Abstract
We examined the role of orbitofrontal (OF) cortex in regulating emotion-attention interaction and the balance between involuntary and voluntary attention allocation. We studied patients with OF lesion applying reaction time (RT) and event-related potential (ERP) measures in a lateralized visual discrimination task with novel task-irrelevant affective pictures (unpleasant, pleasant or neutral) preceding a neutral target. This allowed for comparing the effects of automatic attention allocation to emotional vs neutral stimuli on subsequent voluntary attention allocation to target stimuli. N2-P3a and N2-P3b ERP components served as measures of involuntary and voluntary attention allocation correspondingly. Enhanced N2-P3a amplitudes to emotional distractors and reduced N2-P3b amplitudes to targets preceded by emotional distractors were observed in healthy subjects, suggesting automatic emotional orienting interfered with subsequent voluntary orienting. OF patients showed an opposite pattern with tendency towards reduced N2-P3a responses to emotional distractors, suggesting impaired automatic orienting to emotional stimuli due to orbitofrontal damage. Enhanced N2-P3b responses to targets preceded by any affective distractor was observed in OF patients, suggesting bias towards voluntary target-related attention allocation due to orbitofrontal lesion. Behavioral evidence indicated that LVF attention performance was modulated by emotional stimuli. Specifically, OF patients responded faster to LVF targets subsequent to pleasant emotional distractors. We suggest damage to the orbitofrontal circuitry leads to dysbalance between voluntary and involuntary attention allocation in the context of affective distracters with predisposition to posterior target related processing over frontal novelty and affect related processing. Furthermore, we suggest orbitofrontal influence on emotion- attention interaction is valence and hemisphere dependent.
Introduction
The orbitofrontal cortex has been implicated in signaling the affective value of stimuli to the organism for appropriate choices and actions (O'Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001). While intact orbitofrontal cortex (OF) allows for fine-tuned navigation in social environments, considerable impairment in social and emotional behavior is seen after damage to the orbitofrontal cortex (Beer, John, Scabini, & Knight, 2006; Lovstad et al., 2011). The impairment in real life functioning after orbitofrontal damage is in contrast to the often well preserved cognitive abilities and performance on conventional neuropsychological testing (Cicerone & Tanenbaum, 1997).
Electrophysiological and lesion studies have implicated frontal circuits in involuntary attention and novelty detection (Daffner et al., 1998; Downar, Crawley, Mikulis, & Davis, 2000; Halgren, Marinkovic, & Chauvel, 1998; K. Hartikainen & Knight, 2003; Knight & Scabini, 1998; Lovstad et al., 2011). Similar to novel stimuli, emotional stimuli capture automatic attention (K. M. Hartikainen, Ogawa, & Knight, 2000). Novelty has been suggested to be one dimension of affective space along with valence and arousal (Weierich, Wright, Negreira, Dickerson, & Barrett, 2010). This is supported by evidence that limbic structures including amygdala and orbitofrontal cortex are engaged not only by arousing stimuli of positive or negative valence but also by novel stimuli. This suggests that valence, arousal and novelty all contribute to stimulus salience and significance and modulate allocation of attentional resources. OF cortex is engaged when stimuli are both novel and behaviorally relevant (Ethofer et al., 2009).
We investigated the role of orbitofrontal cortex in emotional modulation of attentional allocation. We studied patients with orbitofrontal lesion applying reaction time and ERP measures in a lateralized visual discrimination task with novel task-irrelevant affective pictures preceding a designated target requiring a behavioral response. We aimed to evaluate the role of orbitofrontal cortex on both involuntary and voluntary attention allocation using a visual attention task with novel affective distractors and neutral targets. The paradigm used in this study was designed to allow for comparing the effects of automatic orienting to emotional vs neutral stimuli on subsequent voluntary orienting. The distracters were not random, but sometimes preceded a target and thus served as a prime to examine the effects of emotionally valenced distracters in responding to subsequent targets.
To index attentional allocation, we measured event-related N2-P3 peak-to-peak amplitude. Novel task-irrelevant stimuli evoke P3 potential with frontal distribution called P3a (Knight, 1984). While P3a reflects activation of neural circuits involved in involuntary attention a more posterior P3b is be generated by neuronal circuits recruited by voluntary attention in target detection tasks (Picton, 1992; Polich, 1998; Soltani & Knight, 2000). In the this study the P3a ERP component served as a measure of involuntary attention allocation while P3b served as a measure of voluntary attention allocation.
