not predicted by CD4 count ≥250 cells/µL among women in Zambia, Thailand and Kenya. HIV Med. 2010;11(10):650–660. doi: 10.1111/j.1468-1293.2010.00873.x. [DOI] [PubMed] [Google Scholar]
- 24.Chande H, Salum I. Prevalence of and factors associated with alcohol consumption in Temeke in August/September, 2002. East Afr J Public Health. 2007;4(2):64–66. [PubMed] [Google Scholar]
- 25.Ouyang DW, Shapiro DE, Lu M, et al. Increased risk of hepatotoxicity in HIV-infected pregnant women receiving antiretroviral therapy independent of nevirapine exposure. AIDS. 2009;23(18):2425–2430. doi: 10.1097/QAD.0b013e32832e34b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore M, Nelson-Piercy C. Pregnancy and the liver. Br J Hosp Med (Lond) 2011;72(11):M170–M173. doi: 10.12968/hmed.2011.72.sup11.m170. [DOI] [PubMed] [Google Scholar]
- 27.Tostmann A, van den Boogaard J, Semvua H, et al. Antituberculosis drug-induced hepatotoxicity is uncommon in Tanzanian hospitalized pulmonary TB patients. Trop Med Int Health. 2010;15(2):268–272. doi: 10.1111/j.1365-3156.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 28.Tostmann A, Boeree MJ, Harries AD, Sauvageot D, Banda HT, Zijlstra EE. Short communication: Antituberculosis drug-induced hepatotoxicity is unexpectedly low in HIV-infected pulmonary tuberculosis patients in Malawi. Trop Med Int Health. 2007;12(7):852–855. doi: 10.1111/j.1365-3156.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- 29.Breen RA, Miller RF, Gorsuch T, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection Thorax. 2006;61:791–794. doi: 10.1136/thx.2006.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungo JR, Jones D, Ashkin D, et al. Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med. 1998;157(6):1871–1876. doi: 10.1164/ajrccm.157.6.9711039. [DOI] [PubMed] [Google Scholar]