Both enhanced (Rule, Shimamura, & Knight, 2002) and reduced novelty (Lovstad et al., 2011) P3s have been reported in orbitofrontal patients. Enhanced novelty responses were observed in a passive movie viewing situation where unpleasant auditory and somatosensory stimuli were randomly delivered (Rule et al., 2002). Diminished responses were observed in an auditory oddball paradigm (Lovstad et al., 2011) requiring an active task. The discrepancies in these findings could be attributed to differences in study design (active vs passive) and topographies of the novelty P3s. A more frontal novelty P3a distribution is observed in an active auditory oddball paradigm using non-emotionally charged distractors, whereas a more posterior scalp distribution is observed in a passive movie viewing situation using disagreeable somatosensory and auditory novel stimuli. The P3 evoked by the novel stimuli in the movie task had a more posterior distribution suggesting greater proportion of parieto-temporal circuits generating the response, thus resembling the P3b with parietal maximum rather than a P3a in scalp topography. The degree to which brain responses to novel stimuli in these two studies were influenced by the emotional context remains to be determined. In the Rule et al (2002) study the stimuli were thought to be emotionally unpleasant since delivery of stimuli unpredictably interfered with the movie. The novel auditory stimuli used in the study by Lovstad et al (2011) included dog barks, laughter and door slams that could be argued to have some emotional significance when presented in an experiment otherwise consisting of monotonous tones. As described earlier, novelty in itself is intricately linked to limbic structures and functions. Novel stimuli can be either rewarding if adding excitement or amusement to an otherwise boring experiment or threatening depending on the stimuli, context, past experiences, mood and personality. In these two previous studies on ERP effects to novel stimuli in orbitofrontal patients there was no comparison of affectively neutral (or less affective) novel stimuli and affectively valenced novel stimuli.
In the current study, we examined the role of the orbitofrontal cortex in involuntary attention allocation to novel affective and affectively neutral distracters as reflected in frontal N2-P3a amplitude and voluntary attention allocation to neutral targets as reflected in parietal N2-P3b. We have previously shown in healthy subjects that task-irrelevant affective stimuli automatically capture attention and temporarily render subsequent targets with less attentional resources (K. M. Hartikainen et al., 2000; K. M. Hartikainen, Ogawa, Soltani, & Knight, 2007). We hypothesized that orbitofrontal cortex biases attentional recourses to novel and emotionally relevant events. We predicted that patients with orbitofrontal lesion would fail to recruit additional resources to affectively valenced novel distractors. We expected reduced frontal P3a to these stimuli, reflecting disruption in limbic novelty circuitry due to orbitofrontal lesions. We predicted a shift towards posterior default attentional settings favoring task-relevant stimuli over task-irrelevant novel emotional stimuli. Consequently, we predicted enhanced posterior P3b amplitude to targets in orbitofrontal patients in the context of emotional distractors.
Methods
Subjects
Seven male patients (mean age 48 ± 18 years) with orbitofrontal lesions (6 bilateral and 1 left unilateral lesions) and eleven age- and sex-matched neurologically intact healthy volunteers (mean age 52 ± 19 years) participated in the study. Inclusion criteria for orbitofrontal patients were a clinical history and CT (computerized tomography) or MRI scan (magnetic resonance imaging) evidence of orbitofrontal damage with no or minimal evidence of brain damage elsewhere in the brain. Brain damage was centered in BA11, 12, 13 and anterior area 47 and there was no damage to either the lateral frontal cortex or the basal forebrain area (Figure 1.). Damage was due to traumatic brain injury (bilateral lesions) or orbital meningioma removal (unilateral lesion). Exclusion criteria were lower than 10th grade education level, lower than 85 IQ level (one standard deviation below the mean), history of learning disability, psychiatric illness or drug abuse. All patients were at least two years post-injury..Patients were recruited from the Veterans Affairs Medical Center in Martinez, California. Those patients that passed the radiological and chart review based on inclusion and exclusion criteria were contacted and asked if they wished to participate in neurological research focused on understanding residual problems related to their neurological event. A neurological and bedside neurobehavioral exam (including test of attention, memory, language, visuospatial and executive function) was performed at least 6 months after brain damage by Dr. Knight. All subjects were right-handed, with normal corrected visual acuity. Visual acuity was measured by Snellen chart. Acuity was at least 20/25 in each eye. Visual fields were intact to bedside testing. Informed consent, approved by both the VA and the University of California, Berkeley Institutional Review Board, were obtained from all the subjects. The study was approved by the Ethics Committee of the Institutional Review Board.
Figure 1.
Lesion reconstruction of seven orbitofrontal patients. The lowest row represents average lesion location. Lesion reconstructions were primarily based on MRI scans. CT scans were obtained for the small number of patients unable to undergo MRI (e.g., due to claustrophobia). The lesion extent was manually transcribed from high resolution 2mm section scans onto sequential axial templates using the MRIcro software (http://www.sph.sc.edu/comd/rorden/mricro.htm). The templates were projected onto a lateral view of the brain by computer software allowing identification of affected Brodmann areas and determination of average lesion extent across patients.
Stimuli and Procedure
An upright or inverted triangle (target) was flashed for 150 ms on either side of the fixation cross, randomly presented in the left or right visual hemifield (Figure 2.) Fifty percent of the targets were upright and the remaining half were inverted. Subjects were asked to respond to the orientation of the triangle, pressing one button with their middle finger if the triangle was pointing up and another button with their index finger if the triangle was pointing down. The subjects were tested over 16 blocks, with 85 trials/block. A brief emotional (pleasant or unpleasant; 150ms stimulus duration) or neutral picture was randomly presented centrally 350 ms prior to the target onset. Three sets (pleasant, unpleasant and neutral) of 48 colored pictures were selected from the International Affective Picture System selected (Center for Research in Psychophysiology, 1999). Each picture was presented equally in its original form and as a mirror image to exclude any possible effects due to image asymmetries. The mean pleasure ratings for pleasant, unpleasant and neutral pictures used in the study were 7.3 ± 0.6, 2.8 ± 0.8, 5.2 ± 0.4, and the mean arousal ratings were 4.7 ± 0.9, 5.8 ± 0.8, 3.5 ± 0.6 respectively. These ratings were based on IAPS norms (Lang, Bradley, & Cuthbert, 1999). The pleasant pictures included photographs of puppies, babies, happy couples, sporting events, beautiful scenery, etc. Unplkeasant pictures consisted of photographs of frightening animals, sad or angry humans, threatening pictures of gun or knife attacks, accident scenes, graveyards, etc. Neutral pictures included photographs of animals, people during daily activities, city scenes, inanimate objects such as hairdryers, etc. Erotic pictures and mutilated people were excluded from the study. On 18% of the trials the targets were not preceded by a picture and on 27% of trials the pictures were not followed by a target. The emotional and neutral stimuli offset was 200 ms prior to the onset of the target. The inter-trial interval was 1500ms.
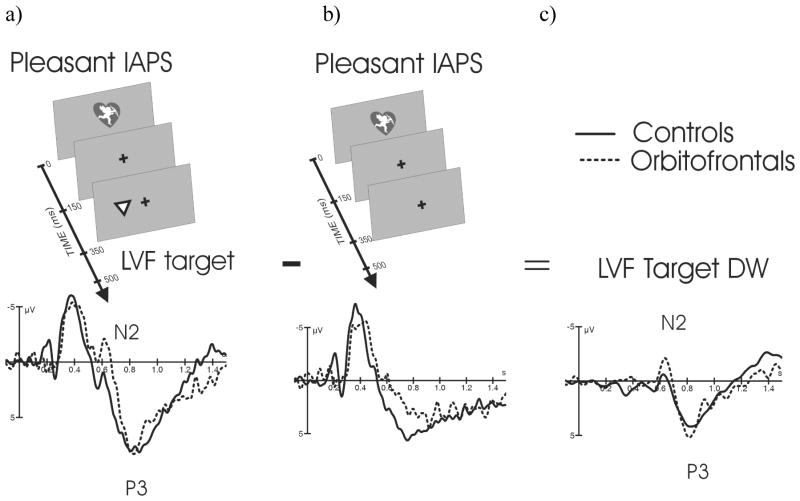
Figure 2.
Paradigm and Difference Waveform (DW). c) LVF Target DWpleasant was created by subtracting b) ERPs to Pleasant IAPS stimuli presented alone from a) ERPs to Pleasant IAPS stimuli (150ms) followed by a subsequent target at 350ms. LVF Target DWpleasant reflects brain's response to LVF targets in the context of pleasant stimuli without the superimposed response to complex visual IAPS stimuli. The other DWs presented in Figure 4 were created in the same manner.
The subjects were seated in a sound attenuated booth facing a computer screen at a distance of one meter. Participants were instructed to keep their eyes on a fixation cross in the middle of the screen throughout the presentation of the stimuli. Subjects were instructed to ignore the pictures and respond to the targets as quickly and accurately as possible. The response hand was counterbalanced.
EEG Analysis
Brain electrical activity was recorded with Ag-AgCl electrodes placed at 30 scalp sites (Fp1, Fp2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, T3, C3, Cz, C4, T4, TP7, CP3, CPz, CP4, TP8, T5, P3, Pz, P4, T6, O1, Oz, O2), referred to the linked mastoid and amplified with a Neuroscan amplifier. Horizontal electro-oculograms were recorded from the outer canthi of each eye, and vertical EOG was recorded from beneath the left eye and Fp1. Impedances were maintained below 5 kΩ. The EEG was amplified (band pass 0.1–80Hz), sampled at 250Hz and digitally stored for off-line analysis. The averaging epochs were 1700 ms, including 250 ms baseline. Trials containing blinks, horizontal eye movements or EMG artifacts were automatically rejected from further analysis.
Difference Waveforms
We used ERP difference waveforms (DW) to assess target-related brain responses to IAPS evoked brain responses. ERPs to identical sets of IAPS stimuli presented alone were subtracted from those presented with a following target. Thus, Target-DWs were controlled for any physical aspects of IAPS stimuli. Six Target-DWs were created to examine the modulatory effects of emotion (3 categories: neutral, pleasant and unpleasant) on hemifield (LVF and RVF) target processing. Figure 2 illustrates how DWs were created.
ERP measurements
We assessed attentional allocation to target processing by measuring the N2-P3 peak to peak amplitude. Theoretical and empirical evidence suggest that P3 amplitude reflects the amount of attentional allocation (Kok, 1997). Since the P3a potential has its maximum in the frontal region we measured the N2-P3 peak to peak amplitudes for the novel stimuli (IAPS) at fronto-central electrode FCZ. Inspection of the grand averaged waveforms were used to determine time windows for the peaks. The novel IAPS stimuli evoked an ERP waveform with N1-P1 and N2-P3a peaks followed by a slow wave (Fig 2.b). The amplitude of the N2 was defined as the lowest negative peak within a time window of 280-480 ms and the amplitude of the P3a-slow wave complex as the highest positive peak 650–1000 ms after the IAPS onset. Likewise since the P3b maximum is at parietal region, the N2-P3b peak to peak amplitudes for the targets were measured at one left and one right centro-parietal electrode, CP3 and CP4. The N2 for the targets was defined as the lowest negative peak within a time window of 260–360 and the P3b s within a time window of 350–700 ms after target onset.
Statistical Analysis
SPSS software program was used for statistical analysis. Repeated measures Analysis of Variance (ANOVA) was performed on ERPs, reaction times (RT) and accuracy. Any significant interaction effects were decomposed by additional ANOVAs. Post-hoc tests were carried out by further ANOVAs or independent sample’s t test.
ERPs to centrally presented IAPS stimuli were analyzed by measuring N2-P3a/slow wave peak-to-peak amplitudes at fronto-central electrode FCZ. The factors were group (Orbitofrontals, Controls) and Emotional Valence (Pleasant, Unpleasant, Neutral). ERPs to targets were analyzed by measuring N2-P3 peak-to-peak amplitudes at CP3 for the left hemisphere and CP4 for the right hemispheres. The factors were group (Orbitofrontals, Controls), Valence (Pleasant, Unpleasant, Neutral), Target Visual Field (LVF, RVF) and Hemisphere (Left (CP3), Right (CP4)). For RT and response accuracy the factors were Group (Orbitofrontals, Controls), Valence (Pleasant, Unpleasant, Neutral) and Target Visual Field (LVF, RVF).
Results
Behavioral results
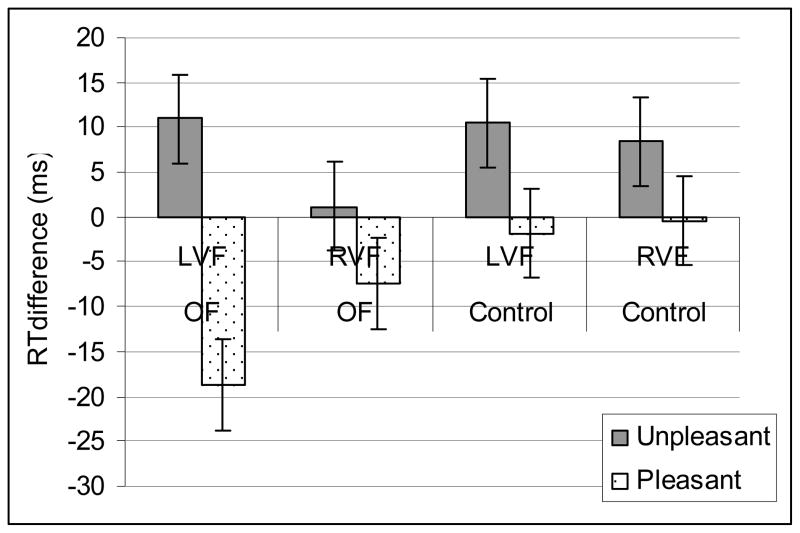
The overall performance of patients with orbitofrontal lesion and healthy controls was comparable (RTs: controls 400 ± 120 ms, orbitofrontal patients 461 ± 147 ms, p = 0.61). Reaction times were slower to the LVF targets 441 ± 125 ms than to RVF targets 422 ± 123 ms [Main ANOVA: Target; F(1,16) = 21.7, p < 0.001] preceded by a distractor. The valence of the distractor modulated the RTs to the targets with pleasant distractor speeding RTs (425 ± 123 ms) and unpleasant distractors slowing down RTs 439 ± 123 ms in comparison to neutral distractors (431 ± 129 ms) [Main ANOVA:Valence; F(2,32) = 11.4, p < 0.001,]. The modulatory effects of emotional distracters on target RTs were significant in the LVF [ Post-hoc ANOVA on LVF targets: Valence F(1,16) = 28.3, p > 0.0001]. The LVF attention performance differed between the groups depending on emotional valence of the distractor (Fig 3) [Group by Valence interaction F(1,16) = 5.6, p < 0.03]. Specifically, orbitofrontal patients responded faster to LVF targets when preceded by pleasant distracters. [Post-hoc ANOVA on LVF targets preceded by pleasant distracters: Group F(1, 16) = 2.2, p < 0.04.] The groups did not differ in right visual field attention performance. The right visual field attention performance was not significantly modulated by emotional distractors (p = 0.13). The task was performed with high accuracy (98 ± 2%) and there was no significant difference in the number of errors between the two groups (controls 1.9 ± 1.6%, orbitofrontal patients 3.1 ± 2.6%, p = 0.14). The number of errors made was not significantly affected by emotional stimuli. There were no significant interaction effects in number of errors between Group, Field and Valence.
Figure 3.
Emotional modulation of attention performance. Pleasant stimuli enhanced LVF attention performance in OF patients. Differential effect of emotional stimuli on reaction times to subsequent targets in the left visual field (LVF) and right visual field (RVF) in comparisons to those preceded by neutral stimuli. RTs to targets preceded by neutral stimuli serve as baseline. Unpleasant stimuli slowed down while pleasant stimuli speeded up reaction times with enhanced effects in the LVF for the pleasant stimuli in the orbitofrontal (OF) patients.
ERP results
ERPS to Novel stimuli
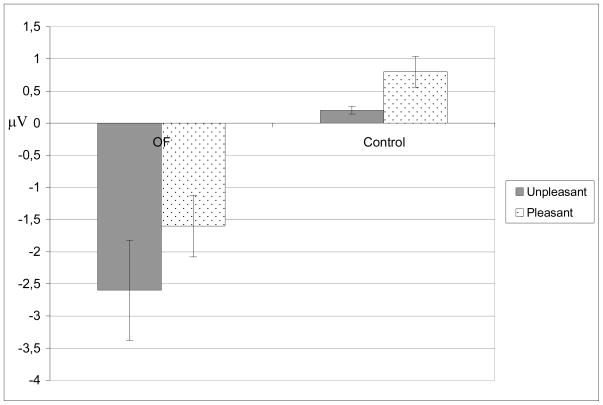
We measured N2-P3a/slow wave peak to peak amplitudes to neutral, unpleasant and pleasant stimuli. The overall amplitudes to Novel IAPS stimuli did not differ between the groups. However, the emotional distractors had an opposite effect on the N2-P3a amplitudes in healthy control group and OF patients as indicated by a significant two-way interaction [Main ANOVA: Group by Valence F(2,30) = 5.4, p < 0.010, Figure 4.] In comparison to neutral distractors, pleasant distractors were associated with enhanced N2-P3a amplitude in healthy controls and decreased N2-P3a amplitude in OF patients [Post-hoc ANOVA: Group by Valence F(1,15) = 7.5, p < 0.014]. In comparison to neutral distractors the effect of unpleasant distractors on N2-P3a amplitude did not quite reach significance [Post-hoc ANOVA: Group by Valence F(1,15) = 4.0, p < 0.06]. Post-hoc analysis on control subjects separately resulted in significant effect of emotion [Post-hoc ANOVA: Valence F(2,20) = 7.9, p = 0.003] with pleasant stimuli evoking enhanced N2- P3a amplitude (21.0 ± 5.8 μV) in comparison to neutral [19.0 ± 5.5 μV, Post-hoc ANOVA: Valence F(1,10) = 4.03, p > 0.003] and to unpleasant stimuli [19.9 ± 5.8 μV, Post-hoc ANOVA: Valence F(1,10) = 6.6, p > 0.027]. The effect of emotional valence in post-hoc analysis on OF patients separetely did not reach significance [Post-hoc ANOVA: Valence F(2,12) = 1.4, p = 0.28 ]. Figure 4. presents the effect of Pleasant and Unpleasant Valence on N2-P3 amplitude to IAPS distractors.
Figure 4.
Reduced attentional allocation to affective distractors in orbitofrontal patients. Effect of valence on N2-P3a amplitude to affectively valenced in comparison to affectively neutral IAPS distractors. There is a significant opposite effect of emotional stimuli to N2-P3a amplitude in orbitofrontal patients and in control subjects in comparison to neutral stimuli.
ERPS to Target stimuli
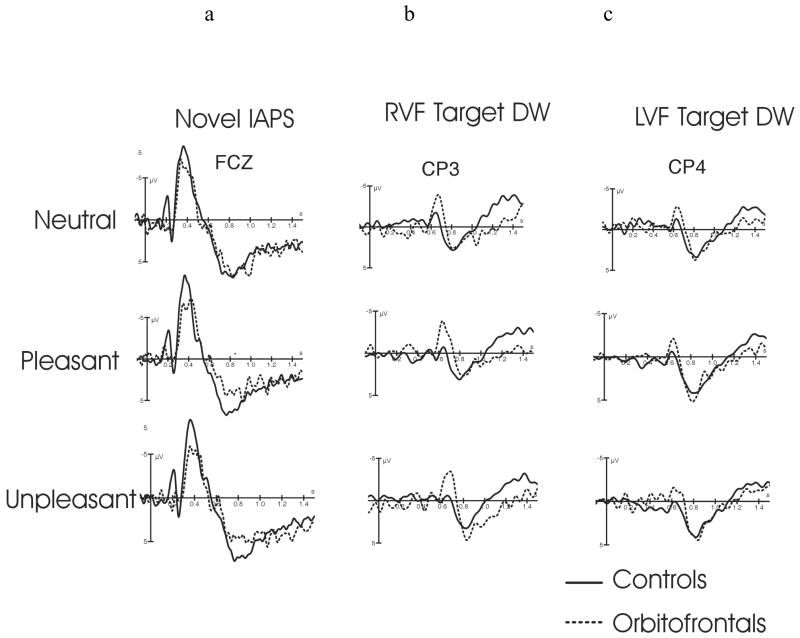
The N2-P3b amplitudes to targets presented alone without preceding IAPS distractor did not differ between the groups (10.0 ± 3.0 uV in controls; 11.4 ± 5.5 uV in OF, p = 0.53). However, the N2-P3b amplitude to targets preceded by IAPS distractor was greater in orbitofrontal patients 14.7 ± 4.3 uV than in control subjects 9.5 ± 4.0 uV [Main ANOVA: Group F(1,15) = 8.2, p < 0.012, Figure 5.]. N2-P3 amplitude to targets was reduced when preceded by affective distractors in comparison to affectively neutral distracters [Main ANOVA: Valence F(2,10) = 4.4, p < 0.02]. Separate analysis within each group revealed that this effect was significant only in healthy control subjects [ANOVA: Valence F(2,20) = 3.67, p < 0.043].
Figure 5.
Modulatory effects of OF cortex on attention allocation. OF lesion had opposite effects on involuntary and voluntary attention allocation. Affective distracters (pleasant and unpleasant, a) evoked reduced N2-P3a amplitudes in orbitofrontal patients in comparison to controls. An opposite pattern was observed for the (b,c) target ERPs preceded by novel IAPS stimuli which showed enhanced N2-P3b potentials in orbitofrontal patients in comparison to controls. This pattern of ERP amplitude modulation suggests reduced attentional allocation to affective distractors and enhanced attentional allocation to target related processing in OF lesion. The ERPs to Targets are subtraction waveforms with preceding IAPS evoked potentials subtracted to reveal superimposed target evoked potentials as shown in Figure 2. Electrode locations for this figure were chosen according to the amplitude maximum with a) ERPs to novel distracters presented at a fronto-central electrode FCZ , while b) ERPs to RVF Targets are presented at a left centro-parietal electrode CP3 and c) LVF Targets at a right centro-parietal electrode CP4.
Discussion
This study provides evidence that attentional allocation based on emotional significance is modulated by orbitofrontal cortex. Healthy control subjects allocated additional attentional resources to distractors of emotional valence, whereas OF patients showed the opposite pattern with less resources allocated to affective distractors. In contrast to the diminished novelty responses to affective distractors, enhanced target responses were observed in OF patients when preceded by affective distracters. This suggests that orbitofrontal cortex regulates the balance of fronto-parietal attention network based on emotional significance of nearby stimuli. Orbitofrontal lesions results in a shift to posterior voluntary attentional activity enhancing task-relevant stimuli over task-irrelevant novel emotional stimuli.
OF cortex together with the temporo-parietal junction has been suggested to coordinate voluntary attentional control settings to determine which stimuli effectively compete for attention (Serences et al., 2005). We have previously shown that in healthy subjects irrelevant affective stimuli capture automatic attention and render subsequent targets with less resources as reflected in slowed RTs and diminished N2-P3b amplitudes to targets preceded by affective stimuli (K. M. Hartikainen et al., 2000; K. M. Hartikainen et al., 2007). While attention allocation to emotional distractors may be disruptive to concurrent task performance, it may be ecologically adaptive to allow for emotional distractors to capture attentional resources to better evaluate whether any action is required.
Parietal N2-P3b ERP amplitudes to targets preceded by IAPS distracters were enhanced in patients with orbitofrontal lesion while the frontal N2-P3a amplitudes to novel affectively valenced distractors were reduced in orbitofrontal patients. We suggest enhanced target N2-P3b amplitudes reflect enhanced voluntary attentional allocation to target processing in the context of emotional distractors. Enhanced recruitment of neural processing resources to task relevant stimuli over task irrelevant stimuli could be a compensatory mechanism allowing overall performance to remain comparable in patients with OF lesion. The shift towards posterior cortical attentional processing after OF damage might facilitate performance in predictable structured testing environments but compromise emotion guided behavior in socially complex unpredictable environments where context-dependent and probabilistic stimulus-reinforcement contingencies dominate successful behavior.
While the overall performance between the healthy subjects and OF patients was comparable, the LVF attention performance differed based on the valence of the preceding distractor. Specifically, the patients were faster to respond to the LVF targets when preceded by pleasant distractors. Previously, we have shown the LVF attention performance to be vulnerable to emotional stimuli competing for right hemisphere attentional resources (K. M. Hartikainen et al., 2007). In the current study, the behavioral effects of emotional stimuli are not fully explained within an attentional resource competition model. The effects of pleasant stimuli could be explained with attentional competition model with less resources allocated to processing of pleasant emotional stimuli allowing for subsequent faster responding in OF patients. However, such effect was not seen with unpleasant stimuli. Therefore, we conclude that the effects of emotional stimuli on response speed are partly independent of attention and may relate to pleasant and unpleasant stimuli having automatic and opposite effects on approach and withdrawal behaviors.
Whether attention is allocated to stimuli with affective value in patients with orbitofrontal damage may depend on the complexity of the affective stimuli, reinforces or punishers. When the affective stimuli are complex or the reward/punishment contingencies are more complicated OFC may be crucial in evaluating how much attentional resources are appropriate. The overall attentional balance in the absence of OF input are biased to stimuli relevant to the task at hand.
Enhanced P3 responses to task-irrelevant, unexpected aversive environmental stimuli has been observed in patients with orbitofrontal damage (Rule et al., 2002). Such inappropriate allocation of attention to repeated irrelevant aversive stimuli after orbitofrontal damage has been suggested to reflect a failure in orbitofrontal inhibitory control functions. Orbitofrontal cortex has been assigned many functions related to inhibition (Hooker & Knight, 2006) that is thought to be a key mechanisms for efficient processing of relevant information with irrelevant and distracting information being suppressed, gated or filtered by frontal circuits (Roberts & Wallis, 2000; Shimamura, 2000). If orbitofrontal cortex was responsible for general suppression of irrelevant affective information, we would expect enhanced ERPs to affectively valenced distractors in patients with OF lesion. On the contrary, ERPs to novel affective distracters were reduced in this study. This finding is in line with other reports supporting orbitofrontal region being engaged by novel and behaviorally relevant stimuli(Lovstad et al., 2011).
The shift towards voluntary attention ERPs in patients with orbitofrontal damage is also in accord with previous literature reporting both diminished peripheral (Bechara, Tranel, Damasio, & Damasio, 1996) and enhanced central emotional responses in OF patients (Rule et al., 2002). We speculate that when the affective value of the stimuli depend on higher-order processing and more complex stimulus-reward/punishment contingencies the attentional control settings are dependent on orbitofrontal input. Such dependence of the attentional control settings on orbitofrontal input derived from complex higher-order processing of reinforcement contingencies could partly explain diminished anticipatory skin conductance response (SCR) in OF patients in the Iowa gambling task. Similarly, the partial voluntary ERP pattern with diminished orbitofrontal input could explain both the preserved SCR to winning or losing (Bechara, 2004) as well as the enhanced brain responses reported to sudden electric writs shocks in OF patients (Rule et al., 2002). Electric wrists shocks are highly arousing and are likely to have a primary punishment value. The brain's ability to respond to such stimuli is preserved even in deep levels of anaesthesia with suppressed cortical activity (K. M. Hartikainen, Rorarius, Perakyla, Laippala, & Jantti, 1995). Future studies with larger orbitofrontal patient group including brain injury control group and using different emotional stimuli with varying levels of complexity will help further elucidate the role of orbitofrontal cortex in emotional modulation of attention.
We suggest intact orbitofrontal cortex regulates allocation of attentional resources based on affective significance rather than task-relevance. For socially appropriate conduct it is crucial to allow flexible allocation of attentional resources to emotional and social cues despite any ongoing task. The shift of attentional balance that allow resources to be efficiently allocated to the task at hand may facilitate patients' performance in experimental tasks and neuropshychological tests. However, the difficulties OF patients encounter in everyday life situations and social interactions may stem partly from inappropriately maintaining attentional resources on the task at hand when novel or emotionally significant events would normally call for deployment of attention to emotional or social cues in order to allow for socially appropriate behavior and successful decision making. Failure to allocate attentional resources to stimuli with emotional significance may be one mechanism for impaired social and emotional conduct in these patients.
Acknowledgments
Supported by the Finnish Academy, The Pirkanmaa Science Foundation and NINDS grant NS 21135
References
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6(2):215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18(6):871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Tanenbaum LN. Disturbance of social cognition after traumatic orbitofrontal brain injury. Arch Clin Neuropsychol. 1997;12(2):173–188. [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Cohen LG, Kennedy BP, West WC, et al. Regulation of attention to novel stimuli by frontal lobes: an event-related potential study. Neuroreport. 1998;9(5):787–791. doi: 10.1097/00001756-199803300-00004. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Kreifelts B, Wiethoff S, Wolf J, Grodd W, Vuilleumier P, et al. Differential influences of emotion, task, and novelty on brain regions underlying the processing of speech melody. J Cogn Neurosci. 2009;21(7):1255–1268. doi: 10.1162/jocn.2009.21099. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106(2):156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Hartikainen K, Knight RT. Lateral and orbital prefrontal contributions to attention. In: Polich J, editor. Detection of Change: Event-related Potential and fMRI Findings. Boston/ Dordrecht/ New York/ London: Kluwer Academic Publishers; 2003. pp. 99–116. [Google Scholar]
- Hartikainen KM, Ogawa KH, Knight RT. Transient interference of right hemispheric function due to automatic emotional processing. Neuropsychologia. 2000;38(12):1576–1580. doi: 10.1016/s0028-3932(00)00072-5. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Ogawa KH, Soltani M, Knight RT. Emotionally arousing stimuli compete for attention with left hemispace. Neuroreport. 2007;18(18):1929–1933. doi: 10.1097/WNR.0b013e3282f1ca18. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Rorarius M, Perakyla JJ, Laippala PJ, Jantti V. Cortical reactivity during isoflurane burst-suppression anesthesia. Anesth Analg. 1995;81(6):1223–1228. doi: 10.1097/00000539-199512000-00018. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Knight RT. The role of lateral orbitofrontal cortex in inhibitory control of emotion. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford University Press; 2006. pp. 307–324. [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59(1):9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. J Clin Neurophysiol. 1998;15(1):3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol Psychol. 1997;45(1–3):19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and affective Raitings. Center for Research in Psychophysiology, U. o; Florida, Gainesfille, FL: 1999. [Google Scholar]
- Lovstad M, Funderud I, Lindgren M, Endestad T, Due-Tonnessen P, Meling T, et al. Contribution of Subregions of Human Frontal Cortex to Novelty Processing. J Cogn Neurosci. 2011 doi: 10.1162/jocn_a_00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9(4):456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 clinical utility and control of variability. J Clin Neurophysiol. 1998;15(1):14–33. doi: 10.1097/00004691-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex. 2000;10(3):252–262. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cogn Affect Behav Neurosci. 2002;2(3):264–270. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16(2):114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. The role of the prefrontal cortex in dynamic filtering. Psychobiology. 2000;(28):207–218. [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14(3–4):199–224. [PubMed] [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49(3):2871–2878. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]